Abstract
Background
Diabetes mellitus is a prevalent disease that endangers human health. Bariatric surgery can effectively relieve insulin resistance with elevated serum bile acids (BAs). 12α-Hydroxylated BAs were previously reported to be associated with insulin resistance. The aim of this study was to analyze changes in 12α-hydroxylated BA composition and possible associated mechanisms in diabetic rats following sleeve gastrectomy (SG).
Methods
SG and sham operations were performed in diabetic rats induced by high-fat diet feeding and streptozotocin. Body weight, food intake, oral glucose tolerance test (OGTT), insulin tolerance test (ITT), and serum BAs were analyzed at corresponding time points. Cholesterol 12α-hydroxylase (CYP8B1) and transcription factor V-Maf Avian Musculoaponeurotic Fibrosarcoma Oncogene Homolog G (MAFG) expression levels were assessed by RT-PCR and western blotting.
Results
Compared with the SHAM group, the SG group displayed significant weight loss from 6 weeks postoperatively, accompanied by decreased food intake from 4 weeks after the operation. At 2 and 12 weeks postoperatively, the areas under the curve of OGTT and ITT were significantly decreased in the SG group. At 12 weeks post-operation, the SG group displayed elevated serum BAs, but the percentage of 12α-hydroxylated BAs was reduced. Furthermore, SG rats exhibited higher MAFG and lower CYP8B1 protein and mRNA levels in the liver (P < 0.05).
Conclusion
The percentage of 12α-hydroxylated bile acids was reduced after SG, which was relevant with the inhibition of CYP8B1 and overexpression of MAFG. These outcomes may play an important role in the improvement of insulin sensitivity following SG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a prevalent disease that endangers human health, and identifying methods to control the disease in a long-term and effective manner is a worldwide problem [1]. Bariatric surgery can not only effectively reduce body weight but also relieve insulin resistance rapidly and permanently, a finding that has been confirmed in clinical studies [2]. Furthermore, bariatric surgery has been included in the guidelines for treatment of T2DM [3, 4]. Sleeve gastrectomy (SG), the most widely used bariatric surgery [5], can dramatically alleviate T2DM [6], but the specific mechanisms remain unclear.
It was confirmed that after multiple bariatric surgical procedures, including SG, serum bile acid (BA) levels were elevated [7,8,9]. BAs, one of the main components of bile juice, are generated from cholesterol under the catalysis of several enzymes such as cholesterol 7α-hydroxylase (CYP7A1) and cholesterol 12α-hydroxylase (CYP8B1). Additionally, BAs participate in the absorption of lipids and fat-soluble vitamins in the gut [10]. Moreover, BAs serve as signaling molecules to activate farnesoid X receptor and G-protein-coupled receptor, thereby inhibiting gluconeogenesis [11], increasing glucagon-like peptide 1 release, and improving body energy metabolism [12]. This indicates that elevated BA levels could improve insulin resistance. BAs include more than 20 components, and each component may have specific effects on body metabolism [13]. It has been demonstrated that 12α-hydroxylated BAs might cause insulin resistance [14]. However, the composition changes and relevant mechanisms of 12α-hydroxylated BAs after SG, which might play a significant role in the mechanism underlying SG-induced improvements in T2DM, remain unclear. The aim of our study was to analyze and explore 12α-hydroxylated BA composition changes and associated mechanisms by following SG in T2DM Wistar rats.
Materials and Methods
Animals
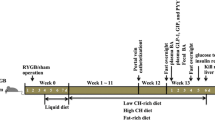
Eight-week-old male Wistar rats (weight ranged 200–220 g, provided by the Laboratory Animal Center of Shandong University [Jinan, China]) were housed in a 12-h light/dark cycle under constant temperature (24 ± 2 °C) and humidity (60 ± 10%) in independent ventilated cages. All rats were given access to clean water and a high-fat diet (HFD, 40% fat, Huafukang Biotech, China) for 1 month to induce insulin resistance and then were injected with streptozotocin (STZ, 35 mg/kg) (Sigma, USA) intraperitoneally. After 72 h, 20 rats with random blood glucose ≥16.7 mmol/l and that satisfied the inclusion criteria were randomly assigned to undergo SG (n = 10) or sham procedures (SHAM, n = 10). All study protocols were approved by the Animal Care and Utilization Committee of Shandong University.
