Abstract
Background
Bile acids (BAs) have emerged as important signaling molecules in regulating metabolism and are closely related to gut microbiota. Bariatric surgery elevates serum BAs and affects gut microbiota universally. However, the specific profiles of postsurgical BA components and gut microbiota are still controversial. The aim of this study is to investigate the serum profiles of BA components and gut microbiota after duodenal-jejunal bypass (DJB).
Method
DJB and SHAM procedures were performed in a high-fat-diet/streptozotocin-induced diabetic rat model. Body weight, energy intake, oral glucose tolerance test, insulin tolerance test, HOMA-IR, serum hormones, serum BAs, expression of BA transporters, and gut microbiota were analyzed at week 2 and week 12 postsurgery.
Results
Compared with SHAM, DJB achieved rapid and durable improvement in glucose tolerance and insulin sensitivity, with enhanced GLP-1 secretion. DJB also elevated serum BAs, especially the taurine-conjugated BAs, with upregulation of BA transporters in the terminal ileum. The phylum level of Firmicutes and Proteobacteria abundance was increased postsurgery, at the expense of Bacteroidetes.
Conclusion
DJB preferentially increases serum taurine-conjugated BAs, probably because of more BA reabsorption in the terminal ileum. The gut microbiota is altered with more Firmicutes and Proteobacteria and less Bacteroidetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery has created a new realm in the treatment of diabetes. Its efficacy has been confirmed as both prominent and durable [1, 2]. However, the unknown intrinsic mechanisms remain a potential threat. It is imperative to elucidate the underlying mechanisms, such that this new treatment could be widely accepted by both patients and doctors with more confidence.
Bile acids (BAs), formerly known as the “intestinal soap”, are now important signaling molecules during bariatric procedures [3]. BAs can activate membrane TGR5 receptor, enhancing glucagon-like peptide 1 (GLP-1) secretion and energy expenditure [4]. BAs are also natural ligands for nuclear receptor, FXR, via which hepatic glucose production and lipid de novo synthesis can be repressed [5, 6]. Besides, serum BA level is elevated following all bariatric surgeries except gastric banding, indicating that BAs, at least partly, mediate the benefits of bariatric surgery [7–10].
BAs represent a compound that contains over 20 individual species. Traditional method (enzyme cycling method) for serum BA measurement only detects total BAs (TBA) without further classification. Nevertheless, during bariatric surgery, BA component analysis may be of great significance, as different BA species have diverse effects on metabolism regulation [11]. For example, chenodeoxycholic acid (CDCA) is a strong agonist of FXR while MCA and UDCA are antagonists. Compared with unconjugated and glycine-conjugated BAs, taurine-conjugated BAs are more potent in activating TGR5 [11]. Additionally, BAs are closely related to gut microbiota. Secondary BA generation needs the 7α-deoxylation functions of certain gut bacteria, and BA gavage has the capacity of altering gut microbiota [12, 13]. One recent study even found that feces transplantation did help improve metabolic disorders [14]. Taken together, exploration into the postsurgical BAs and related gut microbiota changes after bariatric surgery may help us better understand how bariatric surgery works.
In this study, we performed duodenal-jejunal bypass (DJB) and SHAM procedures in a high-fat diet (HFD)/streptozotocin (STZ)-induced diabetic rat model, analyzing the BAs, gut microbiota, and enterohepatic circulation changes postsurgery and discussing potential mechanisms.
Methods
Animals
Eight-week-old male Wistar rats (200 g on average) were purchased from the Laboratory Animal Center of Shandong University (Jinan, China). The rats were individually housed with a 12 h light/dark cycle under constant temperature (24–26 °C) and humidity (50–70 %). All rats were given free access to tap water and fed with HFD (40 % fat, 42 % carbohydrate, and 18 % protein, as a total percentage of calories, Huafukang Biotech, China) for 1 month to induce fat accumulation and insulin resistance. After 12 h fasting, 35 mg/kg STZ (Sigma Aldrich, USA) dissolved in sodium citrate buffer (pH 4.2) was injected intraperitoneally. Seventy-two hours after injection, random blood glucose was measured with a glucometer (Roche Diagnostics, Germany) from tail veins. Twenty rats with random blood glucose ≥16.7 mmol/l were considered diabetic and were matched into SHAM-operated group (n = 10) and DJB group (n = 10). The Animal Care and Utilization Committee of Shandong University approved all the animal procedures.
