Abstract
Background
Roux-en-Y gastric bypass (RYGB) is an effective surgical treatment for type 2 diabetes mellitus (T2DM). The present study aimed to investigate the effects of RYGB on glucose homeostasis, lipid metabolism, and intestinal morphological adaption, as well as hepatic and intestinal gluconeogenesis.
Methods
Twenty adult male T2DM rats induced by high-fat diet and low dose of streptozotocin were randomly divided into sham and RYGB groups. The parameters of body weight, food intake, glucose tolerance, insulin sensitivity, and serum lipid profiles were assessed to evaluate metabolic changes. Intestinal sections were stained with hematoxylin and eosin (H&E) for light microscopy examination. The messenger RNA (mRNA) and protein expression levels of key regulatory enzymes of gluconeogenesis [phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase)] were determined through reverse-transcription PCR (RT-PCR) and Western blotting, respectively.
Results
RYGB induced significant improvements in glucose tolerance and insulin sensitivity, along with weight loss and decreased food intake. RYGB also decreased serum triglyceride (TG) and free fatty acid (FFA) levels. The jejunum and ileum exhibited a marked increase in the length and number of intestinal villi after RYGB. The RYGB group exhibited downregulated mRNA and protein expression levels of PEPCK and G6Pase in the liver and upregulated expression of these enzymes in the jejunum and ileum tissues.
Conclusions
RYGB ameliorates glucose and lipid metabolism accompanied by weight loss and calorie restriction. The small intestine shows hyperplasia and hypertrophy after RYGB. Meanwhile, our study demonstrated that the reduced hepatic gluconeogenesis and increased intestinal gluconeogenesis may contribute to improved glucose homeostasis after RYGB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM), which is characterized by deteriorative insulin secretion function in pancreatic islets and detrimental insulin resistance in insulin-sensitive target organs, comprises approximately 90 % of all cases of diabetes [1]. Currently, the dominating T2DM treatments include lifestyle interventions, pharmacotherapy, and bariatric surgery. A growing body of evidence indicates that bariatric surgery is the most effective treatment for obese T2DM patients; it leads to rapid and sustained weight loss and significant improvement or complete remission of diabetes [2, 3]. Along with the rising prevalence of diabetes, there has been a constant increase in the total number of bariatric surgeries performed worldwide over the past 10 years. In 2013, the most commonly performed bariatric procedure in the world was Roux-en-Y gastric bypass (RYGB), followed by sleeve gastrectomy (SG), and adjustable gastric banding (AGB) [4]. However, the underlying mechanisms that mediate the anti-diabetic effects that occur after RYGB are still poorly understood.
Some of the potential mechanisms of action of RYGB include alterations in hormone secretion, gut microbiota, and bile acid recycling [5]. Recently, a novel function of intestinal morphological adaptation and glucose metabolism reprogramming after RYGB has also emerged to explain the therapeutic effect of bypass surgery [6, 7]. Accumulating evidence suggests that intestinal gluconeogenesis may be involved in the metabolic improvement after bypass surgery [8]. Although gluconeogenesis has been well investigated in the liver and intestine, the role of gluconeogenesis after RYGB is still inconclusive [9]. This study aimed to investigate the effects of RYGB on glucose homeostasis, lipid metabolism, and intestinal morphological adaption, as well as hepatic and intestinal gluconeogenesis in a T2DM rat model. The results of this investigation may help to provide new insights into the underlying metabolic mechanisms of diabetes remission after RYGB.
Materials and Methods
Animals and Diet Protocols
This animal study was approved by the Animal Care and Utilization Committee of Shanghai Sixth People’s Hospital. Twenty 6-week-old male Sprague-Dawley (SD) rats were purchased from Sino-British Sippr/BK Lab Animal Ltd. (Shanghai, China). All animals were kept in individual cages under controlled ambient temperature (24 ± 2 °C) and humidity in a 12-h light/dark cycle. All rats had ad libitum access to tap water and food unless otherwise stated. All rats were fed a high-fat diet (HFD) (60 % fat, 20 % carbohydrate, 20 % protein, as a total percentage of calories; Research Diets, Inc., NJ, USA) for 4 weeks to induce insulin resistance, followed by an intraperitoneal injection of streptozotocin (STZ) (3 mg/kg) (Sigma, USA) to induce a diabetic state. Rats with non-fasting blood glucose ≥16.7 mmol/l after 72 h, measured in duplicate from tail vein blood with a glucometer (Sinocare Inc., Changsha, China), were considered diabetic and randomly assigned to the sham (n = 10) or RYGB group (n = 10). One week after the STZ injection, sham or RYGB surgery was performed.
