Abstract
The leaf extract of Ocimum sanctum was used as a reducing agent for the synthesis of platinum nanoparticles from an aqueous chloroplatinic acid (H2PtCl6·6H2O). A greater conversion of platinum ions to nanoparticles was achieved by employing a tulsi leaf broth with a reaction temperature of 100 °C. Energy-dispersive absorption X-ray spectroscopy confirmed the platinum particles as major constituent in the reduction process. It is evident from scanning electron microscopy that the reduced platinum particles were found as aggregates with irregular shape. Fourier-transform infrared spectroscopy revealed that the compounds such as ascorbic acid, gallic acid, terpenoids, certain proteins and amino acids act as reducing agents for platinum ions reduction. X-ray diffraction spectroscopy suggested the associated forms of platinum with other molecules and the average particle size of platinum nanoparticle was 23 nm, calculated using Scherer equation. The reduced platinum showed similar hydrogen evolution potential and catalytic activity like pure platinum using linear scan voltammetry. This environmentally friendly method of biological platinum nanoparticles production increases the rates of synthesis faster which can potentially be used in water electrolysis applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is gaining tremendous impetus in the present century due to its capability of modulating metals into their nanosize. Research in nanotechnology highlights the possibility of green chemistry pathways to produce technologically important nanomaterials [1, 2]. Nanoparticles can be synthesized using various approaches including chemical, physical and biological. Although chemical method of synthesis requires short period of time for the synthesis of large quantity of nanoparticles, this method requires capping agents for size stabilization of the nanoparticles. Chemicals used for nanoparticles synthesis and stabilization are toxic and lead to non-ecofriendly byproducts. The need for environmental non-toxic synthetic protocols for nanoparticles synthesis leads to the developing interest in biological approaches which are free from the use of toxic chemicals as byproducts. Thus, there is an increasing demand for “green nanotechnology” [3]. Platinum as catalysts enables power generation in fuel cell vehicles, electrocatalysis and chemical synthesis (also in silver–platinum nanoparticles) as a magnetic nanopowder and deposed on silica and carbon nanotubes.
Microbial synthesis of nanoparticles such as sulphate reducing bacteria was used to investigate the enzymatic mechanism for the total bioreduction of platinum (IV) into platinum (0) nanoparticles by cytoplasmic hydrogenase and periplasmic hydrogenase [4]. The bioreduction of H2PtCl6 and PtCl2 into platinum nanoparticles by a hydrogenase enzyme from Fusarium oxysporum was also reported. The triangular platinum nanoparticles appeared as a result of the electron deflection off the metallic surface. The fungus reduced the platinum salt into platinum nanoparticles [5]. The bioreduction of platinum nanoparticles occurred by the resting cells of the metal ion-reducing bacterium Shewanella algae. The platinum deposition process by ion reducers occurred in two steps (1) uptake of PtCl6 2− ions from the aqueous solution into the periplasmic space and (2) the enzymatic reduction of PtCl6 2− ions into elemental platinum with lactate as the electron donor [6].
There are many literatures on the chemical and microbial synthesis of silver, gold and platinum nanoparticles [3, 7–9]. Synthesis of gold nanoparticles using plant extract was the first report in 2002 [7]. In recent years, plant mediated biological synthesis of nanoparticles is gaining importance due to its simplicity and ecofriendliness. The use of plants or their extracts in the synthesis of gold, silver and platinum nanoparticles in a controlled manner for various purposes has been reported [9]. Plants provide a better platform for the nanoparticle synthesis as they are free from toxic chemicals as well as provide natural capping agents [3]. Moreover, use of plant extract also reduces the cost of microorganism isolation and culture media enhancing the cost competitive feasibility over nanoparticles synthesis by microorganisms.
Ocimum sanctum (tulsi) leaves are abundant in tannins like gallic acid, chlorogenic acid and also contain alkaloids, glycosides and saponins along with the volatile oil [10]. The major active constituent of tulsi leaves includes urosolic acid. Because of its medicinal virtues, tulsi is used in ayurvedic. Tulsi also acts as good reducing agents. Gallic acid was responsible for the reduction of silver ions into silver nanoparticles in an aqueous chemical method [11]. Ascorbic acid plays a role in the bioreduction of silver ions into silver nanoparticles [12]. The platinum nanoparticles were synthesized using Diopyros kaki leaf extract which acts as a reducing agent in the ecofriendly extracellular synthesis. This is not an enzyme-mediated process [8]. In present study, the platinum nanoparticles were synthesized from chloroplatinic acid (H2PtCl6) using O. sanctum leaf extract.
