Abstract
Background
Roux-en-Y gastric bypass (RYGB) is an effective surgical treatment for type 2 diabetes mellitus (T2DM). The present study aimed to investigate the effects of RYGB on glucose homeostasis, lipid metabolism, and liver morphological adaption, as well as the changes in bile acids signaling and expression of its target regulatory factors involved in gluconeogenesis, lipogenesis, and fatty acid β oxidation.
Methods
Twenty adult male T2DM rats induced by high-fat diet and a low dose of streptozotocin were randomly divided into sham and RYGB groups. The parameters of body weight, food intake, glucose tolerance, insulin sensitivity, serum lipid profiles, and bile acids level were assessed to evaluate metabolic changes. Liver sections were stained with hematoxylin-eosin (H&E) and oil red O to assess lipid accumulation. The mRNA and protein expression levels of farnesoid X receptor (FXR), small heterodimer partner (SHP), key regulatory factors of gluconeogenesis, lipogenesis, and fatty acid β oxidation (phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase), sterol regulatory element-binding protein-1c (SREBP-1c), peroxisome proliferator-activated receptor-α (PPAR-α)) were determined through RT-PCR and Western blotting, respectively.
Results
RYGB induced significant improvements in glucose tolerance and insulin sensitivity, along with weight loss and decreased food intake. RYGB also decreased serum TG, FFAs, and increased bile acids levels. The lipid droplets in the liver were significantly decreased after RYGB. The RYGB group exhibited downregulated mRNA and protein expression levels of PEPCK, G6Pase, and SREBP-1c and upregulated expression of FXR, SHP, and PPAR-α in the liver.
Conclusions
RYGB ameliorates glucose and lipid metabolism accompanied by weight loss and calorie restriction. The liver exhibited a marked improvement in lipid accumulation after RYGB. The bile acids level, FXR, and its target transcriptional factor SHP expression were elevated. Meanwhile, our study demonstrated that the increased bile acids-FXR signaling, followed by the reduced hepatic gluconeogenesis, lipogenesis, and increased fatty acid β oxidation may contribute to improved metabolic conditions after RYGB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes prevalence continues to increase worldwide, as do the numerous associated comorbidities including cardiovascular disease, metabolic syndrome, and even some cancers [1, 2]. Globally in 2017, more than 451 million people were diabetes and 5 million deaths happened as a result of diabetes and its complications. Type 2 diabetes mellitus (T2DM) comprises approximately 90% of all cases of diabetes and represents a major health and economic burden [3, 4]. The burden of T2DM on quality of life as well as the economy has spurred the development of numerous T2DM treatments that range from behavioral to pharmacologic to surgical [5,6,7]. Currently, bariatric surgery is recommended as the most effective treatment for obese T2DM patients, defined by a body mass index (BMI) greater than 35 kg/m2 [8,9,10]. A lot of clinical trials have demonstrated the superiority of bariatric surgery in terms of sustained weight loss and a significant improvement or complete remission of the metabolic disorder compared with lifestyle intervention and intensive medical therapy [11,12,13,14]. Along with the rising prevalence of diabetes, there has been a constant increase in the total number of bariatric surgeries performed worldwide over the past 10 years [15]. However, the underlying mechanisms that mediate the improvement of metabolic conditions that occur after bariatric surgery are still undetermined.
Some of the potential mechanisms of action of bariatric surgery include alterations in hormone secretion, gut microbiota, and bile acids recycling [16,17,18]. It is well-known that an increase in plasma bile acids is observed after bariatric surgery, and which is frequently proposed to explain the positive metabolic effects of bariatric surgery [19]. Farnesoid X receptor (FXR), belongs to a family of nuclear hormone receptors and is highly expressed in the liver, was the first identified bile acid receptor that regulates various elements of bile acids, glucose, and lipid metabolism. Additionally, it was demonstrated that activation of FXR by agonists in the liver was associated with resolution of hyperglycemia and hyperlipidemia, which is expected as a therapeutic target for metabolic disorders [20,21,22]. Roux-en-Y gastric bypass (RYGB), as the standard metabolic procedure, is the major clinically performed bypass surgery over the past decades [23]. However, it is unclear how RYGB influences the expression of FXR and its related factors in the liver and resolves metabolic disorders. Therefore, the aims of the study were to evaluate the expression of FXR and its related factors after RYGB and to clarify the relationship between bile acids signaling and metabolic improvement in a T2DM rat model. The results of this investigation may help to provide new insights into the underlying metabolic mechanisms of diabetes resolution after RYGB.
Materials and Methods
Animals and Diet Protocols
This animal study was approved by the Animal Care and Utilization Committee of Guangzhou Red Cross Hospital. Twenty 6-week-old male Sprague-Dawley (SD) rats were purchased from Sino-British Sippr/BK Lab Animal Ltd. (Shanghai, China). All animals were kept in individual cages under controlled ambient temperature (24 ± 2 °C) and humidity in a 12-h light/dark cycle. All rats had ad libitum access to tap water and food unless otherwise stated. All rats were fed a high-fat diet (HFD) (60% fat, 20% carbohydrate, 20% protein, as a total percentage of calories; Research Diets, Inc., NJ, USA) for 4 weeks to induce insulin resistance, followed by an intraperitoneal injection of streptozotocin (STZ) (35 mg/kg) (Sigma, USA) to induce a diabetic state. Rats with non-fasting blood glucose ≥ 16.7 mmol/l after 72 h, measured in duplicate from tail vein blood with a glucometer (Sinocare Inc., Changsha, China), were considered diabetic and randomly assigned to the sham (n = 10) or RYGB group (n = 10). One week after the STZ injection, sham or RYGB surgery was performed.
