Abstract
Hydrogen peroxide (H2O2), an active oxygen species, is widely generated in many biological systems and mediates various physiological and biochemical processes in plants. In this study, we demonstrated that exogenous H2O2 was able to improve the tolerance of wheat seedlings to salt stress. Treatments with exogenous H2O2 for 2 days significantly enhanced salt stress tolerance in wheat seedlings by decreasing the concentration of malondialdehyde (MDA), the production rate of superoxide radical (O2 −), and increasing the activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and ascorbate peroxidase (APX), and the concentration of glutathione (GSH) and carotenoids (CAR). To further clarify the role of H2O2 in preventing salt stress damage, CAT and ascorbate (AsA), the specific H2O2 scavengers, were used. The promoting effect of exogenous H2O2 on salt stress could be reversed by the addition of CAT and AsA. It was suggested that exogenous H2O2 induced changes in MDA, O2 −, antioxidant enzymes and antioxidant compounds were responsible for the increase in salt stress tolerance observed in the experiments. Therefore, H2O2 may participate in antioxidant enzymes and antioxidant compounds induced tolerance of wheat seedlings to salt stress. The results also showed that exogenous H2O2 had a positive physiological effect on the growth and development of salt-stressed seedlings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil salinity is one of the major abiotic stress factors for plants growth. Flowers (1999) has estimated that 20–50% of all irrigated crop lands are affected by high salt concentration, resulting in considerable economic losses. Salt stress triggers an increased formation of reactive oxygen species (ROS), which results in cellular damage (Molassiotis et al. 2006; Nasir Kham et al. 2010). To minimize oxidative damage, plants have evolved various enzymatic and non-enzymatic defense mechanisms to detoxify free radicals and reduce oxidative stress. The antioxidant defense systems include enzymatic antioxidants such as glutathione reductase (GR), superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD), whereas non-enzymatic antioxidants include glutathione, ascorbate, α-tocopherol, proline, carotenoids and flavonoids. It has been reported that ROS-induced damage to macromolecules and cell membranes is regulated by H2O2 signaling (Dat et al. 2000; Murphy et al. 2002; Overmyer et al. 2003; Hung et al. 2005). Available information suggests that H2O2 directly regulates the expression of numerous genes, some of which are involved in plant defense and hypersensitive response (Kovtun et al. 2000), antioxidants, cell rescue/defense proteins and signaling proteins such as kinase, phosphatase and transcription factors (Hung et al. 2005; Henry 2008). Hence, H2O2 signaling is of potential significance to any program aimed at improving crop tolerance to environmental stress.
Exogenous application of H2O2 can provide more vigorous root system in wheat (Amjad et al. 2004) and has influence on initial leaf and coleoptile growth in etiolated wheat seedlings (Amjad et al. 2003). Uchida et al. (2002) and Chen et al. (2009) confirmed that H2O2 pretreatment caused oxidative stress at high concentrations, while low level of H2O2 conferred a cytoprotective role. Available information suggests that exogenous H2O2 at low concentration improves chilling tolerance of mung bean and Manila grass (Murphy et al. 2002; Wang et al. 2010) and induces acclimation to salt and heat stresses in rice seedlings pretreated with low level of H2O2 (Uchida et al. 2002). Azevedo Neto et al. (2005) reported that addition of 1 μM H2O2 to the nutrient solution induced salt tolerance by enhanced activities of antioxidants and reduced peroxidation of membrane lipids in leaves and roots of maize as an acclimation response. Recently, important evidence that H2O2 could induce acclimation on salt stress was obtained with wheat seeds pretreated with low concentration of H2O2 (Abdul et al. 2007). However, it is not known if exogenous low level of H2O2 treatment also induces salt stress tolerance in wheat seedlings. The aim of the present investigation was to evaluate the effect of exogenous low level of H2O2 treatment on protecting wheat from salt stress damage using plant morphological and physiological indices.
