Abstract
Background
Duodenal–jejunal bypass (DJB), which is not routinely applied in metabolic surgery, is an effective surgical procedure in terms of type 2 diabetes mellitus resolution. However, the underlying mechanisms are still undefined. Our aim was to investigate the diabetic improvement by DJB and to explore the changes in hepatic insulin signaling proteins and regulatory enzymes of gluconeogenesis after DJB in a non-obese diabetic rat model.
Methods
Sixteen adult male Goto–Kakizaki rats were randomly divided into DJB and sham-operated groups. The body weight, food intake, hormone levels, and glucose metabolism were measured. The levels of protein expression and phosphorylation of insulin receptor-beta (IR-β) and insulin receptor substrate 2 (IRS-2) were evaluated in the liver. We also detected the expression of key regulatory enzymes of gluconeogenesis [phosphoenoylpyruvate carboxykinase-1 (PCK1), glucose-6-phosphatase-alpha (G6Pase-α)] in small intestine and liver.
Results
DJB induced significant diabetic improvement with higher postprandial glucagons-like peptide 1, peptide YY, and insulin levels, but without weight loss. The DJB group exhibited increased expression and phosphorylation of IR-β and IRS-2 in liver, up-regulated the expression of PCK1 and G6Pase-α in small intestine, and down-regulated the expression of these enzymes in liver.
Conclusions
DJB is effective in up-regulating the expression of the key proteins in the hepatic insulin signaling pathway and the key regulatory enzymes of intestinal gluconeogenesis and down-regulating the expression of the key regulatory enzymes of hepatic gluconeogenesis without weight loss. Our study helps to reveal the potential role of hepatic insulin signaling pathway and intestinal gluconeogenesis in ameliorating insulin resistance after metabolic surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery induces profound and durable amelioration of obesity-related comorbid conditions, which provides a new strategy against type 2 diabetes mellitus (T2DM) [1]. As a metabolic procedure, duodenal–jejunal bypass (DJB) has also been proven effective for the treatment of T2DM in rats [2–5]. Moreover, DJB helps to improve glucose tolerance and is an effective treatment for obese or non-obese T2DM subjects [4–6]. The foregut hypothesis has been proposed to explain the role of excluding foregut in diabetes improvement after DJB surgery [2, 7]. However, the mechanisms that mediate the anti-diabetic effects after DJB surgery remain poorly understood.
T2DM is a complex glucose metabolic disease. Improvement in glucose metabolism after bariatric surgery may be achieved via many potential pathways associated with the effects of surgical intervention (Fig. 1). Multiple studies have shown that bariatric surgery improves insulin sensitivity in obese diabetic or obese nondiabetic subjects [8, 9]. Previous studies have also demonstrated that hepatic insulin resistance (reduction of hepatic glucose production) may be attributed to changes of hepatic insulin signaling [10, 11]. In accordance, the key proteins in the insulin signaling pathway such as insulin receptor (IR) and insulin receptor substrate (IRS) have been found to be down-regulated in the liver of diabetic rodents and humans [12–14].
Roux-en-Y gastric bypass (RYGB) up-regulates the expression and phosphorylation of proteins, such as IR and IRS, in hepatic insulin signaling pathway of obese diabetic rat [15]. In contrast, Gavin et al. have found that DJB does not lead to an increase in skeletal muscle insulin signal transduction 3 weeks after surgery in Goto–Kakizaki (GK) rat [16]. However, in non-obese diabetic rats, changes in the levels of the key proteins in hepatic insulin signaling pathway after DJB have not been investigated.
Gut plays a major role in glucose uptake and is involved in the regulation of glucose homeostasis [17, 18]. It has been recognized that glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PCK), the two key regulatory enzymes of gluconeogenesis, are expressed in the small intestine [19–21]. Recent studies have shown that intestinal gluconeogenesis (IG) contributes to the control of glucose and energy homeostasis [22, 23]. Interestingly, Troy et al. have found that IG is enhanced in the remodeled gut after enterogastric anastomosis under fasting state, accompanied by increased hepatic insulin sensitivity. Their finding further supports the idea that the enhancement of IG contributes to the amelioration of insulin resistance [24]. However, no study has been conducted to investigate the effects of metabolic surgery on IG in non-obese diabetic subjects.