Surgical Procedures
Before each procedure, rats were fed 10% Ensure (Abbott, USA) for 2 days and then fasted for 12 h. SG group [15, 16]: rats were anesthetized with intraperitoneal injection of 10% chloral hydrate (3 ml/kg) before procedure. We performed an upper abdominal incision of approximately 5 cm, and the gastric omentum and lesser omentum were then dissected. After ligation and transection of the gastric omental vessels in the pylorus area, we used forceps to clamp the greater curvature in case of hemorrhage. The portion of the stomach outside the clamped area, which was approximately 70% of the whole stomach volume and included the gastric fundus, was resected. The stomach incision was sutured with 5-0 silk suture (Ningbo Medical Needle, China). The abdomen was closed after leakage and hemorrhage was prevented. SHAM group [17]: a laparotomy was performed to expose the stomach and esophagus, and operative time was prolonged to mimic that experienced by the SG rats. Subsequently, the abdominal incision was closed.
Weight and Food Intake
The weight and food intake were measured once per week from the very beginning until the second week post-operation. These parameters were then measured once every 2 weeks until the rats were sacrificed.
Measurements on Remission of T2DM
Oral Glucose Tolerance Test (OGTT)
Glucose was administered by intragastric gavage (1 g/kg) after a 12-h fast. Blood samples were obtained from the tail vein of conscious rats, and blood glucose was measured before and at 10, 30, 60, 90, and 120 min after intragastric gavage. OGTT was performed preoperatively and at 2 and 12 weeks after surgeries.
Insulin Tolerance Test (ITT)
ITT was performed preoperatively and at 2 and 12 weeks after surgeries. After a 12-h fast, blood samples were collected from the caudal vein before and at 10, 30, 60, and 120 min after intraperitoneal injection of insulin (0.5 IU/kg).
Serum BAs
At 12 weeks postoperatively, blood samples (1 ml) were collected from the retrobulbar venous plexus into chilled EDTA tubes containing a dipeptidyl peptidase IV inhibitor and centrifuged (3000 rpm) at 4 °C for 15 min. Serum was collected and stored for further measurements. (1) Total serum BAs were determined by the enzymatic cycling method. (2) Components of BAs, including alpha-muricholic acid (αMCA), beta-muricholic acid (βMCA), cholic acid (CA), deoxycholic acid (DCA), chenodeoxycholic acid (CDCA), ursodeoxycholic acid (UDCA), lithocholic acid (LCA), taurine conjugates (taurine-,T-) of the aforementioned proteins, and hyodeoxycholic acid (HDCA), were measured by high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) [18], which was previously used by our team in another study. The reference standards, including TαMCA, TβMCA, αMCA, and βMCA, were purchased from Steraloids (USA), while all others were from Sigma Aldrich (USA).
Quantitative Real-Time PCR
Total RNA from rat liver was extracted using Trizol reagent (Invitrogen, USA) and reverse transcribed into cDNA in the presence of primers (TOYOBO, Japan). Quantitative real-time reverse transcription PCR was performed in a LightCycler system (Roche Diagnostics, Germany), and the primers used were as follows:
-
CYP8B1: 5′-TCCATATGTCCCGGCAGGTTCTTT-3′ (forward)
-
5′-TGTCAGGGTCCACCAGTTCAAAGT-3′ (reverse)
-
-
MAFG: 5′-CCCAATAAAGGAAACAAGGCC-3′ (forward)
-
5′-GCACCGACATGGTCACCA-3′ (reverse)
-
-
GAPDH: 5′-TCCCTCAAGATTGTCAGCAA-3′ (forward)
-
5′-AGATCCACAACGGATACATT-3′ (reverse)
-
Western Blotting
Liver protein was extracted with RIPA lysis buffer (Beyotime, China) and quantified using the BCA method. Protein from each group was equally loaded on 8% SDS-PAGE gels and separated by electrophoresis. Proteins were then transferred onto PVDF (Millipore, USA). After being blocked in 5% nonfat milk for 2 h, the membranes were incubated with primary antibodies to CYP8B1, V-Maf Avian Musculoaponeurotic Fibrosarcoma Oncogene Homolog G (MAFG), and β-actin (Abcam, USA) overnight at 4 °C condition. The membranes were then incubated with corresponding secondary antibodies in horseradish for 1 h. The protein bands were visualized with ECL solution (Beyotime, China) and imaged on an automated gel imaging analysis system. The gray levels were assessed with ImageJ software (National Institutes of Health, USA).