Surgical Procedures
After a 24-h fast, the rats were anesthetized with an intraperitoneal injection of 1 % sodium pentobarbital solution (5 ml/kg). RYGB operations were initiated by a 4-cm midline incision. The stomach was divided 5 mm below the gastroesophageal junction from the lesser to greater curvature horizontally. The proximal stomach was closed by 4-0 silk suture (Ningbo Medical Needle, China) in a simple interrupted suture technique to create a small gastric pouch, and the distal stomach was closed in a similar fashion. Then, jejunum was transected 10 cm distal to the ligament of Treitz, and the stump was ligated with 4-0 silk suture. The distal limb of jejunum was brought up to the small gastric pouch, and a 7-mm incision was made on the antimesenteric border of the bowel wall and anterior gastric wall along with greater curvature, respectively. The distal limb of jejunum was anastomosed to the small gastric pouch with a side-to-side gastrojejunostomy. The proximal limb of jejunum carrying the biliopancreatic juices was reconnected downward to the Roux limb at a distance of 15 cm from the gastrojejunostomy with a side-to-side jejunojejunostomy. Both the gastrojejunostomy and jejunojejunostomy were performed in a simple interrupted varus suture technique with an about 7-mm anastomosis using 5-0 silk suture (Ningbo Medical Needle, China). Sham surgeries involved the same abdominal incisions and gastrointestinal transections as those in the RYGB group, and reanastomosis was performed at the same sites.
The rats were fed non-residue diets postoperatively at 24 h and followed by standard rodent chow (10 % fat, 70 % carbohydrate, 20 % protein, as a total percentage of calories; Research Diets, Inc., NJ, USA) at four postoperative days, until the study ended.
In all groups, body weight and food intake were measured daily during the first 2 weeks after surgery and then once a week. All rats were starved overnight and euthanatized at 8 weeks postoperatively. Jejunum (the proximal bowel about 17 cm below Treitz ligament in sham group or middle bowel of the Roux limb in RYGB group), ileum (about 10 cm above ileocecal junction in both groups), and liver biopsies were fixed in formalin for routine histology examination or stored at −80 °C after flash frozen in liquid nitrogen for quantitative real-time reverse-transcription PCR (RT-PCR) and Western blotting.
Oral Glucose Tolerance Test and Insulin Tolerance Test
An oral glucose tolerance test (OGTT) and an insulin tolerance test (ITT) were performed at baseline and at two and eight postoperative weeks. For the OGTT, the rats were administered 20 % glucose (1 g/kg) by oral gavage after an overnight fast. Blood glucose was measured using a glucometer at baseline and 30, 60, 90, and 120 min after the glucose administration. For the ITT, the rats were injected intraperitoneally with human insulin (0.5 IU/kg) after an overnight fast, and blood glucose was measured in the same manner as OGTT.
Serum Lipid Profiles
After an overnight fast, blood samples were collected from the tail veins of the conscious rats and placed into chilled tubes containing EDTA solution, and serum was immediately extracted. Serum total cholesterol (TC), triglycerides (TGs), and free fatty acids (FFAs) were measured using enzymatic colorimetric assays (NJJCBIO, Nanjing, China).
Tissue Histological Analysis
The jejunum and ileum of each rat were embedded in paraffin and sectioned. The sections were stained with hematoxylin and eosin (H&E) for light microscopy examination. For assessment of morphological adaptation of intestine, a pathologist who was blinded to other details evaluated all histological sections under microscopy (×100).
