Abstract
Mentha spicata L., as a rich source of phenolic acids, is known for its therapeutic importance. Elicitors play a crucial role in biosynthetic pathways to improve plant secondary metabolites production. The purpose of this study was to investigate the effect of methyl jasmonate (MeJA) as a potent elicitor, on phenolic acids production and the relative changes in expression of their biosynthesis-related key genes (PAL, TAT, C4H, HPPR, and 4CL) in M. spicata hairy root cultures, to elucidate the interrelation between phenolic acids accumulation and the regulation mechanism of gene expression. The results showed that the relative expression levels of phenylpropanoid pathway genes, i.e., PAL, C4H, 4CL, and HPPR in the tyrosine-derived pathway increased 4.04-fold, 3.62-fold, 1.75-fold, and 1.45-fold in comparison to untreated controls, respectively. High-performance liquid chromatography analysis indicated that MeJA dramatically increased rosmarinic acid content (55.44 µg g−1 dry wt, about 11.84-fold) after 6 h exposure to elicitor. Moreover, caffeic acid, chlorogenic acid, and cinnamic acid contents were also enhanced significantly (p < 0.05) in response to MeJA treatment. On the other hand, the application of MeJA had a negative effect on both TAT expression level and lithospermic acid B accumulation. Our results implied that MeJA has a significant impact on RA, CA, CGA, and CIA accumulation, which might be the consequence of gene activation from the phenylpropanoid pathway (PAL, C4H, and 4CL) and HPPR, a key regulatory enzyme of the tyrosine-derived pathway in the elicitor-treated hairy root cultures of M. spicata.
Key message
MeJA is an effective elicitor for stimulating valuable phenolic acids production in M. spicata hairy root cultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-value plant secondary metabolites have gained attention during the last few years due to their pharmaceutical and industrial importance (Guerriero et al. 2018). The biosynthetic pathways of secondary metabolites in plants are regulated by several factors (Narayani and Srivastava 2017). Stress as an important factor regulates the formation of many secondary metabolites (Chezem and Clay 2016; Naik and Al-Khayri 2016). Improving the production of these secondary metabolites through stress induction by using substances that stimulate the plant defense response, is called elicitation (Naik and Al-Khayri 2016; Gonçalves and Romano 2018).
Phenolic acids as aromatic secondary metabolites play an important role in plant defense response (Sircar et al. 2012) and occur in varying quantities in medicinal plants of the Lamiaceae family (Zgórka and Głowniak 2001). Mentha spicata L. with the common name of spearmint or garden mint belongs to the Lamiaceae (Labiatae) family. This aromatic perennial herbaceous plant is widespread in regions with tropical and temperate climates, such as Europe, North America, Australia, and Asia, as well as most parts of Iran (Ay Kee et al. 2017; Dorman et al. 2003; Cirlini et al. 2016). Spearmint as a rich source of phenolic acids with antioxidant (Ay Kee et al. 2017; Dorman et al. 2003; Cirlini et al. 2016; Kanatt et al. 2007; Bimakr et al. 2011), antiviral, antifungal (Bayan and Küsek 2018), antimicrobial (Ay Kee et al. 2017; Dorman et al. 2003; Cirlini et al. 2016) and anti-fever properties possess therapeutic value since ancient centuries (Ay Kee et al. 2017).
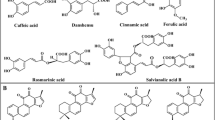
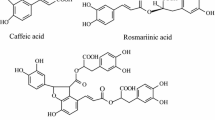
The main phenolic acids such as rosmarinic acid (RA), chlorogenic acid (CGA), caffeic acid (CA), lithospermic acid B (LAB) and t-cinnamic acid (CIA) are mainly produced through the phenylpropanoid pathway. RA and CGA which are CA esters also serve as defense compounds in plants (Petersen et al. 2009). It has been suggested that two aromatic amino acids Phenylalanine and Tyrosine contribute in the biosynthesis of RA via two parallel and conjunct pathways (Fig. 1). The general phenylpropanoid pathway encompasses three steps which are catalyzed by phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H) and hydroxy Cinnamate: CoA Ligase (4CL). The transformation of tyrosine to 4-hydroxyphenyllactic acid is catalyzed by the enzymes of the tyrosine-derived pathway tyrosine aminotransferase (TAT) and 4-hydroxyphenylpyruvate reductase (HPPR). In the next step, 4-coumaroyl-CoA and 4-hydroxyphenyllactic acid as the resultants of the phenylpropanoid and tyrosine-derived pathways are conjugate with each other by rosmarinic acid synthase (RAS). The generation of RA is finalized by hydroxylation activity of 3-hydroxycinnamoyl (3-H) and 3ʹ hydroxycinnamoyl (3ʹ-H) (Kanatt et al. 2007; Bimakr et al. 2011; Bayan and Küsek 2018; Petersen et al. 2009). LAB is considered as a dimer of RA although the detailed biosynthetic pathway of LAB from RA has not been characterized up to the present (Hao et al. 2016; Zhang et al. 2014; Petersen and Simmonds 2003).