Surgical Procedures
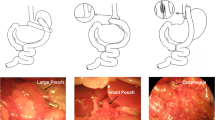
After a 24-h fast, the rats were anesthetized with an intraperitoneal injection of 1% sodium pentobarbital solution (5 ml/kg). RYGB operations were initiated by a 4-cm midline incision. The stomach was divided 5 mm below the gastro-oesophageal junction from the lesser to greater curvature horizontally. The proximal stomach was closed by a 4-0 silk suture (Ningbo medical needle, China) in a simple interrupted suture technique to create a small gastric pouch, and the distal stomach was closed in a similar fashion. Then jejunum was transected 10 cm distal to the ligament of Treitz and the stump was ligated with a 4-0 silk suture. The distal limb of jejunum was brought up to the small gastric pouch, and a 7-mm incision was made on the antimesenteric border of the bowel wall and anterior gastric wall along with greater curvature, respectively. The distal limb of the jejunum was anastomosed to the small gastric pouch with a side-to-side gastrojejunostomy. The proximal limb of the jejunum carrying the biliopancreatic juices was reconnected downward to the Roux limb at a distance of 15 cm from the gastrojejunostomy with a side-to-side jejunojejunostomy. Both the gastrojejunostomy and jejunojejunostomy were performed in a simple interrupted varus suture technique with an about 7 mm of anastomosis using a 5-0 silk suture (Ningbo medical needle, China). Sham surgeries involved the same abdominal incisions and gastrointestinal transections as those in the RYGB group, and reanastomosis was performed at the same sites.
The rats were fed non-residue diets postoperatively at 24 h and followed by standard rodent chow (10% fat, 70% carbohydrate, 20% protein, as a total percentage of calories; Research Diets, Inc., NJ, USA) at four postoperative days until the study ended.
In all groups, body weight and food intake were measured daily during the first 2 weeks after surgery and then once a week. All rats were starved overnight and euthanatized at 8 weeks postoperatively. Liver biopsies were fixed in formalin for routine histology examination or stored at − 80 °C after flash frozen in liquid nitrogen for quantitative real-time RT-PCR and Western blotting.
Oral Glucose Tolerance Test and Insulin Tolerance Test
An oral glucose tolerance test (OGTT) and an insulin tolerance test (ITT) were performed at baseline and at two and eight postoperative weeks. For the OGTT, the rats were administered 20% glucose (1 g/kg) by oral gavage after an overnight fast. Blood glucose was measured using a glucometer at baseline and 30, 60, 90, and 120 min after the glucose administration. For the ITT, the rats were injected intraperitoneally with human insulin (0.5 IU/kg) after an overnight fast, and blood glucose was measured in the same manner as OGTT.
Serum Lipid Profiles and Bile Acids Level
After an overnight fast, blood samples were collected from the tail veins of the conscious rats and placed into chilled tubes containing EDTA solution, and serum was immediately extracted. Serum total bile acids, total cholesterol (TC), triglycerides (TG), and free fatty acids (FFAs) were measured using enzymatic colorimetric assays (NJJCBIO, Nanjing, China).
Liver Histological Analysis
Histological changes in the liver were evaluated using hematoxylin-eosin (H&E) and oil red O staining under a light microscope. A part of the liver sample was embedded in paraffin, and 3-μm-thick sections were stained with H&E. Frozen liver sections were stained with oil red O to reveal intracellular lipids. For assessment of histological changes, a pathologist who was blinded to other details evaluated all histological sections under microscopy (× 400).
Quantitative Real-Time RT-PCR
Total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA). Quantitative real-time reverse-transcription PCR (RT-PCR) was performed in a Light Cycler System (Roche Diagnostics, Mannheim, Germany). Analyses were performed on 1 μg cDNA using the SYBR® Premix Ex Taq Master Mix (Takara, Japan), in a total PCR reaction volume of 10 μl, containing 0.2–0.6 μM of each primer. The following primer pairs were used: FXR:5′-CCACGACCAAGCTATGCAG-3′(forward), 5′-TCTCTGTTTGCTGTATGAGTCCA-3′(reverse); SHP:5′-GCAGCACTGCCTGGAGTC-3′(forward),5′-GTGTGCAATGTGGCAGGA-3′(reverse); PEPCK: 5′-GCCTGTGGGAAAACCAACCT-3′ (forward), 5′-CACCCACACATTCAACTTTCCA-3′ (reverse); G6Pase: 5′-CCCAGACTAGAGATCCTGACAGAAT-3′ (forward), 5′-GCACAACGCTCTTTTCTTTTACC-3′(reverse); SREBP-1c:5′-ACAAGATTGTGGAGCTCAAGG-3′ (forward),5′-TGCGCAAGACAGCAGATTTA-3′ (reverse); PPAR-α:5′-TGCGGACTACCAGTACTTAGGG-3′ (forward),5′-GGAAGCTGGAGAGAGGGTGT-3′ (reverse); β-actin: 5′-ACGGTCAGGTCATCACTATCG-3′ (forward), 5′-GGCATAGAGGTCTTTACGGATG-3′ (reverse).