Quantitative Real-Time RT-PCR
Total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA). Quantitative real-time RT-PCR was performed in a Light Cycler System (Roche Diagnostics, Mannheim, Germany). Analyses were performed on 1 μg cDNA using the SYBR® Premix Ex Taq Master Mix (Takara, China), in a total PCR reaction volume of 10 μl, containing 0.2–0.6 μM of each primer. The following primer pairs were used: PEPCK: 5′-GCCTGTGGGAAAACCAACCT-3′ (forward), 5′-CACCCACACATTCAACTTTCCA-3′ (reverse); G6Pase: 5′-CCCAGACTAGAGATCCTGACAGAAT-3′ (forward), 5′-GCACAACGCTCTTTTCTTTTACC-3′(reverse); β-actin: 5′-ACGGTCAGGTCATCACTATCG-3′ (forward), 5′-GGCATAGAGGTCTTTACGGATG-3′ (reverse).
Western Blotting
Total protein in the liver and intestine tissue was extracted with RIPA lysis buffer containing protease inhibitors (Beyotime, Shanghai, China), and the concentration of protein was determined using a BCA Kit (Beyotime, Shanghai, China). An equal amount of protein was separated using 10 % SDS-PAGE (Beyotime, Shanghai, China). Then, the separated proteins were transferred onto polyvinylidene fluoride membranes (Millipore, USA). Proteins were detected using antibodies against the following: PEPCK (H-300), G6Pase-α (H-60) (Santa Cruz Biotechnology, Santa Cruz, USA), and β-actin (Cell Signaling Technology, USA). After incubation at 4 °C overnight with primary antibody, the membranes were incubated with HRP-conjugated secondary antibody (Cell Signaling Technology, USA) for 60 min. Protein bands were assessed using ECL reagents (Thermo Scientific, USA) and detected by ImageQuant LAS-4000 mini (GE, USA). The band intensity was assessed with ImageJ software (http://rsb.info.nih.gov/ij, National Institutes of Health, USA).
Statistical Analysis
Quantitative data are presented as mean ± standard deviation (SD). Areas under curves (AUCs) for OGTT (AUCOGTT) and ITT (AUCITT) were calculated by trapezoidal integration. For measurements conducted over time, a two-way analysis of variance (ANOVA) with repeated measures was used. For measurements made at one time point, Student’s t test was used for unpaired comparisons. P < 0.05 represented a statistically significant difference. SPSS Version 20.0 was used for the statistical analysis.
Results
Metabolic Parameters
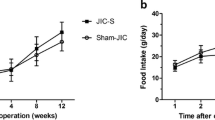
As shown in Fig. 1, there were no significant between-group preoperative differences in body weight, food intake, OGTT, and ITT. In the sham and RYGB groups, body weight reached its lowest value at 1 week postoperatively. In the sham group, body weight was nearly restored to the preoperative values at 2 weeks postoperatively, while the body weight increase in the RYGB group was blunted from week 2 postoperatively. The postoperative body weights in the RYGB group were significantly lower than those in the sham group at week 3 (Fig. 1a). Daily food intake in the RYGB group was significantly decreased postoperatively between weeks 2 and 8 compared with that in the sham group (Fig. 1b).
Body weight, food intake, AUCOGTT, and AUCITT. a Body weight of the rats before and after surgery. The RYGB group showed significant weight loss compared with the sham group from postoperative week 3. b Food intake of the rats before and after surgery. Food intake of the RYGB group was significantly decreased compared with that of the sham group from postoperative week 2. c No difference in the preoperative AUCOGTT values was observed between the sham and RYGB groups. However, the AUCOGTT values were significantly reduced in the RYGB group compared with the sham group at postoperative weeks 2 and 8. d There was no significant preoperative difference in AUCITT between the sham and RYGB groups. However, the AUCITT values for the rats in the RYGB group were decreased at postoperative weeks 2 and 8 compared with the sham group. Asterisk indicates P < 0.05 vs. the sham group
The rats in the RYGB group showed significant improvements in glucose tolerance at postoperative weeks 2 and 8, as demonstrated by the lower values of AUCOGTT (Fig. 1c). Compared with the sham group, the RYGB group demonstrated lower postoperative values of AUCITT at weeks 2 and 8, indicating improved systemic insulin sensitivity (Fig. 1d).
