Abstract
Plants of the genus Haplophyllum produce various secondary metabolites, including lignans, which are the product of the phenylpropanoid pathway. This study investigated the effects of different concentrations of chitin (0, 50.0, 100.0, and 150.0 mg L−1) on the gene expression pattern of the key biosynthetic enzymes in phenylpropanoid pathway and the production of podophyllotoxin, as a medicinal valuable lignan. The cell suspension culture of Haplophyllum virgatum variety virgatum was used as plant material. The effects of chitin were also studied on the changes of fresh weights of the cultured cells. The contents of produced H2O2, malondialdehyde, and the antioxidant enzymes activities (superoxide dismutase, peroxidase, and catalase) were measured under the treatments as well. Increasing the concentration of chitin and duration of elicitor exposure resulted in reducing the malondialdehyde content. Also, podophyllotoxin showed the highest accumulation in 150.0 mg L−1 after 120 h. Transcripts of the studied genes (4CL, CCR, and CAD) showed the highest level at 12 h after treatments, decreasing after 24 h. A time lag was observed between the maximum gene expression of biosynthetic enzymes and the measured lignan contents in different time courses. This study suggests that the introduced optimum concentrations of chitin in this research could be considered an effective biotic elicitor to improve the in vitro production of podophyllotoxin, in this medicinal plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants of the genus of Haplophyllum (Rutaceae), with 70 species, grow chiefly in warm, temperate, and subtropical areas of the northern hemisphere of the Old World. The Iran-Turanian region, especially Iran, Turkey, and Central Asia is the major center of diversity of this genus (Townsend 1986; Parhoodeh et al. 2011). This genus has 30 species in the flora of Iran, of which 14 are endemic, such as H. virgatum Spach. (Mozaffarian 1996; Joharchi 2008). The dominant compounds of H. virgatum essential oil include non-terpene hydrocarbons and monoterpene hydrocarbons such as β-pinene (Mohammadhosseini et al. 2021). The antimicrobial, insecticidal, and antitumor activities of the β-pinene derivatives were reported (Zielinska-Blajet and Feder-Kubis 2020). Rutaceae plants have been applied in traditional medicine to remedy herpes, warts, stomachache, toothache, skin diseases (Bessonova et al. 1989), and testicular cancer (Ea et al. 2008). Plants of this genus contain essential oils, alkaloids, fixed oils, coumarins, sterols, flavonoids, and lignans (Al-Burtamani et al. 2005; Karimi et al. 2013; Mechehoud et al. 2014; Rasulova et al. 2015). The presence of podophyllotoxin, as a lignan, in some Haplophyllum species (Shah et al 2021) prompted this study to investigate this compound in H. virgatum.
Plants produce a variety of organic compounds that do not function in growth and development. Plant secondary metabolites play important roles in the defense mechanism under biotic and abiotic environmental stress. Many secondary metabolites have pharmacological and therapeutic activities. In plants, the phenylpropanoid pathway is related to the biosynthesis of diverse secondary metabolites, including coumarins, flavonoids, lignins, and lignans (Alfermann et al. 2008; Kabera et al. 2014). Phenylpropanoids regulate various physiological processes, including pathogens resistance, environmental stresses, pigmentation, and structural compounds biogenesis (Tanaka et al. 2008; Vogt 2010; Valdes-Lopez and Hernandez 2014). The phenylpropanoid pathway converts phenylalanine to hydroxycinnamoyl-CoA thioesters. These compounds directly synthesize two groups of secondary metabolites, including flavonoids and monolignols. As one of the phenylpropanoid groups, monolignols are present in lignans and lignins. The monolignol biosynthesis pathway starts with the deamination of l-phenylalanine, which results in the formation of trans-cinnamic acid (Supplementary Fig.). Trans-cinnamic acid turns to cinnamaldehyde, via several successive enzymatic reactions (Hano et al. 2006). The enzyme 4-coumarate CoA ligase (4CL; EC6.2.1.12) converts hydroxycinnamic acids to hydroxycinnamoyl CoA thioesters by forming a bond between the carboxyl group of hydroxycinnamic acid and coenzyme A. It is the critical step in the biosynthetic pathway of phenylpropanoids. The cinnamoyl-CoA reductase (CCR; EC 1.2.1.44) catalyzes the reduction of hydroxycinnamoyl CoA thioesters to comparable aldehydes. CCR is an essential enzyme in the rate-limiting step of monolignol biosynthesis (Whetten and Sederoff 1995; Zhou et al. 2010). CAD (EC 1.1.1.195) has an important role in the last stage of monolignol biosynthesis. CAD is a major rate-limiting enzyme in monolignol biosynthesis and catalyzes the NADPH-dependent conversion of hydroxycinnamic aldehydes to hydroxycinnamyl alcohols (monolignols), which are used in lignin and lignan biosynthesis. Lignans, as a large class of secondary metabolites, are composed of phenylpropanoid dimers. These compounds play an important role in human pharmacological and nutritional value (Westcott and Muir 2003; Landete 2012). They also have a preeminent position in plant defense because of their antifeedant activity (Harmatha and Dinan 2003). Podophyllotoxin has shown antiviral activities, and its semisynthetic derivatives, such as teniposide, etoposide, and etopophose, are employed as anticancer drugs (Canel et al. 2001).
Cell suspension culture is an effective technique for studying the effects of different factors on the induction of valuable secondary metabolites (Georgiev et al. 2009; Murthy et al. 2014). In many instances, the chemical synthesis of metabolites is not possible or economically efficient. However, few valuable plant natural products have been commercialized using plant cell cultures (Yue et al. 2016). Thus, the different experimental strategies are used for increasing productivity in plant cell culture, such as elicitation (Murthy et al. 2014). The elicitation technique adds exogenous elicitors in plant cells and tissue cultures. This process triggers the stress response in the suspension culture resulting in a concomitant increase in secondary metabolite biosynthesis. Elicitors are biotic or abiotic based on their origin and can cause biochemical and physiological alteration in plants (Ramirez-Estrada et al. 2016). The fungal polysaccharides, including chitin, act as biotic elicitors. They affect the induction of different secondary metabolites (Namdeo 2007). Chitin is a polymer of N-acetyl glucosamine with the β-(1,4)-glycosidic bond and is effective in the induction of secondary metabolites in plant cell suspension cultures (Gadzovska Simic et al. 2015). Therefore, chitin was investigated to stimulate the lignan production in this study.