Western Blotting
Total protein in the liver tissue was extracted with RIPA lysis buffer containing protease inhibitors (Beyotime, Shanghai, China), and the concentration of protein was determined using a BCA Kit (Beyotime, Shanghai, China). An equal amount of protein was separated using 10% SDS-PAGE (Beyotime, Shanghai, China). Then, the separated proteins were transferred onto polyvinylidene fluoride membranes (Millipore, USA). Proteins were detected using antibodies against the following: phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase) (Santa Cruz Biotechnology, Santa Cruz, USA), farnesoid X receptor (FXR), small heterodimer partner (SHP), sterol regulatory element-binding protein-1c (SREBP-1c), peroxisome proliferator-activated receptor-α (PPAR-α) (Abcam, USA), and β-actin (Cell Signaling Technology, USA). After incubation at 4 °C overnight with a primary antibody, the membranes were incubated with HRP-conjugated secondary antibody (Cell Signaling Technology, USA) for 60 min. Protein bands were assessed using ECL reagents (Thermo Scientific, USA) and detected by ImageQuantLAS-4000 mini (GE, USA). The band intensity was assessed with ImageJ software (http://rsb.info.nih.gov/ij, National Institutes of Health, USA).
Statistical Analysis
Quantitative data are presented as mean ± standard deviation (SD). Areas under curves (AUC) for OGTT (AUCOGTT) and ITT (AUCITT) were calculated by trapezoidal integration. For measurements conducted over time, a two-way analysis of variance (ANOVA) with repeated measures was used. For measurements made at one-time point, a Student’s t test was used for unpaired comparisons. P < 0.05 represented a statistically significant difference. SPSS Version 20.0 was used for the statistical analysis.
Results
Metabolic Parameters
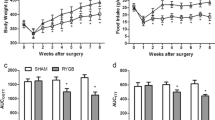
As shown in Fig. 1, there were no significant between-group preoperative differences in body weight, food intake, OGTT, and ITT. In the sham and RYGB groups, body weight reached its lowest value at 1 week postoperatively and was nearly restored to the preoperative values at 2 weeks postoperatively, while the body weight increase in the RYGB group was blunted from week 2 postoperatively. The postoperative body weights in the RYGB group were significantly lower than those in the sham group at week 4 (Fig. 1a). Daily food intake in the RYGB group was significantly decreased postoperatively between weeks 1 and 8 compared with that in the sham group (Fig. 1b).
Body weight, food intake, AUCOGTT, and AUCITT. a Body weight of the rats before and after surgery. The RYGB group showed significant weight loss compared with the sham group from postoperative week 4. b Food intake of the rats before and after surgery. Food intake of the RYGB group was significantly decreased compared with that of the sham group from postoperative week 1. c No difference in the preoperative AUCOGTT values was observed between the sham and RYGB groups. However, the AUCOGTT values were significantly reduced in the RYGB group compared with the sham group at postoperative weeks 2 and 8. d There was no significant preoperative difference in AUCITT between the sham and RYGB groups. However, the AUCITT values for the rats in the RYGB group were decreased at postoperative weeks 2 and 8 compared with the sham group. Asterisk indicates P < 0.05 vs. the sham group
The rats in the RYGB group showed significant improvements in glucose tolerance at postoperative weeks 2 and 8, as demonstrated by the lower values of AUCOGTT (Fig. 1c). Compared with the sham group, the RYGB group demonstrated lower postoperative values of AUCITT at weeks 2 and 8, indicating improved systemic insulin sensitivity (Fig. 1d).
As shown in Fig. 2a, the fasting total bile acids were similar between the sham and RYGB groups before the operation. However, the RYGB group demonstrated significantly higher levels of fasting total bile acids than the sham group at both 2 and 8 weeks after the operation. There were no preoperative differences in the fasting serum TC, TG, and FFAs concentrations between the sham and RYGB groups. The fasting serum level of TC decreased slightly in the RYGB group at postoperative weeks 2 and 8, but there was no significant difference between the sham and RYGB groups (Fig. 2b). At postoperative weeks 2 and 8, the rats in the RYGB group showed significantly lower fasting serum levels of TG and FFAs than those in the sham group (Fig. 2c, d).