Materials and methods
Sample collection and extract preparation
Ocimum sanctum leaves were collected and washed three times with distilled water to remove the dust particles. Leaf broth solution was prepared by boiling a mixture of 5 g of thoroughly washed leaves in 100 mL of sterile distilled water for 5 min. After boiling the mixture, the extract was cooled, the solution was taken and stored at 4 °C. The solution was used within a week of having been prepared.
Biosynthesis of platinum nanoparticles using O. sanctum
The general method for reducing PtCl6 2− ions was to add 10 mL of leaf broth to 190 mL of 1 mM aqueous H2PtCl6·6H2O. The mixture was maintained at 100 °C in a sealed flask to avoid evaporation for 1 h in the hotplate since the temperature catalyses the rate of reduction process. For control experiments, the same amount of platinum solution and plant extract was maintained separately under the same reaction conditions. The reduced platinum solution was sonicated for 30 min to separate platinum nanomaterials from the biomolecules present in tulsi leaf extract. After sonication, solution was filtrated with 0.2 μm (syringe filter). The reduced platinum metals were purified by repeated centrifugation at 5,000 rpm for 30 min and the pellets were washed with distilled water to remove the impurities. Purified platinum nanoparticles were freeze-dried and their structure and composition was analyzed by Energy dispersive absorption X-ray spectroscopy (EDAX), Scanning electron microscope (SEM), Fourier-transform infrared spectroscopy (FTIR) analysis, X-ray diffraction (XRD) analysis and Linear scan voltammetry (LSV).
Characterization of platinum nanoparticles
Energy dispersive absorption X-ray spectroscopy and Scanning electron microscope
Platinum nanoparticle pellets were prepared and adhered to the brass base by means of a carbon tape. The natures of elements were identified by EDAX model: Naron system SIX (Thermo electron corporation). These coupons were examined at different magnifications (12, 15 and 30K) by the SEM (Model, Hitachi, S 3000 H).
X-ray diffraction analysis
The platinum nanoparticles were analyzed by X’pert PRO PAN analyzed X-ray diffractometer with Syn Master 793 software to identify the crystal phase of nanoparticles. The XRD pattern was recorded using computer controlled XRD-system, JEOL, and Model: JPX-8030 with CuKα radiation (Ni filtered = 13418 Å) at the range of 40 kV, 20 A. The PCPDF WIN software program was used to identify the peak table and ultimately for the identification of XRD peak. Average grain or particle size can be calculated using Scherer’s equation:
where, λ is the X-ray wavelength, typically 1.54 Å, K the shape factor, typically 0.9, β the line broadening at half the maximum intensity (FWHM) in radians, θ the Bragg angle, τ the grain size.
Fourier-transform infrared spectroscopy
Fourier-transform infrared spectroscopy was used for the analysis of the reduced platinum. The spectrum was taken in the mid-IR region of 400–4000 cm−1 with 16 scan speed. The spectrum was recorded using attenuated total reflectance (ATR) technique. The samples were mixed with pure KBr crystals in the ratio of 1:100 and the pellets were fixed in the sample holder for the analysis.
Linear scan voltammetry
The reduced platinum and control pure platinum (Alfa Aesar) was coated on glassy carbon electrode used as working electrode. Hg/Hg2SO4 and pure platinum wire as reference and counter electrode and 0.5 M sulphuric acid as electrolyte were used. Initial and final potential as 0 to −0.8 V with scan rate of 1 mV s−1. LSV was carried out in Versa STAT 3 instrument.
Result and discussion
Qualitative analysis for colour change
The high temperature is required for platinum reduction rate faster [8]. In present study, the temperature was maintained at 100 °C and the product about 100% was recovered within an hour. It is well known that 1 g of chloroplatinic acid contains about 40% (0.4 g) of platinum metal ions. The same amount was recovered by plant extract which can be claimed as 100% recovery.