This research has determined whether chitin, as a biotic elicitor, is effective on the phenylpropanoid pathway and podophyllotoxin production in the Iranian endemic plant Haplophyllum virgatum variety virgatum. The influence of chitin on the relative expression of 4CL, CCR, and CAD, the key enzymes acting in the phenylpropanoid pathway, was evaluated to determine how chitin might improve podophyllotoxin production. Supplementary physiological factors, including the activity of antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase (POX), and catalase (CAT), and the production of hydrogen peroxide and malondialdehyde were analyzed to evaluate antioxidant defense mechanisms of treated cells under stressful situations.
Materials and Methods
Plant Materials and Cell Suspension Cultures
The seeds of H. virgatum variety virgatum were collected from the Geno area in the Hormozgan province of Iran (27°27′ N latitude and 56°18′ E longitudes at the altitude of 45 m). The seeds were sterilized with 70.0% (v/v) ethanol for 30 s and 10.0% sodium hypochlorite for 10 min and then washed with sterile distilled water three times. For initiation of callus culture, sterile crushed seeds were cultured as explants on solid B5 medium (Gamborg et al. 1968) supplemented with 0.2 mg L−1 kinetin (Kin), 0.5 mg L−1 α-naphthaleneacetic acid (NAA), and 0.5 mg L−1 indole-3-acetic acid (IAA) at pH 5.7 in darkness at 25°C. The final medium pH was adjusted using a diluted NaOH solution. Cell suspension cultures were established from 2-mo-old fresh friable calluses inoculated in 50 mL liquid B5 medium supplemented with the same explained plant growth regulators. The callus suspension cultures were incubated in darkness at 25°C, shaking 110 rpm. The separated cells (25-d-old) were used as the plant material for further investigation in elicitation experiments (Fig. 1).
The growth curve of cell suspension culture obtained from Haplophyllum virgatum friable calluses. Data represent average values of three replicates ± SD. The vertical bars represent standard errors. Means with different letters are significantly different at P ≤ 0.05 as determined by the Duncan test.
Elicitor Treatment
Treatments of cell suspensions with concentrations of 0, 50.0, 100.0, and 150.0 mg L−1 chitin (Sigma Aldrich, Steinheim, Germany, C7170) were performed when cells were in the mid-log phase of growth. The cells were separated from the liquid media after 8, 12, 24, 48, 72, and 120 h of treatments. The suspension was filtered on a Buchner funnel through screen nylon mesh to remove cell aggregates, then frozen in liquid nitrogen and preserved at − 80 °C for subsequent analyses. The fresh weight changes of treated samples were also compared with the control.
Hydrogen Peroxide Content
H2O2 content was measured based on the procedure reported by Velikova et al. (2000). Approximately 0.5 g of fresh sample was homogenized with 1.0 mL 0.1% (w/v) trichloroacetic acid solution at 4°C. The mixture was centrifuged at 4°C (7300 × g) for 20 min, then 0.5 mL of the supernatant was mixed with 0.5 mL of 10 mM potassium phosphate buffer (pH 7) and 1.0 mL of 1.0 M KI. The absorbance of the mixture was recorded at 390 nm. H2O2 content was calculated using an extinction coefficient of 0.28 µM−1 cm−1 and expressed as μM g−1 FW.
Malondialdehyde Content
The malondialdehyde (MDA) content was measured according Health and Packer (1968). A fresh sample (0.2 g) was homogenized with 0.1% trichloroacetic acid solution (w/v) (2 mL), and the mixture was centrifuged at room temperature (7300 × g) for 10 min. The supernatant (1.0 mL) was mixed with 1.0 mL of 0.5% (w/v) thiobarbituric acid dissolved in 20.0% (w/v) trichloroacetic acid. The resulting mixture was placed in a water bath at 95°C for 30 min. It was immediately cooled in an ice bath, then centrifuged at room temperature (7300 × g) for 10 min. The absorbance of the supernatant was recorded at 532 and 600 nm. The MDA content was measured using 155 mM−1 cm−1 as the extinction coefficient and was reported as μM g−1 FW.
Total Protein Content
Total protein content was measured based on the method of Bradford (1976) using bovine serum albumin. For this, 0.3 g of fresh sample was homogenized at 4°C with 1.0 mL of 50.0 mM Tris–HCl (pH 7.5), then centrifuged at 4°C and 8600 × g for 20 min. The supernatants were used for the designation of protein content and enzyme activity. The protein content was reported as milligrams per gram of FW.
The Activity of Antioxidant Enzymes
The activity of SOD (E.C. 1.15.1.1) was determined using the amount of enzyme that inhibited the photochemical reduction of nitro blue tetrazolium (NBT) (Giannopolitis and Ries 1977). The reaction mixture contained 50.0 mM sodium phosphate buffer (pH 7), 0.1 mM EDTA, 13.0 mM methionine, 10.0 μM riboflavin, 75.0 μM NBT, and 100.0 µL of enzyme extract. The reaction mixture was exposed to light for 15 min, and absorbance was recorded at 560 nm against the non-irradiated sample. The SOD activity was expressed as units per milligram of protein.
The activity of CAT (E.C. 1.11.1.6) was measured based on the decrease of absorbance at 240 nm due to H2O2 decomposition. Approximately 3.0 mL of 50.0 mM sodium phosphate buffer (pH 7), 100.0 µL of H2O2 (1%), and 100.0 µL of enzyme extract were mixed. The final activity of the enzyme is defined in units per mg of protein (Aebi 1984).
The activity of POX (E.C. 1.11.1.7) was measured based on Upadhyaya et al. (1985) method. The reaction mixture contained 2.5 mL of 50.0 mM sodium phosphate buffer (pH 7), 0.1 mL of 5.0 mM H2O2, 0.1 mL of 20.0 mM guaiacol, and 100.0 µL enzyme extract. The POX activity was expressed as a result of the oxidation of guaiacol with increasing absorbance at 470 nm and reported as units per milligram of protein.
Podophyllotoxin Extraction and Analysis
Fresh cells (500 mg) were homogenized in 1.0 mL of ethyl acetate, followed by sonication for 10 min and shaking for 1 h. The supernatant was obtained after centrifugation at room temperature and 5000 × g for 10 min and evaporated until dry. The residue was dissolved with 1.0 mL methanol and filtered via a syringe filter with 0.2-μ pore size (SARTORIUS, Goettingen, Germany) followed by HPLC analysis. The instrument that was used was a Knauer GmbH HPLC system (Berlin, Germany) with a 5-μm C18 vertex column (250 mm × 4.6 mm ID). Column temperature was regulated at 25°C and 20.0 μL of extract injected. The mobile phase was prepared of two HPLC grade solvents (A, acetonitrile 100%; B, 0.01% aqueous phosphoric acid). The gradient elution program was as shown in Table 1. The flow rate was 0.9 mL min−1, and UV detection was adjusted at 290 nm. The calibration curve (concentration against peak surface area) was drawn using standard podophyllotoxin (Sigma-Aldrich, Saint Louis, UK).
