Abstract
Background
Ghrelin is an important factor in the regulation of intake. Most ghrelin is synthesized in the gastric fundus, but this is not the only location. The aim of this experimental study was to analyze the effect of sleeve gastrectomy (removing fundus) on the volume of intake in four experimental models and determine how this relates to changes in weight, plasmatic levels of glycemia, ghrelin, GLP-1, and insulin.
Methods
Sleeve gastrectomy was performed on four experimental models: (1) non-obesity; (2) exogenous obesity caused by excessive calorie intake; (3) genetically determined obesity (Zucker rats); and (4) genetically determined obesity and type 2 diabetes mellitus (Zucker diabetic fatty; ZDF rats). Model 2 had a control group on which sleeve gastrectomy was not performed.
Results
In the non-obese group, there were few changes after intervention, but in model 2, sleeve gastrectomy led to normalization of weight and endocrine–metabolic parameters that were the same as those for non-obese rats. The exception was for GLP-1, which has an anorexigenic effect: GLP-1 remained higher. In Zucker rats, sleeve gastrectomy had a slight effect on all parameters. In ZDF rats, sleeve gastrectomy led to a reduction in intake and a stabilization of weight.
Conclusions
Sleeve gastrectomy is a very good option for exogenous obesity. Normalization of hormonal levels led us to find an extragastric ghrelin production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is the result of multifactorial changes involving both genetic and environmental factors. The physiopathology of obesity from the point of view of intake regulation has led to numerous experimental studies aimed at identifying new forms of regulation. These new forms of regulation are not only found in the gastrointestinal system but also in the adipose tissue (via the metabolism of leptin and insulin) and the central nervous system to finally produce the relevant orexigenic or anorexigenic effect.
The role of ghrelin
In the context of gastrointestinal regulation, it is important to point out the regulation carried out by the gastric hormone ghrelin [1] as a regulator of appetite and, therefore, of weight loss. Ghrelin is the only gastrointestinal hormone with a known orexigenic effect [2]. It is synthesized mainly in the X/A cells of the fundus mucosa and the gastric body, but it has also been shown to be produced, for example, in the intestine, testicles, liver, placenta, and hypophysis [3]. Ghrelin induces an increase in intake in humans and in rats. In the obese, its levels are low, as it decreases as intake increases [4]. It stimulates the secretion of the growth hormone and is high in conditions of malnutrition or anorexia nervosa [5]. The lack of ghrelin suppression after intake could play a part in the origin of obesity [6].
Glucagon-like peptide-1 (GLP-1) and its effects
GLP-1 is an intestinal-regulating peptide of 30 amino acids that secretes at the L cells of the intestinal epithelium. It originates from the family of peptides that are structurally analogous to glucagon–secretin–VIP. The release of GLP-1 takes place because of the arrival of nutrients to the small intestine mediated by the cholinergic system [7]. At the level of the plasma, it is inhibited by the enzyme dipeptidyl dipeptidase IV, which degrades it in a few minutes to inactive products [8]. It stimulates the secretion of insulin by the beta cells in the pancreas with inhibition of glucagon secretion [9] and reduces resistance to insulin and intestinal motility [10]. This has led to several studies into determining its therapeutic possibilities in the field of diabetes mellitus, although analogs of GLP-1 with a longer average life span, such as exendin IV, and inhibitors of dipeptidyl dipeptidase IV must be used [11]. GLP-1 has been seen to have an appetite-inhibiting effect both in humans and in experimental animals. Administration of GLP-1 at the peripheral level has also been seen to have anorexigenic effects both in obese patients and in patients with type-2 diabetes mellitus [12].
Sleeve gastrectomy as a new surgical procedure
Sleeve gastrectomy consists of vertical gastric resection of 80% capacity with exeresis of the fundus and body of the stomach linearly from the Hiss angle to 3–4 cm from the pylorus. This leaves a gastric residual volume ranging from 50 ml [13] to 200 ml [14]. However, as yet, there is no agreement on the optimum residual volume. The operation was created by Hess and Marceau as a restrictive component of biliopancreatic diversion with duodenal switch performed in one stage. Gagner’s [15] group was the first to perform the operation laparoscopically. It has subsequently been used as the first stage of an intervention lasting a few months before full biliopancreatic bypass is performed. It can therefore be considered a treatment of choice as the first stage of malabsorptive surgery for producing a good degree of weight loss for high-risk patients and patients with extremely high levels of morbid obesity. As sleeve gastrectomy is the restrictive technique that leads to the greatest amount of weight loss, it has therefore been reconsidered as another single-procedure bariatric surgical technique. Moreover, as surgical time is short, there are fewer complications, which greatly benefit these patients who have many associated comorbidities. Sleeve gastrectomy is the surgical procedure of choice for super-obese patients with serious comorbidities that are difficult to treat [16].