In this study, we explored whether expressions of hepatic insulin signaling proteins and key regulatory enzymes of IG were up-regulated in non-obese type 2 diabetic rat after DJB surgery. We determined the changes in protein expression and phosphorylation of insulin receptor-beta (IR-β) and IRS-2 in the liver. Meanwhile, protein expression of phosphoenoylpyruvate carboxykinase-1 (PCK1) and G6Pase-α in the small intestine and the liver was also measured. Homeostasis model assessment insulin resistance (HOMA-IR) was also calculated to evaluate insulin resistance [25]. Our results might provide new insight in elucidating the metabolic mechanisms of diabetes resolution after DJB surgery.
Materials and Methods
Animal Model and Diet Protocol
This animal study was approved by the Animal Care and Utilization Committee of Shandong University. Nine-week-old male GK rats (National Rodent Laboratory Animal Resources, Shanghai, China) were acclimated to experiment conditions. All rats were kept in individual cages under standard conditions (constant ambient temperature at 22 °C and humidity at 60 % on a 12-h light/dark cycle) in Shandong University and were fed with 5 % fat rat chow diet (National Rodent Laboratory Animal Resources, Shanghai, China) and water ad libitum before operation. After 2 weeks of acclimatization, 16 GK rats were randomized to two groups: the DJB group and the sham-operated DJB group.
Surgical Techniques
All rats were given non-residue diet for 2 days and fasted overnight pre-operatively. During surgery, the rats were anesthetized with 10 % chloral hydrate solution. Subsequently, DJB was performed as described previously [2]. Specifically, it involved (Fig. 2b) (1) a 4-cm midline epigastric incision, (2) transection of the duodenum just distal to the pylorus and suture of its proximal end using 7-0 silk suture (Ningbo medical needle, China), (3) transection at the plane of the distal jejunum (10 cm from the ligament of Treitz), (4) connecting the distal limb to the pylorus, and (5) a jejunojejunal anastomosis (connecting the proximal limb with jejunum at 10 cm distally).
Operations and intestinal sampling locations. a Sham operation. The locations of four removed segments (jejunum 1, jejunum 2, ileum 1, and ileum 2) of intestine in sham-operated rats corresponded to those of DJB rats. b Duodenal–jejunal bypass (DJB). The locations of four removed segments (jejunum 1, jejunum 2, ileum 1, and ileum 2) of intestine are shown in the figure. The length of each segment is 10 cm
Sham surgeries involved (Fig. 2a) the same abdominal incisions and gastrointestinal transections. Furthermore, no removal was made, and all reanastomosis were done in the same sites as in the DJB procedures. In addition, the operation time of rats in the sham group was prolonged in order to obtain similar operative stress as that of the rats in the DJB group.
All rats were allowed free access to water at 2 h postsurgery. Twenty-four hours after surgery, all rats were given non-residue diet (Ensure, Abbott, USA) for 3 days. Then, normal diet (5 % fat rat chow diet, National Rodent Laboratory Animal Resources, Shanghai, China) was not limited. Weight and food intake were recorded everyday in the first 2 weeks and then once every 2 weeks for the following times. All postoperative complications were recorded carefully.
Biochemical Tests
Hormones
At 2 and 20 weeks postoperation, blood samples were collected from the tail vein of conscious rats into chilled tubes containing a dipeptidyl peptidase IV inhibitor in EDTA solution at baseline and 30, 60, and 120 min after 1 g/kg glucose gavage. After centrifugation (1,006 × g) at 4 °C for 15 min, serum was immediately extracted and stored at −80 °C. Insulin secretion was measured using enzyme-linked immunosorbent assay (ELISA) kits (Millipore, MA, USA). Gut hormones involving glucagon-like peptide 1 (GLP-1), peptide YY (PYY), and glucose-dependent-insulinotropic peptide (GIP) were measured using ELISA kits (Uscn Life Science Inc., Wuhan, China).
