Abstract
Linum album accumulates anti-tumor podophyllotoxin (PTOX) and its related lignans, which were originally isolated from an endangered species Podophyllum. In the present study, we examined the effects of five fungal extracts on the production of lignans in L. album cell cultures. Fusarium graminearum extract induced the highest increase of PTOX [143 μg g−1 dry weight (DW) of the L. album cell culture], while Rhizopus stolonifer extract enhanced the accumulation of lariciresinol up to 364 μg g−1 DW, instead of PTOX. Typical elicitors, such as chitin, chitosan, or methyl jasmonate (MeJA), were shown to be less effective in lignan production in L. album cell cultures. These results verified the advantages of fungal extracts to increase lignan production in L. album cell culture, and suggested potential on-demand metabolic engineering of lignan biosynthesis using differential fungal extracts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Podophyllotoxin (PTOX), originally isolated from the endangered genus Podophyllum, is an important lignan used as a starting material for the semi-synthesis of various PTOX derivatives as anticancer drugs (Canel et al. 2000). The chemical synthesis of PTOX is a multi-step process and its availability from natural sources is limited (Yousefzadi et al. 2010a), which suggests a potential requirement for the development of biotechnological production of PTOX. Plant cell culturing is expected to provide advantages over whole plant cultivation for biosynthetic studies and production of secondary metabolites (Farkya et al. 2004). Linum album, an endemic species in Iran, is also known to accumulate PTOX and its related lignans, and has been targeted as a possible alternative source of PTOX (Petersen and Alfermann 2001; Fuss 2003). The PTOX biosynthesis stems from the production of the central precursor, pinoresinol, which is biosynthesized via the coupling of two coniferyl alcohol molecules (Fig. 1). Pinoresinol is further metabolized into a great diversity of lignans (Fig. 1). Pinoresinol is reduced via lariciresinol to secoisolariciresinol by pinoresinol–lariciresinol reductase (PLR) and subsequently oxidized to matairesinol. The conversion of matairesinol into deoxypodophyllotoxin (DOP), the first aryltetralin lignan, has yet to be well characterized. DOP-7-hydroxylase is thought to be responsible for the hydroxylation at positions 7 of DOP to give PTOX (Federolf et al. 2004).
Recently, a growing body of evidence has verified the positive effects of elicitors on the production of secondary metabolites in in vitro cell cultures of various plant species (Zhao et al. 2005; Ionkova 2007). Elicitation may activate multiple genes responsible for plant defensive responses, leading to enhancement of secondary metabolite production. Induced plant defensive responses involve a network of signal transduction triggered via the recognition of elicitor molecules by specific plant receptors. Lignans are very well known to play an important role in plant defense (Figgitt et al. 1989).
Although elicitation is widely used in plant cell cultures to enhance the yield of compounds, there are only a few reported studies using this approach with PTOX. For instance, methyl jasmonate (MeJA) and salicylic acid (SA) were found to induce PTOX production in cell culture of L. album (Van Fürden et al. 2005; Yousefzadi et al. 2010b). Cell cultures of L. nodiflorum treated with coronalon exhibited tenfold greater production of PTOX (Berim et al. 2005). Similar increases were observed in callus cultures of J. chinensis elicited by chito-ligosacharides (Muranaka et al. 1998). Furthermore, Baldi et al. (2008) showed the increase of the PTOX production by co-culturing L. album cells with axenically arbuscular mycorrhiza-like fungi, Piriformospora indica and Sebacina vermifera. However, the potency of various types of elicitors on PTOX production in cell cultures has yet to be evaluated. In this paper, we present novel effects of fungal elicitors on lignan accumulation in L. album cell cultures.