Metabolic–surgical nexus
Reports that have shown ghrelin to be an important factor in the regulation of intake have encouraged studies relating the levels of ghrelin with the various bariatric surgical techniques [17, 18]. Bariatric surgery anatomically affects not only gastric capacity and the area of absorption of the nutrients and fats but also the pattern of secretion of all the peptides regulating intake. The fact that ghrelin is mainly synthesized in the gastric fundus and that it is a potent orexigenic hormone has led to numerous studies to identify a possible metabolic–surgical nexus. So far, there is no agreement with regard to results on hormonal variations: results depend on the series [19–21]. With sleeve gastrectomy, almost the whole of the gastric fundus is surgically removed, which suggests that after the operation, the levels of ghrelin should, in theory, be low. The good levels of weight loss reported after sleeve gastrectomy may partly be caused by the sharp decline in the levels of ghrelin, which would considerably reduce intake [22]. Increases in the response of other gastrointestinal hormones such as GLP-1 have been reported after biliopancreatic diversion or Roux-en-Y gastrojejunal bypass [23]. This type of response may be caused by the intestinal bolus reaching the level of the small intestine quickly. There is no agreement on GLP-1 ranges of normality in the various physiopathological situations related to body composition. Some authors have reported that in cases of obesity, the levels of GLP-1 are low [24], but low levels are also found in cases of anorexia nervosa [25]. The regulation of GLP-1 also depends on a dipeptidyl peptidase (DPP IV) enzyme, which degrades to GLP-1, thus, reducing its average life span by a few minutes. This enzyme has been shown to be abnormally high in obese subjects [26], and it is recommended that it should be inactivated via a specific protease inhibitor during blood processing for GLP-1 analysis. If this inhibitor is not used, false low levels of GLP-1 may be obtained in obese subjects.
Objective
The general objective of this study was to evaluate the effect on weight, intake, and metabolism (ghrelin, GLP-1, insulin and plasma glycemia levels) of sleeve gastrectomy applied in several physiopathological levels of obesity (obesity caused by increased intake and genetically determined obesity with and without type 2 diabetes mellitus).
Methods
We defined four experimental models, each with ten animals, on which sleeve gastrectomy was performed. Model 2 (cafeteria diet) also included a control group of ten animals that were only administered the cafeteria diet. The total number of cases in the study was therefore 50.
-
Model 1: Non-obese Sprague–Dawley rats
-
Model 2: Sprague–Dawley rats fed a cafeteria diet
-
Model 3: Zucker obese rats (Zucker Fatty)
-
Model 4: Zucker obese type 2 diabetic rats (Zucker diabetic fatty; ZDF)
Chronology of the Procedure
Prior to intervention
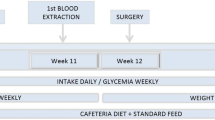
During the second week of stabling before surgery, a blood sample (1.5 ml) was extracted from each animal via the external jugular vein to determine the pre-intervention reference parameters for glucose, GLP-1, total ghrelin, and insulin. Each animal was weighed on each of the 10 days before intervention. Volume of intake was also measured on a daily basis by taking the quantity of feed consumed.
Day of intervention
The animals were weighed. The animals fasted for 8 h. Water was available ad libitum until 1 h before intervention. The animals were 12 weeks old.
After intervention
The animals were fed a liquid diet for 72 h after the surgery. Weight and intake were then measured for the next 2 weeks. Glycemia levels were determined 1 week after intervention from a blood sample taken from the coccygeal vein in the tail. The animals fasted for 6–8 h. Two weeks after surgical intervention, new blood samples were taken from the jugular vein colateral to the first extraction to obtain a second analysis of the biochemical parameters. The animals fasted for 6–8 h. From the day of intervention onwards, in the model 2 control animals (no surgery), the protocol was the same as for the model 2 animals which were intervened. The animals were 14 weeks old. The blood samples were extracted and surgical interventions performed in the Experimental Surgery Laboratory of the Faculty of Medicine and Health Sciences of the Rovira i Virgili University in Tarragona, Spain.
Stabling Conditions and Characteristics
The animals were kept at the animal room of the Faculty of Medicine and Health Sciences of the Rovira i Virgili University in Reus (Tarragona, Spain). All rules regarding temperature, light, diet, drink, etc. for the handling and stabling of animals were followed in accordance with the DOGC (official gazette) of the autonomous government of Catalonia (no. 2.073 10/07/1995) covering the law on the protection of animals for experimentation and other scientific purposes).
Type of feed: Maintenance diet was type AO4 from Panlab®; 3173 kcal/kg.
Water: chlorinated water ad libitum
Cafeteria diet
The cafeteria diet was made up according to the composition of each ingredient and the labels on the products to achieve the desired proportion of carbohydrates, proteins, and fats (45% lipids, 45% carbohydrates, 10% proteins, which is similar to those of other studies in the literature [27]). According to these proportions, the calorie density of the cafeteria diet administered was 6,250 kcal/kg. The diet consisted of madeleines, pork paté, salami, non-roasted peanuts, high-fat cheese, bacon, energy bars, and corn cereals with honey (18–20 g/day).
Liquid diet
Animals were allowed to drink ad libitum for the first 72 h after the intervention. Full oral diet: energy (2 cal/ml) and protein (Resource 2.0®).
Variables Measured
Weight
This was measured using digital scales (accurate to a tenth of a gram) every week after arrival (at 8 weeks old) and then everyday from 10 days before intervention. Weight (in grams) was also measured everyday for 2 weeks after intervention.
Volume of daily intake (g/day)
This was measured every week after arrival and then everyday from 10 days before intervention.
-
Basal glycemia:
Menarini® A glucometer. Glucocard Memory 2. Units in mg/dl.