Oral Glucose Tolerance Test
Oral glucose tolerance test (OGTT) was performed at 2, 4, 8, 12, 16, and 20 weeks after surgery. Blood glucose was measured in conscious rats at baseline and at 10, 30, 60, 120, and 180 min after the administration of 1 g/kg glucose by oral gavage using a glucometer (Roche One Touch® Ultra, Lifescan, Johnson & Johnson, Milpitas, CA, USA).
Insulin Tolerance Test
Insulin Tolerance Test (ITT) was performed at 2, 4, 8, 12, 16, and 20 weeks postoperation by measuring glucose levels at baseline and at 10, 30, 60, 120, and 180 min after injection of 0.5 IU/kg human insulin intraperitoneally in conscious and fasted rats.
Homeostasis Model Assessment Insulin Resistance
At 2 and 20 weeks postoperation, HOMA-IR was calculated to evaluate insulin resistance according to the formula: HOMA − IR = fasting insulin(mU/L) × fasting glucose(mmol/L)/22.5 [25].
Western Blotting
All rats were sacrificed at 20 weeks postoperation. Tissue sampling was performed in anesthetized rats (a peritoneal injection of 10 % chloral hydrate solution). The small intestine was rapidly removed and rinsed with 0.9 % sodium chloride at 4 °C. Four segments (jejunum 1, jejunum 2, ileum 1, and ileum 2) of intestine of rats in the DJB group were removed and separated (Fig. 2a, b). The length of each segment was 10 cm. Meanwhile, the corresponding segments of intestine of sham-operated rats were obtained, too. Subsequently, the mucosa was scraped off with a cold spatula at 4 °C as previously described [19]. The liver was also rapidly removed, immediately frozen in liquid nitrogen, and stored at −80 °C until analysis.
Thirty milligrams of liver tissue and intestinal (jejunum 1, jejunum 2, ileum 1, and ileum 2) mucosa tissue was resuspended in Laemmli sample buffer containing 100 mM dithiothreitol (Beyotime, Shanghai, China). Then, the samples were heated at 100 °C for 3 min and centrifuged at 4 °C for 10 min (13,000 rpm). Protein extracts were resolved on 10 % SDS-PAGE gels (Beyotime, Shanghai, China) and then transferred to PVDF membranes (Millipore, MA, USA). Proteins were detected by the following antibodies: anti-G6Pase-α, anti-IR-β, anti-p-IR-β, anti-IRS-2, anti-p-IRS-2, anti-β-actin (Santa Cruz, CA, USA), and anti-PCK1 (Abcam, MA, USA) antibodies. After incubation at 4 °C overnight with primary antibody, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz, CA, USA) for 1 h. Then, HRP activity was assessed using ECL solution (Millipore, MA, USA) and exposed to film. The image was scanned, and the band intensity was quantified with the ImageJ software (http://rsb.info.nih.gov/ij; National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
All statistical analyses were performed using SPSS version 13.0. The results were reported as mean ± SD. Areas under curves (AUC) for OGTT (AUCOGTT) and ITT (AUCITT) were calculated by trapezoidal integration. Statistical analysis was evaluated using Student's t-test. P < 0.05 represented statistically significant difference in all cases.
Results
General Evaluation of Surgery
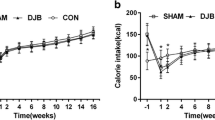
All operations were successful. There was no significant difference in the body weight between the DJB group and the sham group postoperation (Fig. 3a). Meanwhile, no significant difference was observed between the two groups in food intake at any time point after surgery (Fig. 3b). Two DJB rats were found dead due to intestinal obstruction on the 5th and 12th day after operation, respectively. Moreover, one rat in the sham group died from intraperitoneal infection 17 weeks after surgery.