Materials and methods
Establishment of the cell suspension cultures
L. album seeds were collected in Iran and germinated under sterile conditions on MS-medium (Murashige and Skoog) at 25°C in the dark. Shoot cultures were established from seedlings on MS-medium without hormones and incubated under constant irradiation of light. Single seedlings were used to initiate the calli. Explants were subcultured on the surface of solid MS-medium supplemented with 30 g l−1 sucrose, 2 mg l−1 NAA and 0.4 mg l−1 kinetin and subcultured every 10 days. From these calli, TMU-1 cell line was generated (Yousefzadi et al. 2010b). All suspension cultures were incubated on a gyratory shaker at 120 rpm in the darkness at 25°C and subcultured every 10 days.
Elicitation of Linum cell cultures
Fungal elicitors were prepared as described by Farkya et al. (2005) with slight modifications. One-week-old mycelia of Fusarium graminearum, Rhizopus stolonifer, Rhizoctonia solani, Trichoderma viride and Sclerotinia sclerotiorum were harvested and rinsed with sterile distilled water. The collected mycelia were ground under liquid nitrogen and suspented with water to a final concentration of 250 mg ml−1. The suspension was centrifuged at 10,000g for 10 min and then the supernatant was autoclaved for 10 min at 120°C. For elicitation treatments, 500 mg of fresh cells were transferred to 5 ml of the cell culture medium per single well in a 6-well microliter plate, and were supplemented with various types of elicitors at selected concentrations after 7 days of preculture (mid-log phase). The cells were treated with either (1) 0.5, 1 and 2% (v/v) of fungal extracts, (2) 100, 200 and 400 μM of MeJA or (3) 50, 100 and 200 mg l−1 chitin and chitosan. Since we had previously confirmed the maximal production of lignans, cells were harvested for 5 days after elicitation.
Lignans quantification
Dried cells (50 mg) were homogenized in 1 ml of 80% methanol, followed by ethylacetate extraction. The extract was dissolved in methanol again, and filtered through a Millex-LH filter with 0.45 μm pore size (Millipore, Bedford, MA, USA) for reverse-phase high-performance liquid chromatography (HPLC) analysis using the Waters 2960 instruments with 996 photodiode array detector and 4.6 × 150 mm Develosil C30-UG-5 column (Nomura Chemical, Japan). The presence of PTOX and lariciresinol in the samples was verified by mass spectrometry (MS) with commercially available standards (Figs. 2 and 3). All MS data were acquired by an instrument of positive-mode electrospray ionization (ESI) quadrapole time-of-flight (Q-TOF) MS (Micromass-Waters) equipped with a nano flow probe.
Results and discussion
Effect of elicitors on cell growth
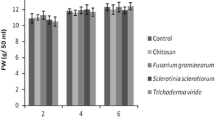
The effect of five fungal elicitors, chitin, chitosan or MeJA on the growth of L. album cells was evaluated by measurement of the dry weight of the cells 5 days after treatment (Fig. 4a–c). All the elicitors were shown to decrease the growth of L. album cell cultures in a dose-dependent manner. S. sclerotiorum extract elicited the most potent growth inhibition; the biomass of L. album cells was reduced to 50% compared to non-elicited control (Fig. 4a). Other fungal extracts decreased the growth of L. album cells by 29–36% (Fig. 4a). As shown in Fig. 4b, c, chitin, chitosan and MeJA also exhibited similar cell growth inhibition (35–40%).