Surgical Procedures
Before surgery, the rats were fed 10 % Ensure (Abbott, USA) for 2 days and then fasted for 12 h. Anesthesia was achieved with 10 % chloral hydrate (3 ml/kg).
-
DJB: The duodenum was transected 1 cm distal to the pylorus, and the stump was closed by 7–0 nylon suture (Ningbo medical, China). Then the jejunum was transected 10 cm distal to the ligament of Treitz, and the distal end was anastomosed to the proximal end of the duodenum (duodenojejunal anastomosis). At last, the biliopancreatic limb was anastomosed to the alimentary limb 15 cm distal to the duodenojejunal anastomosis in a Roux-en-Y fashion.
-
SHAM: The bowels were transected at the sites where enterotomies were performed in DJB, and re-anastomosis was made in situ. The operation time was prolonged similar to that of DJB to acquire similar surgical and anesthetic stress.
During the first 24 h postsurgery, the rats were only given access to water. Then the rats were fed 10 % Ensure for 3 days, followed by the same HFD chow until the end of the study. Body weight and calorie intake were recorded daily. Other parameters were measured at week 2 and 12 postsurgery. All rats were sacrificed after 12 h fasting at week 12. No antibiotics were used.
Oral Glucose Tolerance Test (OGTT)
After 12 h fasting, the rats were administrated with 20 % of glucose (1 g/kg) by intragastric gavage. Blood glucose was measured at T = 0, 10, 30, 60, 90, and 120 min.
Insulin Tolerance Test (ITT) and HOMA-IR
After 12 h fasting, the rats were injected intraperitoneally with human insulin (0.5 IU/kg). Blood glucose was measured at T = 0, 10, 30, 60, 90, and 120 min. Homeostatic model assessment-insulin resistance (HOMA-IR) was calculated to evaluate the insulin resistance according to the formula: HOMA-IR = fasting insulin (mIU/l) × fasting glucose (mmol/l)/22.5 [15].
Blood Sample Preparation
Blood was collected from the retrobulbar venous plexus at T = 0, 10, 30, 60, and 120 min after gavage with 10 % Ensure (1 ml/100 g weight). After centrifugation, the supernatant was stored at −80 °C until analysis. Serum insulin and total GLP-1 were measured using Rat/Mouse Insulin ELISA kits and multi-species GLP-1 total ELISA kits, respectively, (Millipore, USA).
Luminal, Fecal, and Serum Total Bile Acids
Four intestinal segments (0.5 cm each) were excised from the ileum, ascending colon, transverse colon, and descending colon immediately after sacrifice, without prior flushing. TBA from dried feces pellets (500 mg) or intestinal segments was extracted by 1.5 ml 50 % tert-Butyl alcohol for 1 h at 37 °C. After centrifugation, the supernatant was collected for TBA analysis. Serum, luminal, and fecal TBA were measured by Roche Cobas 8000 system using enzyme cycling method.
High-Pressure Liquid Chromatography Coupled With Tandem Mass Spectrometry (HPLC–MS/MS) Detection of Serum Bile Acids
Reverse-phase solid phase extraction (SPE) cartridges (Chromabond C18 cartridges, Germany) were pre-conditioned with 1-ml methanol and 1 ml 0.05 % formic acid. The serum samples (mixed with 1 ml 0.05 % formic acid) were loaded onto the cartridges and rinsed successively with 1 ml 0.05 % formic acid and 1 ml methanol water solution (45:55, V/V). The BAs were then eluted with 1-ml methanol, collected and dried under a nitrogen stream at 60 °C. The residue was dissolved in 200 μl mobile phase (ammonium acetate:methanol = 20:80, V/V), of which 5 μl were injected into the HPLC–MS/MS after mechanical stirring.
