Abstract
Background
In a recent study, we showed a jejuno-ileal circuit (JIC) procedure that effectively improved glucose homeostasis, but the intrinsic mechanism requires further studies. Furthermore, the role of JIC in lipid metabolism is also unknown. Given that adiposity aggravates insulin sensitivity, we hypothesize that the JIC procedure improves fat metabolism and thus further contributes to diabetic remission. The aim of this study was to investigate the effects of JIC surgery on lipid metabolism and glucose homeostasis in a non-obese diabetic rat model.
Methods
Fourteen high-fat diet and low-dose streptozotocin-induced diabetic rats were randomly divided into JIC and sham-JIC groups. Body weight, food intake, glucose tolerance, insulin resistance, serum lipid parameters, glucagon-like peptide 1 (GLP-1), and adipose-derived hormones were measured. At 12 weeks postoperatively, the expressions of hepatic fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) were measured by Western blot. The lipid content of liver was assessed by hematoxylin-eosin staining and Oil Red O staining. The enteroendocrine cells in the distal ileum were examined by immunohistochemical staining.
Results
Relative to the sham group, the JIC rats exhibited significant improvements in glucose tolerance, insulin resistance, and dyslipidemia without weight loss, showing increased GLP-1 and adiponectin and decreased leptin. JIC also reduced the expression of FAS and ACC in the liver, exhibited improved hepatic fat content, and raised the levels of GLP-1 and chromogranin A in the distal gut.
Conclusions
JIC alleviated lipometabolic disorders in hyperglycemic rats, which may contribute to the amelioration of insulin sensitivity and glycemic control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus and lipid dysmetabolism are currently of substantial threat to human health, and the growing prevalence of which are leading to huge burdens on morbidity and mortality [1, 2]. As components of metabolic syndrome, these two sorts of metabolic disorders are not independent diseases but rather interact as both causes and effects. A trial by Imamura et al. [3] indicated that fatty liver is implicated in the occurrence of diabetes, whereas Leite et al. [4] reported that diabetic patients are at a high risk of steatohepatitis. Moreover, insulin resistance gives rise to dysfunctional glucolipid metabolism, which in turn impairs insulin sensitivity [5, 6].
Since traditional methods such as diet, physical activity, and drugs failed to attain long-term therapeutic effect on diabetes and dyslipidemia, there is increased demand for better therapy. Myronovych and colleagues [7] found that mice with vertical sleeve gastrectomy (VSG) get hepatic steatosis relief independently of weight reduction. Similarly, Pezeshki et al. [8] noted that both Roux-en-Y gastric bypass (RYGB) and ileal transposition (IT) improve glycometabolism and lipometabolism in liver and muscle. Hence, metabolic surgery is currently accepted as one of the best way to treat the aforementioned metabolic abnormalities [9, 10].
Recently, we reported a novel surgical procedure [11] which creates a side-to-side anastomosis between the jejunum and distal ileum known as the jejuno-ileal circuit (JIC). Independently of body weight and energy intake, this procedure alleviates hyperglycemia as it accelerates chyme transit and generates gut hormones, in accordance with the well-known “hindgut hypothesis” [12]. The effect of JIC surgery on lipid metabolism, however, is still poorly understood. Given that the JIC procedure, as reported [11], reduces insulin resistance that can worsen dyslipidemia [13] and improves liver function which may be affected by fatty liver [2], we hypothesize that JIC improves lipid homeostasis. To test this hypothesis, we measured adipose-derived hormones, enteroendocrine cells, and liver fat deposition in a widely used high-fat diet (HFD) and low-dose streptozotocin (STZ)-induced diabetic rat model [14]. Due to the interaction between hyperglycemia and dyslipidemia, in addition to recovered insulin sensitivity and enhanced incretin effect, etc., the improved fat metabolism may be one of the plausible explanations for diabetic remission after JIC.
Materials and Methods
Animal Model and Diet Protocol
The animal protocol was approved by the Animal Care and Utilization Committee of Shandong University. Seven-week-old male Wistar rats were housed separately in ventilated cages under controlled temperature and humidity in a 12-h light/dark cycle. All rats had free access to tap water and HFD (40 % of calories as fat; Huafukang Biotech Company, China) for 6 weeks, followed by an intraperitoneal injection of STZ (30 mg/kg; Sigma, USA). Seventy-two hours thereafter, non-fasting blood glucose was measured in duplicate using a glucometer (Roche Diagnostics, Germany) from the tail vein. The rats with non-fasting blood glucose ≥16.7 mmol/L were considered diabetic.