Materials and methods
Plant materials, growth and treatment conditions
Uniform seeds of wheat (Triticum aestivum L. cv. Zhengmai No. 004, obtained from Henan Academy of Agricultural Sciences) were surface sterilized for 3 min by immersion in 0.01% HgCl2, followed by washing for 30 min in flowing water. The seeds were grown in Petri dishes (diameter 18 cm), flushed daily with Hoagland solution, in a growth chamber under a 12-h photoperiod at 400 μmol m−2 s−1 provided by fluorescent lamps, 70% relative humidity and 25°C/18°C (day/night) temperature. Half of the 12-day-old seedlings (with two fully expanded leaves) were treated with Hoagland solution with 150 mM NaCl for 2 days. The other half were allowed to grow with Hoagland solution, which served as the control. Treatment with 150 mM NaCl and 0.05 μM H2O2 was chosen according to our previous work (data not shown). Five Petri plates of 60 seedlings each were regarded as a group of treatments and all experiments were independently repeated at least three times. On the second day of salt stress, leaves were sampled for various analyses. See Table 1 for details about experimental design.
NaCl was directly added to Hoagland solutions, and all the above chemicals were added to Hoagland solutions with or without 150 mM NaCl. All of the solutions were regenerated once a day to maintain identical concentration.
Lipid peroxidation assay
Malondialdehyde (MDA) concentration was measured according to Predieri et al. (1995). Leaf samples (0.3 g fresh weight, FW) were homogenized in 50 mM phosphate buffer (pH 7.8) and then centrifuged for 15 min at 8,000g. A volume of 1 ml of supernatant sample was combined with 2.5 ml thiobarbituric acid (TBA) incubated in boiling water for 20 min and then quickly cooled in an ice bath. The mixture was centrifuged at 10,000g for 5 min and the absorbance of supernatant was monitored at 532 and 600 nm. After subtracting the non-specific absorbance (600 nm), the MDA concentration was determined by its molar extinction coefficient (155 mM−1 cm−1) and the results expressed as μmol MDA g−1 FW.
The production rate of superoxide radical (O2 −) determination
The production rate of O2 − was measured by the modified method as described by Elstner and Heupel (1976). Fresh leaves (0.2 g) were homogenized in 1 ml of 50 mM phosphate buffer (pH 7.8), and the homogenate was centrifuged at 10,000g for 10 min. Then, 0.5 ml of the supernatant was added to 0.5 ml 50 mM phosphate buffer (pH 7.8) and 0.1 ml of 10 mM hydroxylamine hydrochloride. After 1 h reaction at 25°C, the mixture was added to 1 ml of 17 mM sulfanilamide and 1 ml of 7 mM α-naphthylamine at 25°C for 20 min. The specific absorbance at 530 nm was determined. Sodium nitrite was used as a standard solution to calculate the production rate of O2 −.
Enzyme activity determination
Frozen leaves (0.2 g) were homogenized in a mortar and pestle with 2 ml of 50 mM ice-cold phosphate buffer (pH 7.8) containing 1 mM EDTA. The homogenate was centrifuged at 15,000g for 15 min at 4°C. The supernatant was used for assays of the activities of SOD, POD, CAT and APX. All operations were carried out at 4°C.
Activity of superoxide dismutase (SOD) (EC 1.15.1.1) was assayed by measuring its capacity for inhibiting the photoreduction of nitro blue tetrazolium (NBT), as described by Giannopolitis and Ries (1977). The 3 ml reaction mixture contained 50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, 130 mM methionine, 0.75 mM NBT, 0.02 mM riboflavin and 0.1 ml enzyme extract. Riboflavin was added as the last component and the reaction was initiated by placing the tubes under two 20-W fluorescent lamps. The reaction was terminated after 10 min by removing the reaction tubes from the light source. Non-illuminated and illuminated reactions without supernatant served as calibration standards. Absorbance of the reaction mixture was read at 560 nm. One unit of SOD activity (U) was defined as the amount of enzyme required to cause 50% inhibition of the NBT photoreduction rate and the results expressed as unit mg−1 of fresh weight.