The chromatographic separation of BAs was performed on a Zorbax Eclipse XDB-C18 4.6 × 250 mm column (5-μm particle size) fitted on an Agilent 6410 Triple Quadrupole LC/MS system (Agilent technologies, USA). The column was thermostated at 30 °C. The mobile phase consisted of solvent A (ammonium acetate 5 mmol/l) and solvent B (methanol), using a gradient elution (0–20 min, 70–100 % B) with 8 min of balance back to 70 % B. The injection volume was 5 μl with 0.5 ml/min flow rate. The mass spectrometry conditions were as follows: ionization mode, ESI negative; nitrogen drying gas temperature, 350 °C; drying gas flow, 12 l/min; nebulizer, 35 psi; and capillary voltage, 4000 V. Data were acquired by the workstation software (version B.01.04, Agilent) in the multiple reaction monitoring (MRM) mode. Reference standards of αMCA, βMCA, TαMCA, and TβMCA were purchased from Steraloids (USA) and the rest were from Sigma Aldrich (USA).
16S rDNA-Based Study of gut Microbiota
Genomic DNA was extracted from feces pellets using standard methods [16]. Amplicons of the 16S rRNA gene V4 region were sequenced on Illumina MiSeq platform (BGI technology, China).
Real-Time PCR
The expression of asbt, ilbp, and ostα in the small intestine and ntcp and bsep in the liver was analyzed by real-time PCR. RNA was isolated using Trizol reagent (Invitrogen, USA) and converted to complementary DNA (cDNA) using a ReverTra Ace qPCR RT Kit (TOYOBO, Japan). Amplification of messenger RNA (mRNA) was carried out using SYBR Green Real-time PCR Master Mix Kit (TOYOBO, Japan) at a range of temperatures in a Roche Lightcycle 2.0 system. The primers were shown in Supplemental Table 1.
Statistics
Data are mean ± SEM. Area under the curve (AUC) for OGTT and ITT was calculated by trapezoidal integration. Individual BA comparisons were performed by Mann–Whitney U test with Benjamini-Hochberg correction (FDR at 5 %). Microbiota was analyzed using Metastats software (http://metastats.cbcb.umd.edu) with FDR at 5 and 25 %. The rest was evaluated by unpaired Student’s t test. P < 0.05 represented statistically different. All calculations were performed using SPSS version 19.0.
Results
There were 10 rats in each group and all survived until the end of the study.
Body Weight and Energy Intake
No difference was observed in body weight or energy intake before surgery. Due to perioperative caloric restriction and surgical stress, body weight and energy intake in both groups decreased sharply during week 1 postsurgery and gradually increased thereafter (Fig. 1a, b). Energy intake was significantly higher in SHAM group than DJB group at week 11 and 12, probably as a result of the diabetic state (Fig. 1b, P < 0.05 each). No difference in body weight was observed between the two groups after surgery.
Evaluation of DJB
There was no difference in AUC OGTT and AUC ITT presurgery between the two groups. Consistent with our previous report [17], at week 2 and 12, DJB group showed much better glucose tolerance than SHAM group (Fig. 1e), with improvement of insulin resistance, based on lower AUC ITT and HOMA-IR (Fig. 2a, b). GLP-1 secretion was also enhanced after DJB compared with SHAM (Fig. 2e, f). No significant change in insulin secretion was detected.
Luminal, Fecal, and Serum TBA
In both groups, luminal TBA levels were much lower in the ileum and the descending colon than the ascending and transverse colons. Notably, in all intestinal segments, DJB group showed higher TBA levels than SHAM (Fig. 3a, P < 0.05 each). Though at week 12, fecal TBA levels in DJB group appeared higher than SHAM, the difference was not significant (Fig. 3b, P = 0.124). Serum TBA levels were higher with DJB than SHAM at both week 2 and 12 (Fig. 3c, P < 0.01 each).