Serum total bile acids level (a) and lipid profiles (b, c, d) before and after operation. a Serum total bile acids level was significantly increased in the RYGB group compared with the sham group at postoperative weeks 2 and 8. b Serum level of TC decreased slightly in the RYGB groups postoperatively, but there was no significant difference between the sham and RYGB groups. c Serum level of TG was lower in the RYGB group than in the sham group at both 2 and 8 weeks postoperatively. d Serum level of FFAs was lower in the RYGB group than in the sham group at both 2 and 8 weeks postoperatively. TC total cholesterol; TG triglycerides; FFAs free fatty acids. Asterisk indicates P < 0.05 vs. the sham group
Histological Changes in the Liver
The liver in the RYGB group (Fig. 3b) exhibited a marked improvement of steatosis characterized by decreased hepatocyte ballooning compared with the sham group (Fig. 3a). Similarly, the liver in the RYGB group (Fig. 3d) also exhibited a marked alleviation of lipid accumulation characterized by sparser lipid droplets compared with the sham group (Fig. 3c).
Representative microscopic images (× 400) showed histological changes in the liver. The hematoxylin-eosin (H&E) stained sections (a, b) showed hepatic steatosis at eight postoperative weeks. The hepatocyte in the RYGB group (b) exhibited marked improvement of steatosis characterized by decreased hepatocyte ballooning compared with the sham group (a). The oil red O stained sections (c, d) showed lipid accumulation of the liver at eight postoperative weeks. The hepatocyte in the RYGB group (d) exhibited marked alleviation of lipid accumulation characterized by sparser lipid droplets compared with the sham group (c)
Expression of Key Transcriptional Factors Related to Bile Acids
The mRNA expression levels of FXR and SHP in the liver were significantly increased in the RYGB group compared with the sham group (Fig. 4a). In addition, the protein expression levels of FXR and SHP in the liver were also markedly increased in the RYGB group compared with the sham group (Fig. 4b), which was consistent with the results of the corresponding mRNA expression in the liver.
Relative mRNA and protein expression levels of the key transcriptional factors related to bile acids in the liver. a The hepatic mRNA expression levels of FXR and SHP in the RYGB group were significantly higher than those in the sham group at eight postoperative weeks. b The protein expression levels of FXR and SHP were significantly increased in the RYGB group compared with the sham group at eight postoperative weeks. β-actin was used as an internal control. FXR farnesoid X receptor; SHP small heterodimer partner. Asterisk indicates P < 0.05 vs. the sham group
Expression of Key Regulatory Factors of Gluconeogenesis, Lipogenesis, and Fatty Acid β Oxidation
The mRNA expression levels of PEPCK and G6Pase in the liver were significantly decreased in the RYGB group compared with the sham group (Fig. 5a). In addition, the protein expression levels of PEPCK and G6Pase in the liver were also markedly reduced in the RYGB group compared with the sham group (Fig. 5b), which was consistent with the results of the corresponding mRNA expression in the liver.
Relative mRNA and protein expression levels of the key regulatory factors of gluconeogenesis, lipogenesis, and fatty acid β oxidation in the liver. a The mRNA expression levels of PEPCK and G6Pase in the RYGB group were significantly decreased compared with those in the sham group at eight postoperative weeks. b The protein expression levels of PEPCK and G6Pase were significantly decreased in the RYGB group compared with the sham group at eight postoperative weeks. c The mRNA expression level of SREBP-1c in the RYGB group was significantly decreased compared with those in the sham group at eight postoperative weeks. The mRNA expression level of PPAR-α in the RYGB group was significantly increased compared with those in the sham group at eight postoperative weeks. d The protein expression level of SREBP-1c was significantly decreased in the RYGB group compared with the sham group at eight postoperative weeks. The protein expression level of PPAR-α was significantly increased in the RYGB group compared with the sham group at eight postoperative weeks. β-actin was used as an internal control. PEPCK phosphoenolpyruvate carboxykinase, G6Pase glucose-6-phosphatase. SREBP-1c sterol regulatory element-binding protein-1c, PPAR-α peroxisome proliferator-activated receptor-α. Asterisk indicates P < 0.05 vs. the sham group
The mRNA expression level of SREBP-1c in the liver was significantly decreased in the RYGB group compared with the sham group (Fig. 5c). The protein expression level of SREBP-1c in the liver was also markedly decreased in the RYGB group compared with the sham group (Fig. 5d), which was consistent with the result of the corresponding mRNA expression in the liver.
The mRNA expression level of PPAR-α in the RYGB group was significantly increased in the RYGB group compared with the sham group (Fig. 5c). The protein expression level of PPAR-α in the liver was also markedly increased in the RYGB group compared with the sham group (Fig. 5d), which was consistent with the result of the corresponding mRNA expression in the liver.
Discussion
The prevalence of bariatric surgery continues to increase across the globe. All bariatric procedures currently performed are demonstrated effective in the treatment of T2DM and its comorbidities compared to non-surgical interventions [24]. Recent bariatric surgery worldwide survey showed that the most commonly performed procedure in 2014 was sleeve gastrectomy (SG) (45.9%), followed by RYGB (39.6%), and adjustable gastric banding (AGB) (7.4%) [25]. Despite SG having a steep increase all around the world, RYGB still represented the most performed procedure over the past decades. RYGB was formerly the most prevalent bariatric surgery until recently surpassed by SG, and it has been reported with the most significant and sustained improvement of metabolic disorders in many clinical trials [26]. It has also been proven effective in diabetic control in various T2DM rat models [27,28,29]. Consistent with these findings, our present study demonstrated the safety and efficacy of RYGB in HFD/STZ-induced diabetic rats.