Body weight and food intake. a Body weight of rats in two groups before and after surgery. No difference in body weight was observed between DJB and sham groups at all measuring time points. b Food intake of rats in two groups before and after surgery. Food intake of two groups did not differ from each other at all measuring time points
Glucoregulatory Hormones
As shown in Fig. 4a, b, higher GLP-1 levels were detected in the DJB group than in the sham group 2 and 20 weeks postoperation after oral glucose gavages. Furthermore, at 20 weeks postoperation, the rats in the DJB group showed higher fasting GLP-1 levels than those in the sham group. In addition, DJB surgery induced higher levels of fasting and glucose-stimulated PYY secretion at 2 and 20 weeks postoperation (Fig. 4c, d). However, the serum levels of GIP in the DJB group were comparable with those in the sham group (Fig. 4e, f).
Glucose-stimulated GLP-1, PYY, and GIP secretion of rats in two groups at 2 and 20 weeks after surgery. a, b Serum GLP-1 concentrations in response to oral glucose gavage (1 g/kg) at 2 and 20 weeks after surgery. The GLP-1 levels of rats in DJB group were higher than in sham group at 30, 60, and 120 min after oral glucose gavage at 2 weeks postoperation. The GLP-1 levels of rats in DJB group were higher than in sham group at 0, 30, 60, at 120 min at week 20 postoperatively. c, d Serum PYY concentrations after an oral glucose gavage (1 g/kg) at 2 and 20 weeks after surgery. The fasting and glucose-stimulated PYY secretions of rats in DJB group were higher than in sham-operated group at 2 and 20 weeks postoperatively. e, f Glucose-stimulated serum GIP secretion at 2 and 20 weeks after surgery. The GIP levels were not significantly different between two groups. *p < 0.05; **p < 0.01
At 2 weeks postoperation, postprandial insulin levels in the two groups were similar (Fig. 5a). However, at 20 weeks postoperation, the DJB group showed significantly higher levels of insulin than those in the sham group at any measuring time (0, 30, 60, and 120 min) (Fig. 5b).
Glucose-stimulated insulin secretion of rats in two groups after surgery and glucose metabolism parameters. a, b Serum insulin concentrations after an oral glucose gavage (1 g/kg) at 2 and 20 weeks after surgery. The insulin levels of rats between two groups were not significantly different at 2 weeks postoperatively. The serum insulin concentrations of rats in DJB group were higher than in sham group at all measuring times at 20 weeks postoperatively. c, d AUCOGTT and AUCITT. AUCOGTT and AUCITT values of rats in DJB group were lower than in sham group at 2, 4, 8, 12, 16, and 20 weeks postoperatively. e Fasting plasma glucose. The DJB rats showed lower fasting plasma glucose levels than sham-operated rats at 2, 4, 8, 12, 16, and 20 weeks postoperatively. f HOMA-IR. At week 2 and 20 postoperatively, the HOMA-IR values of rats in DJB group were lower than in sham group. *p < 0.05; **p < 0.01
Glucose Metabolism
There were no significant differences in AUCOGTT and AUCITT between the two groups preoperation. However, the rats in the DJB group demonstrated significant improvement in glucose tolerance and insulin tolerance, as shown by lower AUCOGTT and AUCITT values than those in the sham group, at 2, 4, 8, 12, 16, and 20 weeks after operation (Fig. 5c, d).
Compared with the sham-operated rats, the DJB groups showed lower fasting glucose levels at 2, 4, 8, 12, 16, and 20 weeks after surgery (Fig. 5e). Moreover, at 2 and 20 weeks postoperation, the DJB group showed significantly lower HOMA-IR levels than those of the sham group (Fig. 5f), indicating that hepatic insulin resistance was ameliorated after DJB surgery.
Effect of DJB on the Expression of Key Proteins in the Hepatic Insulin Signaling Pathway
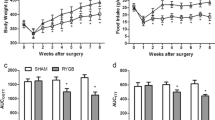
The expression of IR-β protein in liver was detected 20 weeks after operation. In the DJB rats versus sham, both the expression and the phosphorylation levels of IR-β were significantly higher than those in the sham group (Fig. 6a). In addition, as shown in Fig. 6b, the DJB group showed higher levels of expression and phosphorylation of IRS-2 in liver than the sham group at 20 weeks postoperation.