There were no preoperative differences in the fasting serum TC, TG, and FFA concentrations between the sham and RYGB groups. At eight postoperative weeks, the rats in the RYGB group showed significantly lower fasting serum levels of TG and FFAs than those in the sham group (Table 1). The fasting serum level of TC decreased slightly in both groups postoperatively, and there was no significant difference between the sham and RYGB groups (Table 1).
Histological Changes in the Small Intestine
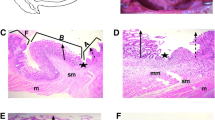
The jejunum in the RYGB group (Fig. 2b) exhibited marked increases in the length and number of intestinal villi compared with the sham group (Fig. 2a). Similarly, the ileum in the RYGB group (Fig. 2d) also exhibited marked increases in the length and number of intestinal villi compared with the sham group (Fig. 2c).
Histological changes in the a, b jejunum and c, d ileum. The H&E-stained sections (×100) showed histological adaptation of the jejunum and ileum at eight postoperative weeks. The jejunum in the RYGB group (b) exhibited marked increases in the length and number of villi compared with the sham group (a). The ileum in the RYGB group (d) also exhibited marked increases in the length and number of villi compared with the sham group (c)
Expression of Key Regulatory Enzymes of Gluconeogenesis
The messenger RNA (mRNA) expression levels of PEPCK and G6Pase in the liver were significantly decreased in the RYGB group compared with the sham group (Fig. 3a). In addition, the protein expression levels of PEPCK and G6Pase in the liver were also markedly reduced in the RYGB group compared with the sham group (Fig. 3b), which was consistent with the results of the corresponding mRNA expression in the liver.
Relative mRNA and protein expression levels of the key regulatory enzymes of gluconeogenesis in the liver. a The hepatic mRNA expression levels of PEPCK and G6Pase in the RYGB group were significantly lower than those in the sham group at eight postoperative weeks. b The protein expression levels of PEPCK and G6Pase were significantly decreased in the RYGB group compared with the sham group at eight postoperative weeks. β-actin was used as an internal control. PEPCK phosphoenolpyruvate carboxykinase, G6Pase glucose-6-phosphatase. Asterisk indicates P < 0.05 vs. the sham group
The mRNA expression levels of PEPCK and G6Pase in the jejunum and ileum were significantly increased in the RYGB group compared with the sham group (Fig. 4a, c). The protein expression levels of PEPCK and G6Pase in the jejunum and ileum were also markedly increased in the RYGB group compared with the sham group (Fig. 4b, d), which was consistent with the results of the corresponding mRNA expression in the jejunum and ileum.
Relative mRNA and protein expression levels of the key regulatory enzymes of gluconeogenesis in the small intestine. a In the jejunum, the mRNA expression levels of PEPCK and G6Pase in the RYGB group were significantly increased compared with those in the sham group at eight postoperative weeks. b In the jejunum, the protein expression levels of PEPCK and G6Pase were significantly increased in the RYGB group compared with the sham group at eight postoperative weeks. c In the ileum, the mRNA expression levels of PEPCK and G6Pase in the RYGB group were significantly increased compared with those in the sham group at eight postoperative weeks. d In the ileum, the protein expression levels of PEPCK and G6Pase were significantly increased in the RYGB group compared with the sham group at eight postoperative weeks. β-actin was used as an internal control. PEPCK phosphoenolpyruvate carboxykinase, G6Pase glucose-6-phosphatase. Asterisk indicates P < 0.05 vs. the sham group
Discussion
Bariatric surgery was originally developed to induce weight loss in morbidly obese patients; however, it subsequently proved to be an effective treatment for T2DM. Current guidelines recommend that bariatric surgery be considered for people with T2DM and a BMI >35 kg/m2 [10]. Interestingly, many studies have indicated that bariatric surgery in non-severely obese patients (BMI of 30–35 kg/m2) may also be superior to medical therapy with respect to T2DM remission and glycemic control [11–13]. Recently, the results of three randomized controlled trials have suggested that RYGB is one of the best treatments for T2DM patients with mild–moderate obesity [14–16]. The precise mechanisms underlying the resolution of diabetes after RYGB have not been determined; therefore, the establishment of a RYGB model for T2DM is still important to investigate the intrinsic mechanisms. In the present study, a non-obese T2DM rat model was induced through a HFD and low dose of STZ, which are considered to closely reproduce the pathogenesis and metabolic characteristics of human T2DM [17]. And, this rat model is widely used to study the mechanisms of remission of T2DM after bypass surgery [18–20]. Our preliminary study demonstrated the safety and efficacy of RYGB in non-obese rats with T2DM.