Rapid and sustained weight loss and caloric restriction effects after RYGB have been shown in various T2DM rat models [28,29,30]. In the present study, RYGB also reduced weight and food intake from postoperative week 2 compared with the sham group, and this effect persisted until the study ended, indicating the rapid and sustained effects of RYGB on weight loss and caloric restriction. Comprised of gastric transection and intestinal bypass, it has been well demonstrated that RYGB limits stomach capacity and decreases digestion. However, it is now accepted that RYGB engenders weight loss and metabolic improvement by mechanisms other than restriction and malabsorption [31]. The multifactorial mechanisms promoting weight loss following RYGB include reduced energy intake, reduced hunger, and reduced neural responsiveness to food cues, all of which are influenced by a series of gastrointestinal tract-derived signals [32].
RYGB has also been proven to be an effective therapy for glycemic control or remission of diabetes in various T2DM rat models and diabetic patients [11, 13, 28, 29]. Despite the effects of weight loss and caloric restriction after RYGB, many studies have demonstrated that the remission of diabetes is a direct consequence of physiological remodel of surgery rather than a secondary effect of weight loss and caloric restriction [33, 34]. Current guidelines recommend that bariatric surgery be considered for people with T2DM and a BMI > 35 kg/m2 [8]. Interestingly, more and more studies have indicated that RYGB in non-severely obese patients (BMI of 30–35 kg/m2) may also be superior to medical therapy with respect to T2DM remission and glycemic control [35, 36]. Even in mild-moderate obesity subjects, RYGB not only leads to significant improvement of glucose homeostasis but also helps patients meet other biochemical goals of diabetes management (i.e., hemoglobin A1C, lipid profiles, and blood pressure) [37,38,39]. Consistent with these results, the present study provided evidence that RYGB improved glucose tolerance and insulin sensitivity, supported by the lower AUCOGTT, and AUCITT values in the RYGB group. Although the effects of body weight loss and caloric restriction might explain some of the improvements in diabetes remission after RYGB, numerous basic and clinical studies have demonstrated multifactorial and complex mechanisms, such as resolve of hepatic steatosis, changes in hormone secretion, gut microbiota, and bile acids signal, all of which may be the important mediators [16, 40, 41].
Bariatric surgery can lead to alleviation of hyperlipidemia and hepatic steatosis [42, 43], while these improvements seem largely dependent on surgery-induced weight loss. Recently, Gero et al. reported that plasma TC, TG, and low-density lipoprotein cholesterol (LDL-C) levels after RYGB were significantly decreased compared with the preoperative levels [44]. Carswell et al. showed that plasma TC and LDL-C levels were reduced from 1 month up to 4 years post-RYGB, and TG level was reduced postoperatively from 3 months up to 4 years compared with the preoperative levels [45]. RYGB could improve lipid metabolism in the early postoperative period (3 months) when the sufficient weight loss was not gained. Therefore, RYGB may influence lipid metabolism regardless of weight change. Our present study also demonstrated that the serum lipid profiles were improved after RYGB at 2 weeks postoperatively. We further investigated hepatic steatosis after RYGB, in accordance with previous studies [46, 47]; there was ameliorated hepatic fat accumulation in HFD/STZ-induced diabetic rats at 8 weeks after RYGB, which may contribute to alleviated insulin resistance, as the liver fat content is positively correlated with hepatic insulin resistance in both diabetic and non-diabetic subjects. However, the precise mechanism by which RYGB ameliorated hyperlipidemia and hepatic steatosis has not been determined.
Bile acids were previously well-known for their important roles in intestinal digestion and absorption of dietary fat, steroids, drugs, and lipophilic vitamins. Recent findings show that bile acids are also important signaling molecules involved in the regulation of glucose, lipid, and energy metabolism [48]. Bile acids directly activate its receptor FXR, which regulates a network of genes involved in bile acid, glucose and lipid metabolism [49]. Our research also showed that fasting serum bile acids were increased after RYGB, and the elevated bile acids level may contribute to the improvement of insulin resistant and hepatic steatosis.
FXR belongs to the nuclear receptor superfamily of transcription factors and is the receptor most dedicated to signaling by bile acids [48, 49]. Small heterodimer partner (SHP), also known as nuclear receptor subfamily 0 group B member 2, which encodes the SHP protein and is a key target gene of FXR. Indeed, most of the suppressive effects of FXR on genes encoding components are predominantly mediated through induction of SHP [50]. To explain the effect of metabolic improvement after RYGB in relation to bile acids, we further investigated the effects of RYGB on the expression of FXR and its target transcriptional factor SHP. Consistent with the elevated serum bile acids level, the expression of FXR and its target transcriptional factor SHP were significantly increased in the RYGB group, which suggested that the bile acids signal in the liver appeared to be enhanced after RYGB.