Surgical Procedures
Fourteen diabetic rats were randomly assigned into the JIC group and sham-JIC group (N = 7 for each). After a 24-h fast, the rats were anesthetized with an intraperitoneal injection of chloral hydrate solution (0.03 g/100 g) and then underwent JIC or sham procedures. JIC surgery was performed as previously described [11]. Specifically, after a 3-cm midline epigastric laparotomy, the jejunum 5 cm distal to the ligament of Treitz and the ileum 15 cm proximal to the ileocecal junction were positioned. Two enterotomies were performed on the above-mentioned intestines, followed by a side-to-side anastomosis. Sham surgery involved the same enterotomies as in the JIC group, but sutures in situ were performed instead of anastomosis. The operative time of sham surgery was prolonged similarly to the JIC group to ensure an equivalent degree of intraoperative trauma.
The rats were only given access to water in the first 24 h postoperatively, with 2 mL 10 % glucose and Ringer’s solution in subcutaneous injection. The rats were then fed 10 % Ensure (Abbott Laboratories, USA) for 2 days, followed by HDF till the scheduled termination date. Body weight and HFD intake were recorded daily in the first 2 weeks after surgery and then twice a week for the rest of the study.
Oral Glucose Tolerance Test
Oral glucose tolerance test (OGTT) was performed preoperatively and at 4 and 12 weeks post-surgery. After an overnight fast, the rats were perorally infused 20 % glucose (1 g/kg). Blood glucose of the tail vein samples was measured before gavage and at 10, 30, 60, 90, and 120 min after infusion.
Insulin Tolerance Test
Insulin tolerance test (ITT) was performed before operation and at postoperative 4 and 12 weeks. Blood glucose was measured in conscious and fasted rats, and then at 10, 30, 60, 90, and 120 min after an intraperitoneal injection with insulin solution (0.5 IU/kg).
Blood Sampling and Serum Parameters
At 4 and 12 weeks postoperatively, the rats were fasted overnight and administrated with 10 % Ensure solution (1 mL/100 g). Blood samples were collected from retrobulbar venous plexus into chilled tubes at baseline and 10, 30, 60, and 120 min after intragastric gavage of Ensure. After centrifugation (1006×g) at 4 °C for 15 min, the supernatant was immediately collected and stored at −80 °C for future analysis. The levels of plasma triglyceride, cholesterol, free fatty acid (FFA), and total bile acids (TBA) were measured under an automatic biochemical analyzer (Hitachi, Japan). Serum glucagon-like peptide 1 (GLP-1), adiponectin, and leptin were tested using enzyme-linked immunosorbent assay kits (Millipore, USA).
Tissue Sampling
All rats were sacrificed at postoperative 12 weeks. The liver was removed and fully washed with sterile saline solution, cut into small pieces, immediately fixed with 10 % formalin or frozen in liquid nitrogen, and stored at −80 °C in Eppendorf tubes for later analysis. The distal ileum was rapidly removed and gently rinsed, then fixed and embedded in paraffin.
Western Blotting
Liver total protein was extracted by RIPA lysis buffer (Beyotime, China). Equivalent amounts of samples were loaded on SDS-PAGE gels (Beyotime, China) and separated by electrophoresis. The following primary antibodies were used: anti-fatty acid synthase (anti-FAS; Abcam, USA), anti-acetyl-CoA carboxylase (anti-ACC; Cell Signaling Technology, USA), and anti-GAPDH (Proteintech, China). After incubation at 4 °C overnight, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (ZSGB-BIO, China) for 1 h. The protein bands were then visualized by ECL solution (Millipore, USA) and detected by FluorChem E System (Alpha Innotech, USA). The band intensity was quantified with ImageJ software.
Hematoxylin-Eosin Staining and Oil Red O Staining
Paraffin-embedded hepatic samples underwent hematoxylin-eosin (HE) staining, while dried frozen sections of liver tissue were fixed by formalin and then utilized for Oil Red O staining. The frozen sections were immersed into freshly prepared Oil Red O solution (Solarbio, China) for 10 min under 60 °C. After rinses using isopropanol and distilled water, hematoxylin (ZSGB-BIO, China) was used to stain the nuclei. Finally, the sections were observed and photographed under an Olympus X71 microscope (Olympus, Japan) using the OLYMPUS DP2-BSW software.