Rapid and sustained weight loss effects after RYGB have been shown in various T2DM rat models [21, 22]. A similar weight-reducing effect was also observed for RYGB in our study, and this effect persisted until the study ended. Comprised of gastric transection and intestinal bypass, it has been well demonstrated that RYGB limits stomach capacity and reduces food intake [23]. In the present study, RYGB also significantly reduced food intake from postoperative week 2 compared with the sham group, indicating the rapid and sustained effect of RYGB on caloric restriction.
In addition to the effects of weight loss and caloric restriction, RYGB also leads to glycemic control or remission of T2DM in more than 70 % of patients [14–16]. Consistent with these results, the present study provided evidence that RYGB induced anti-diabetic effects beginning at postoperative week 2, supported by lower AUCOGTT and AUCITT values. The OGTT has been the main method of diagnosing diabetes for decades; it detects early diabetes efficiently, as well as subjects with impaired glucose tolerance, according to the mechanisms of glucose homeostasis [24]. This study showed that RYGB lowered the postoperative values of AUCOGTT and improved glucose tolerance. Regarding the ITT, the study showed that RYGB lowered the postoperative values of AUCITT and improved insulin sensitivity.
Previous studies demonstrated that serum lipid profiles were also improved after bypass surgery in both human and T2DM rat model [15, 25]. Our research showed that serum TG and FFA levels were significantly decreased 8 weeks after RYGB, which may help to alleviate insulin resistance because excessive serum FFAs are ultimately esterified into TG and both of which promote insulin resistance [26]. These findings raise the possibility that RYGB may improve glucose homeostasis by modulating lipid metabolism.
Several studies previously described that the Roux limb displays morphological changes after RYGB [27, 28]. In accordance with previous studies, we confirmed that the jejunum and ileum both exhibited hyperplasia and hypertrophy after RYGB. However, the importance of the morphological adaptation of the small intestine in terms of the metabolic effects of RYGB remains largely unknown. As direct contact with undigested nutrients appears to be the most potent factor in inducing mucosal adaptation of the small intestine [29], the number of enteroendocrine cells increased passively as the intestine adaptation and that the increased total number of L and I cells is likely to contribute to the higher circulating levels of GLP-1, PYY, and CCK, potentially leading to metabolic improvement after RYGB [7]. The role of intestinal glucose metabolism in T2DM remains largely unknown. A recent study showed that the Roux limb exhibits reprogramming of intestinal glucose metabolism to meet the increased bioenergetic demands of intestinal remodeling changes after RYGB [6]. Saeidi et al. proposed that the reprogramming of intestinal glucose metabolism renders the intestine a major organ for glucose disposal, contributing to glucose homeostasis after RYGB. Therefore, we hypothesized that the morphological and glucose metabolism adaptation of the intestine may occur in response to its advance exposure to undigested nutrients and that it may play an important role in improving glucose homeostasis.