The possibility that FXR activation inhibits hepatic gluconeogenesis to lower fasting plasma glucose has been extensively investigated in vitro and in vivo models; bile acids administration and FXR activation decreased fasting plasma glucose level in diabetic mice [21]. PEPCK and G6Pase are key regulatory enzymes of gluconeogenesis, and regulation of these enzymes is important in the control of endogenous glucose production. It is well-known and documented that the impaired suppression of gluconeogenesis in the liver promotes hepatic glucose production and aggravates insulin resistance [51]. In this study, the mRNA and protein expression levels of PEPCK and G6Pase were decreased in the liver after RYGB, which may contribute to the improved insulin sensitivity and glucose tolerance resulting from RYGB. Given that a majority of studies concluded that FXR activation repressed PEPCK and G6Pase in primary hepatocytes and in liver cell lines by inducing SHP, the downregulation of hepatic gluconeogenesis may be involved in the enhance bile acids-FXR signaling after RYGB.
A role for FXR in regulating lipid metabolism and alleviating hepatic steatosis has been well delineated in recent years [52]. Liver lipid content is mainly regulated by SREBP-1c, which is a critical transcription factor that regulates lipid biosynthesis and regulates the expression of several genes involved in de novo lipogenesis [53]. Watanabe et al. reported that the activation of FXR by bile acids induced SHP expression and suppressed the expression of SREBP-1c [54]. In addition, activation of FXR induces the expression of PPAR-α, which is a key regulator of fatty acid β oxidation and stimulates the rate-limiting enzyme expression in fatty acid β oxidation [55]. Thus, activation of FXR can suppress hepatic de novo lipogenesis and promotes fatty acid β oxidation, limiting hepatic lipid accumulation. In this study, liver fat accumulation was significantly alleviated in the RYGB group than the sham group. Similarly, the mRNA and protein expression levels of FXR, SHP, and PPAR-α in the liver were significantly increased in the RYGB group compared with the sham group. The mRNA and protein expression level of SREBP-1c was significantly lower in the RYGB group than the sham group. Therefore, elevated bile acids followed by the activation of FXR and induction of SHP in the liver may contribute to resolved liver fat accumulation after RYGB through changed mRNA and protein expression involved in lipogenesis and fatty acid β oxidation.
RYGB alters the enterohepatic bile acid circulation, resulting in increased serum bile acids level as well as upregulated FXR signaling. Recent rodent studies also reported that bile acids are increased after SG and that FXR is essential for the positive effects of bariatric surgery on weight loss and glycemic control [56]. As bile acid signaling through FXR has been demonstrated to be a critical mediator of the beneficial effects of bariatric surgery, the potential downstream targets responsible for these effects have also gained attention. A series of studies suggested that gut microbiota influences glucose and lipid metabolism via bile acids—dependent modulation of FXR signaling. One hypothesis is that bile acid signaling regulates metabolism via alterations in gut microbiota [57]. Another FXR target gene of interest is the gut-derived hormone fibroblast growth factor-15/19 (FGF15/19) (in mouse and human ortholog FGF19), which has been identified as an important potential mediator of the beneficial effects of bariatric surgery [58]. However, the exact route by which bile acids-FXR signaling contributes to glucose and lipid homeostasis remains unclear. Bozadjieva et al. have hypothesized that although bile acids and FXR signaling are potent mediators of metabolic function, unidentified downstream targets are the main mediators behind the benefits of bariatric surgery [59]. Further studies should be required to determine the concrete mechanism and develop pharmacotherapies that target these signaling pathways to treat metabolic disorders associated with T2DM.
The preliminary study about the change of bile acids signaling after RYGB has some limitations. Firstly, we did experiment in a rodent model which was different from humans, although this HFD/STZ-induced diabetic rat is a generally accepted T2DM rat model. Secondly, we changed the feeding regime from HFD to normal chow in both groups postoperatively which might produce better anti-diabetic and anti-fat accumulation effects in RYGB-operated rats compared with continual HFD. Thirdly, we only studied the FXR expression in the liver, as some studies reported that the changed FXR expression in the intestine might also contribute to the improved metabolism after RYGB. The last but not the least, although the expression of FXR and its related factors were accordingly changed after RYGB, this study did not functionally evaluate the FXR pathway using the antagonists. Further experimental and clinical studies are necessary to investigate the relationship between bile acids signaling and metabolic improvement in the future.
The present study demonstrated improvements in glucose homeostasis and lipid metabolism after RYGB accompanied by the elevated bile acids level, FXR, and its target transcriptional factor SHP expression. We also demonstrated that gluconeogenic enzymes and key transcriptional factor of lipogenesis were attenuated, and a key regulator of fatty acid β oxidation was increased in the liver after RYGB. Taken together, the findings in the current study suggest that the increase of plasma bile acids level followed by the activation of FXR and induction of SHP, which may result in improvement of hepatic insulin sensitivity and hepatic steatosis through regulating the key factors involved in hepatic gluconeogenesis, lipogenesis and fatty acid β oxidation. These results suggest that the effects of RYGB on metabolic improvement may be associated with the influence of the bile acids-FXR pathway in the liver in a T2DM rat model.
References
Wild SH, Walker JJ, Morling JR, et al. Cardiovascular disease, cancer, and mortality among people with type 2 diabetes and alcoholic or nonalcoholic fatty liver disease hospital admission. Diabetes Care. 2018;41(2):341–7.