Catalase (CAT) activity (EC 1.11.1.6) was determined using the method of Cakmak and Marschner (1992), with minor modifications. The 3 ml reaction solution consisted of 100 mM phosphate buffer (pH 7.0), 0.1 μM EDTA, 0.1% H2O2 and 0.1 ml enzyme extract. The reaction was initiated by adding the enzyme extract. The decrease of H2O2 was monitored at 240 nm and quantified by its molar extinction coefficient (36 M−1 cm−1).
Analysis of peroxidase (POD) (EC 1.11.1.7) capacity was based on oxidation of guaiacol using hydrogen peroxide (Zhang and Kirham 1994). The enzyme extract (0.02 ml) was added to the reaction mixture containing 0.02 ml guaiacol solution and 0.01 ml hydrogen peroxide solution in 3 ml of phosphate buffer solution (pH 7.0). The addition of enzyme extract started the reaction, and the increase in absorbance was recorded at 470 nm for 5 min. Enzyme activity was quantified by the amount of tetraguaiacol formed using its molar extinction coefficient (26.6 mM−1 cm−1).
The activity of ascorbate peroxidase (APX) (EC 1.11.1.1) was assayed according to Nakano and Asada (1981). The reaction mixture (3 ml) contained 50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, 0.5 mM ascorbate, 0.1 mM H2O2 and 0.1 ml enzyme extract. The reaction was started by the addition of H2O2 and ascorbate oxidation was measured at 290 nm for 3 min. Enzyme activity was quantified using the molar extinction coefficient for ascorbate (2.8 mM−1 cm−1).
The determination of antioxidant compounds concentration
Fresh leaves (0.2 g) were homogenized in ice-cold 5% (w/v) trichloroacetic acid and centrifuged at 15,000g for 15 min. After centrifuging, to 0.2 ml supernatant, 2.6 ml phosphate buffer (pH 7.7) and 0.2 ml DTNB (2.51 mg ml−1) were added. After 5 min at 30°C, the absorbance was determined at 412 nm. Glutathione (GSH) content was calculated based on a standard curve (Ellman 1959).
Ascorbic acid (AsA) concentration was measured according to Tonamura (1978). Fresh leaves (0.2 g) were homogenized in ice-cold 2 ml 10% metaphosphoric acid. After centrifugation at 15,000g for 10 min, to 0.5 ml of supernatant, 1 ml of citric acid–phosphoric acid buffer (pH 2.3) and 1 ml of 2,6-dichlorophenol indophenol (30 mg l−1) were added. After 30 s, the absorbance was determined at 524 nm. AsA concentration was expressed as mg g−1 FW.
Leaf samples (0.3 g, fresh weight) were ground in 10 ml of 80% acetone. The homogenate was centrifuged at 2,700g for 10 min. The absorbance of supernatant was recorded at 663.2, 646.8 and 470 nm. The contents of carotenoids (xanthophylls and β-carotene) were calculated with the formulae of Lichtenthaler (1987).
Growth parameter
Green plant parts and roots were oven dried at 65°C until constant weight and weighted using electronic scale as biomass (g). Plant height and root length were also measured.
Statistical analysis
The experiment was a completely random design with five replications. All data obtained were subjected to a one-way analysis of variance (ANOVA), and the mean differences were compared by Duncan’s multiple range test. Comparisons with P < 0.05 were considered significantly different. In all the figures, the spread of values is shown as error bars representing standard errors of the means.
Results
Effect of exogenous H2O2 on MDA concentration and the production rate of O2 − in wheat seedlings under salt stress
Compared to the control (CK), salt stress caused a significant increase (P < 0.05) in the concentration of MDA and the production rate of O2 − in wheat seedlings. In contrast, exogenous H2O2 combined with 150 mM NaCl treatment caused a decrease (P < 0.05) in the concentration of MDA and the production rate of O2 − in wheat seedlings (Fig. 1). These results indicated that exogenous H2O2 treatment significantly protected wheat seedlings from damage by salt stress. To further clarify the role of H2O2 in preventing salt stress-induced oxidative damage, CAT and AsA, the specific H2O2 scavengers, were used. The protective effect of H2O2 on salt stress-induced lipid peroxidation could be reversed by the addition of 100 U ml−1 CAT and 2 mM AsA (Fig. 1). Treatments with CAT and AsA alone did not have any effect on lipid peroxidation and the production rate of O2 − in wheat seedlings compared with the control.
