Abstract
The aims of this study were to investigate the role of sulphate-reducing bacteria in facilitating Pt removal from aqueous solutions and to investigate the role of a hydrogenase enzyme in Pt reduction in vitro. To avoid precipitation of Pt as Pt sulphide, a resting (non-growing) mixed culture was used. A pH-dependent rate of Pt removal from aqueous solution was observed, indicating that metal speciation was the main factor for its removal from solution. The maximum initial concentration of Pt(IV) that the cells can effectively remove from solution was 50 mg/l, while the maximum capacity was only 4 mg of Pt per gram of resting biomass. Transmission electron microscopy and energy dispersive X-ray analyses indicated that Pt was being precipitated in the periplasm, a major area of hydrogenase activity in the cells. In vitro investigation of Pt reduction with hydrogen as the electron donor showed that 49% was removed within 1 h when a relatively pure hydrogenase extract was used, 31% was removed with a cell-free soluble extract and 70% removed by live cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulphate-reducing bacteria (SRB) are economically, environmentally and biotechnologically important organisms. Their unique ability to couple the oxidation of H2 or simple organic acids such as lactic acids to metal reduction in lieu of sulphate as a final electron acceptor has been reportedly extensively [e.g. Rh(III); Ngwenya and Whiteley 2006; Fe(III), U(VI); Lovely et al. 1993); Cr(VI); Lovely and Philips 1994; Tc(VII), Pd(II); Lloyd et al. 1998a, b) and Mo(VI); Tucker et al. 1998]. SRB reduction of these metals can be used to develop and design treatment systems employing SRB for bioremediation.
Several SRB-based technologies have been successfully developed to treat heavy metal contaminated effluents. These include the Paques THIOPAQ system (Barnes et al. 1991), the Budel–Dorplein in the Netherlands (Barnes et al. 1991) and the Biomet Mining Corporation biosulphide process (Rowley et al. 1997) which precipitate metals as their metal sulphides. In addition, SRB have additional important roles in facilitating metal reduction by binding metal ions directly by means of extracellular polymers within their cell walls (Baldwin and Jalali 2000; Gadd et al. 1999; Gadd and White 2000). Membrane uptake of metal ions and enzymatic processes for the reduction of metals has furthermore been reported in SRB (Macaskie et al. 1994, 2001).
During this present study, a mixed SRB culture was used, as it offers advantages over a pure culture in environmental biotechnology in that it is less liable to contamination from other organisms. In addition, because it comprises a number of populations with varying optima for culture variables, e.g. nutrient concentrations, temperature, redox potential and pH (Gadd and White 1996; Song et al. 1998), a mixed culture is able to adapt to minor changes in conditions.
A review of the role of enzymes in bioremediation technology has recently appeared (Whiteley and Lee 2006). The present study was aimed at the enzymatic reduction of Pt(IV) to its elemental form Pt(0) as an effective method for the recovery of Pt from aqueous solutions. Two analytical techniques were used to aid the understanding of Pt(IV) reduction by resting cells. First, an X-ray microanalysis technique with the help of X-ray fluorescence was used for the identification of Pt within previously exposed cells to a Pt solution. Second, transmission electron microscopy (TEM) was used to establish the distribution and localisation of precipitates within cells.
Materials and methods
Analytical-grade reagents were used except where stated otherwise. Gases were purchased from Afrox (South Africa); membrane disc filters were purchased from Osmonics (South Africa); potassium hexachloroplatinate, [K2PtCl6] was purchased from Fluka; Pt atomic absorption spectroscopy standard solution was purchased from Wirsam Scientific. All glassware used for all experiments performed were pre-washed in 15% nitric acid to eliminate the interference from other metal ions and rinsed with deionized water. For pH adjustment, 1 M HCl and 1 M NaOH solutions were used as required. Culture shaking was performed on a Labcon orbital shaker. Centrifugation was in a Beckman J2-21 centrifuge with a JA-20 rotor or an Eppendorf 5810R centrifuge. Absorbances were measured using a Shimadzu UV visible 160A recording spectrophotometer or PowerWave X (Bio-Tek, Instrumental, South Africa). Morpholinepropane sulphonic acid (MOPS), sodium cholate and fluorescein diacetate were obtained from Sigma-Aldrich (South Africa). Fluorescence determination was by a Perkin-Elmer fluorescence spectrophotometer 203.