Effect of fungal elicitors (a), chitosan and chitin (b) and methyl jasmonate (MeJA) (c) on growth of l. album cells. Fungal extracts [0.5, 1 and 2% (v/v)], chitosan and chitin (50, 100 and 200 mg/l) and MeJA (100, 200 and 400 μM) were added after 7 days of growth and cultivated for a further 5 days. Data represent average values from 3 separate experiments ± SD
Effect of elicitors on lignan accumulation
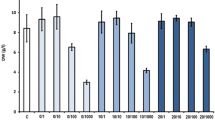
We then examined the effect of fungal elicitors on the accumulation level of PTOX and lariciresinol, an intermediate in PTOX biosynthesis in L. album cells. We also assessed lignans in liquid media but a significant amount of lignan was not detected. HPLC analysis demonstrated that all the fungal elicitors tested in our study stimulated the production of PTOX at apparent optimal concentration of 1% (v/v) (Fig. 5a; Supplemental Table 1). In particular, the F. graminearum extract was shown to induce the highest amount of PTOX (142 μg g−1 DW). Moreover, the treatment of L. album cells with R. solani resulted in the accumulation of PTOX at 94 μg g−1 DW. These levels of PTOX were 7.9- and 5.2-fold higher than that of non-elicited control (17.8 μg g−1 DW), respectively. A striking feature is that the PTOX induction by the fungal extracts (Fig. 5a) was much more prominent than the induction by prevailing elicitors such as chitin, chitosan or MeJA (up to 40 μg g−1 DW of PTOX) (Fig. 5b, c; d Supplemental Tables 2 and 3). Collectively, we conclude that the extract of F. graminearum is the most potent for the PTOX production among tested elicitors in this study.
Effect of fungal elicitors (a), chitosan and chitin (b) and methyl jasmonate (MeJA) (c) on PTOX production. Fungal extracts [0.5, 1 and 2% (v/v)], chitosan and chitin (50, 100 and 200 mg/l) and MeJA (100, 200 and 400 μM) were added after 7 days of growth and cultivated for a further 5 days. Data represent average values from 3 separate experiments ± SD
Intriguingly, the L. album cell culture treated with R. stolonifer at 2% (v/v) and R. solani extracts at 1% (v/v) exhibited 8.5-fold (307 μg g−1 DW) and 7.4-fold (267 μg g−1 DW) greater accumulation of lariciresinol, respectively, compared with that of the control (35 μg g−1 DW) (Fig. 6a–c; Supplemental Table 1), showing the usefulness of these fungal strains for the lariciresinol production over other fungal elicitors. In contrast, the treatment with chitin, chitosan and MeJA led to the accumulation of only 87 μg g−1 DW lariciresinol, indicating that the extracts of R. solani and R. stolonifer are more effective elicitors for lariciresinol production than chitin, chitosan, MeJA., and other fungal extracts.
Effect of fungal elicitors (a), chitosan and chitin (b) and methyl jasmonate (MeJA) (c) on lariciresinol production. Fungal extracts [0.5, 1 and 2% (v/v)], chitosan and chitin (50, 100 and 200 mg/l) and MeJA (100, 200 and 400 μM) were added after 7 days of growth and cultivated for a further 5 days. Data represent average values from 3 separate experiments ± SD
Taken together, these results show that fungal extracts induced prominent and species-specific induction of the PTOX and lariciresinol production in L. album cell culture as novel elicitors, compared to other types of elicitors including chitin, chitosan and MeJA.
This is the first report of enhancement of accumulation of PTOX and lariciresinol in cell suspension cultures of L. album by fungal extracts. The present study also highlights the specificity of the differential fungal strains for the production of lignans in Linum cells. This is consistent with the report that fungal elicitors of Botrytis cinerea, Phoma exigua and Fusarium oxysporum triggered the accumulation of monolignols differentially in flax cells (Hano et al. 2006). Moreover, elicitation of secondary metabolites frequently varies among different varieties within the same species. For example, only one out of ten separate lines of cell suspension cultures derived from different varieties of L. album has been reported to increase PTOX content in response to MeJA treatment (approximately 500 μg g−1 DW) (van Fürden et al. 2005), and MeJA only induced the accumulation of PTOX up to 40 μg g−1 DW in the cell cultures in the present study (Fig. 5c). These findings indicate that elucidation of the optimal combination of varieties of L. album and elicitors is required to maximize induction of lignans. In other words, unprecedented selective lignan production is likely to be developed on the basis of certain L. album variety–fungal extract pairs. The specific and diverse effects of fungal elicitors, as observed in this study, are highly likely to be implicated with unique modes of recognition upon interactions with fungi and the complexity of elicitor signal transduction, and the resulting defense responses in plants (Imre et al. 1998). Characterization of active compounds for the induction of lignan biosynthesis from crude fungal extracts is expected to pave the way for the clarification of the molecular mechanisms underlying plant cells-fungi recognition and the resultant responses.