Effect of exogenous H2O2 on the regulation of MDA concentration and the production rate of O2 − in wheat seedlings under salt stress for 2 days. Different treatment represents: Hoagland solutions (control, CK), 150 mM NaCl (N), 150 mM NaCl + 0.05 μM H2O2 (N + H), 150 mM NaCl + 0.05 μM H2O2 + 100 U ml−1 CAT (N + H + C), 150 mM NaCl + 0.05 μM H2O2 + 2 mM AsA (N + H + A), Hoagland solutions + 0.05 μM H2O2 (CK + H), Hoagland solutions + 100 U ml−1 CAT (CK + C), Hoagland solutions + 2 mM AsA (CK + A). Bars are mean ± standard error of six replicates. Means with different letters above bars were significantly different at 0.05 level according to Duncan’s multiple range test
Effect of exogenous H2O2 on antioxidant enzymes in wheat seedlings under salt stress
Salt stress alone caused a significant decrease (P < 0.05) in the activities of SOD, POD, CAT and APX compared with the control. In contrast, a more remarkable increase (P < 0.05) in the activities of SOD, POD, CAT and APX was observed in the seedlings with exogenous H2O2 combined with 150 mM NaCl (Fig. 2). Figure 2 also showed that the effect of exogenous H2O2 was blocked by H2O2 scavenger CAT and AsA. Treatments with CAT, AsA and H2O2 alone had no effect on the activities of SOD, POD, CAT and APX.
Effect of exogenous H2O2 on SOD, APX, CAT and POD activities in wheat seedlings under salt stress for 2 days. See notes to Fig. 1
Effect of exogenous H2O2 on antioxidant compounds in wheat seedlings under salt stress
Salt stress caused a significant decrease (P < 0.05) in the concentration of GSH and CAR in wheat seedlings (Table 2), while the concentration of GSH and CAR of H2O2 treated wheat seedlings were significantly enhanced as compared to 150 mM NaCl stress alone. Table 2 shows that CAT and AsA significantly decreased the 0.05 μM H2O2-induced increase in GSH and CAR concentrations under 2 days of salt stress. The AsA concentration was not affected either by salt stress or by exogenous 0.05 μM H2O2 treatment. No significant change in AsA concentration (Table 2) was observed in different treatments.
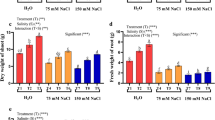
Effect of exogenous H2O2 on the growth and development of wheat seedlings under salt stress
Significant reduction (P < 0.05) was observed in seedling growth (plant height, dry weight, root length and root dry weight) under salt stress compared to the control. However, exogenous H2O2 remarkably promoted wheat seedling growth under salt stress. The promoting effect of exogenous H2O2 was reversed in the presence of the H2O2 scavenger CAT and AsA (Fig. 3). These results confirmed that exogenous H2O2 was responsible for the enhancement of adaptive responses of wheat seedlings against salt stress.