Reactor design and operation
An experimental sulphidogenic bioreactor (10,000 ml) containing modified postgate medium B (Postgate 1984) was set up as described (Ngwenya and Whiteley 2006) and was seeded with a 750-ml inoculum of a mixed consortium of sulphate-reducing bacteria obtained from the Environmental Biotechnological Research Unit BioSure process in Grahamstown, South Africa. The bioreactor was covered with aluminium foil to minimise exposure to light and was kept anaerobic by purging with N2/CO2 (80:20%) for 15 min followed by H2 (99%) for 5 min at room temperature. A 750-ml inoculum was taken from this stock bioreactor and used as a starter for a smaller 5,000-ml bioreactor which was used for all experiments carried out during the research. This was connected to a reservoir containing a solution of sulphate (2,000 mg/l) as the final electron acceptor. The carbon source for the reactor was fresh raw primary sewage sludge obtained from the primary clarifiers of the Grahamstown sewage plant. In order that the sulphate was not limiting within the reactor, the influent sulphate and chemical oxygen demand (COD) concentrations were both 1,000 mg/l, resulting in a COD/SO4 ratio of 1:1. The mean pH of the reactor was 6.31, and it was operated at ambient temperatures (20–22°C). A gas trap containing a solution of zinc acetate was installed to dissolve any released hydrogen sulphide.
Analytical procedures
All analyses were carried out in duplicate and values reported as the means with standard deviations. Samples used for sulphate, sulphide and CODSoluble were filtered through 0.45-μm cellulose acetate plus. CODTotal, CODSoluble and CODParticulate were determined using the Merck Spectroquant reagent kit as per manufacturer’s instructions. Before COD determination, all samples were acidified with concentrated HCl to pH 2 to remove any dissolved sulphide. Sulphide was determined with a Merck Spectroquant reagent kit, and absorbance read at 665 nm. Pt stock solution (1,000 mg/l) was prepared by dissolving 1 g potassium hexachloroplatinate in 1,000 ml milli-Q water, and a standard calibration curve for Pt at 266 nm was prepared by making appropriate dilutions of the stock solution. The concentrations of Pt ions in the solution were analysed on a GBC 909 atomic absorption spectrophotometer (GBC Scientific Equipment, Dandenong, Australia), with air-acetylene flame using Pt 10 mA hollow cathode lamp at 266 nm.
Pt reduction by cells
During mid-stationary phase, cells were harvested by centrifugation (5,000 × g, 10 min, 25°C), washed in H2O then resuspended anaerobically in MOPS–NaOH buffer (20 mM, pH 7.0, 100 ml) pre-equilibrated under oxygen-free nitrogen at a biomass density of 5 g/l (wet weight) in a flask sealed with butyl rubber stopper.
Initial Pt concentration
Cells [5 g/l (wet weight)] in MOPS–NaOH buffer (20 mM, pH 7.0, 1.0 ml) were challenged with Pt(IV)Cl4 (1.0 ml) at different initial concentrations of 10, 25, 50, 75 and 100 mg/l with hydrogen as the electron donor. Duplicate samples (300 μl) were taken at timed intervals and analysed for Pt.
Heat-killed cells
Cells [5 g/l (wet weight)] in MOPS–NaOH buffer (20 mM, pH 7.0, 1.0 ml) were heated at 80°C in a water bath for 30 min followed by purging with oxygen-free nitrogen (5 min) before Pt(IV)Cl4 (50 mg/l, 1.0 ml) was introduced in the presence of hydrogen as the electron donor. Duplicate samples (300 μl) were withdrawn at timed intervals and analysed for Pt.