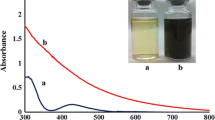
Qualitative analysis of the colour change of the platinum (IV) solution from light yellow to brown, indicative of the formation of platinum (II) and from brown to black, indicative of the formation of platinum (0) (Fig. 1) [5, 13, 14]. The same amount of platinum solution and plant leaf extract was maintained separately under the same reaction conditions for control experiments. The colour change was not observed. Yageshini et al. [5] reported the rapid bioreduction of PtCl2 with a 30% reduction after 2 h, 70% after 4 h, and over 90% after 8 h using plant extract. In present study, the bioreduction of chloroplatinic acid with reaction temperature of 100 °C using tulsi leaf extract to reduce the reaction time with greater efficiency is reported. The rapid conversion of silver and gold was noted within 11 and 3 min respectively at a temperature of 95 °C using Magnolia leaf broth. The rate of platinum nanoparticle synthesis increased with increases in reaction temperature. At a reaction temperature of either 25 or 60 °C, 20% of platinum ions were converted to platinum nanoparticles. Increasing the reaction temperature to 95 °C improved the level of conversion to almost 100% [15] which supports with the present observation. It is also reported that the synthesis of gold nanotriangles using lemongrass extract significantly at high temperatures [15]. The relatively low rate of platinum nanoparticle synthesis is possibly due to a difficulty in initially forming platinum nuclei, indicating that achieving close to 100% conversion to platinum nanoparticles requires longer reaction times and higher temperatures than those required for either gold or silver nanoparticles [8].
Energy dispersive absorption X-ray spectroscopy and Scanning electron microscope
It is evident from the EDAX spectrum (Fig. 2 ) that the reduced platinum with some trace elements were obtained using tulsi extract. Platinum content of about 71.56% was present (Table 1). The presence of trace elements such as carbon, oxygen, sodium and calcium are due to the components from tulsi leaf extract. The bioreduction of platinum nanoparticles using sulphate-reducing bacteria showed the high peaks of platinum with some trace amounts of chlorine and sulphur [4]. It is also noticed that the high peaks of platinum with copper, chlorine, lead, oxygen and uranium while using S. algae [7].
The SEM images (Fig. 3a, b, c) showed the aggregates of reduced platinum nanoparticles. This aggregates formation may be due to the high temperature and the components present in tulsi leaf extract. The nanoparticles produced were much larger with the majority of nanoparticles being rectangular and triangular in shape. The nanoparticles were aggregated, and thus it was very difficult to distinguish one shape from the other which supports with the observation made by Yageshni et al. [5]. It appeared as though there was some extrapolymeric substance that coated the nanoparticles kept them closely attached to each other, spherical nanoparticles were produced by the bioreduction of H2PtCl6 and these appeared to be monodispersed and varying in size. The results indicated that in addition to pH and temperature, the oxidation state of the platinum salt played an important role in the mechanism and formation of the nanoparticles though the size and shape of the particles was uncontrolled [5].
XRD analysis
Figure 4 shows the typical XRD diffraction peaks corresponding to reduced platinum. Intense peaks were observed at 40.0737°, 46.5736°, 67.8481° and 81.6412° corresponding to PtO2, K2(PtCl4), Pt and PtCl2. The results of XRD patterns were interpreted using PCPDF WIN software. The broadening of the Bragg peaks indicates the formation of nanoparticles. Full width at half maximum (FWHM) data were used with Scherer’s formula to determine the average particle size [16]. The average particle size estimated was approximately 23 nm. SEM observation shows the size of aggregated particles due to the temperature effect which are larger than 23 nm calculated using Scherer’s equation.
FTIR analysis
FTIR analysis was used to characterize the synthesized platinum nanoparticles and to identify the possible interaction of proteins with the nanoparticles (Fig. 5). FTIR spectrum for reduced platinum, intense bands were observed at 3398.18, 2925.40, 2357.98, 1619.17, 1398.82, 1312.80, 1110.08, 775.60 and 661.17 cm−1. With these peaks assigned as OH stretching in alcohols, CH3, CH2 and CH stretching of alkanes, P–H vibration of phosphine, C=C groups or aromatic rings, OH bending (in-plane) in alcohols, C=O stretching vibrations of carboxylic acid, C–C–C bending of aldehydes of ketones, N–H wagging in amines and C–H deformation stretching vibrations in alkynes, respectively.