LC–MS analysis was used to confirm the presence of podophyllotoxin in H. virgatum cell suspension culture extracts. For this purpose, LC–MS analyses were performed on the standard podophyllotoxin and the lignan extracts. The device used was Waters Alliance 2695 HPLC-Micromass Quattro micro API Mass Spectrometer with positive-mode electrospray ionization (ESI) (Fig. 5).
Relative Expression of 4CL, CCR, and CAD
Fresh samples were powdered with liquid nitrogen. Total RNA extraction was performed using the RNX-plus kit (SinaClon, Tehran, Iran) according to the constructor’s instructions. The quantity of total RNA was assessed using spectrophotometer analysis (BMG Labtech Spectrostar, Ortenberg, Germany). Agarose gel electrophoresis (1.0%) was used to evaluate the quality of RNA. The cDNA Synthesis Kit (KiaGeneFanavar Aria, Tehran, Iran) was used for cDNA synthesis from 1.0 μg RNA in a final volume of 20.0 μL according to the instruction provided by the constructor.
Real-time quantitative PCR (RT-qPCR) (Rotor-Gene 6000, QIAGEN, Germantown, MD) was used to determine the expression of 4CL, CCR, and CAD genes. RT-qPCR was performed by 0.5 μL of cDNA being added to the solution, including 0.5 μL of each primer, 5.0 μL of Green-2-Go qPCR Mastermix (Bio Basic, Toronto, Canada), and 3.5 μL double-distilled water for a final volume of 10.0 μL. The real-time program is a combination of the primary denaturation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 s, followed by annealing temperature (Tubulin, 57.4°C; 4CL, 58°C; CCR, 56.5°C; and CAD, 57.4°C) for 30 s, and 72°C for 30 s. To confirm the single amplified product of the gene, a single distinct peak of melting curve analysis was used at 72 to 95 °C. Tubulin was used as an interior control to normalize the RT-qPCR data. The 2−ΔΔCT method was used for calculating the relative gene expression. The design of primers based on conserved sequences in the Rutaceae family. The Oligo 7 program was used for primer designing (Table 2).
Statistical analysis
All data were obtained from three independent replications. SPSS software (Ver.16) was used to compare the mean differences, and one-way analysis of variance (ANOVA) was done to analyze the data. The significance of differences was defined by Duncan’s tests at the P ≤ 0.05 level. A heatmap diagram was drawn using the Pearson correlation coefficient and Phyton 3.7.4 software (https://www.phyton.org) between all parameters.
Result and Discussion
Effect of Elicitor on Growth
The influence of the elicitor on the growth of H. virgatum cells was monitored by the fresh weight measurement of the cells after treatment with and without chitin (Fig. 2). An increase of fresh weights was observed in the control samples during the experiment (0 mg L−1 chitin between 8 and 120 h). In the concentration of 50.0 mg L−1 chitin, no significant increment of cell fresh weight was observed during the investigation. However, a substantial increase in the fresh weight was observed in the presence of 100.0 and 150.0 mg L−1 chitin after 120 h and 48 h, respectively. Gadzovska Simic et al. (2015) found that biomass production decreased by using chitin as an elicitor while it increased by pectin and had no change in the presence of dextran, which is contrary to the results of this study. The observed differences between reactions to different exogenous elicitors could depend on the difference in plant species, elicitor types, and elicitor concentrations (Baldi et al. 2008). Winkler et al. (2017) demonstrated that chitin in the form of tetramer induced the expression of genes related to vegetative growth, developmental processes, and carbon and nitrogen metabolism while its octamer form led to the activation of immune and defense responses of the plant. Supposedly, chitin oligomers bearing different lengths activated different responses in the plants. A challenge in synthesizing secondary metabolites through tissue culture is improving their products without reducing the biomass. In this study, treatment with chitin increased lignan production without affecting growth probably by changing the expression of the key genes in the phenylpropanoid pathway.
Fresh weight (FW) changes in cell suspension culture of Haplophyllum virgatum under different concentrations of chitin (0, 50.0, 100.0, 150.0 mg L−1) after 8, 12, 24, 48, 72, and 120 h time courses. Data represent average values of three replicates ± SD. The vertical bars represent standard errors. Means with different letters are significantly different at P ≤ 0.05 as determined by the Duncan test.
Effect of Elicitor on H 2 O 2 Content
Elicitors induce H2O2 production at the early stages of treatment (Lin et al. 2005). In this study, the H2O2 contents decreased initially (in the 8 and 12 h time courses) and then increased in the 24 and 48 h time courses. The decreasing trend was observed at 72-h time course again (Fig. 3(a)). At 120 h, no significant difference was observed between the control and different concentrations of chitin. Sharma and Dubey (2005) showed drought stress decreased H2O2 content in rice seedlings, which may be related to the scavenging of H2O2 by increasing guaiacol peroxidase activity. An increase in H2O2 production was observed when root suspension cultures of Panax ginseng (Ali et al. 2007), hairy root cultures of Silybum marianum (Khalili et al. 2014), and shoot cultures of Melissa officinalis (Fooladi vanda et al. 2019) were treated with salicylic acid and methyl jasmonate, Ag+, and chitosan, respectively. Toghyani et al. (2020) reported that H2O2 content increased immediately after gamma irradiation in Chlorella vulgaris and decreased over 48 h after treatment. The reduction of hydrogen peroxide content and the high activity of antioxidants coincided, which led to a decrease in MDA levels. The balance between the activity of H2O2-producing enzymes and its scavenging enzymes in cells has an important function in the content of the reactive oxygen species (ROS) and defense mechanisms against oxidative stress (Creissen et al. 1994; Paciolla et al. 2016).
The content of (a) H2O2, (b) MDA, and (c) podophyllotoxin in cell suspension culture of Haplophyllum virgatum under different concentrations of chitin (0, 50.0, 100.0, 150.0 mg L−1) after 8, 12, 24, 48, 72, and 120 h time courses. Data represent average values of three replicates ± SD. The vertical bars represent standard errors. Means with different letters are significantly different at P ≤ 0.05 as determined by the Duncan test.