Luminal TBA (a), fecal TBA (b), serum TBA (c), mRNA relative expression of bile acid transporters in the liver (ntcp and bsep) and the terminal ileum (asbt, ilbp, and ostα) (d), Pearson correlation analysis of mean colonic TBA and feces Bacteroidetes abundance (e), Pearson correlation analysis of mean colonic TBA and feces Proteobacteria abundance (f). SHAM vs. DJB, *P < 0.05, **P < 0.01, ***P < 0.001
BA Transporters
The BA transporters, asbt, ilbp, and ostα, showed increased expression in DJB group (Fig. 3d, P < 0.05 each), which might indicate more active BA reabsorption in the ileum after DJB. Bsep displayed a decreasing expression tendency (P = 0.051). The expression of ntcp was not altered.
Serum BA Composition
Absolute Value
In both groups, clearly, CA was the most abundant BA species. At week 2, TαMCA, TCDCA, TUDCA, and TLCA were only in trace amount, but significantly higher with DJB than SHAM (Fig. 4a, P < 0.05 each). At week 12, all the taurine-conjugated species, plus αMCA, βMCA, CDCA, UDCA, and LCA, were higher in DJB group than SHAM (Fig. 4b, P < 0.05 each), with TUDCA increased the most (36-fold), followed by TLCA and TαMCA (19-fold both).
Percentage
As shown in Fig. 4 clearly, CA accounted for over 50 % of TBA in SHAM group while this percentage was less than 40 % after DJB at week 2 and 12 (P < 0.05, DJB vs. SHAM at week 12). Interestingly, though the absolute value of some BA species elevated at week 2, the relative percentages were unchanged at all (Fig. 4c, P > 0.05 for all). In contrast, at week 12, the percentages of taurine-conjugated BAs (except TDCA and TCDCA) were significantly higher in DJB group than SHAM (Fig. 4d, P < 0.05 each), with TLCA, TUDCA, and TCA increased the most (6-fold all).
Subgroup Analysis
The 12α-hydroxylated BAs and non-12α-hydroxylated BAs were only increased after DJB at week 12 (Fig. 5a, P < 0.05 each), without any difference between the two groups in the ratio at either week 2 or 12 (Fig. 5b).
12α-hydroxylated BAs (CA, DCA, TCA, and TDCA) and non-12α-hydroxylated BAs at week 2 and 12 (a), ratio of 12α-hydroxylated and non-12α-hydroxylated BAs (b), conjugated BAs and unconjugated BAs at week 2 and 12 (c), ratio of conjugated and unconjugated BAs (d), FXR agonists (CA, TCA, DCA, TDCA, CDCA, TCDCA, LCA, and TLCA) and FXR antagonists (αMCA, TαMCA, βMCA, TβMCA, UDCA, and TUDCA) at week 2 and 12 (e), ratio of FXR agonists and antagonists (f), primary BAs and secondary BAs at week 2 and 12 (g), ratio of primary and secondary BAs (h). SHAM vs. DJB, *P < 0.05, **P < 0.01
Conjugated BAs were found elevated in DJB group than SHAM at week 12 (Fig. 5c, P < 0.01). No statistically significant change was observed in unconjugated BAs. The ratio of conjugated and unconjugated BAs was higher in DJB group at week 12 (Fig. 5d, P < 0.05).
Both FXR agonists and antagonists showed increase in DJB group at week 12 (Fig. 5e, P < 0.05). The ratio of FXR agonists and antagonists in DJB group was decreased compared with SHAM at week 2 (Fig. 5f, P < 0.05), but this difference was no longer significant at week 12.
Primary BAs were increased in DJB group at week 12 (Fig. 5g, P < 0.05). No alteration was detected in the ratio of primary and secondary BAs (Fig. 5h).