The biosynthesis pathway of caffeic acid, chlorogenic acid, rosmarinic acid and lithospermic acid B. PAL → phenylalanine ammonia-lyase; TAT → tyrosine aminotransferase; C4H → cinnamic acid 4-hydroxylase; HPPR → 4-hydroxyphenylpyruvate reductase; 4CL → hydroxycinnamate coenzyme A ligase; RAS → rosmarinic acid synthase
Induction of hairy root by wild-type Agrobacterium rhizogenes, a gram-negative soil bacterium, is a high-efficiency biotechnological approach to produce active plant ingredients. Hairy root cultures provide many advantages as a persistent source for the production of valuable secondary metabolites such as rapid growth rate, lateral branching, biochemical and genetic stability, and capability to synthesize secondary metabolites at comparable values to natural plant roots (Zhang et al. 2014; Md Setamam et al. 2014; Pistelli et al. 2010).
Elicitors are one of the momentous factors which trigger the defense responses in plants, leading to the accumulation of target secondary metabolites (Naik and Al-Khayri 2016). Phytohormones as efficient elicitors stimulate growth and secondary metabolite production in hairy root cultures (Sharifi et al. 2014; Liang et al. 2013; Kim et al. 2013). Application of phytohormones such as indole-3-butyric acid (IBA) (Sharifi et al. 2014; Marsh et al. 2014), abscisic acid (ABA), and gibberellic acid 3 (GA3) (Liang et al. 2013) in hairy root cultures has been reported in several literatures. Compared to other phytohormones, IBA is responsible for secondary root formation in hairy root cultures (Kim et al. 2012). Methyl jasmonate (MeJA) as a signal molecule has an important role in the signal transduction pathway. It is considered as a potent abiotic elicitor which is applied exogenously in plant cell and tissue cultures to induce the production of desirable secondary compounds (Narayani and Srivastava 2017; Naik and Al-Khayri 2016; Lijavetzky et al. 2008; Xing et al. 2018; Gai et al. 2019; Pilaisangsuree et al. 2018). MeJA has been widely used to study the regulation of secondary metabolism in hairy root and cell cultures of several medicinal plants such as Salvia miltiorrhiza (Zhang et al. 2014; Xiao et al. 2009; Xing et al. 2018) and Agastache rugosa (Kim et al. 2013). Several studies have reported that MeJA (100 µM) positively affecting the rosmarinic acid and lithospermic acid B accumulation, as well as the expression levels of the related key genes in Salvia miltiorrhiza hairy root cultures (Xiao et al. 2009; Zhang et al. 2014; Xing et al. 2018). In another report, MeJA had an important impact on the activation of the phenylpropanoid pathway genes in addition to the induction of RA in A. rugosa suspension cultures (Kim et al. 2013). Despite the availability of several types of research about improving the production of phenolic compounds in some species of the Lamiaceae family by using elicitors such as MeJA, to the best of our knowledge, there have been no studies investigating the effect of MeJA on phenolic acids production in hairy root cultures of Mentha spicata.
In the current study, after evaluation of the IBA effect on hairy root growth, MeJA was used as an elicitor to investigate its effect on desired phenolic acids production in M. spicata hairy root cultures. Furthermore, the relative changes in expression of several genes (PAL, TAT, C4H, HPPR, and 4CL) encoding key enzymes in the two phenylpropanoid and tyrosine-derived pathways were monitored to elucidate the interrelation between desired phenolic acids accumulation in response to MeJA stress and the regulation mechanism of gene expression.
Materials and methods
Agrobacterium rhizogenes growth
Agrobacterium rhizogenes strain A13 was provided by the bank of microbes at NIGEB (Tehran, Iran). The bacterial strain initiated from glycerol stocks was cultured in 5 ml of liquid Luria–Bertani (LB) medium on a rotary shaker (180 rpm) at 28 °C for 48 h. The bacterial strain was streaked on solidified LB medium and incubated at 28 °C for 48 h.
Plant and establishment of hairy root culture
The cuttings of M. spicata were collected from Kerman, Iran and after approval by voucher specimens; they were grown in vitro at the phytotron of Iran National Institute of Genetic engineering and Biotechnology. Internode segments were surface sterilized with ethanol 70% (3 min) and 1% (v/v) commercial bleach containing one drop of Tween 20% (3–4 min) followed by three times rinse with sterile double distilled water. Hairy root induction was carried out by infection of explants with the strain A13 of A. rhizogenes according to our previous results (Yousefian et al. 2020). Briefly, internode segments were directly punctured using a needle infected by bacterial paste and then inoculated on full-strength MS medium containing 30 g L−1 sucrose and solidified with 8 g L−1 agar (pH 5.7) at 25 °C in the dark. After 48 h, the explants were washed with 250 mg L−1 cefotaxime (Exir pharmaceutical co. Iran) solution to eradicate the remaining bacteria and followed by drying on sterile filter paper, they were transferred to full-strength MS solid medium containing 250 mg L−1 cefotaxime (pH 5.7). The emerging hairy roots at the injured sites were moved to Petri dishes containing full-strength MS solid medium supplemented with 250 mg L−1 cefotaxime. After approximately 2 weeks, hairy root lines that showed superior growth and lateral branching were inoculated in 50 ml of half-strength MS liquid medium with an initial biomass of 0.3 g. The flasks were incubated at 25 °C on a gyratory shaker at 110 rpm in the dark. Hairy roots were sub-cultured at 14-day intervals into fresh medium while the concentration of cefotaxime was halved each time.