-
Basal insulinemia:
Sensitive Rat Insulin RIA Kit (Linco®). Processing for RIA.
-
Basal total ghrelin:
Ghrelin (total) RIA Kit (Linco®). Processing for RIA.
-
Basal active GLP-1:
GLP-1 (Active) RIA kit (Linco®). Processing for RIA.
Excess weight lost
Change in weight observed/excess weight [28] = (initial weight − current weight/initial weight − ideal weight) × 100.
Excess weight lost corrected for age
Percentage of excess weight lost taking into account the change in excess weight because of age: (Initial excess weight − final excess weight/initial excess weight) × 100
Information about the theoretical and ideal weights of each rat model was kindly supplied by Charles River Laboratories
kcal Consumed
Amount of standard feed consumed in g/day × 3.173 (kcal/g of feed).
Cafeteria diet
Amount of standard feed consumed in g/day × 3.173 (kcal/g of feed) + g of cafeteria diet consumed per day × 6.25 (kcal/g of cafeteria diet).
Protocol for Anesthesia and Killing
All surgical interventions were performed and blood samples extracted under general anesthetic induced by pharmaceuticals. Anesthesia was administered by intramuscular injection of 20 mg/kg of Zoletil 20® (tiletamine and zolazepam) and 0.01 mg/kg of atropine. In the final stage, the animal was killed by administration of an overdose of Zoletil®.
Surgical Intervention
Sleeve gastrectomy (Fig. 1): 4 cm mid-laparotomy. Farabeuf retractors were positioned and the structures were identified. The stomach was carefully tractioned from the body so that it could be released from the liver, the left lobe of which largely covers it, and the gastrohepatic ligaments sectioned. To dissect the greater curve, ligation with 6/0 silk of the short vessels towards the spleen and of the gastroepiploic vessels in the region of the antrum was needed. This arrangement defined the line of incision for the longitudinal sleeve gastrectomy. This line covered the entire rumen and much of the gastric fundus. Two bulldog forceps were positioned to limit the flow of gastric content during subsequent suturing. After exeresis, 5/0 polypropylene was used to stitch the wound from the fundus to the antrum with a double line of continuous suture. The tightness of the sealed wound was tested by pressure or by injection of physiological serum using orogastric probe.
Post-surgical Care
During the first 72 h, the animals were given a liquid diet. A total of 5 cc of glucosaline serum was injected subcutaneously every 24 h for the first 48 h, after which, they were given standard animal feed.
Sample Processing
All blood samples were taken in cold conditions, as is required for the total ghrelin and GLP-1 molecules, which are highly thermolabile. They were centrifugated under refrigeration conditions (2–4°C) and frozen at −70°C. The samples were determined and processed by radioimmunoassay at the Obesity Unit Laboratory of the Department of Endocrinology and Diabetes of the Hospital Clinic Universitari in Barcelona. A total of 240 μl is needed to detect insulin and ghrelin. To determine GLP-1, total blood must first be treated with an inhibitor (dipeptidyl dipeptidase DDP-IV) at the moment of extraction. Dipeptidyl dipeptidase IV is a peptide in the serum that causes the degradation of GLP-1. The amount of plasma needed is 320 μl.
Statistical Data Analysis
To calculate the number of cases (n), we use the formula for comparing the means for paired data and analysis of variance for each of the main variables. Variables were analyzed using Sample Power 2.0. To compare means in the same model, we used the Student’s–Fisher t test with n − 1 degrees of freedom under the assumption of normality for the population of the difference variable. When this assumption of normality did not hold, we used the non-parametric Wilcoxon test for paired data. To compare the means in two different models, we used the Student’s–Fisher t and Wilcoxon test. To analyze the data, we used SPSS-PC version 13.1 software.
Results
Changes in weight
In model 1 (SD rats), a progressive loss in weight is observed with maximal loss on the fourth day. The weight loss observed in week 13 is statistically significant (p < 0.05), but was recovered in week 14. In model 2 (cafeteria diet rats), and for up to 1 week post-intervention, a progressive increase in weight is observed that is much higher than the standard increase in weight for this type of rat. After intervention, progressive loss in weight is observed, which reaches a maximum on the fourth post-intervention day (p < 0.05). The weights of the rats in this group did not regain their pre-intervention levels. The excess weight loss is 76.4%, and the excess weight corrected for age is 116%. Model 2 (cafeteria control rats) progressively increased in weight even after their cafeteria diet was removed. Differences between these two models are statistically significant (p < 0.001; Fig. 2). In model 3 (Zucker rats), a progressive loss in weight after intervention is observed with a maximal weight loss on the first post-intervention day. The loss in weight observed in week 13 is not statistically significant, but the increase observed in week 14 is (p < 0.05). Unlike model 2, the weight is recovered after the second post-intervention day. In model 4 (ZDF rats), a progressive loss in weight is observed, which peaks on the seventh post-intervention day (p < 0.05) and increases in week 14 (p < 0.05). In this model, the post-intervention weight is not only lower than the theoretical weight; it also reaches its ideal level. Excess weight loss in Zucker fatty rats was −18.8%, which means that they did not decrease their weight but increased it. When corrected for age, the excess weight loss in these rats is 7.3%. In Zucker diabetic fatty rats, the loss in excess weight is 12.1%. When corrected for age, this is 107.6%. Table 1 shows the average weights in weeks 12, 13, and 14.