Effect of Elicitor on MDA Content
The environmental stresses increase ROS production and thus cause peroxidation of membrane lipids and damage to the cell membrane (Ali et al. 2006). Lipid peroxidation is associated with MDA as the marker, which has been used to evaluate the sensitivity of the plant to oxidative stress (Blokhina et al. 2003; Li et al. 2011). The MDA content was decreased upon treatment with chitin in a concentration-dependent manner at 8- and 12-h time courses. However, no changes in lipid peroxidation were observed in the treated samples at 24, 48, 72, and 120 h in comparison to the control (0 mg L−1 chitin) (Fig. 3(b)). In contrast to the results in this study, the MDA content increased in Allium cepa L. and Pisum sativum under NaCl treatment (Ahmad et al. 2008; El-baky et al. 2003) and Cnidium officinale exposed to MeJA (Ho et al. 2020). The significant increase in MDA level appeared to be derived from the lower activity of the antioxidant enzymes. Salicylic acid (SA) increased the activity of the antioxidant enzymes, such as SOD, POX, and CAT, which protected plants against ROS generation and lipid peroxidation (Hayat et al. 2010). Similar to the results in this study, using chitosan under salinity stress decreased the MDA levels and increased the activity of antioxidant enzymes in Oryza sativa (González et al. 2015). Based on this study’s findings, the decrease in MDA content under chitin treatment was caused by the effective antioxidant system.
Effect of Elicitor on Protein Content and Antioxidant Enzyme Activities
Based on the results in this study, total protein content did not show a significant difference with the control in 8 to 48 h after chitin treatments. Still, after 72 h, the protein content at 150.0 mg L−1 of chitin was significantly decreased (Fig. 4(a)).
Total protein contents and antioxidant enzyme activities in cell suspension culture of Haplophyllum virgatum under different concentrations of chitin (0, 50.0, 100.0, 150.0 mg L−1) after 8, 12, 24, 48, 72, and 120 h time courses. (a) Total protein contents; (b) SOD activities; (c) CAT activities; (d) POX activities. Data represent average values of three replicates ± SD. The vertical bars represent standard errors. Means with different letters are significantly different at P ≤ 0.05 as determined by the Duncan test. SOD, superoxide dismutase; CAT, catalase; POX, peroxidase.
In 120-h time course, the protein contents increased in response to 50.0 and 100.0 mg L−1 chitin then decreased again in response to 150.0 mg L−1 chitin. It seems that the effect of chitin on protein content started at higher time courses. The increase in protein content in response to stress may be because new proteins’ biosynthesis is related to defensive mechanisms (Poonam et al. 2013).
Decreased protein content might be because of reduced protein biosynthesis (Hall and Flowers 1973) or increased protein hydrolysis by catabolic enzymes, such as proteases (Davies 1987). The next cause is protein denaturation and oxidation, which leads to increased proteolysis (Mourato et al. 2012).
The elicitation affects the induction of ROS, such as O2− and H2O2. A variety of antioxidant enzymes for ROS depletion in plant cells were reported, including SOD, POX, and CAT (Foyer et al. 1991; Mittler 2002; Dynowski et al. 2008). SOD acts as the first line of defense against oxidative damage and scavenges superoxide radicals (Mittler 2002). SOD catalyzes the conversion of superoxide anion to H2O2 and molecular oxygen (Giannakoula et al. 2010). According to the results, the SOD activity significantly increased with increasing chitin concentration (Fig. 4(b)). Higher levels of SOD activity were related to the concentration of 150.0 mg L−1 chitin at the time courses of 72 and 120 h, which was about two- and threefold higher than control samples, respectively. Increased SOD production and activity has led to improved plant oxidative stress tolerance (Gupta et al. 1993). Yin et al. (2002) and Sun et al. (2004) showed scavenging of the superoxide anions by chitosan and SOD. The scavenging ability of chitosan seems to be related to its structure because it contains hydroxyl and amine agents that react with ROS (Xie et al. 2001; Li et al. 2002; Sun et al. 2004). This study’s results are in accordance with previous reports, which have shown that SOD activity increased when plants were under different environmental stresses, such as metal toxicity and drought (Sharma and Dubey 2005; Mishra et al. 2011). The flax plants treated with SA, mainly when applied with pathogen, increased SOD activity. This increment could prevent the attack of superoxide radicals to biological molecules (Belkadhi et al. 2013). Based on this study’s findings, with increasing chitin concentration and duration of exposure, the MDA content decreased, which is consistent with an increase in SOD activity.
One group of antioxidant enzymes that scavenge hydrogen peroxide is peroxidases. POX catalyzes the decomposition of hydrogen peroxide by the oxidation of phenolic and indolic compounds (Giannakoula et al. 2010). In this study, POX activity declined when cells were exposed to chitin, especially in the concentration of 100.0 mg L−1 (Fig. 4(c)). There were no significant differences between the control samples at the examined times. However, different environmental stresses induced guaiacol peroxidase activity (Shah et al. 2001; Moussa and Abdel-Aziz 2008). Increased activity of POX via elicitation has been described in many studies (Xu et al. 2007, Gharechahi et al. 2013, Khataee et al. 2020). Fooladi vanda et al. (2019) did not report a significant increase in guaiacol peroxidase activity under higher doses of chitosan. It could be due to the scavenging of superoxide radicals in the presence of high doses of chitosan. Due to the role of peroxidase in the plant antioxidant system, its increase in stress is predictable. However, the decrease in the activity of this enzyme in some concentrations can be due to the excessive accumulation of oxygen radicals (Demiral and Turkan 2005).
Catalase is another group of antioxidant enzymes that decompose hydrogen peroxide (Sofo et al. 2005). In this study, catalase activity was enhanced at 50.0 and 100.0 mg L−1 chitin concentrations with increasing the exposure time. Still, such activity was reduced at 150.0 mg L−1 (Fig. 4(d)). Environmental stress conditions may increase or decrease catalase activity based on stress type, intensity, and duration (Sharma and Dubey 2005; Moussa and Abdel-Aziz 2008). Chitosan stimulated the catalase activity in Triticum aestivum (Ma et al. 2014) and Melissa officinalis (Fooladi vanda et al. 2019). Saisavoey et al. (2014) showed that treating the cell culture of Pueraria mirifica with methyl jasmonate decreased CAT activity. However, the glutathione peroxidase activity was increased. It could suggest that the peroxidase compensated H2O2 scavenge. H2O2 accumulation under stress can be one of the factors leading to catalase inactivation (Harinasut et al. 2003).
Effect of Elicitor on Podophyllotoxin Content
The contents of produced podophyllotoxin in suspension cells under chitin treatment were measured by HPLC where podophyllotoxin (Sigma-Aldrich) was used as standard. The results show that chitin treatments could significantly affect the rate of podophyllotoxin accumulation (Fig. 3(c)). Analysis of standard podophyllotoxin (Sigma-Aldrich) by electrospray ionization mass spectrometry (ESI/MS) showed a mass of 416 (M + 1) for this compound (Fig. 5a). As shown in Fig. 5b, in a similar analysis of the lignan extract obtained from the H. virgatum suspension cells, the presence of this compound was confirmed.