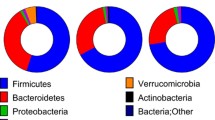
Feces Microbiota
As shown in the pie charts (Fig. 6b, d), the feces bacteria population in both groups was dominated by Bacteroidetes, Firmicutes, and Proteobacteria at the phylum level. In SHAM group, Bacteroidetes was most abundant at both week 2 and 12. However, after DJB, both Firmicutes and Proteobacteria expanded, at the expense of Bacteroidetes.
Relative feces microbiota composition of each rat in SHAM group (S1 to S10) and DJB group (D1 to D10) at week 2 at the phylum level (a), pie charts of microbiota composition at week 2 (b), relative feces microbiota composition of each rat at week 12 (c), pie charts of microbiota composition at week 12 (d)
At an FDR of 5 %, compared with SHAM, Bacteroidetes showed decreased abundance at both week 2 and 12 in DJB group (P < 0.05 both) with Firmicutes increased at only week 2 (P = 0.019). No alteration was observed in other phyla. At an FDR of 25 %, in addition to Bacteroidetes, Firmicutes was found increased at both week 2 and 12 (P = 0.019 and 0.186, respectively). The abundance of Proteobacteria was increased at week 2 in DJB group as well (P = 0.186).
In the correlation analyses, we found a negative correlation between mean TBA levels in the colon and Bacteroidetes abundance (Fig. 3e, r = −0.73, P < 0.001). Also, a positive correlation was found between the mean colonic TBA levels and Proteobacteria abundance (Fig. 3f, r = 0.62, P < 0.001).
Discussion
In this study, we performed DJB and SHAM procedures in a diabetic rat model and, for the first time, demonstrated that (i) DJB preferentially elevated serum taurine-conjugated BAs as well as TBA in the terminal ileum and the colon; (ii) within the enterohepatic circulation, the expression of ileal BA transporters, asbt, ilbp, and ostα, was upregulated, which might account for the elevated serum BAs; (iii) at the phylum level, Firmicutes and Proteobacteria were both increased at the expense of Bacteroidetes.
BAs are synthesized in the liver, secreted into the duodenum, and recycled in the terminal ileum. BAs that cannot be reabsorbed by hepatocytes are spilt into the systemic circulation. Thereby, serum BAs can be treated as a reflection of excessive BA loading burden in the BA enterohepatic circulation. In humans, BAs are mainly glycine-conjugated while in rodents taurine-conjugated BAs predominate [18, 19]. Following bariatric surgeries, serum BAs are universally elevated, even after SG, a procedure without any rearrangement of the small intestine [20]. The mechanisms are unclear. In RYGB, the BA production in the liver is repressed while in BPD it’s largely increased [9]. This discrepancy may suggest distinct explanations for different procedures. One hypothesis attributes elevated serum BAs to more BA reabsorption in the distal gut (mainly the terminal ileum). After bariatric surgery, the route of BA recycling is shortened, leading to faster interaction between the BAs and the terminal ileum and probably more reabsorption. One strong evidence comes from the IT procedure. The interpositioned terminal ileum retains the capacity of BA absorption, despite a certain level of “jejunization”, increasing serum BAs, and decreasing distal luminal BA levels [21]. For SG, despite the intact gut, the gastric emptying is accelerated, which also expedites the contact of BAs and the distal gut [22]. The procedure used in this study is similar to RYGB. In fact, DJB was initially designed to investigate the mechanisms of RYGB [23]. Due to its prominent effects, DJB has now been used as a clinical procedure [24]. In the present study, we found increased serum TBA at both week 2 and 12. And among the hepatic BA transporters, we detected a decreased expression of bsep (P = 0.051), without any change in ntcp. More importantly, we did detect increased expression of asbt, ilbp, and ostα, all of which mediate BA reabsorption in the terminal ileum. Consistent with RYGB, we also found decreased BA production in the liver (unpublished data). Taken together, we presume that the elevated serum BAs after DJB depends on increased reabsorption, rather than more production of BAs. Additionally, the luminal BA levels were higher in the terminal ileum and the colon after DJB than SHAM, without difference in fecal BA excretion. This finding indicates that the colon might also be potent in absorbing excessive luminal BAs, though this process might depend on passive absorption [25].