Unnikrishnan R, Pradeepa R, Joshi SR, et al. Type 2 diabetes: demystifying the global epidemic. Diabetes. 2017;66(6):1432–42.
Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Cuschieri S. Type 2 diabetes - an unresolved disease across centuries contributing to a public health emergency. Diabetes Metab Syndr. 2019;13(1):450–3.
Ivers NM, Jiang M, Alloo J, et al. Diabetes Canada 2018 clinical practice guidelines: key messages for family physicians caring for patients living with type 2 diabetes. Can Fam Physician. 2019;65(1):14–24.
Buchwald H, Buchwald JN. Metabolic (bariatric and nonbariatric) surgery for type 2 diabetes: a personal perspective review. Diabetes Care. 2019;42(2):331–40.
Thrasher J. Pharmacologic management of type 2 diabetes mellitus: available therapies. Am J Med. 2017;130(6S):S4–S17.
Dixon JB, le Roux CW, Rubino F, et al. Bariatric surgery for type 2 diabetes. Lancet. 2012;379(9833):2300–11.
Dixon JB, Zimmet P, Alberti KG, et al. Bariatric surgery: an IDF statement for obese type 2 diabetes. Surg Obes Relat Dis. 2011;7(4):433–47.
Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:5934.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376(7):641–51.
Simonson DC, Halperin F, Foster K, et al. Clinical and patient-centered outcomes in obese patients with type 2 diabetes 3 years after randomization to Roux-en-Y gastric bypass surgery versus intensive lifestyle management: the SLIMM-T2D study. Diabetes Care. 2018;41(4):670–9.
Ikramuddin S, Korner J, Lee WJ, et al. Lifestyle intervention and medical management with vs without Roux-en-Y gastric bypass and control of hemoglobin A1c, LDL cholesterol, and systolic blood pressure at 5 years in the diabetes surgery study. JAMA. 2018;319(3):266–78.
Yan Y, Sha Y, Yao G, et al. Roux-en-Y gastric bypass versus medical treatment for type 2 diabetes mellitus in obese patients: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95(17):s.
Angrisani L, Santonicola A, Iovino P, et al. IFSO worldwide survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783–94.
Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2(2):152–64.
Stefanidis A, Oldfield BJ. Neuroendocrine mechanisms underlying bariatric surgery: Insights from human studies and animal models. J Neuroendocrinol. 2017;29(10).
Makaronidis JM, Batterham RL. Potential mechanisms mediating sustained weight loss following Roux-en-Y gastric bypass and sleeve gastrectomy. Endocrinol Metab Clin N Am. 2016;45(3):539–52.
Mazidi M, de Caravatto PP, Speakman JR, et al. Mechanisms of action of surgical interventions on weight-related diseases: the potential role of bile acids. Obes Surg. 2017;27(3):826–36.
Bilz S, Samuel V, Morino K, et al. Activation of the farnesoid X receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am J Physiol Endocrinol Metab. 2006;290(4):716–22.
Zhang Y, Lee FY, Barrera G, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103(4):1006–11.
Ma K, Saha PK, Chan L, et al. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116(4):1102–9.
Lee WJ, Almalki O. Recent advancements in bariatric/metabolic surgery. Ann Gastroenterol Surg. 2017;1(3):171–9.
Yu J, Zhou X, Li L, et al. The long-term effects of bariatric surgery for type 2 diabetes: systematic review and meta-analysis of randomized and non-randomized evidence. Obes Surg. 2015;25(1):143–58.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg. 2017;27(9):2279–89.
Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8:CD003641.
Seyfried F, Bueter M, Spliethoff K, et al. Roux-en Y gastric bypass is superior to duodeno-jejunal bypass in improving glycaemic control in Zucker diabetic fatty rats. Obes Surg. 2014;24(11):1888–95.
Xu B, Yan X, Shao Y, et al. A comparative study of the effect of gastric bypass, sleeve gastrectomy, and duodenal-jejunal bypass on type-2 diabetes in non-obese rats. Obes Surg. 2015;25(10):1966–75.
Zhou D, Jiang X, Jian W, et al. Comparing the effectiveness of total gastrectomy and gastric bypass on glucose metabolism in diabetic rats. Obes Surg. 2016;26(1):119–25.
Yan Y, Zhou Z, Kong F, et al. Roux-en-Y gastric bypass surgery suppresses hepatic gluconeogenesis and increases intestinal gluconeogenesis in a T2DM rat model. Obes Surg. 2016;26(11):2683–90.
Buchwald H. The evolution of metabolic/bariatric surgery. Obes Surg. 2014;24(8):1126–35.
Pucci A, Batterham RL. Mechanisms underlying the weight loss effects of RYGB and SG: similar, yet different. J Endocrinol Invest. 2019;42(2):117-28.
Mahawar KK, Sharples AJ. Contribution of malabsorption to weight loss after Roux-en-Y gastric bypass: a systematic review. Obes Surg. 2017;27(8):2194–206.
Mahawar KK. Gastric bypass is not a “restrictive and malabsorptive” procedure. Obes Surg. 2016;26(9):2225–6.