Immunohistochemical Staining
Paraffin-embedded intestinal samples were sectioned at 5 μm thickness. Sections were subjected to antigen retrieval in Tris/EDTA buffer (1:50; ZSGB-BIO, China) and blocked by goat serum, then incubated with anti-chromogranin A (CgA) antibody (1:100) and anti-GLP-1 antibody (1:1000; abcam, USA) as primary antibodies. After overnight storage at 4 °C incubation, HRP-conjugated secondary antibody was used before staining DAB and hematoxylin (ZSGB-BIO, China). Brown-yellow granules viewed under a microscope were considered as positive expression, and the mean density was measured by Image-Pro Plus.
Statistical Analysis
The results were expressed as mean ± standard deviation. Areas under curves (AUC) for OGTT (AUCOGTT) and ITT (AUCITT) were calculated by trapezoidal integration. Differences between groups were evaluated by Student’s t test except for GLP-1 concentration after Ensure administration, which was analyzed by two-way ANOVA. P < 0.05 represented statistically significant difference. SPSS 19.0 was used for statistical analysis.
Results
Operative Results
All surgeries were performed successfully. Before the end point, however, one rat undergoing JIC was dead because of leakage, and a sham-operated rat died from exhaustion.
Body Weight and Food Intake
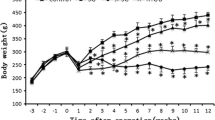
During the entire experiment, there were no significant differences between groups in both body weight and food intake either before or after surgeries (Fig. 1).
Glucose Metabolism and Insulin Resistance
As shown in Fig. 2, no difference in AUCOGTT and AUCITT was found between JIC and sham groups before operation. At 4 and 12 weeks after surgery, AUCOGTT and AUCITT were markedly decreased in the JIC group when compared with sham, however, indicating improvements of glucose tolerance and insulin sensitivity.
AUCOGTT and AUCITT. a AUCOGTT values of rats before and at 4 and 12 weeks after surgery. No difference was found between the JIC and sham groups preoperatively. At 4 and 12 weeks postoperatively, the values were significantly lower in the JIC group than in the sham group. b AUCITT values of rats before and at 4 and 12 weeks after surgery. No difference was observed between groups preoperatively. At 4 and 12 weeks postoperatively, the values were significantly lower in the JIC group relative to the sham group. **P < 0.01 vs. sham. N = 6 in each group
Serum Hormone Parameters
Figure 3a, b shows that the rats in the JIC group exhibited significantly elevated fasting and postprandial concentrations of plasma GLP-1 at 4 and 12 weeks after surgery. At the same time, fasting serum leptin was significantly decreased in the JIC group relative to sham (Fig. 3c). At postoperative 12 weeks, the levels of fasting adiponectin of JIC rats were higher than those of sham as shown in Fig. 3d.
Plasma levels of GLP-1, leptin, and adiponectin. a, b Ensure-stimulated GLP-1 levels at postoperative 4 and 12 weeks. As compared to sham, the GLP-1 level was significantly higher in the JIC group at both 4 and 12 weeks after surgery. c Concentration of fasting serum leptin at 4 and 12 weeks after surgery. At 4 weeks postoperatively, JIC exhibited significant lower levels of fasting leptin than sham. At 12 weeks after surgery, the difference became more significant. d Concentration of fasting serum adiponectin at 12 weeks post-surgery. JIC exhibited significant higher levels of fasting adiponectin than sham. *P < 0.05; **P < 0.01 vs. sham. N = 6 in each group
Serum Lipid Profiles and Bile Acid
At 4 weeks postoperatively, no difference was observed in cholesterol, free fatty acid (FFA), and total bile acid (TBA) concentrations between JIC and sham groups, while fasting triglyceride level was decreased in the JIC group. At 12 weeks post-surgery, the rats who underwent the JIC procedure showed lower fasting levels of triglyceride and FFA and higher levels of TBA than those in the sham group (Table 1).
Hepatic Lipid Content
According to HE and Oil Red O staining (Fig. 4), the rats with sham operation exhibited a higher degree of fat accumulation in liver, while JIC resulted in remarkable improvement of hepatic steatosis. The Western blots of key enzymes involving hepatic lipogenesis are shown in Fig. 5. The expressions of FAS and ACC were significantly lower in the JIC group as compared to sham.