This study’s results showed that the contents of podophyllotoxin were increased in response to chitin 72 h and 120 h after the beginning of the treatment in a concentration dependent manner (Fig. 3(c)). The highest content of podophyllotoxin (245.886 ± 2.165 µg g−1 FW) was observed after 120 h under 150 mg L−1 chitin. Studies have shown that the treatment of Linum album with methyl jasmonate increases the amount of podophyllotoxin compared to the control (Fuss 2003; Van Fürden et al. 2005). Esmaeilzadeh Bahabadi et al. (2011) demonstrated the accumulation of podophyllotoxin was increased in L. album cell cultures. Chitosan and chitin oligomers increased the production of podophyllotoxin in Linum album cell culture by fungal extracts, such as chitin, chitosan, and methyl jasmonate (Esmaeilzadeh Bahabadi et al. 2014). In some reports, lignan compounds were metabolized and entered into another pathway (Renouard et al. 2014; Corbin et al. 2017). Tokunaga et al. (2005) demonstrated the incorporation of pinoresinol into lignin polymers in isolated Zinnia elegans mesophilic cells. Tashackori et al. (2019) showed that pinoresinol was increased in PLR-suppressed roots of Linum album after treatment with fungal cell wall. Under the same treatment of fungal cells, the content of podophyllotoxin was decreased. It appears that the metabolites of the phenylpropanoid pathway shifted to lignin accumulation. Factors, such as elicitor concentration, time to add elicitor in culture medium, and duration of elicitor exposure, affect the induction of secondary metabolites (Vasconsuelo and Boland 2007).
Effect of Elicitor on the Expression of 4CL, CCR, and CAD Genes
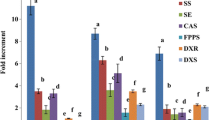
As mentioned before, the effects of chitin on transcription levels of 4CL, CCR, and CAD in H. virgatum cell suspensions were studied by qRT-PCR method (Fig. 6). The expression of 4CL was increased in the treatments with 100.0 and 150.0 mg L−1 chitin after 12 h. However, 4CL expression decreased at 24 h (Fig. 6(a)). The transcript level of 4CL in treated cells with 50.0 mg L−1 chitin was similar to the control. The maximum 4CL expression (2.806 ± 0.098) attained in the 100 mg L−1 chitin-treated samples after 12 h was nearly 2.7 times more than the control (1.050 ± 0.024). The transcripts of 4CL were accumulated by wounding mature Arabidopsis leaves after 2 h. It increased after 6 h by infection of Arabidopsis leaves with a Pseudomonas syringae pv. maculicola strain containing avrB (Lee et al. 1995). Di et al. (2012) showed the increment of 4CL expression under methyl jasmonate treatment until 8 h, and then decreased at 12 h, which is consistent with this current study’s findings. Methyl jasmonate treatment also increased the relative expression of 4CL in cell cultures of Agastache rugosa and hairy root cultures of Mentha spicata (Kim et al. 2013; Yousefian et al. 2020).
The relative expression levels of (a) 4CL, (b) CCR, and (c) CAD genes in cell suspension culture of Haplophyllum virgatum under different concentrations of chitin (0, 50.0, 100.0, 150.0 mg L−1) after 12 and 24 h time courses. Data represent average values of three replicates ± SD. The vertical bars represent standard errors. Means with different letters are significantly different at P ≤ 0.05 as determined by the Duncan test. 4CL, 4-coumarate CoA ligase; CCR, cinnamoyl-CoA reductase; CAD, cinnamoyl alcohol dehydrogenase.
In Nicotiana tabacum (Piquemal et al. 1998) and Arabidopsis thaliana (Goujon et al. 2003), decreased lignin accumulation due to decreased CCR expression showed that CCR is a rate-limiting enzyme in the biosynthesis of monolignol. In this study, the CCR expression increased significantly in all used concentrations of chitin after 12 h. It decreased significantly after 24 h, yet they were still more than the control (Fig. 6(b)). The highest CCR expression observed after 12 h under 150.0 mg L−1 chitin concentration, which was 17 times more than the control. Hano et al. (2006) observed Botrytis cinerea and Fusarium oxysporum extracts activated the flax CCR gene expression. Esmaeilzadeh Bahabadi et al. (2014) showed that the expression levels of CCR increased by chitosan and chitin oligomers reaching a peak 3 d after treatment.
Similar to 4CL and CCR, the CAD gene expression reached a peak at 12 h and decreased at 24 h (Fig. 6(c)). The CAD gene expression significantly increased in 100.0 mg L−1 (10.1-fold) and 150.0 mg L−1 (7.8-fold) chitin treatments in comparison with control after 12 h. The highest expression (2.565 ± 0.334) was recorded in the cells treated with 100.0 mg L−1 chitin at 12 h. Brill et al. (1999) showed induction of CAD expression after using salicylic acid and wounding in Medicago sativa leaves. The fungal elicitors upregulated CAD gene expression in Linum usitatissimum cell suspension cultures (Hano et al. 2006). Consistent with the current study’s findings, Esmaeilzadeh Bahabadi et al. (2014) showed increased CAD expression under chitin treatment on day 3 and then decreased after 5 d in the cell culture of Linum album.
Heatmap Diagram Analysis
The heat map diagram between all studied parameters in the cell suspension culture of H. virgatum under chitin treatment is in Fig. 7. H2O2 content showed the highest positive correlation with MDA content. This study’s observations confirmed that increasing the content of reactive oxygen species could lead to cell damage. Among antioxidant enzymes, H2O2 showed the highest negative correlation with catalase activity. However, it had a positive correlation with peroxidase activity. High levels of H2O2 appear to deactivate the catalase enzyme. MDA content indicated a positive correlation with POX activity and a negative correlation with SOD and CAT activities. Like the current study’s results, some studies have shown a direct correlation between increased SOD activity and decreased oxidative damage. When SOD activity is high, scavenging of reactive oxygen species, especially superoxide radicals, which damage the membrane, performs. There was a positive correlation between podophyllotoxin content and the expression of studied genes, although the correlation coefficient is less than 0.5. Therefore, it seems that lignin production could be more than lignan production in H. virgatum suspension cells. Increasing the expression of upstream genes in the phenylpropanoid pathway can increase the podophyllotoxin content in the downstream pathway. The highest positive correlation was observed between the expression levels of 4CL and CAD genes. All studied genes showed negative correlations with growth rate, POX and CAT activities, and H2O2 and MDA contents. 4CL has no correlation with SOD activity while CAD and CCR have positive correlations with this enzyme activity. These findings can be considered proof of the role of phenylpropanoids in the defense mechanisms of this plant in the way that increasing biosynthesis of these substances could decrease the need for other defense mechanisms.