Hsu CC, Almulaifi A, Chen JC, et al. Effect of bariatric surgery vs medical treatment on type 2 diabetes in patients with body mass index lower than 35: five-year outcomes. JAMA Surg. 2015;150(12):1117–24.
Chong K, Ikramuddin S, Lee WJ, et al. National differences in remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery-subgroup analysis of 2-year results of the diabetes surgery study comparing Taiwanese with Americans with mild obesity (BMI 30-35 kg/m2). Obes Surg. 2017;27(5):1189–95.
Cummings DE, Cohen RV. Bariatric/metabolic surgery to treat type 2 diabetes in patients with a BMI <35 kg/m2. Diabetes Care. 2016;39(6):924–33.
Rao WS, Shan CX, Zhang W, et al. A meta-analysis of short-term outcomes of patients with type 2 diabetes mellitus and BMI ≤ 35 kg/m2 undergoing Roux-en-Y gastric bypass. World J Surg. 2015;39(1):223–30.
Muller-Stich BP, Senft JD, Warschkow R, et al. Surgical versus medical treatment of type 2 diabetes mellitus in nonseverely obese patients: a systematic review and meta-analysis. Ann Surg. 2015;261(3):421–9.
Pop LM, Mari A, Zhao TJ, et al. Roux-en-Y gastric bypass compared with equivalent diet restriction: mechanistic insights into diabetes remission. Diabetes Obes Metab. 2018;20(7):1710–21.
Berggren J, Lindqvist A, Hedenbro J, et al. Roux-en-Y gastric bypass versus calorie restriction: support for surgery per se as the direct contributor to altered responses of insulin and incretins to a mixed meal. Surg Obes Relat Dis. 2017;13(2):234–42.
Klebanoff MJ, Corey KE, Chhatwal J, et al. Bariatric surgery for nonalcoholic steatohepatitis: a clinical and cost-effectiveness analysis. Hepatology. 2017;65(4):1156–64.
Vix M, Diana M, Liu KH, et al. Evolution of glycolipid profile after sleeve gastrectomy vs. Roux-en-Y gastric bypass: results of a prospective randomized clinical trial. Obes Surg. 2013;23(5):613–21.
Gero D, Favre L, Allemann P, et al. Laparoscopic Roux-en-Y gastric bypass improves lipid profile and decreases cardiovascular risk: a 5-year longitudinal cohort study of 1048 patients. Obes Surg. 2018;28(3):805–11.
Carswell KA, Belgaumkar AP, Amiel SA, et al. A systematic review and meta-analysis of the effect of gastric bypass surgery on plasma lipid levels. Obes Surg. 2016;26(4):843–55.
He B, Piao D, Yu C, et al. Amelioration in hepatic insulin sensitivity by reduced hepatic lipid accumulation at short-term after Roux-en-Y gastric bypass surgery in type 2 diabetic rats. Obes Surg. 2013;23(12):2033–41.
He B, Liu L, Yu C, et al. Roux-en-Y gastric bypass reduces lipid overaccumulation in liver by upregulating hepatic autophagy in obese diabetic rats. Obes Surg. 2015;25(1):109–18.
Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap--bile acids in metabolic control. Nat Rev Endocrinol. 2014;10(8):488–98.
Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11(1):55–67.
Li G, Thomas AM, Hart SN, et al. Farnesoid X receptor activation mediates head-to-tail chromatin looping in the Nr0b2 gene encoding small heterodimer partner. Mol Endocrinol. 2010;24(7):1404–12.
Sharabi K, Tavares CD, Rines AK, et al. Molecular pathophysiology of hepatic glucose production. Mol Asp Med. 2015;46:21–33.
Arab JP, Karpen SJ, Dawson PA, et al. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology. 2017;65(1):350–62.
Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–31.
Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113(10):1408–18.
Pineda Torra I, Claudel T, Duval C, et al. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17(2):259–72.
Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–8.
Liu H, Hu C, Zhang X, et al. Role of gut microbiota, bile acids and their cross-talk in the effects of bariatric surgery on obesity and type 2 diabetes. J Diabetes Investig. 2018;9(1):13–20.
DePaoli AM, Zhou M, Kaplan DD, et al. FGF19 analogue as a surgical factor mimetic that contributes to metabolic effects beyond glucose homeostasis. Diabetes. 2019:181305.
Bozadjieva N, Heppner KM, Seeley RJ. Targeting FXR and FGF19 to treat metabolic diseases-lessons learned from bariatric surgery. Diabetes. 2018;67(9):1720–8.
Funding
This work was supported by the Science and Technology Project of Guangzhou Health and Family Planning Commission (Grant No. 20181A011027).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
They authors declare that they have no conflict of interest.
Statement of Human and Animal Rights
All applicable institutional and national guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yan, Y., Sha, Y., Huang, X. et al. Roux-en-Y Gastric Bypass Improves Metabolic Conditions in Association with Increased Serum Bile Acids Level and Hepatic Farnesoid X Receptor Expression in a T2DM Rat Model. OBES SURG 29, 2912–2922 (2019). https://doi.org/10.1007/s11695-019-03918-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-03918-0