Preparation and treatment of IBA
IBA (Duchefa Biochemie, Netherland) was dissolved in NaOH, filter-sterilized (Orange scientific 0.2 µm) and added in 50 ml of autoclaved half-strength MS liquid medium (pH 5.7) with a final concentration of 0.3 mg L−1. To draw growth curve, hairy roots were cultured in half-strength MS medium supplemented with IBA for approximately 5 months. Half-strength MS medium without IBA was considered as control culture. Each treatment was performed in three independent flasks. The growth index was calculated by the following formula (Bauer et al. 2009):
MeJA preparation and treatment
MeJA (Sigma-Aldrich, USA) was dissolved in distilled water and filter-sterilized through 0.2 µm filters (Orange scientific). The stock solution (100 mM) was added to autoclaved half-strength MS liquid medium (pH 5.7) (50 µl in 50 ml medium) to give a final concentration of 100 µM.
MeJA treatment was performed on day 87 post-inoculation (the end of the exponential growth phase) and hairy roots were transferred into fresh medium supplemented with filter-sterilized MeJA (with a final concentration of 100 µM). Half-strength MS medium without MeJA was used for control roots. Three independent biological replicates were considered for experiments. Based on a time-course study, about 1 g of the hairy roots was harvested from the culture medium at 0, 3, 6, 12, 24, and 48 h for RNA isolation. The remaining part was harvested for extraction at 0, 3, 6, 12, 24, 48, 72 and 96 h post-treatment. We presumed that induction of gene expression at earlier time points may induce the production of some phenolic acids at later time points. Fresh weight (FW) was determined after washing the hairy roots by distilled water and blotted dry by filter paper. Dry weight (dry wt) of hairy roots was measured after lyophilisation.
Nucleic acid isolation and cDNA synthesis
Genomic DNA of M. spicata transformed hairy roots and non-transformed roots were extracted by the modified CTAB method (Khan et al. 2007) to confirm the presence of the rolB gene by PCR (non-transformed roots served as negative control) (The sequences of primers are listed in Table S1, in supplementary data).
Total RNA was extracted at selected times based on the method described by Esmaeili et al. (2016) (Fig. S3). The concentration and the quality of RNA were assessed using NanoDrop™ 2000 spectrophotometer (Thermo Scientific, USA) and agarose gel electrophoresis. Immediately after erasing the genomic DNA contamination (Fermentas DNase, USA), cDNA was synthesized from DNase-treated RNA by using SuPrime Script RTase according to the manufacturer’s instruction of GeNet Bio kit (Daejeon, Korea).
Molecular cloning of partial sequences of MsPAL, MsTAT, and Ms4CL genes
Given that the sequences of key genes involved in phenolic acids biosynthesis such as PAL, TAT, and 4CL have not been reported in M. spicata yet, nucleotide sequences of the desired genes from some other species in the family Lamiaceae being available in NCBI (https://www.ncbi.nlm.nih.gov/), were retrieved. Multiple sequence alignment was carried out and suitable primers were designed using Oligo7 software (Rychlik 2007) based on the conserved regions of the sequences. Cloning of the desired part of the genes was performed using the TA-cloning kit (Thermo Scientific, Lithuania). Distinct vectors comprising partial sequences of MsPAL, MsTAT, and Ms4CL genes were sequenced. Appropriate primers were designed to detect the transcript levels of MsC4H and MsHPPR genes, based on the full-length sequences of mentioned genes in M. spicata which have been cloned previously in our laboratory (Genebank accession numbers: MH208309 and MG893898).
Quantitative real-time PCR
Real-time PCR reactions to detect gene expression levels of PAL, TAT, C4H, HPPR, and 4CL, were performed using a micPCR system (BioMolecular systems, Australia). (Gene-specific primers are listed in Table S1, in supplementary data). The reaction was carried out in a total volume of 10 µl, containing 5 µl of 2X real-time PCR master mix including SYBR green I (Biofact™, South Korea), 0.4 µM of each specific primer and 1 µl of 1/20 diluted cDNA (approximately 50 ng) as a template. Quantitative real-time PCR (qRT-PCR) conditions were as follows: pre-denaturation at 94 °C for 5 min (1 cycle), denaturation at 94 °C for 30 s, annealing for 30 s at a given temperature and the final step of collection fluorescence at 72 °C for 30 s (40 cycles). A part of the β-actin gene was used as an internal control (Genebank accession number: KM044035.1) and the relative changes in gene expression were quantified by the comparative CT method (Livak and Schmittgen 2001). The reactions were conducted in triplicate and the results are expressed as mean ± SD.