Effect of DJB on the expression of key proteins in the hepatic insulin signaling and the key regulatory enzymes of hepatic gluconeogenesis. a, b Effect of DJB on IR-β and IRS-2 expression and their phosphorylation states. DJB rats showed higher expression and phosphorylation of IR-β in liver than those of sham group at postoperative week 20. Compared with sham-operated rats, significant increases occurred in expression and phosphorylation of IRS2 in liver in DJB rats at postoperative week 20. c, d Effect of DJB on key regulatory enzymes of hepatic gluconeogenesis (PCK1 and G6Pase-α). At postoperative week 20, DJB rats exhibited lower expression of PCK1 and G6Pase-α in liver than the sham-operated rats. *p < 0.05; **p < 0.01
Effect of DJB on the Expressions of the Key Regulatory Enzymes of Gluconeogenesis
At 20 weeks postoperation, the DJB rats exhibited lower levels of PCK1 expression in liver than the sham-operated rats (Fig. 6c). Similarly, a significant difference in the expression of hepatic G6Pase-α was observed between the two groups (Fig. 6d).
By contrast, DJB surgery induced higher expressions of PCK1 and G6Pase-α in small intestine. Figure 7 shows the changes of PCK1 and G6Pase-α in jejunum 1, jejunum 2, ileum 1, and ileum 2 at 20 weeks postoperation. The rats in the DJB group showed higher levels of PCK1 expression in the four segments of small intestine than the sham-operated rats (Fig. 7a). Increases in the expression levels of G6Pase-α were detected in all four intestinal segments, but statistically significant differences were detected between the two groups only, in jejunum 1 and ileum 2 (Fig. 7b).
Effect of DJB on the expression of the key regulatory enzymes of intestinal gluconeogenesis. a Effect of DJB on expression of PCK1 in small intestine. At 20 weeks postoperation, DJB rats showed higher expression of PCK1 in the four segments of small intestine (jejunum 1, jejunum 2, ileum 1, and ileum 2) than sham-operated rats. b Effect of DJB on expression of G6Pase-α in small intestine. DJB rats exhibited higher expression of G6Pase-α in jejunum 1 and ileum 2 than sham-operated rats. *p < 0.05; **p < 0.01
Discussion
Duodenal–jejunal bypass surgery has been proven effective in the control of diabetes for non-obese type 2 diabetic subjects [2, 5, 6, 26]. However, the underlying molecular mechanisms are still unclear. In the present study, we investigated the effects of DJB to further understand the mechanisms of diabetic improvement after metabolic surgery in GK rats. To our knowledge, this is the first study on the changes of hepatic insulin signaling proteins and key regulatory enzymes of intestinal gluconeogenesis after DJB surgery in a non-obese T2DM rat model.
The present study provided evidence that DJB surgery induced sustained anti-diabetic effects, supported by the lower HOMA-IR, OGTT, and ITT values in the DJB group. However, no differences in the insulin levels were observed between the two groups at 2 weeks after operation. Moreover, the DJB group did not show lower body weight or less food intake than the sham group. Similarly, previous studies showed that DJB procedure improves glucose tolerance without weight loss [2, 4]. Therefore, improved insulin sensitivity and diabetes improvement after DJB may be independent of weight loss. Furthermore, our data suggest that the early improvement in insulin sensitivity is due to the amelioration of hepatic insulin resistance.
In addition, we evaluated the levels of several intestinal hormones. Rats in the DJB group demonstrated increased glucose-stimulated GLP-1 and PYY levels. Similar results have been found on rats after other metabolic surgeries [27–29]. Furthermore, previous studies have shown that elevated GLP-1 and PYY can ameliorate insulin resistance [28, 29]. Although Salinari et al. have observed a decrease in GIP levels after bariatric surgery [30], no differences in plasma GIP levels between DJB and sham groups are observed in our study, which is consistent with the results of some previous studies [31, 32]. These results indicate that GIP may not be involved in the anti-diabetic effect after bypass of the foregut.