Changes in intake
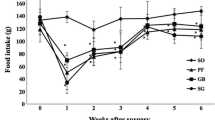
In model 1 (non-obese rats), the levels of intake are maintained, as the average daily intake before and after intervention is the same. In the second week after intervention, intake even increases and by week 14 goes from 73 to 84.4 kcal/day. After intervention in model 2, levels of intake decrease sharply from an average of 150.2 kcal/day to an average of 63.1 kcal/day in week 13 and to an average of 72 kcal/day in week 14 (after intervention, the cafeteria diet was removed). Intake is clearly lower in the obese group than in the model non-obese group (p < 0.05). When the hypercaloric (cafeteria) diet was removed from the diet of Sprague–Dawley rats (model 2 controls), intake dropped to much lower levels than its initial value. If we analyze the means and standard deviations for this group and their levels of intake per week, we see that their levels of intake were the same as those of the non-obese rats in model 1, which did undergo surgery. The intake of the rats in model 3 (Zucker fatty) practically did not change in the first week after intervention. In this model, in the second week after intervention, intake increased by 22% (p < 0.05). As with the change in weight after intervention, intake in this model of obesity was unaffected by surgical intervention. In model 4 (Zucker diabetic fatty), the pre-intervention level of intake was much higher than with the other models (because of diabetes). Average intake before intervention was 128.5 kcal/day. In the first week after intervention, average intake dropped by 51% to 65.7 kcal/day, and in the second week, it was 107.9 kcal/day, which is a 16.03% drop in intake with respect to the initial level. This result is statistically significant (p < 0.05).
Changes in plasma glycemia levels
In model 1 (non-obese rats), there are no significant changes. In model 2 (obese SD rats), there is a drop in glycemia levels in the first week after intervention (p < 0.05). These animals were hyperglycemic at the beginning of the study because of the exogenous obesity produced by the cafeteria diet. In the second week after intervention, the animals become normoglycemic. However, in the control model 2 rats, plasma glycemia levels did not improve when the cafeteria diet was removed. In model 3 (Zucker fatty), the levels of glycemia were high, but normalized 1 week after intervention. Two weeks after intervention, on the other hand, they increased but still remained lower than pre-operational levels. In model 4 (Zucker diabetic fatty), the initial hyperglycemic levels were very high. There was a considerable and highly significant improvement (p < 0.001) 1 week after intervention, although the values did not reach normoglycemic levels. In week 14, the glycemia levels were not significantly different from pre-intervention levels. The changes in glycemic levels are represented in Table 2.
Changes in plasma ghrelin levels
In model 1 rats, ghrelin levels increased after intervention, but not significantly. However, there was a considerable and statistically significant increase in the ghrelin levels of model 2 rats. Although the ghrelin levels of rats in model 2 were initially much lower than those in model 1, after intervention, they were practically identical in both models. On the other hand, the ghrelin levels of model 2 control rats, which were as low as those on which surgery was performed, did not change (Fig. 3). In both models of Zucker rats (model 3: Zucker fatty and model 4: Zucker diabetic fatty), the pre-intervention levels were much lower than in the previous models. After surgery, there was a statistically significant increase (p < 0.05) in ghrelin in both model 3 and model 4. This increase was much higher in the rats of model 4, whose initial values were much lower than those of model 3. After intervention, plasma ghrelin levels were very similar in both groups (Fig. 4; Table 2).
Changes in plasma GLP-1 levels
The GLP-1 levels of the non-obese rats in model 1 were initially much lower than those of the obese Sprague–Dawley rats. After intervention, the levels were slightly lower than their initial levels, but this was not statistically significant. In model 2 rats, the base GLP-1 levels were higher than in the non-obese group (p > 0.05). After intervention, the levels of GLP-1 in model 2 also decreased, although considerably more than in model 1 (p < 0.05). Model 2 control rats had initial levels that were similar to those of the non-control group. These levels also fell after the cafeteria diet had been suspended (p < 0.05). Importantly, the decrease in GLP-1 levels of rats in model 1 was similar to that of the non-control rats in model 2, whereas the decrease in GLP-1 levels of rats in the control group was much sharper. If we compare the initial and final values of the two groups in model 2, we find that there are no statistically significant differences in week 11, but that there are statistically significant differences in week 14. Rats on which surgery was not performed have significantly lower GLP-1 levels (Fig. 3). In model 3 (Zucker fatty) and model 4 (Zucker diabetic fatty), the initial levels in both of these models were higher than in both of the previous models. After intervention, the GLP-1 plasma levels in model 4 decreased (p < 0.05), but not in model 3 (Fig. 4; Table 2).