Extraction and HPLC analysis of phenolic acids
Freeze-dried hairy root samples, harvested at specific times, were finely powdered by a mortar and pestle. 50 mg of the tissue was extracted twice with 10 ml of 60% ethanol under sonication (Elma Ultrasonics, Germany) at 40 °C for 30 min followed by centrifuge at 10,000 rpm for 30 min. The supernatant was filtered through a 0.2 µm filter (Orange scientific) and the solvent was evaporated by rotary evaporator (Buchi rotavapor, Switzerland) at 40 °C. Extracts were dissolved in 1 ml of HPLC grade ethanol for HPLC analysis. To construct standard curves, a multi-level calibration method was performed. Standard solutions of RA, CA, LAB, CGA and CIA (purchased from Sigma-Aldrich, USA) were prepared at five concentrations in the range of 50, 25, 12.5, 6.25 and 1.5 mg L−1. Phenolic acids quantification was conducted on a Knauer HPLC system (Berlin, Germany) equipped with a K-1001 HPLC pump, a Rheodyne injector including 20 μL sample loops model 7725i and a K-2800 photodiode array detector (PDA). A stainless steel Reversed-Phase C18 column (150 mm × 4.6 mm, 5 µm particle size, 100 A, Beckman, USA) was used to perform chromatography assessments at room temperature and the chromatographic data were monitored by EZChrom Elite software. The flow rate was 0.7 ml min−1. Desired phenolic acid compounds were detected at 275, 300 and 320 nm and the injection volume of the sample was 20 µl. Solvent (A): HPLC-grade water with 0.02% trifluoroacetic acid (TFA) and solvent (B): 0.02% TFA in methanol, were used as the mobile phase. The following gradient elution was applied for separation of five major phenolic acids: 0–5 min, 5% solvent B which reaches to 15% within 20 min; 20–25 min, 35% solvent B; 25–45 min, 55% solvent B, and at 45–55 min solvent B reaches to 100%.
Statistical analysis
Graph-Pad Prism 8 statistical software was used to analyze hairy root cultures treated with MeJA at each time point. Phenolic acids accumulation and gene expression data were analyzed by the two-way ANOVA, and subsequently, with the post-hoc Tukey’s test. Results were designated significant when the p-value < 0.05 (0.01 < p < 0.05*, p < 0.01**).
Results
Effect of IBA on hairy root growth in liquid medium
The growth rate of hairy roots was improved by the treatment of IBA into liquid half-strength MS medium. After approximately 5 months, the biomass of hairy roots in medium supplemented with 0.3 mg L−1 IBA increased 27.7-fold compared to a 5.2-fold increase of biomass in the control medium without IBA (Fig. 2). Moreover, as shown in Fig. 3, the application of IBA caused more lateral branching of hairy roots (Fig. 3).
Molecular confirmation of M. spicata transformed hairy roots by PCR
The presence of the rolB gene in A. rhizogenes transformed roots was confirmed by amplification of a 430 bp fragment through PCR analysis using specific primers. A. rhizogenes plasmid was used as a positive control. The expected fragment was not amplified in the genomic DNA of non-transformed roots as the negative control (Supplementary Fig. S1).
Partial cDNA clone sequencing of MsPAL, MsTAT, and Ms4CL genes
The designed primers mentioned above (Table S1, in supplementary data), was used for amplification and cloning of the partial sequences of PAL, TAT and 4CL genes in M. spicata (MsPAL, MsTAT, and Ms4CL) with lengths of 146, 145 and 147 bp, respectively. Two clones of each gene were randomly sequenced. Sequencing of the inserts showed that both clones from each of the MsPAL, MsTAT, and Ms4CL genes had identical nucleotide sequences. The partial cDNA sequences of MsPAL, MsTAT, and Ms4CL genes were submitted to the NCBI EST database and their accession numbers are JZ977300.1, JZ977299.1, and JZ977301.1, respectively. A BLAST search revealed that the partial nucleotide sequences of MsPAL, MsTAT, and Ms4CL genes had high homology with PAL, TAT and 4CL genes from other species of the Lamiaceae family. Multi-alignment analysis indicated that MsTAT shares 92% identity to SkhTAT from Satureja khuzistanica (KY682078.1) and SmTAT from Salvia miltiorrhiza (DQ334606.1). Besides, MsPAL from M. spicata shares 97% identity to SkhPAL from Satureja khuzistanica (KY682076.1) and 91% identity to SmPAL from Salvia miltiorrhiza (DQ408636.1). Finally, Ms4CL shares 86% identity to Sm4CL from Salvia miltiorrhiza (AY237163.1) (Supplementary Fig. S2).