Statistical Analysis
Data are expressed as mean ± standard error and analyzed by SPSS version 22.0 software. The areas under the curves (AUC) of OGTT (AUCOGTT) and ITT (AUCITT) were calculated using the trapezoidal rule. Student’s t test was used for comparison between two groups, while Mann-Whitney U test was used for comparison of the BA components in two groups. P < 0.05 was considered statistically significant in all cases.
Results
SG and sham surgeries were successfully conducted in the respective groups, and all rats survived until the end of the study.
Body Weight and Food Intake
As displayed in Fig. 1, there was no statistical difference in body weight or food intake between the SG and SHAM groups at the beginning of the study (P > 0.05). These values reached the lowest levels at 1 week after the operation. From 6 weeks post-operation, SG group weights were significantly lower than those of the SHAM group (P < 0.05, Fig. 1a). Similarly, food intake was also lower in the SG group than that of the SHAM group from 4 weeks after the operation (P < 0.05, Fig. 1b).
Glucose Homeostasis
As shown in Fig. 2, there were no differences in preoperative AUCOGTT or AUCITT values between the SG and SHAM groups (P > 0.05). However, these values were significantly lower in the SG group than those in the SHAM group at 2 and 12 weeks after the operation (P < 0.05). These findings indicated that glucose metabolism and insulin resistance in the SG group were significantly improved during the early postoperative stage.
Serum BAs
As shown in Fig. 3, total serum BAs in the SG group were significantly higher than those in the SHAM group (P < 0.05) at week 12 after the operation.
In the SG group, the absolute value of βMCA, UDCA, CDCA, and all taurine-conjugated BAs increased (P < 0.05, Fig. 4a), while the percentage of CDCA, TαMCA, TβMCA, TCA, and TDCA elevated and CA decreased compared with the SHAM group in serum BAs (P < 0.05, Fig. 4b). No significant differences between the two groups were shown in other kinds. According to whether belonging to 12α-hydroxylated BAs, those BAs are divided into 12α-hydroxylated BAs (CA, DCA, TCA, and TDCA) and non-12α-hydroxylated BAs. Our study found that the concentrations of both categories were significantly elevated in the SG group (P < 0.05, Fig. 4c). However, the percentage of 12α-hydroxylated BAs in total BAs was reduced in the SG group compared with that of the SHAM group (P < 0.05, Fig. 4d).
The Expression of MAFG and CYP8B1 in the Liver
Since we observed decreased 12α-hydroxylated BA expression in the liver, we investigated CYP8B1, which can reflect 12α-hydroxylated BA synthesis, and MAFG, which plays a role in CYP8B1 regulation [19]. At week 12 after operation, both MAFG messenger RNA (mRNA) and protein levels were elevated in the SG group relative to those in the SHAM group (P < 0.05, Fig. 5a, c). In contrast, CYP8B1 mRNA and protein levels were lower in the SG group (P < 0.05, Fig. 5b, c).
Discussion
SG can effectively reduce body weight and induce T2DM remission, but the specific mechanisms have remained elusive. In this study, we observed that the reduction in blood glucose and improvements in insulin resistance occurred earlier than the reductions in body weight and food intake. These findings indicate that early remission effect of T2DM following SG is not dependent on the effects of weight loss, consistent with previous research [20, 21].
Serum BAs reflect whole-body BA levels. Thus, analysis of serum BAs allows us to research BA levels and function as signaling molecules in rodent models [22]. In this study, we observed that total serum BA levels were elevated following SG, consistent with our previous study [18] and Cummings’ research [9]. CYP8B1 expression following the SG procedure was downregulated, consistent with a report by Myronovych et al. [23]. These findings revealed that BA synthesis metabolism was inhibited after SG procedure. This suggests that elevated total serum BA levels were not due to increased BA synthesis but rather may be due to increased intestinal BA uptake. It has also been reported that accelerated gastric emptying after SG could lead to faster BA absorption in the distal ileum, which might elevate total serum BA levels [24].