Cell-free soluble extract
Cells [5 g/l (wet weight)] in MOPS–NaOH buffer (20 mM, pH 7.0, 25 ml) were lysed by sonication (30-s cycle, 4 min, 4°C) followed by centrifugation (15,000 × g, 20 min, 4°C). The supernatant was set aside, and the cell debris was resuspended in sodium cholate (3%) in MOPS–NaOH buffer (20 mM, pH 7.0, 25 ml) to release any membrane-bound enzyme. After stirring at 4°C overnight, the suspension was centrifuged (15,000 × g, 20 min, 4°C), dialysed against H2O and the supernatants combined.
This soluble cell-free extract containing hydrogenase activity (2.0 ml; specific activity = 30 U/mg; Rashamuse 2003) was added to a flask containing Pt(IV)Cl4 (50 mg/l, 2.0 ml) with hydrogen, as the electron donor, used throughout the incubation period (1 h). Duplicate samples (100 μl) were removed at timed intervals, and residual Pt concentration was monitored by atomic absorption.
Purified hydrogenase
Purified hydrogenase (2.5 ml; specific activity = 109.5 U/mg; Rashamuse 2003) was added to a flask containing Pt(IV)Cl4 (50 mg/l, 2.0 ml) with hydrogen, as the electron donor, used throughout the incubation period (1 h). Duplicate samples (100 μl) were removed at timed intervals, and residual Pt concentration was monitored by atomic absorption.
pH
The effect of pH on the reduction of Pt from solution by a resting consortium was studied between pH 2 and 7 by adjusting the pH of a Pt control solution (16 mg/l; pH 4) with either HCl or NaOH. The solutions were then filtered through a Millipore filtering system (25 mm diameter, 0.45 μm cellulose-acetate plus disc filter) and analysed for Pt. Washed cells (5 g/l) in MOPS–NaOH buffer (20 mM, pH 7.0, 1.0 ml) were then added into each of the different Pt solutions using hydrogen as the electron donor. The removal of unprecipitated Pt was monitored at timed intervals using an atomic absorption spectrophotometer.
Electron donors
To determine the optimal electron donors for Pt reduction, washed cells (5 g/l) in MOPS–NaOH buffer (20 mM, pH 7.0, 200 ml) were treated separately with different electron donors (lactate, ethanol and hydrogen) at a final concentration of 10 mM. A flask with no electron donor was used as a reference control. To each flask was added Pt(IV)Cl4 (50 mg/l), and duplicate samples (3 ml) were withdrawn at regular intervals and analysed for Pt.
Equilibrium sorption isotherm
Washed cells (5 g/l) in MOPS–NaOH buffer (20 mM, pH 7.0, 100 ml) were challenged with Pt(IV)Cl4 solutions in concentrations ranging from 10–70 mg/l and incubated at room temperature (3 h), with hydrogen as the electron donor. Duplicate samples (3 ml) were removed and analysed for Pt. The maximum amount of Pt removal (q max) from solution by biomass was calculated according to the Langmuir model (Ngwenya and Whiteley 2006).
Analysis of Pt-loaded cells
Transmission electron microscopy
Samples (100 μl) of cells challenged with Pt(IV)Cl4 (50 mg/l) in Eppendorf tubes were harvested (13,000 × g, 10 min, 25°C) and the supernatant discarded. Bacterial pellets were then prepared for TEM as previously described (Ngwenya and Whiteley 2006).
Energy dispersive X-ray microanalysis
The concentration ratios of the metals in the cells were obtained using energy dispersive X-ray (EDX) microanalysis in a 120 kV Philips EM420 coupled to an EDAX-DX-4 energy dispersive X-ray system. Quantification was achieved using the thin foil model, which includes the absorption correction and with a theoretical k factor calculated according to Zaluzec (Williams and Carter 1996). Only the metallic elements present in the spectrum were used in the quantification, and the atomic percentages obtained were then used to calculate the concentration ratios of the various metals present in the cells. Due to the higher acceleration voltage used for EDX microanalysis, 250 nm thick sections were cut and collected onto copper grids. The presence of intracellular elements was confirmed through examining the carbon-coated section using a Philips EM 420 TEM at an acceleration voltage of 120 kV. The sites of the metal ion deposition, localisation and evidence of ion exchange were confirmed from EDX spectra obtained from the EDAX-DX-4 energy dispersive X-ray system.