In the BA composition analysis, we found a marked elevation of serum taurine-conjugated BAs at week 12, based on both absolute value and relative percentage. The unconjugated BAs also showed increase in absolute value but the percentage did not change at all except CA. These changes in serum BA composition parallel those after RYGB, suggesting the possibly shared mechanisms between the two procedures in affecting BA metabolism [9]. As serum BAs roughly resemble the BA profiles in the terminal ileum [26], the elevated taurine-conjugated BAs indirectly reflect the upregulation of the ileal BA transporter asbt, which has a higher affinity for taurine-conjugated BAs over unconjugated species [25, 27]. Taurine-conjugated BAs have particular effects on regulating metabolism, especially in activating TGR5 receptor. TGR5 is expressed in many tissues and has a series of physiological benefits when activated, including increasing energy expenditure and GLP-1 secretion, improving glucose tolerance and insulin sensitivity, accelerating gastric emptying, and so forth [3]. BAs are the most potent activators of TGR5, with the taurine-conjugated forms further increasing the potency [11]. One of the major taurine-conjugated BAs, TCA, can even stimulate GLP-1 secretion and increase fullness via rectal administration, confirming the benefits of TCA in the large intestine [28]. This might also explain the enhanced GLP-1 secretion after DJB. The taurine-conjugated BAs, accordingly, might be a potential therapeutic target for the treatment of diabetes. In addition to taurine-conjugated BAs, taurine per se also plays a role in the regulation of metabolism [29]. Human studies have revealed that the body taurine content is lower in obese and diabetic states [30, 31]. And animal study has demonstrated that taurine supplementation prevents obesity and improves glucose tolerance [32]. The present study cannot provide evidence for taurine metabolism. Nevertheless, CA or UDCA gavage in rodents could result in increased serum TCA or TUDCA levels [12, 33], which suggests that the taurine conjugation process appears to be more active with additional BAs in the gut. Whether the taurine conjugation in the liver is altered after bariatric surgery warrants future studies.
We also measured the ratio of 12α-hydroxylated and non-12α-hydroxylated BAs, which was previously reported negatively correlated with insulin sensitivity [34, 35]. However, we found the ratio unaltered after DJB. Another study even found the ratio increased after RYGB [36]. Consequently, we presume the early improvement of insulin sensitivity after DJB may also depend on other mechanisms, such as jejunal nutrient sensing [37]. FXR is another natural receptor of BAs. In this study, we found the FXR agonists and antagonists were both increased after DJB at week 12, but the ratio was decreased at week 2 and tended to be lower at week 12 (P = 0.073), compared with SHAM. Although it has been proved that FXR is a molecular target for the effects of SG, we cannot draw any conclusions from the current results. Hence, future studies concentrating on luminal and hepatic BA composition analysis seem necessary, since most BA/FXR-mediated metabolic benefits are initiated in the liver or the small intestine [5, 38].