An important result of the present study was that DJB surgery up-regulated the expression and phosphorylation of the proteins in the hepatic insulin signaling pathway, including IR-β and IRS-2 in the liver in GK rats. There are very few studies on changes of insulin signaling transduction after metabolic surgery. Bonhomme et al. found that IR is up-regulated in the liver after RYGB surgery in an obese diabetic animal model and that RYGB induces a time-related increase in p-IR and pIRS1/2 in liver [15]. Another study has shown that the increased expression of IR-β and IRS-1 in the myocardium after ileal transposition is associated with myocardial insulin sensitivity [33]. In contrast, Gavin et al. have found that DJB does not increase glucose disposal and insulin signal transduction in skeletal muscle 3 weeks after surgery in GK rats [16]. We speculated that the different results between their study and ours might be related with the time when insulin signal transduction was detected. DJB may not induce a significant increase in the expression of some proteins in the insulin signal pathway early after surgery in GK rats. It has been proven that the down-regulation of IRS-2 signaling is related to type 2 diabetes, especially insulin resistance [13, 34, 35]. Our results provided evidence to support that hepatic insulin signaling was involved in the amelioration of hepatic insulin resistance.
We also investigated the effect of DJB surgery on the key regulatory enzymes of gluconeogenesis in GK rats. We firstly detected the expression of PCK1 and G6Pase-α in the liver. PCK1, also called phosphoenolpyruvate carboxykinase, is well known for its exclusive function in the regulation of gluconeogenesis [20, 36]. Previous studies have demonstrated that the over-expression of PEPCK in the liver of mice aggravates insulin resistance [37]. G6Pase is the other crucial gluconeogenesis enzyme in the control of glucose homeostasis [17]. Trinh et al. have found that the over-expression of G6Pase in liver is sufficient to perturb the regulation of endogenous glucose production and possibly contributes to insulin resistance [38]. Given that hepatic gluconeogenesis plays a role in the development of insulin resistance, the down-regulation of hepatic gluconeogenesis may be involved in the regulation of insulin resistance by DJB surgery. As expected, our study displayed that DJB surgery caused decreases in the protein expressions of PCK1 and G6Pase-α in the liver of GK rats.
In the present study, we also observed changes in the key regulatory enzymes of gluconeogenesis in the small intestine after DJB surgery. The DJB rats showed higher levels of PCK1 expression in the four segments of small intestine than the sham rats. The expression of G6Pase-α increased in all four intestinal segments, although statistical significant differences were only detected in jejunum 1 and ileum 2 between the two groups. It is well accepted that the small intestine also contributes to the endogenous glucose production through the role of IG [17, 18]. Recently, a novel function of IG in the control of glucose homeostasis has been confirmed [22, 23]. Furthermore, Troy et al. have found that enhanced intestinal gluconeogenesis after gastric bypass is linked to improved insulin sensitivity in mice [24]. Our results indicated that DJB surgery increased the expression of PCK1 and G6Pase-α in the small intestine in GK rats, and the results supported the hypothesis that the up-regulated IG might be a potential contributor to ameliorate insulin resistance after metabolic surgery.
However, the exact route by which IG contributes to glucose homeostasis remains unclear. Mithieux has hypothesized that the enhancement of IG after gastric bypass contributes to ameliorating insulin resistance [39], but this hypothesis has been challenged [40]. In addition, another study showed that hepatic vagal afferent fibers is not the only pathway to transmit the hepatic glucose signaling to brain after RYGB in Sprague–Dawley rats [41]. Further studies should be conducted to determine the concrete mechanism.
In summary, we provided a valuable rat model for studying the mechanisms of diabetes improvement in the absence of weight loss. Our findings indicated that DJB up-regulated the key regulatory enzymes of gluconeogenesis in small intestine and the key proteins in the hepatic insulin signaling pathway while down-regulating the key regulatory enzymes of gluconeogenesis in liver in GK rats. These results support the potential role of hepatic insulin signaling and IG in ameliorating insulin resistance after metabolic surgery.