Influence of Pt(IV) on cell membrane integrity
Membrane permeability of the cells was determined by the fluorescence techniques based on Slavik (1982). Washed cells (5 g/l) in MOPS–NaOH buffer (20 mM, pH 7.0, 100 ml) were treated with fluorescein diacetate (2% in acetone w/v, 0.2 ml) under an atmosphere of oxygen-free nitrogen. Solutions of Pt(IV)Cl4 in concentrations of 10, 25 and 50 mg/l were injected through the rubber stopper into each respective tube. The suspensions were incubated (room temperature, 1 h) while being purged with hydrogen and then centrifuged (4,000 × g, 15 min, 25°C) and the fluorescence of the supernatant recorded at an emission wavelength of 520 nm after excitation at 435 nm.
Results
Pt reduction by cells
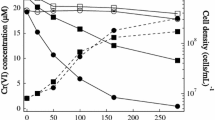
Reduction of different initial Pt concentrations by the cells was carried out to determine the concentration at which cells could remove the metal effectively. Where the initial Pt concentration was less than or equal to 50 mg/l, the Pt remaining in the solution had dropped to 5 mg/l or less (90% decrease) after 60 min (data not shown). In the flasks with initial Pt concentrations of 75 and 100 mg/l, the Pt in the solution reached a steady state after 40 min at 40 and 80 mg/l (47% and 20% decrease), respectively. As a result, 50 mg/l was used as the initial Pt concentration in all subsequent experiments. This concentration was considered to be the maximum sublethal concentration for which the cells can effectively remove Pt, and any concentration above this was likely to stimulate loss of bacterial viability.
Pt reduction by live cells in comparison with heat-killed cells, cell-free soluble extract and purified hydrogenase over time is shown (Fig. 1). Considerably more Pt was removed from solution in the flask with live cells compared to the heat-killed cells and cell-free extracts. After 10 min, 52% of the initial Pt concentration (50 mg/l) was removed in the flask with live cells, while an equilibrium reduction of 70% was reached after 60 min. In addition, heat-killed cells also showed low percentage reduction of Pt, reaching equilibrium at 22% reduction after 1 h. A reasonably moderate Pt(IV) reduction of 31% was observed by the soluble cell-free extract within 60 min, while with a pure hydrogenase extract (Rashamuse 2003), in the presence of hydrogen, a fairly significant Pt(IV) reduction of 49% was seen after the same incubation period (Fig. 1). These results strongly indicate that viable cells facilitates high percentage Pt reduction from solution.
Pt(IV) reduction by the cells was evaluated at a range of pH values (2.0, 3.0, 4.0, 5.0, 6.0 and 7.0), with the initial pH of the Pt solution acting as a control at pH 4. The pH studies showed no substantial pH sensitivity in Pt(IV) reduction by cells at below pH 4, with only 22% reduction taking place after 30 min and 38% after 2 h; at pH 4 the reduction was between 30 and 42% over the same time span (data not shown). On the other hand, a significantly high percentage Pt reduction of 60% after 30 min and 83% after 2 h occurred at pH 7.0 (data not shown).
Hydrogen, lactate and ethanol were chosen as the electron donors for Pt reduction because they have previously been shown as suitable electron donors for rhodium reduction (Ngwenya and Whiteley 2006). Equilibrium percentage Pt reductions for different electron donors, after 3 h incubation, were 75, 65 and 53% for H2, lactate, and ethanol, respectively (Fig. 2). The control flask with no electron donor gave results with the lowest Pt reduction of 23%.
The maximum Pt capacity (q max) was evaluated by plotting the equilibrium sorption isotherm (Langmuir model) into the obtained data (Fig. 3). The initial Pt concentration utilised in this experiment ranged from 10–70 mg/l at a pH value of 7.0. The q max of the mixed culture for Pt was determined to be 4 mg/g Pt (wet weight biomass).