CDCA and CA are generated from cholesterol under the action of CYP7A1 and several other enzymes in human hepatic microsome, while the generation of CA requires the assistance of CYP8B1. In humans, the primary BAs were glycine- or taurine-conjugated CA and CDCA [25]. In rodents, most CDCA is transformed into MCA [26]. Over 95% of MCA and CA combine with taurine and constitute the main component of BAs in rodents [27]. DCA is a secondary bile acid generated from CA under the action of intestinal microflora. CA, TCA, DCA, and TDCA belong to the family of 12α-hydroxylated BAs because of the participation of CYP8B1 in their generation process. Biddinger et al. [28] observed that serum 12α-hydroxylated BA levels were increased compared with those of the control group. Haeusler’s study [14] revealed that human insulin resistance was relevant with the elevated level of serum 12α-hydroxylated BAs. Kaur et al. [29] determined that lack of CA induced by CYP8B1 deficiency led to improved insulin resistance in Cyp8b1−/− mice. In summary, there was a significant correlation between 12α-hydroxylated BAs and insulin resistance. Based on analysis of BA composition, we found a reduced proportion of CA in BAs but elevated absolute levels. Additionally, we observed a decreased percentage of 12α-hydroxylated BAs following the SG procedure. In our previous study [18], we observed enhanced conjugation of BAs in the liver to reveal the elevated taurine-conjugated BAs following SG in a diabetic rat model. However, this could not explain the increased absolute value but decreased percentage of 12α-hydroxylated BAs after surgery. These results indicate that downregulated CYP8B1 expression after SG decreased the proportion of 12α-hydroxylated BAs, thus leading to a weakened role of 12α-hydroxylated BAs in total BAs. These outcomes might be crucial for amelioration of postoperative insulin resistance. However, Dutia et al. [30] observed that there was a higher proportion of 12α-hydroxylated BAs in total BAs 2 years after Roux-en-Y gastric bypass procedure, which was contrary to our study. However, there was lack of change in the ratio of 12α-hydroxylated/non-12α-hydroxylated BAs at 1 year after RYGB in patients with T2DM in a study by Sachdev and colleagues [31]. In addition, Zhang et al. [32] found the ratio unaltered after duodenal-jejunal bypass. The results are varied, and we speculate that this discrepancy may be due to the different selection of experimental subjects and surgical procedures, which remain unclear.
We observed that CYP8B1 expression was reduced following SG, but the mechanism remains unclear. De Aguiar Vallim et al. [19] reported that MAFG could inhibit BA metabolism by suppressing Cyp8b1 activity. MAFG is a member of the MAF transcription factor family and encoded by the MAFG gene. De Aguiar Vallim et al. confirmed that overexpression of liver MAFG inhibited expression of CYP8B1, decreased CA levels, and increased MCA levels in mice. Furthermore, knocking out the MAFG gene elevated the level of CYP8B1 and CA. Based on the conclusions above, we measured the expression of MAFG in rat liver after operation and found that the transcription and expression level of liver MAFG in the SG group were higher than those of the SHAM group, indicating that the expression of MAFG was elevated in diabetic rats after SG. There have been no studies investigating the correlation between the expression of MAFG and bariatric surgery to date.
In conclusion, our study demonstrated that the absolute value level of 12α-hydroxylated BAs was elevated but its percentage was reduced after SG, which might be relevant with the inhibition of CYP8B1 by overexpression of MAFG. These may play an important role in the improvement of insulin sensitivity after SG.
References
Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–21.
Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–84.
Standards of medical care in diabetes-2016: summary of revisions. Diabetes Care. 2016;39 Suppl 1:S4-5.
Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39(6):861–77.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery worldwide 2013. Obes Surg. 2015;25(10):1822–32.
Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252(2):319–24.
Kohli R, Bradley D, Setchell KD, et al. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98(4):E708–12.
Han H, Hu C, Wang L, et al. Duodenal-jejunal bypass surgery suppresses hepatic de novo lipogenesis and alleviates liver fat accumulation in a diabetic rat model. Obes Surg. 2014;24(12):2152–60.
Cummings BP, Bettaieb A, Graham JL, et al. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 2012;153(8):3620–32.