Heatmap diagram generated from obtained data in this study, which shows correlations between different studied parameters in cell suspension culture of Haplophyllum virgatum under chitin treatment. The red and blue parts indicate positive and negative correlation, respectively. MDA, malondialdehyde; SOD, superoxide dismutase; POX, peroxidase; CAT, catalase; PTOX, podophyllotoxin; 4CL, 4-coumarate ligase; CCR, cinnamoyl CoA reductase; CAD, cinnamoyl alcohol dehydrogenase.
Conclusions
This current study showed chitin, as a biotic elicitor, affects the secondary metabolite production in H. virgatum. By comparing the time of lignans production with the time of gene expression, a time interval was recorded between maximum lignans contents and maximum gene expression. According to this study’s results, the expression of genes 4CL, CCR, and CAD involved in the upstream pathway of phenylpropanoid metabolism positively correlated with podophyllotoxin content. Study of downstream genes of the lignan biosynthesis pathway can provide more information on the relationship between chitin treatment and the content of other lignans. Chitin as an efficient ecofriendly biotic elicitor, at optimal concentrations, could improve the production of valuable secondary metabolites in this medicinal plant.
Data Availability
Detailed data are reserved by the authors and could not be made public. Detailed data will be provided upon request.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmad P, John R, Sarwat M, Umar S (2008) Responses of proline, lipid peroxidation and ntioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int J Plant Prod 2:353–366
Al-Burtamani SKS, Fatope MO, Marwah RG, Onifade AK, Al-Saidi SH (2005) Chemical composition, antibacterial and antifungal activities of the essential oil of Haplophyllum tuberculatum from Oman. J Ethnopharmacol 96:107–112
Alfermann AW, Fuss E, Bayindir U (2008) Hinokinin biosynthesis in Linumcorym bulosum Reichenb. Plant J Cell Mol Boil 55:810–820
Ali MB, Hahn EJ, Paek KY (2007) Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules 12:607–621
Ali MB, Yu KW, Hahn EJ, Paek KY (2006) Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep 25:613–620
Baldi A, Jain A, Bisaria VC (2008) Co-culture of arbuscular mycorrhiza-like fungi (Piriformospora indica and Sebacina vermifera) with plant cells of Linum album for enhanced production of podophyllotoxin: a first report. Biotechnol Lett 30:1671–1677
Belkadhi A, De Haro A, Soengas P, Obregon S, Cartea ME, Djebali W, Chaïbi W (2013) Salicylic acid improves root antioxidant defense system and total antioxidant capacities of flax subjected to cadmium. OMICS J Integr Biol 17:398–406
Bessonova IA, Kurbanov D, Yunusov SY (1989) Components of Haplophyllum ramosissimum. Chem Nat Compd 25:39–40
Blokhina O, Virolainen E, Fagerstedt (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Brill EM, Abrahams S, Hayes CM, Jenkins CLD, Watson JM (1999) Molecular characterisation and expression of a wound-inducible cDNA encoding a novel cinnamyl-alcohol dehydrogenase enzyme in Lucerne (Medicago sativa L.). Plant Mol Biol 41:279–291
Canel C, Dayan FE, Ganzera M, Khan IA, Rimando A, Burandt CLJ, Moraes RM (2001) High yield of podophyllotoxin from leaves of Podophyllum peltatum by in situ conversion of podophyllotoxin 4-o-β-glucopyranoside. Planta Med 67:97–99
Corbin C, Drouet S, Mateljak I, Markulin L, Decourtil C, Renouard S, Lopez T, Doussot J, Lamblin F, Auguin D, Laine E, Fuss E, Hano C (2017) Functional characterization of the pinoresinol-lariciresinol reductase-2 gene reveals its roles in yatein biosynthesis and flax defense response. Planta 246:405–420
Creissen GP, Edwards EA, Mullineaux PM (1994) Glutathione reductase and ascorbate peroxidase. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defense systems in plants. CRC Press, Boca Raton, USA, pp 343–364
Davies JA (1987) Protein damage and degradation by oxygen radicals. I General Aspects J Biol Chem 262:9895–9901
Demiral T, Turkan I (2005) Comparative lipid peroxidation antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53:247–257
Di P, Hu Y, Xuan H, Xiao Y, Chen J, Zhang L, Chen W (2012) Characterization and the expression profile of 4-coumarate: CoA ligase (Ii4CL) from hairy roots of Isatis indigotica. Afr J Pharmacy Pharmacol 6:2166–2175
Dynowski M, Schaaf G, Loque D (2008) Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem J 414:53–61
Ea S, Giacometti S, Ciccolini J, Akhmedjanova V, Aubert C (2008) Cytotoxic effects of haplamine and its major metabolites on human cancer cell lines. Planta Med 74:1265–1268
El-baky A, Hanna H, Amal AM, Hussein MM (2003) Influence of salinity on peroxidation, antioxidant enzymes and electrophoretic patterns of protein and isoenzymes in leaves of some onion cultivars. Asian J Plant Sci 2:1220–1227
Esmaeilzadeh Bahabadi S, Sharifi M, Murata J, Satake H (2014) The effect of chitosan and chitin oligomers on gene expression and lignans production in Linum album cell cultures. J Medicinal Plants 13:46–53
Esmaeilzadeh Bahabadi S, Sharifi M, Safaie N, Murata J, Yamagaki T, Satake H (2011) Increased lignan biosynthesis in the suspension cultures of Linum album by fungal extracts. Plant Biotechnol Rep 5:367–373
Fooladi vanda G, Shabani L, Razavizadeh R (2019) Chitosan enhances rosmarinic acid production in shoot cultures of Melissa officinalis L. through the induction of methyl jasmonate. Bot Stud 60:1–10
Foyer CH, Lelandais M, Galap C, Kunert KJ (1991) Effect of elevated cytosolic glutathione reductase activity on the cellular glutathione pool and photosynthesis in leaves under normal and stress conditions. Plant Physiol 97:863–872
Fuss E (2003) Lignans in plant cell and organ cultures: an overview. Phytochem Rev 2:307–320
Gadzovska Simic S, Tusevski O, Maury S, Delaunay A, Laine E, Joseph C, Hage’ge D (2015) Polysaccharide elicitors enhance phenylpropanoid and naphtodianthrone production in cell suspension cultures of Hypericum perforatum. Plant Cell Tiss Org Cult 122:649–663