Effect of MeJA on the expression profile of genes related to the phenylpropanoid and tyrosin-derived pathways in M. spicata hairy roots
In order to investigate the effect of MeJA treatment on expression levels of genes related to the phenolic acids biosynthetic pathway, real-time qRT-PCR was performed. The relative expression levels of MsPAL, MsC4H, Ms4CL, MsTAT, and MsHPPR genes were analyzed at 3, 6, 12, 24, and 48 h after treatment with MeJA. As shown in Fig. 4, statistically significant changes were detected in the expression levels of all genes in response to elicitor treatment. A significant increase (about 4.04-fold of control) in the transcription value of MsPAL, the entry point enzyme of the phenylpropanoid pathway, was observed at 12 h post-MeJA treatment. The results showed an increase in the transcription value of MsC4H (the second enzyme in the phenylpropanoid pathway) shortly (at 3 h) after the MeJA application. Moreover, the maximum expression levels of MsC4H and Ms4CL reached about 3.62 and 1.75-fold higher than the control at 6 h post-treatment, respectively. It would appear that the expression of Ms4CL was reinduced at 24 and 48 h followed by a reduction at 12 h post-treatment. Apart from MsTAT (the first enzyme in the tyrosine-derived pathway), Fig. 4 clearly indicates that the transcription value of all genes significantly stimulated after 6 h of exposure to MeJA. Additionally, the maximum transcription level of MsHPPR at 6 h, reached about 1.45-fold of the control. Thereafter, the expression of MsPAL, MsHPPR, and MsC4H dropped at later time points. In comparison, the expression level of MsTAT at 3 and 12 h remained lower than the control and it was suppressed at 6, 24 and 48 h after initial exposure to MeJA.
Effect of MeJA treatment on expression levels of related genes in the phenylpropanoid and tyrosin-derived pathways in M. spicata hairy roots cultures. Green columns and blue columns represent the untreated control and the MeJA treatment, respectively. The vertical bars represent standard deviations (n = 3) and the asterisks indicate statistically significant differences at p-value < 0.05 (0.01 < p < 0.05*, p < 0.01**) between the expression levels in the MeJA treated cultures and that in the corresponding controls. (Color figure online)
Effect of MeJA on phenolic acids accumulation in M. spicata hairy roots
The time-course analysis of five phenolic acids contents, including RA, CA, CGA, LAB, and CIA in M. spicata hairy roots treated by 100 µM MeJA is presented in Fig. 5. MeJA induced significant changes in the accumulation levels of phenolic acids. Effect of MeJA on CIA and CGA accumulation levels was conspicuous at later stages of the elicitation (48, 72, and 96 h after elicitor treatment) with the maximum level of 1.98-fold (43.06 µg g−1 dry wt) and 1.7-fold (15.56 µg g−1 dry wt) higher than the control at 72 h after MeJA treatment, respectively. CA and RA were significantly (p < 0.05) induced shortly (at 3 h) after the MeJA application. The maximum contents of CA were observed at 3 h (183.49 µg g−1 dry wt) and 6 h post-treatment (159.19 µg g−1 dry wt) about 1.7 and 1.47-fold of control, respectively. Despite significant fluctuations, the RA accumulation was higher than the control samples at every time point, with maximum level reaching about 11.84-fold (55.44 µg g−1 dry wt) more than the control sample at 6 h post-treatment. In other words, MeJA is a potent inducer of RA. Of the five quantified phenolic acids, MeJA treatment had a negative effect (p < 0.05) on LAB accumulation, the minimum content (24.21 µg g−1 dry wt, about 2.38-fold lower than the control) was observed at 24 h post-treatment.
Effect of MeJA on phenolic acids accumulation in M. spicata hairy root cultures. Green columns and blue columns represent the untreated control and the MeJA treatment, respectively. The vertical bars represent standard deviations (n = 3) and the asterisks indicate statistically significant differences at p-value < 0.05 (0.01 < p < 0.05*, p < 0.01**) between the phenolic acids accumulation in the MeJA treated cultures and that in the corresponding controls. (Color figure online)
Correlation between gene expression levels and phenolic acids accumulation
To study the possible correlation between the expression profiles of the key genes (MsPAL, MsC4H, Ms4CL, MsTAT, and MsHPPR) involved in phenolic acids biosynthetic pathway and five phenolic acids (RA, CA, CGA, LAB, and CIA) accumulation in MeJA-treated hairy root cultures, a correlation analysis using Pearson’s correlation coefficient (r) was performed (Table 1).
Table 1 indicates that the expression profiles of MsPAL and MsC4H are positively correlated with RA synthesis in M. spicata hairy root cultures in the presence of MeJA. Moreover, significantly positive correlations between MsC4H and CA accumulation, and MsTAT and LAB production were observed.