To establish the site for Pt deposition, thin sections of cells that had been incubated in the absence (control) and in the presence of Pt were viewed using TEM (Fig. 4). The control did not show any precipitates within the periplasmic region or in the outer membrane surface (Fig. 4a). Whole cells incubated with Pt exhibited electron dense precipitates principally within the periplasmic region (Fig. 4b–d). A close examination of the thin section of a cell challenged with Pt for 12 h (Fig. 4c) revealed that some Pt precipitates were being formed on the outer margin of the membrane surface.
TEM of thin sections of cells before and after challenged with Pt(IV)Cl4 (50 mg/l) at different time intervals: a control (no platinum), b 6 h, c 12 h, d 18 h. Hydrogen was supplied as the electron donor. Electron dense precipitates (indicated by the arrows) at the periphery of the cells were analysed by energy dispersive X-ray diffraction to confirm the presence of the platinum (Bar = 50 nm)
To confirm that the electron dense precipitates observed in the periplasmic region of the cells (Fig. 4) contained Pt, sections were analysed by EDX (Fig. 5a). Some elements observed in the spectra originated from external sources, e.g. Cu was from the copper grid used to coat the cells, while Cl was provided by metal solution. The close analysis of the spectra (Fig. 5b–d) showed a varied response to Pt presumably as a result of different exposure time. While EDX microanalysis is a useful technique for analysis of biological samples, it does not provide the oxidation (valence) state of the precipitated reduction products. As a result, it is not known at this stage whether the Pt(IV) had been reduced to Pt(II) or Pt(0).
Energy dispersive X-ray diffraction analysis spectra of the thin sections of cells after different time intervals showing strong copper and platinum peaks of the black precipitate formed during Pt reduction. (a) Control (no Pt), (b) 6 h (c) 12 h and (d) 18 h. These spectra were analysed from sections in Fig. 4
The effect of Pt(IV) on plasma membrane integrity of the cells is shown in (Fig. 6) and, marked by an increase in the fluorescence intensity in solution, is indicative that exposure to Pt(IV) has resulted in an enhanced membrane permeability. The effect of Pt(IV) on cell membrane permeability was measured using a fluorescence technique (Slavik 1982) based on the hydrolytic conversion of non-fluorescent, non-polar fluorescein diacetate (which can pass through a cell membrane) into a highly fluorescent product called fluorescein. Under normal circumstances, fluorescein shows very low permeability across the cell plasma membrane. In the current experiments, the high fluorescence in the surrounding medium (Fig. 6), after incubation of the cells with different Pt(IV) concentrations, is an indication of the permeability of the membrane.
Discussion
Resting cell conditions with hydrogen as the electron donor were utilised for Pt reduction throughout the study to separate any Pt reduction from any growth-related processes such as precipitation of Pt as Pt sulphide (via hydrogen sulphide produced during sulphate reduction).
The rapid Pt reduction by live cells suggests that the Pt-binding ligands might be on the cell wall surface. This suggestion is further supported by the observation of relatively poor Pt reduction by heat-killed cells, which indicates non-active Pt reduction by the biomass. This type of mechanism, associated with non-viable biomass in which metal ions either bind directly to functional groups on the bacterial cell walls or to anionic sites associated with extracellular polymeric substances in the cell wall, has also been noted in the studies on biosorption of Zn(II) and Cu(II) by non-available biomass of D. desulfuricans (Tabak et al. 2000). In contrast, the diffusion and internalisation of Pt into the cell is also taking place, as Pt reduction, in the presence of live cells, was followed by a typical slow secondary metal reduction between 10 and 40 min before equilibrium was reached after 60 min. An enzyme-dependent and hydrogen-dependent Cr(VI) (Lovely and Philips 1994) and U(VI) (Lovely et al. 1993) reduction by Desulfovibrio vulgaris showed that the soluble cell extracts accounted for a recovery of 86 and 95%, respectively, for the two metals, suggesting a direct involvement of a hydrogenase enzyme in the metal reduction.