HE and Oil Red O staining of liver at 12 weeks postoperatively. a HE of JIC. b HE of sham. JIC rats showed significantly reduced ballooning degeneration of hepatocytes relative to the sham group. c Oil Red O of JIC. d Oil Red O of sham. JIC rats exhibited slighter red pigmentation as compared to the sham group
Endocrine Function of the Distal Ileum
The immunohistochemical (IHC) staining of GLP-1 and endocrine cells in the distal ileum is shown in Fig. 6. Markedly increased positive areas of GLP-1 were identified in the JIC group compared with those in the sham-operated group. Similarly, in the JIC group, CgA expressed in higher quantities than those in the sham group, which suggests an increased number of enteroendocrine cells.
IHC staining of GLP-1 and CgA in the distal ileum (original magnification ×400). a GLP-1 staining of JIC. b GLP-1 staining of sham. c CgA staining of JIC. d CgA staining of sham. e Optical density of IHC. Rats in the JIC group exhibited significant higher expressions of intestinal GLP-1 and CgA than those of the sham group. *P < 0.05 vs. sham. N = 6 in each group
Discussion
There is increasing evidence that diabetic subjects suffer from disordered lipid metabolism [4, 15]. Due to the growing epidemic of diabetes and severe consequences of lipometabolic disturbances (especially fatty liver disease), researchers are exploring the connections between the two. In the present study, we investigated the metabolic alteration of lipids after a novel JIC procedure that operates on the hindgut hypothesis.
Our findings show that lipid metabolism and glycemic steady state were improved after JIC surgery, which is similar to current bariatric procedures such as RYGB [16, 17], VSG [16, 18], IT [19], duodenal-jejunal bypass (DJB) [20], and biliopancreatic diversion [17]. HFD and STZ-induced non-obese diabetic rats are used not only for diabetic research [14, 21] but also for hyperlipidemia and hepatic steatosis models [22, 23]. In these models, the weight-independent beneficial effects of JIC to glucose and lipid metabolism included the following: (1) AUCOGTT fell off, demonstrating ameliorative glucose tolerance; (2) decrease of AUCITT indicated the recovery of insulin sensitivity; and (3) abnormal lipid homeostasis improved, manifesting as reduced blood triglyceride and liver fat accumulation.
Insulin resistance was thought to be crucial in the occurrences of diabetes and impaired lipometabolism [24]. In both patients and rats, bariatric surgeries were reported to alleviate hyperglycemia, dyslipidemia, and hepatic steatosis by recovering insulin sensitivity [5, 25, 26]. Our results are in agreement with these studies, showing that insulin resistance was significantly improved as early as 4 weeks after JIC surgery.
In accordance with previous observations [18, 26, 27], our HE staining showed extensive ballooning degeneration of hepatocytes in sham, and the Oil Red O staining of JIC rats’ liver exhibited less red pigmentation, indicating that JIC surgery effectively reduced hepatic lipid content. Our results also show that the protein expressions of hepatic FAS and ACC, the key regulatory enzymes of lipogenesis in liver, were downregulated after JIC. This is consistent with alleviation of fatty liver after JIC, which is supported by Han et al. [20] and Ramzy et al. [28]. Numerous studies revealed that hepatic fat accumulation is closely related to insulin resistance [5, 29]. Consequently, along with restored adipose infiltration in liver, insulin sensitivity was increased and glucose control was improved after JIC operation.
We speculate that the elevated secretion of GLP-1 also accounts for reduced insulin resistance and improved metabolic homeostasis after surgery. It is possible for the undigested nutrients to reach the terminal intestine quickly following JIC and hence accelerate the secretion of GLP-1. However, the weight gain did not differ between the two groups, as it seems that GLP-1 is not the key factor of weight control [30, 31]. GLP-1 has impacts on stimulating insulin secretion and enhancing insulin sensitivity [32]. Although some researchers point that GLP-1 plays a limited role or is even not required in diabetic regression after RYGB or VSG [30, 33], many studies [19, 20, 34] demonstrated that serum GLP-1 was markedly increased after metabolic surgeries, with or without weight loss, in line with our data. Furthermore, Ding et al. [35] observed that GLP-1 receptor agonist reversed hepatic steatosis in a mouse model, demonstrating that GLP-1 has a direct effect on relieving liver steatosis in addition to the effect on glucose metabolism. The same conclusion was presented by Ben-Shlomo and coworkers [36].
The IHC staining was supportive of the proposed hypothesis. The increased GLP-1-positive granules in JIC of ileal specimens support elevated production, which was consistent with the change of plasma concentration. CgA has been reported many times as an indication of enteroendocrine cells. Following IT surgery, Hansen et al. [37] and Patriti et al. [12] found high expressions of CgA by IHC staining and PCR, respectively. In our study, ileal CgA staining presented a stronger expression after JIC, indicating a larger amount of intestinal endocrine cells which are closely related to gut hormone secretion.