BAs are also closely related to gut microbiota. It has been confirmed that BAs are necessary growth factors for some bacteria [39], and BA transformation (e.g., secondary BA generation) also depends on gut bacteria [13]. In recent studies, gut microbiota were also found related to obesity and diabetes [40, 41]. Furthermore, bariatric surgery also altered the composition of gut microbiota as early as 1 week postsurgery and these changes stabilized after 5 weeks [14]. However, diverging results in gut microbiota alterations have been observed after bariatric surgery. Zhang et al. found that Firmicutes were dominant in both normal weight and overweight individuals and were reduced after RYGB [42]. Furet el al. reported increased Bacteroidetes after RYGB, at the expense of Firmicutes [43]. In a rodent study, nevertheless, both Bacteroidetes and Firmicutes were decreased after RYGB, with Proteobacteria more abundant [44]. In our study setting, we observed decreased Bacteroidetes but increased Firmicutes and Proteobacteria abundance. This result differs from the previous studies but parallels a recently reported BA gavage experiment, in which Firmicutes and Proteobacteria predominated with Bacteroidetes decreased after CA gavage [12]. Additionally, the TCA level was also elevated after CA gavage, indicating a more active taurine conjugation process, which was consistent with the present study. Therefore, unlike RYGB, DJB represents a novel gut microbiota profile. And this profile might reflect an adaption of gut microbiota to increased luminal BA levels, since we also discovered the correlationship between gut microbiota and the colonic BA levels. Interestingly, similar microbiota profiles were also observed in obese individuals and HFD-induced obese state [41, 45], making the microbiota profile after DJB more like a hazard instead of a benefit. One possible explanation is that the HFD per se leads to more BAs present in the gut that might affect microbiota migration [46].
This study has several limitations. First, the results from the present study were based on a diabetic rat model and, accordingly, might or might not be relevant to humans. However, as bariatric surgery is effective across species, and rats and humans share most bile acid species, as well as the predominant gut microbiota, we presume that findings in the present study could help to better understand how bariatric surgery affects bile acids and gut microbiota migration, and point to taurine-conjugated BAs as a potential therapeutic target. Second, the measurements of BA transporters were confined at the transcriptional level. Since the posttranscriptional regulation exists, BA transporters should be further analyzed at the protein level. In addition, the changes in serum glycine-conjugated BAs were not evaluated which need to be elucidated in the future.
Conclusion
DJB preferentially elevates serum taurine-conjugated BAs with increased expression of BA transporters in the terminal ileum. Luminal BAs are also elevated, with more abundance of Firmicutes and Proteobacteria at the expense of Bacteroidetes.
SG sleeve gastrectomy, IT ileum translocation, RYGB Roux-en-Y gastric bypass, BPD biliopancreatic bypass, BA bile acid, asbt apical sodium dependent bile acid transporter, ilbp ileal lipid binding protein, ostα organic solute transporter alpha, bsep bile salt export pump, ntcp sodium-taurocholate cotransporting polypeptide, αMCA alpha muricholic acid, βMCA beta muricholic acid, CA cholic acid, DCA deoxycholic acid, CDCA chenodeoxycholic acid, UDCA ursodeoxycholic acid, LCA lithocholic acid, HDCA hyodeoxycholic, the prefix T indicates conjugation with taurine.
References
Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370(21):2002–13.
Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77.
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–9.
Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113(10):1408–18.
Ma K, Saha PK, Chan L, et al. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116(4):1102–9.
Cummings BP, Bettaieb A, Graham JL, et al. Bile-acid-mediated decrease in endoplasmic reticulum stress: a potential contributor to the metabolic benefits of ileal interposition surgery in UCD-T2DM rats. Dis Model Mech. 2013;6(2):443–56.
Han H, Wang L, Du H, et al. Expedited biliopancreatic juice flow to the distal gut benefits the diabetes control after duodenal-jejunal bypass. Obes Surg. 2015;25(10):1802–9.
Ferrannini E, Camastra S, Astiarraga B, et al. Increased bile acid synthesis and deconjugation after biliopancreatic diversion. Diabetes. 2015;64(10):3377–85.
Kohli R, Bradley D, Setchell KD, et al. Weight loss induced by roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98(4):E708–12.
de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17(5):657–69.
Islam KB, Fukiya S, Hagio M, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141(5):1773–81.
Swann JR, Want EJ, Geier FM, et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4523–30.
Liou AP, Paziuk M, Luevano Jr JM, et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Salonen A, Nikkila J, Jalanka-Tuovinen J, et al. Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J Microbiol Methods. 2010;81(2):127–34.
Hu C, Zhang G, Sun D, et al. Duodenal-jejunal bypass improves glucose metabolism and adipokine expression independently of weight loss in a diabetic rat model. Obes Surg. 2013;23(9):1436–44.