Changes in plasma insulin levels
In non-obese rats, the plasma insulin levels were practically unchanged after intervention. In model 2 rats, however, the Insulin levels were practically twice as high as those of the non-obese model 1 rats (p < 0.05). The situation was similar for the pre-intervention plasma insulin levels of model 2 control rats. After intervention, the plasma insulin levels of rats in model 2 (non-controls) decreased considerably, reaching the levels of rats in model 1 (p < 0.001). On the other hand, the levels of rats in the control group decreased less considerably after the cafeteria diet was removed, doubling the average insulin levels of model 2 rats on which surgery was performed. Model 3 rats initially had hyperinsulin levels, and after intervention, these levels decreased, but were not statistically significant. The basal insulin levels of model 4 rats were lower than those of model 3 rats. These levels increased slightly after intervention, but the increase was not statistically significant. This was the only model in which plasma Insulin levels did not decrease after intervention (Table 2).
Correlation between the variables
Few correlations have been observed between weight, calorie intake and ghrelin, GLP-1, insulin, and glycemia levels in the four models. In model 1, there is a negative correlation (r = −0.57; p < 0.05) between intake and pre-intervention plasma ghrelin levels. This negative correlation disappeared after intervention and even became positive (r = 0.44), although this result is not statistically significant. In model 1, we also observed a strong negative correlation between pre-intervention ghrelin and insulin levels (r = −0.78; p < 0.01) that disappeared after intervention. In model 2, before intervention, GLP-1 had a strong negative correlation with insulin (r = −0.81; p < 0.01) that also disappeared after intervention. In the other models, no other statistically significant results were found.
Post-intervention morbidity and mortality
Three eventrations (model 4; ZDF) and one dehiscence of the laparotomy suture with wound infection (model 3; Zucker fatty) were observed. Mortality depended on the type of model: five rats (33%) in model 4 (ZDF), one rat (9.9%) in model 2 (Sprague–Dawley cafeteria diet), and one rat (9.9%) in model 3 (Zucker fatty). None of the rats died during the operation. In the group of ZDF rats, mortality occurred in the first 24 h after the operation. In the other groups, mortality occurred at the start of solid intake (72 h after intervention). Necropsies determined dehiscence of the suture of the stomach with free peritoneal fluid in rats in models 2 and 3. No evidence of this was found by necropsy performed on any of the five rats in model 4.
Discussion
Changes in weight
When sleeve gastrectomy was performed on non-obese rats, the initial loss of weight was subsequently recovered. We should bear in mind that this gastrectomy was performed on healthy rats that were not carrying excess weight. Nor did they have any metabolic history of changes in intake, which may explain why weight after intervention returned to its initial values. Few studies have been conducted into the effect of sleeve gastrectomy on patients with low levels of obesity and its effectiveness in the long term. Studies therefore need to be made into the benefits of this type of surgery for this type of patient [29]. When sleeve gastrectomy was performed on exogenously obese rats (especially because of a high-fat diet), the initial weight loss was only partly recovered, and the curve was much more gradual. The initial weight was not totally regained, and the loss in weight was quite substantial. As with various series of humans, the loss in excess weight was more than 50% [13], which indicates that this type of intervention for this type of metabolic condition is highly effective. This technique appears not to be wholly effective for the type of obesity in experimental model 3 (Zucker fatty). Nevertheless, there was a certain degree of excess weight loss if corrected for age. These rats appear not to be a reference model for this type of surgery. Although the rats in model 4 also had genetic-type obesity, the effect of intervention was good. All these suggest that the genetic contribution to the obesity of a patient should be evaluated before surgery is performed, as it may influence the effectiveness of the surgery. An obesity risk index, which has been described to quantify the genetic contribution to a patient’s morbid obesity, could be included as one of the patient’s pre-intervention variables [30].
Changes in intake
Sleeve gastrectomy seems to have had an effect only on exogenously obese rats and not on normal weight rats, which actually increase their intake after intervention because of a catch-up phenomenon. This phenomenon may be caused by the fact that the model is not a pathological one, unlike other animal models with associated pathologies for which this phenomenon has not been observed [31]. Sleeve gastrectomy had no effect on the calorie intake of rats in model 1 (non-obesity), which suggests that the difference in the pre- and post-intervention levels of intake of model 2 cafeteria rats on which surgery was performed was basically caused by the surgery. In the genetically obese rats, the influence of intake depended on whether the rats were highly hyperglycemic or moderately hyperglycemic. In model 3, there was no decrease in intake. In the case of model 4, there was a clear decrease in intake that was accompanied by a decrease in excess weight. In Zucker diabetic fatty rats, stress caused by surgery undergone by rats with poor metabolic control may have had an important effect on their intake. It is striking that 2 weeks after intervention, weight had not been regained, that it had reached its ideal values and that, as we will see later, this was not associated with better metabolic control [32].
Changes in plasma glycemia levels
In non-obese rats, no clear hypoglycemic response was observed. In model 2, the hyperglycemia caused by their exogenous obesity became normoglycemia 2 weeks after intervention. In the control group, suppression of the cafeteria diet did not improve plasma glycemia levels. This suggests that sleeve gastrectomy had an effect on the metabolic state of these animals, although this may be caused only by the changes in weight because of intervention [33]. In model 3 (Zucker fatty), the glycemia levels were high, but normalized 1 week after intervention and recovered in week 14. These rats were genetically obese, and the recovery of their glycemia levels may be related to the fact that the excess weight lost was not very high. As the rats in model 4 (Zucker diabetic fatty) were initially extremely hyperglycemic, they had a clear dysfunction of their pancreatic β cells [34]. Although the intervention led to a considerable and significant improvement in their hyperglycemia in the first week after intervention, the fact that the diabetes of these animals was genetic means that the return to the high pre-intervention levels of glycemia observed in week 14 is partly unavoidable.