References
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76.
Rubino F, Marescaux J. Effect of duodenal–jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239(1):1–11.
Ramos AC, Galvao Neto MP, de Souza YM, et al. Laparoscopic duodenal–jejunal exclusion in the treatment of type 2 diabetes mellitus in patients with BMI < 30 kg/m2 (LBMI). Obes Surg. 2009;19(3):307–12.
Kindel TL, Yoder SM, Seeley RJ, et al. Duodenal–jejunal exclusion improves glucose tolerance in the diabetic, Goto–Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg. 2009;13(10):1762–72.
de Luis D, Domingo M, Romero A, et al. Effects of duodenal–jejunal exclusion on beta cell function and hormonal regulation in Goto–Kakizaki rats. Am J Surg. 2012;204(2):242–7.
de Moura EG, Martins BC, Lopes GS, et al. Metabolic improvements in obese type 2 diabetes subjects implanted for 1 year with an endoscopically deployed duodenal–jejunal bypass liner. Diabetes Technol Ther. 2012;14(2):183–9.
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244(5):741–9.
Castagneto-Gissey L, Mingrone G. Insulin sensitivity and secretion modifications after bariatric surgery. J Endocrinol Investig. 2012;35(7):692–8.
van Dielen FM, Nijhuis J, Rensen SS, et al. Early insulin sensitivity after restrictive bariatric surgery, inconsistency between HOMA-IR and steady-state plasma glucose levels. Surg Obes Relat Dis. 2010;6(4):340–4.
Smith U, Axelsen M, Carvalho E, et al. Insulin signaling and action in fat cells: associations with insulin resistance and type 2 diabetes. Ann N Y Acad Sci. 1999;892:119–26.
Fick LJ, Belsham DD. Nutrient sensing and insulin signaling in neuropeptide-expressing immortalized, hypothalamic neurons: a cellular model of insulin resistance. Cell Cycle. 2010;9(16):3186–93.
Rondinone CM, Wang LM, Lonnroth P, et al. Insulin receptor substrate (IRS) 1 is reduced and IRS-2 is the main docking protein for phosphatidylinositol 3-kinase in adipocytes from subjects with non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1997;94(8):4171–5.
Withers DJ, Gutierrez JS, Towery H, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391(6670):900–4.
Suzuki R, Tobe K, Aoyama M, et al. Both insulin signaling defects in the liver and obesity contribute to insulin resistance and cause diabetes in Irs2(-/-) mice. J Biol Chem. 2004;279(24):25039–49.
Bonhomme S, Guijarro A, Keslacy S, et al. Gastric bypass up-regulates insulin signaling pathway. Nutrition. 2011;27(1):73–80.
Gavin TP, Sloan 3rd RC, Lukosius EZ, et al. Duodenal–jejunal bypass surgery does not increase skeletal muscle insulin signal transduction or glucose disposal in Goto–Kakizaki type 2 diabetic rats. Obes Surg. 2011;21(2):231–7.
Croset M, Rajas F, Zitoun C, et al. Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes. 2001;50(4):740–6.
Mithieux G. New data and concepts on glutamine and glucose metabolism in the gut. Curr Opin Clin Nutr Metab Care. 2001;4(4):267–71.
Rajas F, Bruni N, Montano S, et al. The glucose-6 phosphatase gene is expressed in human and rat small intestine: regulation of expression in fasted and diabetic rats. Gastroenterology. 1999;117(1):132–9.
Rajas F, Croset M, Zitoun C, et al. Induction of PEPCK gene expression in insulinopenia in rat small intestine. Diabetes. 2000;49(7):1165–8.
Mithieux G, Bady I, Gautier A, et al. Induction of control genes in intestinal gluconeogenesis is sequential during fasting and maximal in diabetes. Am J Physiol Endocrinol Metab. 2004;286(3):E370–5.
Mithieux G. The new functions of the gut in the control of glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2005;8(4):445–9.