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Georgiev MI, Weber J, Maciuk A (2009) Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl Microbiol Biotechnol 83:809–823
Gharechahi J, Khalili M, Hasanloo T, Hosseini Salekdeh G (2013) An integrated proteomic approach to decipher the effect of methyl jasmonate elicitation on the proteome of Silybum marianum L. hairy roots. Plant Physiol Biochem 70:115–122
Giannakoula A, Moustakasa M, Syrosb T, Yupsanisb T (2010) Aluminum stress induces up-regulation of an efficient antioxidant system in the Al-tolerant maize line but not in the Al-sensitive line. Env Exp Bot 67:487–494
Giannopolitis CN, Ries SK (1977) Superoxide dismutases II. Purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiol 59:315–318
González LM, Guerrero YR, Rodríguez AF, Vázquez MN (2015) Effect of seed treatment with chitosan on the growth of rice (Oryza sativa L.) seedlings cv. INCA LP-5 in saline medium. Cult Trop 36:136–142
Goujon T, Ferret V, Mila I, Pollet B, Ruel K, Burlat V, Joseleau JP, Barrie`re Y, Lapierre C, Jouanin L (2003) Down regulation of the AtCCR1 gene in Arabidopsis thaliana: effects on phenotype, lignins and cell wall degradability. Planta 217:218–228
Gupta S, Heinen JL, Holaday AS, Burke JJ, Allen RD (1993) Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA 90:1629–1633
Hall JL, Flowers TJ (1973) The effects of NaCl on protein synthesis in the halophyte Suaeda maritima. Planta 110:361–368
Hano C, Addi M, Bensaddek L, Cronier D, Baltora-Rosset S, Doussot J, Maury S, Mesnard F, Chabbert B, Hawkins S, Laine E, Lamblin F (2006) Differential accumulation of monolignol derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 223:975–989
Harinasut P, Poonsopa D, Roengmongkol K, Charoensataporn R (2003) Salinity effects on antioxidant enzymes in mulberry cultivar. Sci Asia 29:109–113
Harmatha J, Dinan L (2003) Biological activities of lignans and stilbenoids associated with plant-insect chemical interactions. Phytochem Rev 2:321–330
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous SA under changing environment: a review. Environ Exp Bot 68:14–25
Health RL, Packer L (1968) Photoperoxidatin in isolated chloroplasts. I. Kinetics and stochiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Ho TT, Murthy HN, Park SY (2020) Methyl jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. Int J Mol Sci 21:716
Joharchi M (2008) Rutaceae. In: Assadi M, Maassoumi AA, Babakhanlou P, Mozaffarian V (eds) Flora of Iran. Research Institute of Forests and Rangelands (RIFR), Tehran, pp 8–75
Kabera JN, Semana E, Mussa AR, He X (2014) Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. J Pharm Pharmacol 2:377–392
Karimi F, Yousefzadi M, Mirjalili MH, Rahmani N, Zaeifi M (2013) Chemical composition of the essential oil of Haplophyllum virgatum var. virgatum from Iran. Chem Nat Compd 49:148–149
Khalili M, Hasanloo T, Safdari Y (2014) Hydrogen peroxide acts as a secondary messenger for production of silymarin in Ag+ elicited Silybum marianum hairy root cultures. J Med Plant by Prod 1:35–40
Khataee E, Karimi F, Razavi K (2020) Different carbon sources and their concentrations change alkaloid production and gene expression in Catharanthus roseus shoots in vitro. Funct Plant Biol 48:40–53
Kim YB, Kim JK, Uddin MR, Xu H, Park WT, Tuan PA, Li X, Chung E, Lee JH, Park SU (2013) Metabolomics analysis and biosynthesis of rosmarinic acid in Agastache rugosa Kuntze treated with methyl jasmonate. PLoS ONE 8:e64199
Landete JM (2012) Plant and mammalian lignans: a review of source, intake, metabolism, intestinal bacteria and health. Food Res Int 46:410–424
Lee D, Ellard M, Wanner LA, Davis KR, Douglas CJ (1995) The Arabidopsis thaliana 4- coumarate: CoA ligase (4CL) gene: stress and developmentally regulated expression and nucleotide sequence of its cDNA. Plant Mol Biol 28:871–884
Li JT, Qiu ZB, Zhang XW, Wang LS (2011) Exogenous hydrogen peroxide can enhance tolerance of wheat seedlings to salt stress. Acta Physiol Plant 33:835–842
Li WJ, Jiang X, Xue PH, Chen SM (2002) Inhibitory effects of chitosan on superoxide anion radicals and lipid free radicals. Chin Sci Bull 47:887–889
Lin W, Hu X, Zhang W, Rogers WJ, Cai W (2005) Hydrogen peroxide mediates defence responses induced by chitosans of different molecular weights in rice. J Plant Physiol 162:937–944
Ma LJ, Li YY, Yu CM, Wang Y, Li XM, Li N, Chen Q, Bu N (2014) Germination and physiological response of wheat (Triticum aestivum) to pre-soaking with oligochitosan. Int J Agric Biol 16:766–770
Mechehoud Y, Chalard P, Figueredo G, Marchioni E, Benayache F, Benayache S (2014) Chemical composition of the essential oil of Haplophyllum tuberculatum (Forssk.) L.A. Juss. from Algeria. Res J Pharm Biol Chem Sci 5:1416–1419
Mishra S, Jha AB, Dubey RS (2011) Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 248:565–577
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mohammadhosseini M, Venditti A, Frezza C, Serafini M, Bianco A, Mahdavi B (2021) The genus Haplophyllum juss.: phytochemistry and bioactivities. Molecules 26:4664
Mourato M, Reis R, Martins LL (2012) Characterization of plant antioxidative system in response to abiotic stresses: a focus on heavy metal toxicity. In: Montanaro G, Dichio B (eds) Advances in selected plant physiology aspects. InTech, Rijeka, pp 23–44
Moussa R, Abdel-Aziz SM (2008) Comparative response of drought tolerant and drought sensitive maize genotypes to water stress. Aust J Crop Sci 1:31–36
Mozaffarian V (1996) A dictionary of Iranian plant names. Farhang Moaser, Tehran, p 671
Murthy HN, Lee EJ, Paek KY (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss Org Cult 118:1–16
Namdeo AG (2007) Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn Rev 1:69–79
Paciolla C, Paradiso A, de Pinto MC (2016) Cellular redox homeostasis as central modulator in plant stress response. In: Gupta D, Palma J, Corpas F (eds) Redox State as a Central Regulator of Plant-Cell Stress Responses. Springer Cham, Switzerland, pp 1–23