Effect of exogenous H2O2 on the growth and development of wheat seedlings under salt stress for 2 days. See notes to Fig. 1. Bars are mean ± standard error of 18 replicates. Means with different letters above bars were significantly different at the 0.05 level according to Duncan’s multiple range test
Discussion
Plant growth and development (plant height, dry weight, root length and root dry weight) were inhibited by salt stress (Fig. 3). However, it was observed that exogenous H2O2 treatment decreased the deleterious effect of salt stress on the growth of wheat. A significant increase (P < 0.05) in the seedlings’ growth under salt stress (plant height, dry weight, root length and root dry weight) was observed under exogenous H2O2 treatment, which was concomitant with the decreased production rate of O2 − and MDA concentration (Fig. 1). The content of MDA, a product of lipid peroxidation, has been considered an indicator of oxidative damage (Meloni et al. 2003; Wang et al. 2009, 2010). Exogenous H2O2 treatment could prevent lipid peroxidation and thus protect the cells from the deleterious effect of salt stress. Fedina et al. (2009) also reported that 1 μM H2O2 pretreatment notably decreased MDA and endogenous H2O2 concentration in the barley seedling under salt stress. There were other reports suggesting that exogenous H2O2 treatments prevented the increase of MDA and endogenous H2O2 concentration in plants (Prasad et al. 1994; Uchida et al. 2002; Azevedo Neto et al. 2005; Wang et al. 2009, 2010).
Superoxide radicals (O2 −) are toxic byproducts of oxidative metabolism and can interact with H2O2 to form highly reactive hydroxyl radicals (OH·), which are thought to be primarily responsible for oxygen toxicity in the cell (Bowler et al. 1992; Vaidyanathan et al. 2003). The dismutation of O2 − into H2O2 and oxygen is an important step in protecting the cell, and is catalyzed by SOD. We have shown that SOD activity increased in wheat seedlings treated with exogenous H2O2 (Fig. 2a), suggesting that exogenous H2O2-treated seedlings had a better O2 − radical scavenging ability. It has been shown that salt tolerance is directly related to the increase in SOD activity (Shalata et al. 2001; Badawi et al. 2004). SOD initiates detoxification of O2 − by forming H2O2, which also being toxic must be eliminated by conversion to H2O in subsequent reactions. In plants, a number of enzymes viz. CAT, APX, POD, glutathione-S-transferase (GST) and glutathione reductase (GR) regulate intracellular H2O2 levels (Noctor and Foyer 1998; Yasar et al. 2008; Wang et al. 2010). Our results demonstrated that exogenous H2O2 could raise APX, CAT and POD activities and the concentration of GSH and CAR in seedlings under salt stress. Our results are similar to those of Nasir Kham et al. (2010), who suggested that exogenous calcium chloride treatment of linseed under salt stress significantly increased the activities of POD and CAT. Thus, our results suggest that the coordination of APX, POD and CAT activities with SOD activity plays a central protective role in the O2 − and H2O2 scavenging process (Dionisio-Sese and Tobita 1998; Fedina et al. 2009), and the active involvement of these enzymes is related, at least in part, to salt-induced oxidative stress tolerance in wheat treated with exogenous H2O2. Shalata and Tal (1998) suggested that the ability of plant tissues to mobilize enzymatic defense against uncontrolled lipid peroxidation might be an important facet of their salt tolerance. Wang et al. (2010) also proposed that increased expression of antioxidative system (CAT, POD, APX, GR and GST) has been associated with decreased oxidative damage in different chilling tolerant species. Because of higher activities of APX, CAT and POD and higher concentrations of GSH and CAR in H2O2-treated seedlings, free radical production could be eliminated quickly. It prevented lipid peroxidation and MDA production, and thus cells of the plant were protected from salts tress damage.
Catalase (CAT) is an H2O2 scavenger (Li et al. 2007, 2009). Zhang et al. (2001) found that H2O2 was involved in ABA-induced stomatal closure in vicia faba L. and exogenous CAT could abolish the function of H2O2. Ascorbic acid (AsA) also is an H2O2 scavenger and one of the most important reducing substrates for H2O2 removal in cells (Noctor and Foyer 1998). Li et al. (2009) demonstrated that the promoting effect of exogenous H2O2 in the formation and growth of adventitious roots can be removed or inhibited by H2O2 scavengers or inhibitors (CAT and AsA). Here, exogenous CAT and AsA were used to demonstrate the role of exogenous H2O2 on salt stress tolerance of wheat seedlings. When seedlings were treated with 100 U CAT (CK + CAT) or 2 mM AsA (CK + AsA), seedling growth and lipid peroxidation, and activity of antioxidant enzymes and the concentration of antioxidant compounds showed no significant difference when compared with the control (Figs. 1, 2, 3; Table 2). However, when 100 U CAT and/or AsA was used in combination with H2O2, the promoting effect of exogenous H2O2 on salt stress was eliminated by 100 U CAT and/or 2 mM AsA. These results suggest that exogenous CAT and AsA itself had no effects on the seedling growth and development, but that it could eliminate the promoting effect of exogenous H2O2 via scavenging or inhibiting.