The present study proposes that pH-dependent Pt reduction by SRB could be due to both the various functional groups on the bacterial cell walls as well as the chemistry of Pt itself. The functional groups capable of metal sorption are usually basic (e.g. carboxyl, phosphate, amine groups etc.), which are deprotonated at high pH values. As the pH increases, more functional groups are dissociated and become available for ([PtCl6]2− or [PtCl4]2−) ion reduction due to less competition from protons (Tabak et al. 2000). In the presence of an alkaline solution, Pt has been reported to form a range of hydroxide complexes containing halide, such as [PtX n(OH)6-n]2− where X = Cl−, Br− or I−, thus, facilitating Pt precipitation (Greenwood and Earnshaw 1989). The increase in pH of the solution from about pH 4 results in increased Pt(IV) reduction, suggesting that the interaction of Pt(IV) with alkali solution in the absence of biomass is likely to be covalent.
High percentage Pt reduction by the cells was much greater when H2 was provided as the electron donor, compared to lactate and ethanol which is consistent with other findings (Ngwenya and Whiteley 2006; Lovely et al. 1993; Lloyd et al. 1998a, b; Tucker et al. 1998). The reasons for this are twofold: First, the relatively small diffusible H2 molecule can readily cross the outer membrane and enter the periplasm region where it can be oxidised by soluble or membrane-bound hydrogenases. In contrast, lactate dehydrogenase (and alcohol dehydrogenase) are typically located in the cytoplasmic membrane or cytoplasm, and transport of lactate (or alcohol) to these sites may be rate limiting. Also, electrons from these processes feed through reduced nicotinamide adenine dinucleotide before entering the electron transport chain, adding additional steps that can slow the overall process. A second explanation is that electrons liberated during oxidation of H2 enter a separate and perhaps less complex electron transport chain than when lactate or ethanol is the electron donor (Wildung et al. 2000). Nevertheless, a H2–Pt-dependent reduction in this present study provides the first line of evidence that a hydrogenase enzyme could be involved.
The value of q max is the measure of the binding capacity of the biomass to the metal of interest. The maximum removal capacity of Cd(II) and Cr(VI) by live cells of Bacillus laterosporus was found to be 84 and 34 mg/g, respectively (Zoubolis et al. 2004), while a mixed SRB culture was effective in removing a maximum of 66 mg rhodium per gram of biomass (Ngwenya and Whiteley 2006). Equilibrium sorption isotherm studies indicated that the reduction of Pt by cells was a result of both the depletion of adsorbable Pt species in solution and the saturation of cells with Pt (Fig. 3).
The Pt precipitates observed in this study were not surprising, based on the fact that bacteria are excellent nucleation sites for mineral formations due to their high surface-to-volume ratio and the electronegative surface functional groups (e.g. carboxyl, phosphoryl and hydroxyl groups). As Pt(IV) is being reduced to either Pt(II) or Pt(0), it is free to bind stoichiometrically to these sites, and once bound, the cell wall acts as a template for further heterogeneous nucleation precipitates. In addition, the presence of clearly visible Pt precipitates observed within the periplasmic region also suggests that a proportion of Pt was reduced as a Pt complex with organic ligands found on the cell wall.