Parhoodeh P, Rahmani M, Hashim NM, Sukari MA, Lian GEC (2011) Lignans and other constituents from aerial parts of Haplophyllum Villosum. Molecules 16:2268–2273
Piquemal J, Lapierre C, Myton K, O’Connell A, Schuch W, Grima- Pettenati J, Boudet AM (1998) Down-regulation of cinnamoyl- CoA reductase induces significant changes of lignin profiles in transgenic tobacco plants. Plant J 13:71–83
Poonam S, Kaur H, Geetika S (2013) Effect of jasmonic acid on photosynthetic pigments and stress markers in Cajanus cajan (L.) Millsp. seedlings under copper stress. Am J Plant Sci 4:817
Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusido R, Palazon J (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21:182
Rasulova KA, Kodirova DR, Bobakulov KM, Abdullaev ND (2015) Griffithine, a new furanoquinolone alkaloid from: Haplophyllum griffithianum. Chem Nat Compd 51:743–745
Renouard S, Tribalatc MA, Lamblin F, Mongelard G, Fliniaux O, Corbin C, Marosevic D, Pilard S, Demailly H, Gutierrez L, Hano C, Mesnard F, Lain´e E, (2014) RNAi-mediated pinoresinol lariciresinol reductase gene silencing in flax (Linum usitatissimum L.) seed coat: Consequences on lignans and neolignans accumulation. J Plant Physiol 171:1372–1377
Saisavoey T, Thongchul N, Sangvanich P, Karnchanatat A (2014) Effect of methyl jasmonate on isoflavonoid accumulation and antioxidant enzymes in Pueraria mirifica cell suspension culture. J Med Plant Res 8:401–407
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Shah Z, Gohar UF, Jamshed I, Mushtaq A, Mukhtar H, Zia-UI-Haq M, Toma SI, Manea R, Moga M, Popovici B (2021) Podophyllotoxin: history, recent advances and future prospects. Biomolecules 11:603
Sharma P, Dubey RS (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul 46:209–221
Sofo A, Dichio B, Xiloyannis C, Masia A (2005) Antioxidant defences in olive trees during drought stress: changes in activity of some antioxidant enzymes. Funct Plant Biol 32:45–53
Sun T, Xie WM, Xu PX (2004) Superoxide anion scavenging activity of graft chitosan derivatives. Carbohydr Polym 58:379–382
Tanaka Y, Sassaki N, Akemi O (2008) Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54:733–749
Tashackori H, Sharifi M, Chashmi NA, Fuss E, Behmanesh M, Safaie N (2019) RNAi-mediated silencing of pinoresinol lariciresinol reductase in Linum album hairy roots alters the phenolic accumulation in response to fungal elicitor. J Plant Physiol 232:115–126
Toghyani MA, Karimi F, Hosseini Tafreshi SA, Talei D (2020) Two distinct time dependent strategic mechanisms used by Chlorella vulgaris in response to gamma radiation. J Appl Phycol 32:1677–1695
Tokunaga N, Sakakibara N, Umezawa T, Ito Y, Fukuda H, Sato Y (2005) Involvement of extracellular dilignols in lignification during tracheary element differentiation of isolated Zinnia mesophyll cells. Plant Cell Physiol 46:224–232
Townsend CC (1986) Taxonomic revision of the genus Haplophyllum (Rutaceae). Hooker’s Icones Plantarum, vol 40–43. Kew Publishing, 2000, p 366. https://books.google.com/books/about/Taxonomic_Revision_of_the_Genus_Haplophy.html?id=bChVngEACAAJ
Upadhyaya A, Sankhla D, Davis TD, Sankhla N, Smith BN (1985) Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J Plant Physiol 121:453–461
Valdes-Lopez O, Hernandez G (2014) Phenylpropanoids as master regulators: state of the art and perspectives in common bean (Phaseolus vulgaris). Front Plant Sci 5:336
Van Fürden B, Humburg A, Fuss E (2005) Influence of methyl jasmonate on podophyllotoxin and 6-methoxypodophyllotoxin accumulation in Linum album cell suspension cultures. Plant Cell Rep 24:312–317
Vasconsuelo A, Boland R (2007) Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci 172:861–875
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci 151:59–66
Vogt T (2010) Phenylpropanoid biosynthesis. Mol Plant 3:2–20
Westcott ND, Muir AD (2003) Flax seed lignan in disease prevention and health promotion. Phytochem Rev 2:401–417
Whetten R, Sederoff RR (1995) Lignin biosynthesis. Plant Cell 7:1001–1013
Winkler AJ, Dominguez-Nunez JA, Aranaz I, PozaCarrion C, Ramonell K, Somerville S, Berrocal-Lobo M (2017) Short chain chitin oligomers: promoters of plant growth. Mar Drugs 15:1–21
Xie WM, Xu PX, Liu Q (2001) Antioxidant activity of water-soluble chitosan derivatives. Bioorg Med Chem Lett 11:1699–1701
Xu C, Zhao B, Ou Y, Wang X, Yuan X, Wang Y (2007) Elicitor-enhanced syringin production in suspension cultures of Saussurea medusa. World J Microb Biot 23:965–970
Yin XQ, Lin Q, Zhang Q, Yang LC (2002) O2- scavenging activity of chitosan and its metal complexes. Chin J Appl Chem 19:325–328
Yousefian Sh, Lohrasebi T, Farhadpour M, Haghbeen K (2020) Effect of methyl jasmonate on phenolic acids accumulation and the expression profile of their biosynthesis-related genes in Mentha spicata hairy root cultures. Plant Cell Tiss Org Cult 142:285–297
Yue W, Ming QL, Lin B, Rahman K, Zheng CJ, Han T, Qin LP (2016) Medicinal plant cell suspension cultures: pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit Rev Biotechnol 36:215–232
Zhou R, Jackson L, Shadle G, Nakashima J, Temple S, Chen F, Dixon RA (2010) Distinct cinnamoyl CoA reductases involved in parallel routes to lignin in Medicago truncatula. Proc Natl Acad Sci 107:17803–17808
Zielinska-Blajet M, Feder-Kubis J (2020) Monoterpenes and their derivatives-recent development in biological and medical applications. Int J Mol Sci 21:7078
Acknowledgements
The authors would like to thank Shahed University for providing the facilities necessary to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Torabi, S., Karimi, F. & Razavi, K. Phenylpropanoid biosynthetic gene expression in cell suspension culture of Haplophyllum virgatum Spach. under chitin treatment. In Vitro Cell.Dev.Biol.-Plant 59, 49–60 (2023). https://doi.org/10.1007/s11627-023-10327-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-023-10327-7