The postoperative alterations of serum fasting leptin and adiponectin are also in agreement with previous findings [38, 39]. We surmise that, in spite of no change in body weight after JIC, there might be possible influences to body composition, which may vary adipose-derived hormones. As products of adipocytes, leptin induces metabolic disorders and insulin resistance [40], while adiponectin improves lipometabolism and insulin sensitivity [41, 42]. Individuals with insulin resistance or metabolic syndrome usually have a higher level of leptin and a lower level of adiponectin [43, 44]. The decreased circulating leptin and increased plasma adiponectin indicated an improvement of insulin sensitivity and lipid homeostasis after JIC.
Additionally, the improvement of metabolic homeostasis following JIC may be partly attributed to the elevated serum bile acid. Some literatures on RYGB [45, 46], VSG [7], IT [47], and DJB [48, 49] demonstrated that bile acid is of great importance in regulating glucose and lipid homeostasis after metabolic surgery. Similarly, in the current study, JIC exerted a rise of plasma TBA.
There are several limitations in this study. First, although we found that the serum levels of adipocytokines changed after surgery, their expressions in visceral fat were not tested. Second, we did not measure body fat mass by magnetic resonance. Third, the concentrations and composition changes of bile acid in liver and intestinal lumen need further study. Currently, JIC surgery is just an experimental procedure and far from translating to humans.
In conclusion, JIC achieved an immediate and durable remission of hyperglycemia, hyperlipidemia, and hepatic steatosis with constant body weight and food consumption in diabetic rats. The improvements of hepatic lipogenesis, GLP-1 secretion, adipokine expressions, and bile acid concentration may contribute to, at least partly, the resolution of insulin resistance and metabolic disorders.
References
Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med. 2007;356:213–5.
Angulo P. Obesity and nonalcoholic fatty liver disease. Nutr Rev. 2007;65(6):57–63.
Imamura Y, Uto H, Hiramine Y, et al. Increasing prevalence of diabetes mellitus in association with fatty liver in a Japanese population. J Gastroenterol. 2014;49(10):1406–13.
Leite NC, Villela-Nogueira CA, Pannain VL, et al. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: prevalences and correlated factors. Liver Int. 2011;31(5):700–6.
Mathurin P, Gonzalez F, Kerdraon O, et al. The evolution of severe steatosis after bariatric surgery is related to insulin resistance. Gastroenterology. 2006;130(6):1617–24.
Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362(9388):951–7.
Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity. 2014;22(2):390–400.
Pezeshki A, Chelikani PK. Effects of Roux-en-Y gastric bypass and ileal transposition surgeries on glucose and lipid metabolism in skeletal muscle and liver. Surg Obes Relat Dis. 2014;10(2):217–28.
Rabl C, Campos GM. The impact of bariatric surgery on nonalcoholic steatohepatitis. Semin Liver Dis. 2012;32(1):80–91.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370(21):2002–13.
Wang Y, Zhang X, Liu T, et al. Jejunum-ileum circuit procedure improves glucose metabolism in diabetic rats independent of weight loss. Obesity. 2015. In Press.
Patriti A, Aisa MC, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery. 2007;142(1):74–85.
Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87(7):3023–8.
Srinivasan K, Viswanad B, Asrat L, et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–20.
Porepa L, Ray JG, Sanchez-Romeu P, et al. Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. CMAJ. 2010;182(11):E526–31.
Griffo E, Nosso G, Lupoli R, et al. Early improvement of postprandial lipemia after bariatric surgery in obese type 2 diabetic patients. Obes Surg. 2014;24(5):765–70.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–73.
Kawano Y, Ohta M, Hirashita T, et al. Effects of sleeve gastrectomy on lipid metabolism in an obese diabetic rat model. Obes Surg. 2013;23(12):1947–56.
Wang TT, Hu SY, Gao HD, et al. Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann Surg. 2008;247(6):968–75.
Han H, Hu C, Wang L, et al. Duodenal-jejunal bypass surgery suppresses hepatic de novo lipogenesis and alleviates liver fat accumulation in a diabetic rat model. Obes Surg. 2014;24(12):2152–60.
Reed MJ, Meszaros K, Entes LJ, et al. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metab Clin Exp. 2000;49(11):1390–4.