Li T, Chiang JY. Bile acids as metabolic regulators. Curr Opin Gastroenterol. 2015;31(2):159–65.
Fiorucci S, Mencarelli A, Palladino G, et al. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci. 2009;30(11):570–80.
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–9.
De Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17(5):657–69.
Haeusler RA, Astiarraga B, Camastra S, et al. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62(12):4184–91.
Sun D, Liu S, Zhang G, et al. Sub-sleeve gastrectomy achieves good diabetes control without weight loss in a non-obese diabetic rat model. Surg Endosc. 2014;28(3):1010–8.
Pereferrer FS, Gonzalez MH, Rovira AF, et al. Influence of sleeve gastrectomy on several experimental models of obesity: metabolic and hormonal implications. Obes Surg. 2008;18(1):97–108.
Bruinsma BG, Uygun K, Yarmush ML, et al. Surgical models of Roux-en-Y gastric bypass surgery and sleeve gastrectomy in rats and mice. Nat Protoc. 2015;10(3):495–507.
Wu Q, Zhang X, Zhong M, et al. Effects of bariatric surgery on serum bile acid composition and conjugation in a diabetic rat model. Obes Surg. 2016;26(10):2384–92.
De Aguiar Vallim TQ, Tarling EJ, Ahn H, et al. MAFG is a transcriptional repressor of bile acid synthesis and metabolism. Cell Metab. 2015;21(2):298–310.
Burkard I, von Eckardstein A, Rentsch KM. Differentiated quantification of human bile acids in serum by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2005;826(1–2):147–59.
Trung VN, Yamamoto H, Yamaguchi T, et al. Effect of sleeve gastrectomy on body weight, food intake, glucose tolerance, and metabolic hormone level in two different rat models: Goto-Kakizaki and diet-induced obese rat. J Surg Res. 2013;185(1):159–65.
Zwicker BL, Agellon LB. Transport and biological activities of bile acids. Int J Biochem Cell Biol. 2013;45(7):1389–98.
Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring). 2014;22(2):390–400.
Braghetto I, Davanzo C, Korn O, et al. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19(11):1515–21.
Nagana Gowda GA, Shanaiah N, Cooper A, et al. Bile acids conjugation in human bile is not random: new insights from (1)H-NMR spectroscopy at 800 MHz. Lipids. 2009;44(6):527–35.
Denk GU, Kleiss CP, Wimmer R, et al. Tauro-beta-muricholic acid restricts bile acid-induced hepatocellular apoptosis by preserving the mitochondrial membrane potential. Biochem Biophys Res Commun. 2012;424(4):758–64.
Shonsey EM, Wheeler J, Johnson M, et al. Synthesis of bile acid coenzyme a thioesters in the amino acid conjugation of bile acids. Methods Enzymol. 2005;400:360–73.
Biddinger SB, Haas JT, Yu BB, et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med. 2008;14(7):778–82.
Kaur A, Patankar JV, de Haan W, et al. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes. 2015;64(4):1168–79.
Dutia R, Embrey M, O'Brien S, et al. Temporal changes in bile acid levels and 12alpha-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes. Int J Obes. 2016;40(3):554.
Sachdev S, Wang Q, Billington C, et al. FGF 19 and bile acids increase following Roux-en-Y gastric bypass but not after medical management in patients with type 2 diabetes. Obes Surg. 2016;26(5):957–65.
Zhang X, Wang Y, Zhong M, et al. Duodenal-jejunal bypass preferentially elevates serum taurine-conjugated bile acids and alters gut microbiota in a diabetic rat model. Obes Surg. 2016;26(8):1890–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All study protocols were approved by the Animal Care and Utilization Committee of Shandong University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement of Human and Animal Rights
All applicable institutional and national guidelines for the care and use of animals were followed.
Funding
The National Natural Science Foundation of China (no. 81270888/H0713, no. 81370496/H0308) and the Fundamental Research Funds of Shandong University (no. 2014QLKY22).
Rights and permissions
About this article
Cite this article
Wang, M., Wu, Q., Xie, H. et al. Effects of Sleeve Gastrectomy on Serum 12α-Hydroxylated Bile Acids in a Diabetic Rat Model. OBES SURG 27, 2912–2918 (2017). https://doi.org/10.1007/s11695-017-2714-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2714-6