In this study, we also observed postoperative gluconeogenic enzyme changes in the liver and intestine. The mRNA and protein expression levels of PEPCK and G6Pase were increased in the intestine but decreased in the liver after RYGB. PEPCK and G6Pase are key regulatory enzymes of gluconeogenesis, and regulation of these enzymes is important in the control of endogenous glucose production. It is well known and documented that the impaired suppression of gluconeogenesis in the liver promotes hepatic glucose production and aggravates insulin resistance [30]. Hepatic insulin resistance, which is characterized by impaired suppression of hepatic gluconeogenesis and glucose output by insulin, plays a crucial role in the pathogenesis of hyperglycemia and glucose intolerance [31]. Given that hepatic gluconeogenesis plays an important role in glycemic control, the downregulation of hepatic gluconeogenesis may be involved in the improved insulin sensitivity and glucose tolerance resulting from RYGB.
Because RYGB induces morphological changes in the intestinal tract, we reasoned that the expression levels of PEPCK and G6Pase increased to adapt to the changes triggered by RYGB and that the increased expression levels of PEPCK and G6Pase may benefit glucose homeostasis. An intestinal/hepatic regulation hypothesis has been proposed that the shunting of nutrient delivery to distal small intestine after bypass surgery enhances intestinal gluconeogenesis, which activates hepatoportal glucose sensor and decreases food intake and suppresses hepatic glucose production, then improves glucose homeostasis [32]. Recently, Soty et al. found that intestinal gluconeogenesis can signal the hypothalamus and, thus, is capable of modulating numerous functions associated with whole body metabolism that are under the control of the hypothalamus [33]. Thus, the findings in the current study suggest that increased intestinal gluconeogenesis may result in increased portal glucose concentration and decreased hepatic gluconeogenesis, as well as translate a neural signal to the hypothalamus and modulate metabolism throughout the body. These findings are consistent with the findings of previous studies that have demonstrated that enhanced intestinal gluconeogenesis after bypass surgery is responsible for ameliorating hyperglycemia in rats [34, 35].
The present study demonstrated improvements in glucose homeostasis and lipid metabolism accompanied by weight loss and calorie restriction, as well as hyperplasia and hypertrophy of the small intestine after RYGB. The present study also demonstrated that gluconeogenic enzymes were attenuated in the liver and increased in the intestine after RYGB. Together, these study results suggest that RYGB surgery suppresses hepatic gluconeogenesis and stimulates intestinal gluconeogenesis, which may then improve glucose homeostasis. Further studies are needed to determine the role that morphological and metabolic adaption of the small intestine plays in glucose homeostasis after RYGB.
References
Kharroubi AT, Darwish HM. Diabetes mellitus: the epidemic of the century. World J Diabetes. 2015;6(6):850–67.
Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934.
Muller-Stich BP, Senft JD, Warschkow R, et al. Surgical versus medical treatment of type 2 diabetes mellitus in nonseverely obese patients: a systematic review and meta-analysis. Ann Surg. 2015;261(3):421–9.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25(10):1822–32.
Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2(2):152–64.
Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341(6144):406–10.
Mumphrey MB, Patterson LM, Zheng H, et al. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol Motil. 2013;25(1):e70–9.
Mithieux G. Nutrient control of energy homeostasis via gut-brain neural circuits. Neuroendocrinology. 2014;100(2-3):89–94.
Hayes MT, Foo J, Besic V, et al. Is intestinal gluconeogenesis a key factor in the early changes in glucose homeostasis following gastric bypass? Obes Surg. 2011;21(6):759–62.
SAGES Guidelines Committee. SAGES guideline for clinical application of laparoscopic bariatric surgery. Surg Obes Relat Dis. 2009;5(3):387-405.
Serrot FJ, Dorman RB, Miller CJ, et al. Comparative effectiveness of bariatric surgery and nonsurgical therapy in adults with type 2 diabetes mellitus and body mass index <35 kg/m2. Surgery. 2011;150(4):684–91.
Cohen RV, Pinheiro JC, Schiavon CA, et al. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care. 2012;35(7):1420–8.
Boza C, Munoz R, Salinas J, et al. Safety and efficacy of Roux-en-Y gastric bypass to treat type 2 diabetes mellitus in non-severely obese patients. Obes Surg. 2011;21(9):1330–6.
Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomised, controlled trial. Lancet Diabetes Endocrinol. 2015;3(6):413–22.
Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149(7):716–26.
Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014;149(7):707–15.
Srinivasan K, Viswanad B, Asrat L, et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–20.
Jurowich CF, Rikkala PR, Thalheimer A, et al. Duodenal-jejunal bypass improves glycemia and decreases SGLT1-mediated glucose absorption in rats with streptozotocin-induced type 2 diabetes. Ann Surg. 2013;258(1):89–97.
Li M, Li H, Zhou Z, et al. Duodenal-jejunal bypass surgery ameliorates glucose homeostasis and reduces endoplasmic reticulum stress in the liver tissue in a diabetic rat model. Obes Surg. 2015. doi:10.1007/s11695-015-1816-2
Jurowich CF, Otto C, Rikkala PR, et al. Ileal interposition in rats with experimental type 2 like diabetes improves glycemic control independently of glucose absorption. J Diabetes Res. 2015;2015:490365.
Xu B, Yan X, Shao Y, et al. A comparative study of the effect of gastric bypass, sleeve gastrectomy, and duodenal-jejunal bypass on type-2 diabetes in non-obese rats. Obes Surg. 2015;25(10):1966–75.
Bhutta HY, Rajpal N, White W, et al. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PLoS One. 2015;10(3):e0122273.
Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141(3):950–8.
Bartoli E, Fra GP, Carnevale Schianca GP. The oral glucose tolerance test (OGTT) revisited. Eur J Intern Med. 2011;22(1):8–12.
Han H, Hu C, Wang L, et al. Duodenal-jejunal bypass surgery suppresses hepatic de novo lipogenesis and alleviates liver fat accumulation in a diabetic rat model. Obes Surg. 2014;24(12):2152–60.
Sun Y, Li W, Hou X, et al. Triglycerides and ratio of triglycerides to high-density lipoprotein cholesterol are better than liver enzymes to identify insulin resistance in urban middle-aged and older non-obese Chinese without diabetes. Chin Med J. 2014;127(10):1858–62.
Spak E, Bjorklund P, Helander HF, et al. Changes in the mucosa of the Roux-limb after gastric bypass surgery. Histopathology. 2010;57(5):680–8.
le Roux CW, Borg C, Wallis K, et al. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252(1):50–6.
Taqi E, Wallace LE, de Heuvel E, et al. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg. 2010;45(5):987–95.
Sharabi K, Tavares CD, Rines AK, et al. Molecular pathophysiology of hepatic glucose production. Mol Aspects Med. 2015; 46:21–33.
Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2003;285(4):E685–92.
Troy S, Soty M, Ribeiro L, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8(3):201–11.
Soty M, Penhoat A, Amigo-Correig M, et al. A gut-brain neural circuit controlled by intestinal gluconeogenesis is crucial in metabolic health. Mol Metab. 2015;4(2):106–17.
Kim M, Son YG, Kang YN, et al. Changes in glucose transporters, gluconeogenesis, and circadian clock after duodenal-jejunal bypass surgery. Obes Surg. 2015;25(4):635–41.
Sun D, Wang K, Yan Z, et al. Duodenal-jejunal bypass surgery up-regulates the expression of the hepatic insulin signaling proteins and the key regulatory enzymes of intestinal gluconeogenesis in diabetic Goto-Kakizaki rats. Obes Surg. 2013;23(11):1734–42.
Acknowledgments
This work was supported by the Key Project of Shanghai Health and Family Planning Commission (Grant No. 201440026).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
All authors declare that we have no conflict of interest.
Statement of Informed Consent
Informed consent does not apply to this study.
Statement of Human and Animal Rights
All applicable institutional and national guidelines for the care and use of animals were followed.
Additional information
Yong Yan and Zhou Zhou contributed equally to this work. Cheng Hu and Xueli Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yan, Y., Zhou, Z., Kong, F. et al. Roux-en-Y Gastric Bypass Surgery Suppresses Hepatic Gluconeogenesis and Increases Intestinal Gluconeogenesis in a T2DM Rat Model. OBES SURG 26, 2683–2690 (2016). https://doi.org/10.1007/s11695-016-2157-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2157-5