Mithieux G, Andreelli F, Magnan C. Intestinal gluconeogenesis: key signal of central control of energy and glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2009;12(4):419–23.
Troy S, Soty M, Ribeiro L, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8(3):201–11.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Donglei Z, Liesheng L, Xun J, et al. Effects and mechanism of duodenal–jejunal bypass and sleeve gastrectomy on GLUT2 and glucokinase in diabetic Goto–Kakizaki rats. Eur J Med Res. 2012;17(1):15.
Shin AC, Zheng H, Townsend RL, et al. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151(4):1588–97.
van den Hoek AM, Heijboer AC, Voshol PJ, et al. Chronic PYY3-36 treatment promotes fat oxidation and ameliorates insulin resistance in C57BL6 mice. Am J Physiol Endocrinol Metab. 2007;292(1):E238–45.
Mingrone G. Role of the incretin system in the remission of type 2 diabetes following bariatric surgery. Nutr Metab Cardiovasc Dis. 2008;18(8):574–9.
Salinari S, Bertuzzi A, Asnaghi S, et al. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32(3):375–80.
Pacheco D, de Luis DA, Romero A, et al. The effects of duodenal–jejunal exclusion on hormonal regulation of glucose metabolism in Goto–Kakizaki rats. Am J Surg. 2007;194(2):221–4.
Clements RH, Gonzalez QH, Long CI, et al. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70(1):1–4. discussion 4-5.
Yan Z, Chen W, Liu S, et al. Myocardial insulin signaling and glucose transport are up-regulated in Goto–Kakizaki type 2 diabetic rats after ileal transposition. Obes Surg. 2012;22(3):493–501.
Valverde AM, Burks DJ, Fabregat I, et al. Molecular mechanisms of insulin resistance in IRS-2-deficient hepatocytes. Diabetes. 2003;52(9):2239–48.
Hatakeyama H, Kanzaki M. Molecular basis of insulin-responsive GLUT4 trafficking systems revealed by single molecule imaging. Traffic. 2011;12(12):1805–20.
She P, Burgess SC, Shiota M, et al. Mechanisms by which liver-specific PEPCK knockout mice preserve euglycemia during starvation. Diabetes. 2003;52(7):1649–54.
Xu H, Yang Q, Shen M, et al. Dual specificity MAPK phosphatase 3 activates PEPCK gene transcription and increases gluconeogenesis in rat hepatoma cells. J Biol Chem. 2005;280(43):36013–8.
Trinh KY, O'Doherty RM, Anderson P, et al. Perturbation of fuel homeostasis caused by overexpression of the glucose-6-phosphatase catalytic subunit in liver of normal rats. J Biol Chem. 1998;273(47):31615–20.
Mithieux G. A synergy between incretin effect and intestinal gluconeogenesis accounting for the rapid metabolic benefits of gastric bypass surgery. Curr Diab Rep. 2012;12(2):167–71.
Hayes MT, Foo J, Besic V, et al. Is intestinal gluconeogenesis a key factor in the early changes in glucose homeostasis following gastric bypass? Obes Surg. 2011;21(6):759–62.
Shin AC, Zheng H, Berthoud HR. Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism. Ann Surg. 2012;255(2):294–301.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81270888/H0713), Specialized Research Fund for the Doctoral Program of Higher Education (no. 20100131110049), Shandong Provincial Outstanding Medical Academic Professional Program, Natural Science Foundation of Shandong Province grants (no. ZR2012HQ030), and Graduate Independent Innovation Foundation of Shandong University (no. yzc11068).
Conflict of Interest
We declare that all authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dong Sun and Kexin Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sun, D., Wang, K., Yan, Z. et al. Duodenal–Jejunal Bypass Surgery Up-Regulates the Expression of the Hepatic Insulin Signaling Proteins and the Key Regulatory Enzymes of Intestinal Gluconeogenesis in Diabetic Goto–Kakizaki Rats. OBES SURG 23, 1734–1742 (2013). https://doi.org/10.1007/s11695-013-0985-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-013-0985-0