This study suggests that Pt(IV) reduction by SRB involves both surface phenomena and diffusion, the latter most likely as a result of increased membrane permeability
References
Baldwin SA, Jalali K (2000) The role of sulphate reducing bacteria in copper removal from aqueous sulphate solutions. Water Res 34:797–806
Barnes LJ, Janssen FJ, Sherren J, Versteegh JH, Koch RO, Scheeren PJH (1991) A new process for the microbial recovery of sulphate and heavy metals from contaminated waters extracted by a geohydrological control system. Chem Eng Res Des 69:184–186
Gadd GM, White C (1996) A comparison of carbon/energy and complex nitrogen sources for bacterial sulphate-reduction: potential application to bioprecipitation of toxic metals as sulphides. J Ind Microbiol 17:116–123
Gadd GM, White C (2000) Copper accumulation by sulphate reducing bacterial biofilms. FEMS Microbiol Lett 183:313–318
Gadd GM, White C, Bridge AMT (1999) Extracellular metal binding activity of the sulphate reducing bacterium Desulfococcus multivorans. J Microbiol 145:2987–2995
Greenwood NN, Earnshaw A (1989) Chemistry of the elements. Permagon, Oxford, UK, pp 56–109
Lloyd JR, Nolting HF, Sole VA, Bosecker K, Macaskie LE (1998a) Technetium reduction and precipitation by sulphate reducing bacteria. Geomicrobiol J 15:43–56
Lloyd JR, Yong P, Macaskie LE (1998b) Enzymatic recovery of elemental palladium by using sulphate-reducing bacteria. Appl Environ Microbiol 64:4607–4609
Lovely DR, Philips EJP (1994) Reduction of chromate by Desulfovibrio vulgaris and its c3 cytochrome. Appl Environ Microbiol 60:726–728
Lovely DR, Roden EE, Philips EJP, Woorward JC (1993) Enzymatic iron and uranium reduction by sulphate reducing bacteria. Mar Geol 113:41–53
Macaskie LE, Jeong BC, Tolley MR (1994) Enzymatically accelerated biomineralization of heavy metals: application to the removal of americium and plutonium from aqueous flows. FEMS Microbiol Rev 14:351–368
Macaskie LE, Williams DR, Mabbett AN, Lloyd JR (2001) Metal reduction by sulphate reducing bacteria: physiological diversity and metal specificity. Hydrometallurgy 59:327–337
Ngwenya N, Whiteley CG (2006) Recovery of rhodum (III) from solutions and industrial wastewaters by a sulphate reducing consortium. Biotechnol Prog 22:1604–1611
Postgate JR (1984) The sulphate reducing bacteria, 2nd edn. Cambridge University Press, Cambridge, UK
Rashamuse KJ (2003) The bioaccumulation of Pt(IV) from aqueous solutions using sulphate reducing bacteria: role of a hydrogenase enzyme. MSc Thesis, Rhodes University, Grahamstown, South Africa
Rowley MV, Warkentin DD, Sicotte V (1997) Site demonstration of biosulfide process at the former Britannia Mine. In: Proceedings of the Fourth International Conference on Acid Rock Drainage. Vancouver, BC, Canada
Slavik J (1982) Intracellular pH of yeast cells measured with fluorescein probe. FEBS Lett 140:22–26
Song YC, Piak BC, Shin HS, La SJ (1998) Influence of electron donor and toxic materials on the activity of sulphate reducing bacteria for the treatment of electroplating wastewater. Water Sci Technol 38:187–194
Tabak HH, Harmon SM, Utgikar VP, Chen BY (2000) Studies on biosorption of zinc(II) and copper (II) on Desulfovibrio desulfuricans. Int Biodeterior Biodegrad 46:11–18
Tucker MD, Barton LL, Thompson BM (1998) Removal of U and Mo from water by immobilized Desulfovibrio desulfuricans in column reactors. Biotechnol Bioeng 60:90–96
Whiteley CG, Lee D-J (2006) Enzyme technology and bioremediation. Enzyme Microb Technol 38:291–316
Wildung RE, Gorby YA, Krupka KM, Hess NJ, Li SW, Plymalc JP, Mckinley JP (2000) Effect of electron donor and solution chemistry and products of dissimilatory reduction of technetium by Shewanella putrefaciens. Appl Environ Microbiol 66:2451–2460
Williams DB, Carter CB (1996) Transmission electron microscopy. Plenum, New York, USA, p 599
Zoubolis AI, Loukidou MX, Matis KA (2004) Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process Biochem 39:909–916
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rashamuse, K.J., Whiteley, C.G. Bioreduction of Pt (IV) from aqueous solution using sulphate-reducing bacteria. Appl Microbiol Biotechnol 75, 1429–1435 (2007). https://doi.org/10.1007/s00253-007-0963-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-0963-3