Discussion
Secondary metabolites are bioactive chemical compounds that are synthesized by plants in response to biotic and abiotic stresses and elicitors (Guerriero et al. 2018). Among the different classes of plant secondary metabolites, phenolic compounds comprise the largest group (Hussein and El-Anssary 2018). Phenolic acids are ubiquitous compounds (Hussein and El-Anssary 2018; Mandal et al. 2010) with a wide range of pharmacological effects, such as antimicrobial, antioxidant (Ghasemzadeh and Ghasemzadeh 2011), antiviral (Ziaková and Brandšteterová 2003) and anti-inflammatory (Xing et al. 2018) activities. The antioxidant activity of phenolic acids such as rosmarinic acid can provide protection against many oxidative stress-related diseases like cancer. These compounds are used in cosmetology and dermatology due to the health-promoting activities (Petersen and Simmonds 2003; Soto et al. 2015). Phenolic acids are produced as a defense response to protect plants from adverse environmental conditions (Ghasemzadeh and Ghasemzadeh 2011) and present abundantly in plants belonging to the Lamiaceae family, (Ziaková and Brandšteterová 2003) especially M. spicata. (Ay Kee et al. 2017).
Scientific and commercial attention to medicinal plants has been increased due to their pharmaceutical importance. Therefore, growing demand for exploitation of wild plant populations has put many medicinal plant species at risk of extinction (Singh and Dwivedi 2018). Plant tissue culture techniques such as hairy root induction by A. rhizogenes and elicitation to promote the accumulation of high-value secondary metabolites provide an efficient method for the production of important pharmaceutical compounds without a negative impact on biodiversity and natural resources (Singh and Dwivedi 2018; Gonçalves and Romano 2018). In the current study, hairy root cultures of M. spicata were successfully established by using A. rhizogenes strain A13. Whereas it has been confirmed that the rolB gene has an essential role in hairy root induction (Nourozi et al. 2014; Pavlova et al. 2014), the presence of this gene in hairy root lines was detected by polymerase chain reaction (PCR) analysis. Optimization of the hairy roots culture conditions and the application of phytohormones offer opportunities for obtaining high-yield lines and large-scale production of hairy roots biomass (Sharifi et al. 2014). Auxin phytohormones play a central role in root development and growth (Park et al. 2016; Overvoorde et al. 2010). Based on several published literatures among the plant growth regulators of auxin, IBA as an auxin-type hormone is greatly responsible for secondary root formation in hairy root cultures (Kim et al. 2012). It has been reported that IBA accelerates the growth and increases the biomass of hairy roots in Tribulus terrestris (Sharifi et al. 2014) and Scutellaria lateriflora, respectively (Marsh et al. 2014). Yang et al. (2010) evaluated the effect of various concentrations of auxins and polyamines on hairy root growth and rosmarinic acid production in Nepeta cataria and reported that IBA was the most effective auxin-type hormone which increased both growth rate and RA content (Yang et al. 2010).
In order to obtain sufficient biomass of hairy roots for further MeJA treatment, hairy root lines with superior growth and lateral branching were transferred to half-strength MS liquid medium supplemented with 0.3 mg L−1 IBA. In our experiments, application of 0.3 mg L−1 IBA significantly improved the growth rate of M. spicata’s hairy roots. The positive effect of IBA on the growth rate of hairy roots was in accordance with previous studies (Sharifi et al. 2014).
The capability of MeJA as an important signaling molecule to enhance the production of various secondary metabolites by inducing the expression of related genes has been well documented (Lijavetzky et al. 2008; Xiao et al. 2009; Ruan et al. 2019; Xing et al. 2018). Some reports have indicated that MeJA increases the accumulation of phenolic acids. MeJA enhanced rosmarinic acid and lithospermic acid B in Salvia miltiorrhiza hairy root cultures (Xiao et al. 2009; Zhang et al. 2014). Besides, the application of MeJA in Coleus blumei (Bauer et al. 2009), Coleus forskohlii hairy root cultures and Agastache rugosa Kuntze cell cultures stimulated RA accumulation (Xiao et al. 2009; Kim et al. 2013). Mizukami et al. (1993) reported a tenfold increase of RA content in the MeJA-treated cells of Lithospermum erythrorhizon (Mizukami et al. 1993). In this study, in order to elucidate the regulatory mechanism of phenolic acids production in response to MeJA treatment in hairy root cultures of M. spicata, the phenolic acids accumulation, as well as the expression level of several related key genes, were investigated at different time points. The results showed that the treatment with MeJA (100 µM) greatly enhanced the RA content about 11.84-fold more than the control sample. The expression profile analysis of phenolic acid biosynthesis-related genes indicated that the increased relative expression levels of MsPAL and MsC4H at 6 h post-treatment correlated with the induced RA accumulation. According to Table 1, significant positive correlations between the two phenylpropanoid pathway genes (MsPAL and MsC4H) and RA content were observed.