Changes in plasma ghrelin levels
The most striking thing is that contrary to what we expected, ghrelin levels increased in all groups. This contradicts the hypothesis that this surgical technique is able to affect the production of Ghrelin. Some authors in the literature conclude that the significant decreases in ghrelin levels after sleeve gastrectomy [22, 35] are caused by the fundus. In our animal models, not only is this result not observed but the opposite happens. We found that the levels of ghrelin were significantly lower in obese rats than in non-obese rats. After intervention, on the other hand, we observed significant increases in ghrelin that, in the case of the Sprague–Dawley obese rats, were at the same level as non-obese rats. After intervention, ghrelin normalized. This has an inverse parallelism with that observed in anorectic subjects where ghrelin levels are higher but decrease to normal levels once weight is normalized [36]. In the case of obese rats (all three models analyzed), the low levels of intake produced by the surgical intervention may be responsible for the increase in ghrelin, as happens in cases of anorexia. However, this would require ghrelin to be produced by other organs to compensate for the theoretical lack of production by the fundus. Many studies in the literature have reported a significant reduction in ghrelin in morbidly obese patients after a Roux-en-Y gastrojejunal bypass [17, 20, 37]. Also, in studies of Scopinaro’s biliopancreatic diversion by Scopinaro’s own group, although ghrelin levels decrease initially but tend to increase subsequently and, in the long term, (12 months) tend to be even higher than pre-intervention levels [38]. This seems to support the hypothesis that the lack of contact between food and the mucosa of the stomach is responsible for the decrease in ghrelin levels [39]. Adami, a member of Scopinaro’s group, suggests that ghrelin increases because of the stimulus produced by the food in the stomach, which, in this type of surgery, is not excluded (unlike the gastrojejunal bypass where it is excluded and the ghrelin level is therefore low). However, this is contradictory, as several authors have observed increases in ghrelin after a Roux-en-Y gastrojejunal bypass [40]. This cannot be corroborated by our animal models because ghrelin levels increased, although sleeve gastrectomy involved the exeresis of almost the whole fundus. Other studies with animal models in which the stomach has neither been operated on nor excluded have reported a decrease in ghrelin levels of as much as 30% after a duodenojejunal bypass, which suggests that this hormone is regulated by the small intestine [41].
Changes in plasma GLP-1 levels
The first striking thing about the pre-intervention levels of GLP-1 is that the values in non-obese rats are lower than those in obese rats. Some authors report that GLP-1 levels are low in cases of obesity [24]. However, levels are also observed to be low in cases of anorexia nervosa. It has been suggested that this is caused by a lack of adaptation in a state of hyperintake that would make obese subjects maintain inappropriately low levels of GLP-124. The regulation of GLP-1 also depends on the enzyme, dipeptidyl peptidase (DPP IV), which degrades to GLP-1 in a few minutes [8]. This enzyme is high in obese subjects and, when blood is processed to analyze GLP-1, it is recommended that it be inactivated by a specific protease inhibitor. However, in some studies, this inhibitor is not administered [42], and it is in these cases that base GLP-1 values have been similar for obese and non-obese subjects. In our study, not only are the values not lower but they are clearly higher. However, our values are similar to those of other studies that used animal models [43]. The key to this apparent contradiction may lie in the levels of DPP-IV, which, as we have seen, are higher in obese subjects. If the inhibitor is not applied quickly after the blood is extracted, there may be a rapid degradation of GLP-1 that wrongly produces low results, as some authors have concluded [26]. On the other hand, the higher GLP-1 values in obese subjects could explain the contradiction of finding low GLP-1 values in both anorectic and obese subjects (in theory, these levels could be high to counter-regulate the hyperintake), as is demonstrated in our study and confirmed by others [43]. Our results show that the post-intervention levels of GLP-1 tend to be lower than pre-intervention levels. We have not found this result in the literature on bariatric surgery, but we have in studies in which weight loss is caused by a hypocaloric diet [44]. The responses found after a Roux-en-Y gastrojejunal bypass show an increase in both basal GLP-1 and GLP-1 after stimulation by intake [23, 45]. The same occurs in studies on biliopancreatic diversion [46]. This result could be expected because the mucosa of the small intestine is hyperstimulated by the food caused by a topographical change in certain segments when the surgical procedure is performed [47]. In the case of restrictive surgery, studies on the levels of GLP-1 in patients who underwent vertical banded gastroplasty found no significant differences between pre- and post-intervention levels [48]. The fact that our post-intervention levels were lower than pre-intervention levels may be caused by a reduction in the volume of intake (the surgical operation is restrictive) but with no malabsorption, which would lead to a reduction in the signal that stimulates the secretion of GLP-1 by the small intestine. Nevertheless, we found that the group of rats that experienced the greatest reduction was the control group on which surgery was not performed. This group, then, also presents a sharp reduction in calorie intake, so we can attribute the fact that GLP-1 did not decrease more in the other experimental groups to the surgical intervention.