Sharma AK, Bharti S, Ojha S, et al. Up-regulation of PPARgamma, heat shock protein-27 and -72 by naringin attenuates insulin resistance, beta-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br J Nutr. 2011;106(11):1713–23.
Vornoli A, Pozzo L, Della Croce CM, et al. Drug metabolism enzymes in a steatotic model of rat treated with a high fat diet and a low dose of streptozotocin. Food Chem Toxicol. 2014;70:54–60.
Leclercq IA, Da Silva MA, Schroyen B, et al. Insulin resistance in hepatocytes and sinusoidal liver cells: mechanisms and consequences. J Hepatol. 2007;47(1):142–56.
Makinen J, Hannukainen JC, Karmi A, et al. Obesity-associated intestinal insulin resistance is ameliorated after bariatric surgery. Diabetologia. 2015;58(5):1055–62.
He B, Chen L, Yu C, et al. Roux-en-Y gastric bypass increases hepatic and peripheral insulin sensitivity in rats with type 2 diabetes mellitus. Surg Obes Relat Dis. 2014;10(3):485–93.
de Meijer VE, Le HD, Meisel JA, et al. Repetitive orogastric gavage affects the phenotype of diet-obese mice. Physiol Behav. 2010;100(4):387–93.
Ramzy AR, Nausheen S, Chelikani PK. Ileal transposition surgery produces ileal length-dependent changes in food intake, body weight, gut hormones and glucose metabolism in rats. Int J Obes (Lond). 2014;38(3):379–87.
Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133(2):496–506.
Wilson-Perez HE, Chambers AP, Ryan KK, et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes. 2013;62(7):2380–5.
Ye J, Hao Z, Mumphrey MB, et al. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol. 2014;306(5):R352–62.
Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359(9309):824–30.
Vetter ML, Wadden TA, Teff KL, et al. GLP-1 plays a limited role in improved glycemia shortly after Roux-en-Y gastric bypass: a comparison with intensive lifestyle modification. Diabetes. 2015;64(2):434–46.
Jimenez A, Casamitjana R, Flores L, et al. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256(6):1023–9.
Ding X, Saxena NK, Lin S, et al. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43(1):173–81.
Ben-Shlomo S, Zvibel I, Shnell M, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54(6):1214–23.
Hansen CF, Vassiliadis E, Vrang N, et al. The effect of ileal interposition surgery on enteroendocrine cell numbers in the UC Davis type 2 diabetes mellitus rat. Regul Pept. 2014;189:31–9.
Hu C, Zhang G, Sun D, et al. Duodenal-jejunal bypass improves glucose metabolism and adipokine expression independently of weight loss in a diabetic rat model. Obes Surg. 2013;23(9):1436–44.
Lindegaard KK, Jorgensen NB, Just R, et al. Effects of Roux-en-Y gastric bypass on fasting and postprandial inflammation-related parameters in obese subjects with normal glucose tolerance and in obese subjects with type 2 diabetes. Diabetol Metab Syndr. 2015;7:12.
Moon HS, Dalamaga M, Kim SY, et al. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev. 2013;34(3):377–412.
Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–51.
Dubois SG, Heilbronn LK, Smith SR, et al. Decreased expression of adipogenic genes in obese subjects with type 2 diabetes. Obesity. 2006;14(9):1543–52.
Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5.
Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86(5):1930–5.
Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17(9):1671–7.
Pournaras DJ, Glicksman C, Vincent RP, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153(8):3613–9.
Kohli R, Kirby M, Setchell KD, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):G652–60.
Han H, Wang L, Du H, et al. Expedited biliopancreatic juice flow to the distal gut benefits the diabetes control after duodenal-jejunal bypass. Obes Surg. 2015;25(10):1802–9.
Kashihara H, Shimada M, Kurita N, et al. Duodenal-jejunal bypass improves diabetes and liver steatosis via enhanced glucagon-like peptide-1 elicited by bile acids. J Gastroenterol Hepatol. 2015;30(2):308–15.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81270888/H0713, no. 81370496/H0308, no. 81300286/H0308, no. 81471019/H0712).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Statement of Informed Consent
Informed consent does not apply in this study.
Statement of Human and Animal Rights
All applicable institutional and national guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, X., Zhong, M. et al. Improvements of Glucose and Lipid Metabolism After Jejuno-ileal Circuit Procedure in a Non-obese Diabetic Rat Model. OBES SURG 26, 1768–1776 (2016). https://doi.org/10.1007/s11695-015-1997-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1997-8