References
Baldi A, Jain A, Bisaria VC (2008) Co-culture of arbuscular mycorrhiza-like fungi (Piriformospora indica and Sebacina vermifera) with plant cells of Linum album for enhanced production of podophyllotoxins: a first report. Biotechnol Lett 30:1671–1677
Berim A, Spring O, Conrad J, Maitrejean M, Boland W, Petersen M (2005) Enhancement of lignan biosynthesis in suspension cultures of Linum nodiflorum by coronalon, indanoyl-isoleucine and methyl jasmonate. Planta 222:769–776
Canel C, Moraes RM, Dayan FE, Ferreira D (2000) Molecules of interest: podophyllotoxin. Phytochemistry 54:115–120
Farkya S, Bisaria VS, Sirvastava AK (2004) Biotechnological aspects of the production of the anticancer drug podophyllotoxin. Appl Microbiol Biotechnol 65:504–519
Farkya S, Julka A, Mehra R, Datta V, Srivastava AK, Bisaria VS (2005) Enhanced production of secondary metabolites by biotic elicitors in plant cell suspension cultures. In: 5th Asia Pacific Biochemical Engineering Conference. Jeju Island, Korea
Federolf K, Alfermann AW, Fuss E (2004) Aryltetralin-lignan formation in two different cell lines of Linum album: deoxypodophyllotoxin 6-hydroxylase, a key enzyme for the formation of 6-methoxypodophyllotoxin. Phytochemistry 68:1397–1406
Figgitt DP, Denever SP, Dewick PM, Jackson DE, Willians P (1989) Topoisomerase II: a potential target for novel antifungal agents. Biochem Biophys Res Commun 160:257–262
Fuss E (2003) Lignans in plant cell and organ cultures: an overview. Phytochem Rev 2:307–320
Hano C, Addi M, Bensaddek D, Cronier S, Laine E (2006) Differential accumulation of monolignol-derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 223:975–989
Imre E, Somssich IE, Hahlbrok K (1998) Pathogen defence in plant—a paradigm of biological complexity. Trends Plant Sci 3:86–90
Ionkova I (2007) Biotechnological approaches for the production of lignans. Phcog Rev 1:57–68
Muranaka T, Miyata M, Ito K, Tachibana S (1998) Production of podophyllotoxin in Juniperus chinensis callus cultures treated with oligosaccharides and a biogenetic precursor. Phytochemistry 49:491–496
Petersen M, Alfermann AW (2001) The production of cytotoxic lignans by plant cell cultures. Appl Microbiol Biotechnol 55:135–142
Van Fürden B, Humburg A, Fuss E (2005) Influence of methyl jasmonate on podophyllotoxin and 6-methoxypodophyllotoxin accumulation in Linum album cell suspension cultures. Plant Cell Rep 24:312–317
Yousefzadi M, Sharifi M, Behmanesh M, Moyano E, Bonfill M, Cusido RM, Palazon J (2010a) An approach to the biotechnological production of podophyllotoxin: a potential natural product for clinical anticancer drugs. Eng Life Sci 4:281–292
Yousefzadi M, Sharifi M, Behmanesh M, Moyano E, Palazon J (2010b) Salicylic acid improves podophyllotoxin production in cell cultures of Linum album by increasing the expression of genes related with its biosynthesis. Biotechnol Lett 32:1739–1743
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Acknowledgments
This work was supported by Tarbiat Modares University and Suntory Foundation for Life Sciences, Bioorganic Research Institute.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bahabadi, S.E., Sharifi, M., Safaie, N. et al. Increased lignan biosynthesis in the suspension cultures of Linum album by fungal extracts. Plant Biotechnol Rep 5, 367–373 (2011). https://doi.org/10.1007/s11816-011-0190-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-011-0190-3