Changes in plasma insulin levels
Sleeve gastrectomy had no significant effect on the insulin levels of non-obese rats (we may consider these rats to be healthy). On the other hand, the pre-intervention insulin levels of rats fed the cafeteria diet were significantly higher than the post-intervention levels. This suggests that in this group, the metabolic effect of the cafeteria diet was sufficient to reproduce a resistance to insulin and hyperglycemia that is equivalent to the metabolic syndrome [49]. The response of this group after surgical intervention means that the levels of insulin were similar to those of the non-obese group (i.e., to the group of healthy rats). This contrasts especially with the response of model 2 control rats on which surgery was not performed. In model 3 (Zucker fatty), surgical intervention had little effect on the levels of plasma insulin, although there was a non-significant tendency for the levels to decrease. We should point out that the insulin levels in this group were higher than those in model 4 because in this latter model, the production of insulin was exhausted, which led to diabetes. It appears that sleeve gastrectomy led to a re-equilibrium of metabolism, which improved the state of hyperinsulinism and increased the insulin levels of diabetic rats (model 4 ZDF) whose reduced insulin reserves were responsible for their metabolic decompensation.
Conclusions
Sleeve gastrectomy is an excellent option for exogenous morbid obesity and can lead to weights, intakes, and hormonal parameters that are the same as those of non-obese rats. It also seems that it is a good option to control weight despite a genetic basis. The levels of ghrelin and GLP-1 can be explained as an attempt to compensate the loss of weight via an orexigenic or anorexigenic effect. Further studies are needed to determine if the normalization of ghrelin levels could be explained by a hypothetical extragastric synthesis.
References
Heung Man L, Guiyun W, Englander E, Kojima M, Greeley G. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 2002;143(1):185–90.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth hormone releasing acylated peptide from stomach. Nature 1999;402:656–60.
Tena-Sempere M, Barreiro ML, González LC, Gaytan F, Zhang FP, Caminos JE, et al. Novel expression and functional role of ghrelin in rat testis. Endocrinology 2002;143:717–25.
Shiiya T, Nakazato M, Mizuta M, Date Y. Plasma ghrelin levels in lean and obese humans and the effect of glucose on secretion. J Endocrinol Metab 2002;87(1):240–4.
Otto B, Cuntz U, Fruehauf E, Wawarta R. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nerviosa. Eur J Endocrinol 2001;145(5):669–73.
English PJ, Ghatei MA, Malik IA, Bloom SR. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab 2002;87(6):2984.
Balks HJ, Holst JJ, Von zur Muhlen A, Brabant G. Rapid oscillations in plasma glucagon-like peptide-1 (GLP-1) in humans: cholinergic control of GLP-1 secretion via muscarinic receptors. J Clin Endocrinol Metab 1997;82:786–90.
Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide-1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995;136:3585–96.
Fehmann HC, Goke R, Goke BB. Cell and molecular biology of the incretin hormones glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide. Endocr Rev 1995;16:390–410.
Gutniack M, Orskov C, Holst JJ. Antidiabetogenic effect of GLP-1 amide in normal subjects and patients with diabetes mellitus. N Engl J Med 1992;326:1316–22.
Ahren B, Simonsson E, Larsson H, Landin-Olsson M, Torgeirsson H, Jansson PA. Inhibition of dipeptidyl peptidase IV improves metabolic control over a 4-week study period in type 2 diabetes. Diabetes Care 2002;25:869–75.
Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rorer B, Lareida J, et al. Glucagon-like peptide-1 promotes satiety and reduces foodintake in patients with diabetes mellitus type 2. Am J Physiol 1999;276:R1541–4.
Baltasar A, Serra C, Pérez N, Bou R, Bengochea M, Ferri L. Laparoscopic sleeve gastrectomy: a multipurpose bariatric operation. Obes Surg 2005;15:1124–8.
Gagner M, Rogula T. Laparoscopic reoperative sleeve gastrectomy for poor weight loss after biliopancreatic diversion with duodenal switch. Obes Surg 2003;13:649–54.
Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg 2000;10:514–23.
Almogy G, Crookes PF, Anthone GJ. Longitudinal gastrectomy as a treatment for the high risk superobese patient. Obes Surg 2004;14:492–7.
Cummings D, Weigle D, Frayo R, Breen P, Ma M, Dellinger E, et al. Plasma ghrelin levels after diet induced weight loss or gastric bypass surgery. N Engl J Med 2002;346:1623–30.
Rubino F, Gagner M. Weight loss and plasma ghrelin levels. N Engl J Med 2002;347:1379–81.
Stoeckli R, Chanda R, Langer I, Keller U. Changes of body weight and ghrelin plasma levels after gastric banding and gastric bypass. Obes Res 2004;12:346–50.
Leonetti F, Silecchia G, Iacobellis G, Ribaudo MC, Zappaterreno A, Tiberti C, et al. Different plasma ghrelin levels after laparoscopic gastric bypass and adjustable gastric banding in morbid obese subjects. J Clin Endocrinol Metab 2003;88:4227–31.
Adami GF, Cordera R, Andraghetti G, Camerini GB, Marinari G, Scopinaro N. Changes in serum ghrelin concentration following biliopancreatic diversion for obesity. Obes Res 2004;12:684–7.
Langer F, Reza M, Bohdjalian A, Felberbauer F, Zacherl J, Wenzl E, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg 2005;15:1024–9.
Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marín JL, et al. Glucagon-like peptide-1, Peptide YY, Hunger and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 2006;91(5):1735–40.
Huda M, Wilding J, Pinkney J. Gut peptides and the regulation of appetite. Obes Rev 2006;7:163–82.
Tomasik PJ, Sztefko K, Starzyk J. Cholecystokinin, glucose dependent insulinotropic peptide and glucagon-like peptide 1 secretion in children with anorexia nervosa and simple obesity. J Pediatr Endocrinol Metab 2004;17:1623–31.
Lugari R, Dei Cas A, Ugolotti D, Barilli AL, Camellini C, Ganzerla GC. Glucagon-like peptide 1 (GLP-1) secretion and plasma dipeptidyl peptidase IV (DPP-IV) activity in morbidly obese patients undergoing biliopancreeatic diversion. Horm Metab Res 2004;36:111–5.
Llado I, Rodriguez S, Pujol E, Monjo M, Estrany E, Roca P, et al. Gender effects on adrenergic receptors expression and lipolysis in white adipose tissue of rats. Obes Res 2002;10:296–305.
Deitel M, Greenstein R. Recommendations for reporting weight loss. Obes Surg 2003;13:159–60.
Baltasar A, Serra C, Pérez N, Bou R, Bengochea M, Ferri L. Laparoscopic sleeve gastrectomy: a multipurpose bariatric operation. Obes Surg 2005;15:1124–8.
Thirlby R, Randall J. A genetic obesity risk index for patients with morbid obesity. Obes Surg 2002;12:25–9.
Adelman R, Holliday M. Improved growth in growth retarded uremic rats with use of calorie supplementation. Clin Nephrol 1977;8(1):298–303.
Pories J, Swanson S, MacDonald K. Who would have thought it: an operative proves to be the most effective therapy for adult onset diabetes mellitus. Ann Surg 1995;222:339–52.
Greenway S, Greenway F, Klein S. Effects of obesity surgery on non-insulin-dependent diabetes mellitus. Arch Surg 2002;137:1109–17.
Tokuyama Y, Sturis J, DePaoli AM, Takeda J, Stoffel M, Tang J, et al. Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes 1995 Dec;44(12):1447–57.
Cohen R, Uzzan B, Bihan H, Khochtali I, Reach G, Catheline JM. Ghrelin levels and sleeve gastrectomy in super-super-obesity. Obes Surg 2005;15:1501–2.
Otto B, Cuntz U, Fruehauf E, Wawarta R. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nerviosa. Eur J Endocrinol 2001;145(5):669–73.
Stoeckli R, Chanda R, Langer I, Keller U. Changes of body weight and ghrelin plasma levels after gastric banding and gastric bypass. Obes Res 2004;12:346–50.
Adami GF, Cordera R, Andraghetti G, Camerini GB, Marinari G, Scopinaro N. Changes in serum ghrelin concentration following biliopancreatic diversion for obesity. Obes Res 2004;12:684–7.
Adami G, Cordera R, Marinari G, Lamerini G, Andraghetti G, Scopinaro N. Plasma ghrelin levels in short term following biliopancreatic diversion. Obes Surg 2003;13:889–92.
Holdstock C, Engstrom BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab 2003;88:3177–83.
Rubino F, Zizzari P, Tomasetto C, Bluett M, Forgione A, Vix M, et al. The role of the small bowel in the regulation of circulating Ghrelin levels and food intake in the obese Zucker rat. Endocrinology 2005;146:1745.
Verdig C, Toubro S, Buemann B, Lysgard MJ, Juul HJ, Astrp A. The role of postprandrial releases of Insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord 2001;25:1206–14.
Berghofer P, Peterson RG, Schneider K, Fehmann HC, Goke B. Incretin hormone expression in the gut of diabetic mice and rats. Metabolism 1997;46(3):261–7.
Adam T, Lejeune M, Westerterp-Plantenga M. Nutrient-stimulated glucagon-like peptide 1 release after body weight loss and weight maintenance in human subjects. Br J Nutr 2006;95(1):160–7.
Le Roux C, Aylwin S, Batterham R, Borg C, Coyle F, Prasad V, et al. Gut hormones profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 2006;243(1):104–8.
Valverde I, Puente J, Martín-Duce A, Molina L, Lozano O, Sancho V, et al. Changes in glucagon-like peptide 1 (GLP-1) secretion after biliopancreatic diversion or vertical banded gastroplasty in obese subjects. Obes Surg 2005;15:387–97.
Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg 2002;236(5):554–9.
Kellum JM, Kuemmerle MD, Dorisio TM, Engle RN, Wolf MA. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg 1990;211(6):763–77.
Caro J. Clinical review 26: insulin resistance in obese and nonobese men. J Clin Endocrinol Metab 1991;73:691–5.
Acknowledgements
The authors thank Charles Rives Laboratories® for the information about the standard anthropometric values of the different rat models.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sabench Pereferrer, F., Hernàndez Gonzàlez, M., Feliu Rovira, A. et al. Influence of Sleeve Gastrectomy on Several Experimental Models of Obesity: Metabolic and Hormonal Implications. OBES SURG 18, 97–108 (2008). https://doi.org/10.1007/s11695-007-9351-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-007-9351-4