It has been known that H2O2 acts as a second messenger in response to wounding, heat, chilling and drought stresses in rice, maize and mung bean seedlings (Prasad et al. 1994; Uchida et al. 2002; Li et al. 2009; Wang et al. 2010). Therefore, salt stress causes cellular redox imbalances, and wheat seedlings can make use of common pathways and components in the stress–response relationship (Pastori and Foyer 2002). This may explain why H2O2 was required as a signaling molecule and could enhance tolerance of wheat seedlings to salt stress. Although we have not yet investigated the effects of exogenous H2O2 treatment on the later stages of wheat growth and grain yields, this method has the potential to increase plant growth and decrease yield losses under saline conditions.
In summary, this study has demonstrated that the ROS-induced decrease in root and shoot growth and development of wheat seedlings under saline conditions can be ameliorated by exogenous H2O2 treatment. The results support the hypothesis that exogenous H2O2 treatment can enhance tolerance of wheat seedlings to salt stress by enhancing the production of enzymatic and non-enzymatic antioxidants and decreasing lipid peroxidation. The promoting effects of H2O2 on seedling to salt stress can be removed or inhibited by H2O2 scavengers. The mechanism by which H2O2 treatment may protect against salt stress will be investigated further.
References
Abdul W, Mubaraka P, Sadia G, Shahzad MA (2007) Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164:283–294
Amjad H, Salman AM, Nayyer I, Rubina A, Shafqat F (2003) Influence of hydrogen peroxide on initial leaf and coleoptile growth in etiolated wheat seedlings (Triticum aestivum L.). Asian J Plant Sci 2(15–16):1121–1125
Amjad H, Shafqat F, Nayyer I, Rubina A (2004) Influence of exogenous application of hydrogen peroxide on root and seedling growth on wheat (Triticum aestivum L.). Int J Agric Biol 6(2):366–369
Azevedo Neto AD, Prisco JT, Eneas-Filho J (2005) Hydrogen peroxide pre-treatment induces stress acclimation in maize plants. J Plant Physiol 162:1114–1122
Badawi GH, Yamauchi Y, Shimada E, Sasaki R, Naoyoshi K, Tanaka K (2004) Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci 166:919–928
Bowler C, Van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Chen XY, Ding X, Xu S, Wang R, Shen WB (2009) Endogenous hydrogen peroxide plays a positive role in the upregulation of heme oxygenase and acclimation to oxidative stress in wheat seedling leaves. J Integr Plant Biol 51(10):951–960
Dat JF, Vandendede F, Vranova E, Montagu MV, Inze D, Breusegem FV (2000) Dual action of the active oxygen species during plant stress response. Cells Mol Life 57:779–795
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammonium-chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Fedina S, Nedeva D, Çiçek N (2009) Pre-treatment with H2O2 induces salt tolerance in barley seedling. Biol Plant 53(2):321–324
Flowers TJ (1999) Salinisation and horticultural production. Sci Hortic A 78:1–4
Giannopolitis CN, Ries SK (1977) Superoxide dismutase. 1. Occurrence in higher plants. Plant Physiol 59:309–314
Henry JF (2008) Use and abuse of exogenous H2O2 in studies of signal transduction. Free Rad Biol Med 42(7):926–932
Hung SH, Yu CW, Lin CH (2005) Hydrogen peroxide functions as a stress signal in plants. Bot Bull Acad Sin 46:1–10
Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97:2940–2945
Li SW, Xue LG, Xu SJ, An LZ (2007) Hydrogen peroxide involvement in formation and development of adventitious roots in cucumber. Plant Growth Regul 52:173–180
Li SW, Xue LG, Xu SJ, An LZ (2009) Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ Exp Bot 65:63–71