Shonsey EM, Wheeler J, Johnson M, et al. Synthesis of bile acid coenzyme a thioesters in the amino acid conjugation of bile acids. Methods Enzymol. 2005;400:360–73.
Sjovall J. Dietary glycine and taurine on bile acid conjugation in man; bile acids and steroids 75. Proc Soc Exp Biol Med Soc Exp Biol Med. 1959;100(4):676–8.
Nakatani H, Kasama K, Oshiro T, et al. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metab Clin Exp. 2009;58(10):1400–7.
Kohli R, Kirby M, Setchell KD, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):G652–60.
Braghetto I, Davanzo C, Korn O, et al. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19(11):1515–21.
Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239(1):1–11.
Klein S, Fabbrini E, Patterson BW, et al. Moderate effect of duodenal-jejunal bypass surgery on glucose homeostasis in patients with type 2 diabetes. Obesity. 2012;20(6):1266–72.
Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res. 2007;24(10):1803–23.
Sayin SI, Wahlstrom A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17(2):225–35.
Craddock AL, Love MW, Daniel RW, et al. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol. 1998;274(1 Pt 1):G157–69.
Wu T, Bound MJ, Standfield SD, et al. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes, Obes Metab. 2013;15(5):474–7.
Murakami S. The role of taurine in the pathogenesis of obesity. Mol Nutr Food Res. 2015;19.
Rosa FT, Freitas EC, Deminice R, et al. Oxidative stress and inflammation in obesity after taurine supplementation: a double-blind, placebo-controlled study. Eur J Nutr. 2014;53(3):823–30.
Merheb M, Daher RT, Nasrallah M, et al. Taurine intestinal absorption and renal excretion test in diabetic patients: a pilot study. Diabetes Care. 2007;30(10):2652–4.
Tsuboyama-Kasaoka N, Shozawa C, Sano K, et al. Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology. 2006;147(7):3276–84.
Kohli R, Setchell KD, Kirby M, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154(7):2341–51.
Kaur A, Patankar JV, de Haan W, et al. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes. 2014;22.
Haeusler RA, Astiarraga B, Camastra S, et al. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62(12):4184–91.
Dutia R, Embrey M, O’Brien CS, Haeusler RA, Agenor KK, Homel P, et al. Temporal changes in bile acid levels and 12alpha-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes. Int J Obes. 2015;39(5):806–13.
Breen DM, Rasmussen BA, Kokorovic A, et al. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med. 2012;18(6):950–5.
Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21(2):159–65.
Van Eldere J, Celis P, De Pauw G, et al. Tauroconjugation of cholic acid stimulates 7 alpha-dehydroxylation by fecal bacteria. Appl Environ Microbiol. 1996;62(2):656–61.
Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103.
Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3.
Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–70.
Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–57.
Li JV, Ashrafian H, Bueter M, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60(9):1214–23.
Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84.
Reddy BS. Diet and excretion of bile acids. Cancer Res. 1981;41(9 Pt 2):3766–8.
Acknowledgments
The National Natural Science Foundation of China (no.81270888/H0713, no.81370496/H0308, no.81300286/H0308) supported this work, Natural Science Foundation of Shandong Province (grant no. ZR2009CM051), and the Taishan Scholar Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Statement of informed consent
Inform consent does not apply in this article.
Statement of human and animal rights
All applicable institutional and national guidelines for the care and use of animals were followed.
Funding
The National Natural Science Foundation of China (no.81270888/H0713, no.81370496/H0308, no.81300286/H0308) supported this work, Natural Science Foundation of Shandong Province (grant no. ZR2009CM051), and the Taishan Scholar Foundation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(XLSX 42 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Wang, Y., Zhong, M. et al. Duodenal-Jejunal Bypass Preferentially Elevates Serum Taurine-Conjugated Bile Acids and Alters Gut Microbiota in a Diabetic Rat Model. OBES SURG 26, 1890–1899 (2016). https://doi.org/10.1007/s11695-015-2031-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-2031-x