The deamination of phenylalanine by the entry point enzyme of the phenylpropanoid pathway, PAL, generates CIA (Hao et al. 2016; Zhang et al. 2014; Petersen and Simmonds 2003; Petersen et al. 2009) which is converted by cinnamic acid 4-hydroxylase (C4H) and p-coumarate 3-hydroxylase (C3H) to CA as an important intermediate of the phenylpropanoid pathway (Lin and Yan 2012). CA accumulation was significantly (p < 0.05) induced shortly (at 3 h) after the MeJA application, while the CIA content was not affected at the early stages of the elicitation. This might be attributed to its role as a precursor being transformed to produce more RA and CA. Moreover, a significant positive correlation was observed between the expression level of MsC4H and CA accumulation at 3 h and 6 h after MeJA treatment (Table 1). CGA is an ester of CA and their biosynthesis share the general phenylpropanoid pathway (Petersen et al. 2009). Elevated levels of CGA content in MeJA-treated hairy roots at later time points was observed in parallel with the reduction of CA accumulation. Increased production of CGA may results in more precursor transformation (the maximum content was observed at 72 h post-treatment).
The significant increase of MsPAL transcription value at 12 h post-treatment coincided with a decrease in MsC4H and Ms4CL expression levels at the same time. It can be interpreted as a feedback inhibition of RA, the final product of the phenylpropanoid pathway. As shown in Fig. 1, 4CL is responsible for the reaction that generates 4-Coumaroyl-CoA as the substrate for the branch pathway responsible for the CGA synthesis. The reinduction of Ms4CL at 24 and 48 h may affect the production of CGA at later stages of MeJA elicitation.
The enhanced transcription value of MsHPPR from the tyrosine-derived pathway was consistent with the expression level changes of MsPAL and RA accumulation. On the other hand, the application of MeJA had a negative effect on both MsTAT expression level and LAB accumulation which remained lower than the control at every time points. The maximum content of LAB (57.60 µg g−1 dry wt, approximately equal value to the control sample) was observed at 96 h post-treatment. The results of the correlation analysis also confirmed the significant positive correlation of LAB content and MsTAT expression level (Table 1). This result is supported by a report from Xiao et al. (2009) who studied the correlation between the phenolic acids accumulation and the expression profile of six related genes. They concluded that TAT expression is mainly correlated with LAB accumulation, while HPPR expression is more correlated with RA content, in the statistical sense (Xiao et al. 2009). Furthermore, the suppression of MsTAT expression at 6 h after the MeJA treatment may be due to the feedback inhibition. High-level production of RA, the final product of the tyrosine-derived pathway, within 6 h may inhibit the action of MsTAT, the entry point enzyme of the pathway, to prevent the further synthesis of this metabolite. The observations in the current study are coincident with the report from Kim et al. (2013) who indicated that MeJA treatment in cell suspension cultures of A. rugosa increased the transcript levels of phenylpropanoid biosynthetic genes, ArPAL, Ar4CL, and ArC4H, which resulted in enhanced RA accumulation (Kim et al. 2013). In another report by Mizukami et al. (1993), elicitation with MeJA in L. erythrorhizon cell suspension cultures induced PAL and HPPR activities rapidly, whereas TAT activity was slightly increased (Mizukami et al. 1993).
Our findings in this study indicated that the application of MeJA increased the relative expression levels of MsPAL, MsC4H, and Ms4CL, key genes in the phenylpropanoid pathway. In comparison, the transcript of MsHPPR in the tyrosine-derived pathway was more sensitive to the elicitor treatment relative to MsTAT.
Conclusion
For the first time, we investigated the effect of MeJA on phenolic acids production, as well as the relative changes in expression levels of several genes (MsPAL, MsTAT, MsC4H, MsHPPR, and Ms4CL) encoding key enzymes in the two phenylpropanoid and tyrosine-derived pathways in hairy root cultures of M. spicata. MeJA induced a significant increase in RA, CA, CGA, and CIA contents. Preceding the phenolic acids accumulation, the relative expression levels of MsPAL, MsC4H, and Ms4CL, and MsHPPR were enhanced. The present investigation suggests MeJA as an effective elicitor for stimulating valuable phenolic acids production in M. spicata hairy root cultures.