Lichtenthaler HK (1987) Chlorophylls and carotenoids pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Molassiotis A, Sotiropoulos T, Tanou G, Diamantidis G, Therios I (2006) Boron-induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 (Malus domestica Borkh). Environ Exp Bot 56:54–62
Murphy TM, Sung WW, Lin CH (2002) H2O2 treatment induces glutathione accumulation and chilling tolerance in mung bean. Funct Plant Biol 29:1081–1087
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nasir Kham M, Manzer HS, Mohammad F, Naeem M, Masroor M, Kham A (2010) Calcium chloride and gibberellic acid protect linseed from NaCl stress by inducing antioxidative defense system and osmoprotectant accumulation. Acta Physiol Plant 32:121–132
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol Plant Mol Biol 49:249–279
Overmyer K, Brosche H, Kangasjarvi J (2003) Reactive oxygen species and hormonal control of cell death. Trends Plant Sci 8:335–342
Pastori GM, Foyer CH (2002) Common components, networks, and pathways of cross-tolerance to stress. The central role of redox and abscisic acid-mediated controls. Plant Physiol 129:460–468
Prasad TK, Anderson MK, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6:65–74
Predieri S, Norma MA, Krizek DT (1995) Influence of UV-B radiation on membrane lipid composition and ethylene of evolution in ‘Doyenne d’Hiver’ pear shoots grown in vitro under different photosynthetic photo fluxes. Environ Exp Bot 35:152–260
Shalata A, Tal M (1998) The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol Plant 104:169–174
Shalata A, Mittova V, Volokita M, Guy M, Tal M (2001) Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon Pennellii to salt-dependent oxidative stress: the root antioxidative system. Physiol Plant 112:487–494
Tonamura B (1978) Test reactions for a stopped flow apparatus regulation of 2, 6-D and potassium ferricynide by l-ascorbic acid. Anal Biochem 84:370–383
Uchida A, Jagendorf AT, Hibino T, Takabe T (2002) Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci 163:515–523
Vaidyanathan H, Sivakumar P, Chakrabarty R, Thomas G (2003) Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.) differential response in salt-tolerant and sensitive varieties. Plant Sci 165:1411–1418
Wang Y, Yang ZM, Zhang QF, Li JL (2009) Enhanced chilling tolerance in Zoysia matrella by pre-treatment with salicylic acid, calcium chloride, hydrogen peroxide or 6-benzylaminopurine. Biol Plant 53(1):179–182
Wang Y, Li JL, Wang JZ, Li ZK (2010) Exogenous H2O2 improves the chilling tolerance of manilagrass and mascarenegrass by activating the antioxidative system. Plant Growth Regul 61(2):195–204
Yasar F, Ellialtioglu S, Yildiz K (2008) Effect of salt stress on antioxidant defense systems, lipid peroxidation and chlorophyll content in green bean. Russ J Plant Physiol 55(6):782–786
Zhang JX, Kirham MB (1994) Osmotic stress-induced changes in activities of superoxide dismutase, catalase and peroxidase in wheat species. Plant Cell Physiol 35:785–791
Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126:1438–1448
Acknowledgments
This work was supported by the Key Subject of Biochemistry and Molecular Biology of Henan Province and Opening Foundation of Key Laboratory of Resource Biology and Biotechnology in Western China (Northwest University), Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Filek.
Rights and permissions
About this article
Cite this article
Li, JT., Qiu, ZB., Zhang, XW. et al. Exogenous hydrogen peroxide can enhance tolerance of wheat seedlings to salt stress. Acta Physiol Plant 33, 835–842 (2011). https://doi.org/10.1007/s11738-010-0608-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0608-5