Terpenoids are believed to be the surface-active molecules stabilizing the nanoparticles and reaction of the metal ions is possibly facilitated by reducing sugars and or terpenoids present in the neem leaf broth. The O. sanctum leaves contain ascorbic acid which may play a role in bioreduction of silver ions into silver nanoparticles [12]. The mechanism for the reduction of Ag ions to silver could be soluble antioxidative substances like ascorbate present in plants. Ascorbic acid is a reducing agent can reduce the species leading to the formation of ascorbate radical [17]. Gallic acid is used as a reducing and stabilizing agent, the oxidation reaction of phenol groups in gallic acid was responsible for the reduction of silver ions and the produced quinoid compound with a ketoenol-system could be absorbed on the surface of silver nanoparticles accounting for their stabilization [11]. Compared to all the other compounds, the major bioactive compounds are found as salanin, nimbin, azadirone and azadirachtins are responsible for the synthesis of gold nanoparticles [18]. Proteins and aminoacids have a tendency to reduce silver ions to silver nanoparticles [19]. Biomolecules as reducing agents are found to have a significant advantage over their counterparts as protecting agents [14]. The presence of carboxylic acid, amines, phosphine indicates the presence of ascorbic acid, gallic acid, terpenoids, certain proteins and amino acids present in tulsi leaf extract which acts as reducing agents for platinum ions reduction.
Linear scan voltammetry
In Fig. 6 the graph (a and b) shows the potential (V) for hydrogen evolution and catalytic activity of reduced platinum and pure platinum (control). The hydrogen generation potential cathodically for pure platinum and reduced platinum starts at −0.387 and −0.5283 V. The steep increase in hydrogen generation potential cathodically for pure platinum and reduced platinum starts at −0.668 and −0.639 V were found to be similar.
Conclusion
The synthesis of platinum by biological route is an alternative ecofriendly method. The compounds such as ascorbic acid, gallic acid, terpenoids, certain proteins and amino acids present in tulsi leaf extract act as reducing agents for platinum ions reduction. Although the average particle of the reduced platinum size was in the range of 23 nm with irregular shape which showed similar hydrogen generation potential like pure platinum. Further study is in progress in applying the reduced platinum for water electrolysis.
References
Govindaraju K, Tamilselvan S, Kiruthiga V, Singaravelu G (2010) J Biopest 3:394–399
Nanda A, Saravanan M (2009) Nanomedicine 5:452–456
Garima S, Riju B, Kunal K, Ashish RS, Rajendra PS (2010) J Nanopart Res 1–8. doi:10.1007/s11051-010-0193-y
Riddin T, Govender Y, Whiteley CG (2009) Enzym Microb Technol 45:267–273
Yageshni G, Tamsyn R, Mariekie G, Chris GW (2009) Biotechnol Lett 31:95–100
Yasuhiro K, Kaori O, Norizon S, Toshiyuki N, Shinsuke N, Hajime H et al (2007) J Biotechnol 128:648–653
Gardea TJL, Parsons JG, Gomez E, Peralta VJ, Troiani HE, Santiago P et al (2002) Nano Lett 2:397–401
Jae YS, Eun YK, Beom SK (2010) Bioprocess Biosyst Eng 33:159–164
Vineet K, Sudesh KY (2008) J Chem Technol Biotechnol 84:151–157
Shankar M, Bijay RM, Sushil CM (2009) Indian J Physiol Pharmacol 53:291–306
Wang M, Chen Q, Jiang C, Yang D, Liu X, Xu S (2007) Colloids Surf A Physicochem Eng Asp 30173–30179
Sondi I, Goia DV, Matijevic EJ (2008) Colloid Interface Sci 260:75–78
Liu Z, Ling XY, Su X, Lee JY (2004) J Phys Chem B 108:8234–8240
Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X et al (2007) Nanotechnology 18:105104–105114
Rai A, Chaudhary M, Ahmad A, Bhargava S, Sastry M (2007) Mater Res Bull 42:1212–1220
Klug HP, Alexander LE (1974) X-ray diffraction procedures for polycrystalline and amorphous materials. Wiley, New York
Naheed A, Seema S, Singh VN, Shamsi SF, Anjum F, Mehta BR (2010) Biotechnol Res Int 10:1–8
Thirumurugan A, Jiflin GJ, Rajagomathi G, Neethu Anns T, Ramachandran S, Jaiganesh R (2010) Int J Biological Technol 1:75–77
Sastry M, Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan M et al (2010) Colloids Surf B Biointerf 28:313–318
Acknowledgments
The authors would like to express their thanks to Instrumentation Division of CECRI for analyzing the samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soundarrajan, C., Sankari, A., Dhandapani, P. et al. Rapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applications. Bioprocess Biosyst Eng 35, 827–833 (2012). https://doi.org/10.1007/s00449-011-0666-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0666-0