References
Ay Kee L, Bakr Shori A, Salihin Baba A (2017) Bioactivity and health effects of Mentha spicata. Integr Food Nutr Metab. https://doi.org/10.15761/IFNM.1000203
Bauer N, Kiseljak D, Jelaska S (2009) The effect of yeast extract and methyl jasmonate on rosmarinic acid accumulation in Coleus blumei hairy roots. Biol Plant 53:650–656. https://doi.org/10.1007/s10535-009-0118-8
Bayan Y, Küsek M (2018) Chemical composition and antifungal and antibacterial activity of Mentha spicata L. volatile oil. Cienc e Investig Agrar 45:64–69. https://doi.org/10.7764/rcia.v45i1.1897
Bimakr M, Rahman RA, Taip FS, Ganjloo A, Salleh LM, Selamat J, Hamid A, Zaidul ISM (2011) Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod Process 89:67–72. https://doi.org/10.1016/j.fbp.2010.03.002
Chezem WR, Clay NK (2016) Regulation of plant secondary metabolism and associated specialized cell development by MYBs and bHLHs. Phytochemistry 131:26–43. https://doi.org/10.1016/j.phytochem.2016.08.006
Cirlini M, Mena P, Tassotti M, Herrlinger KA, Nieman KM, Dall’Asta C, Del Rio D (2016) Phenolic and volatile composition of a dry spearmint (Mentha spicata L.) extract. Molecules. https://doi.org/10.3390/molecules21081007
Dorman HJD, Koşar M, Kahlos K, Holm Y, Hiltunen R (2003) Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J Agric Food Chem 51:4563–4569. https://doi.org/10.1021/jf034108k
Esmaeili F, Shiran BJ, Fallahi H, Mirakhorli N, Budak H, Martínez-Gómez P (2016) In silico search and biological validation of microRNAs related to drought response in peach and almond. Funct Integr Genomics 17:189–201. https://doi.org/10.1007/s10142-016-0488-x
Gai Q, Jiao J, Wang X, Zang Y, Niu L, Fu Y (2019) Elicitation of Isatis tinctoria L. hairy root cultures by salicylic acid and methyl jasmonate for the enhanced production of pharmacologically active alkaloids and flavonoids. Plant Cell Tissue Organ Cult 137:77–86. https://doi.org/10.1007/s11240-018-01553-8
Ghasemzadeh A, Ghasemzadeh N (2011) Flavonoids and phenolic acids: role and biochemical activity in plants and human. J Med Plant Res 5:6697–6703. https://doi.org/10.5897/JMPR11.1404
Gonçalves S, Romano A (2018) Production of plant secondary metabolites by using biotechnological tools. Second Metab Sources Appl. https://doi.org/10.5772/intechopen.76414
Guerriero G, Berni R, Muñoz-Sanchez JA, Apone F, Abdel-Salam EM, Qahtan AA, Alatar AA, Cantini C, Cai G, Hausman JF, Siddiqui KS, Hernández-Sotomayor SMT, Faisal M (2018) Production of plant secondary metabolites: examples, tips and suggestions for biotechnologists. Genes (Basel). https://doi.org/10.3390/genes9060309
Hao G, Jiang X, Feng L, Tao R, Li Y, Huang L (2016) Cloning, molecular characterization and functional analysis of a putative R2R3-MYB transcription factor of the phenolic acid biosynthetic pathway in S. miltiorrhiza Bge. f. alba. Plant Cell Tissue Organ Cult 124:151–168. https://doi.org/10.1007/s11240-015-0883-3
Hussein RA, El-Anssary AA (2018) Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. Herb Med. https://doi.org/10.5772/intechopen.76139
Kanatt SR, Chander R, Sharma A (2007) Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat. Food Chem 100:451–458. https://doi.org/10.1016/j.foodchem.2005.09.066
Khan S, Irfan QM, Kamaluddin AT, Abdin MZ (2007) Protocol for isolation of genomic DNA from dry and fresh roots of medicinal plants suitable for RAPD and restriction digestion. Afr J Biotechnol 6:175–178. https://doi.org/10.5897/AJB06.612
Kim SJ, Cha MS, Lee EJ, Kim IH, Kwon JE, Kang SC, Park TH (2012) In vitro induction of hairy root from isoflavones-producing Korean wild arrowroot Pueraria lobata. J Plant Biotechnol 39:205–211. https://doi.org/10.5010/JPB.2012.39.3.205
Kim YB, Kim JK, Uddin MR, Xu H, Park WT, Tuan PA, Li X, Chung E, Lee JH, Park SU (2013) Metabolomics analysis and biosynthesis of rosmarinic acid in Agastache rugosa Kuntze treated with methyl jasmonate. PLoS ONE 8:e64199. https://doi.org/10.1371/journal.pone.0064199
Liang Z, Ma Y, Xu T, Cui B, Liu Y, Guo Z, Yang D (2013) Effects of abscisic acid, gibberellin, ethylene and their interactions on production of phenolic acids in Salvia miltiorrhiza bunge hairy roots. PLoS ONE 8:e72806. https://doi.org/10.1371/journal.pone.0072806
Lijavetzky D, Almagro L, Belchi-Navarro S, Martínez-Zapater JM, Bru R, Pedreño MA (2008) Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res Notes 1:132. https://doi.org/10.1186/1756-0500-1-132
Lin Y, Yan Y (2012) Biosynthesis of caffeic acid in Escherichia coli using its endogenous hydroxylase complex. Microb Cell Fact 11:42
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Mandal SM, Chakraborty D, Dey S (2010) Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav 5:359–368. https://doi.org/10.4161/psb.5.4.10871
Marsh Z, Yang T, Nopo-Olazabal L, Wu S, Ingle T, Joshee N, Medina-Bolivar F (2014) Effect of light, methyl jasmonate and cyclodextrin on production of phenolic compounds in hairy root cultures of Scutellaria lateriflora. Phytochemistry 107:50–60. https://doi.org/10.1016/j.phytochem.2014.08.020
Md Setamam N, Jaafar Sidik N, Abdul Rahman Z, Che Mohd Zain CR (2014) Induction of hairy roots by various strains of Agrobacterium rhizogenes in different types of Capsicum species explants. BMC Res Notes 7:414. https://doi.org/10.1186/1756-0500-7-414
Mizukami H, Tabira Y, Ellis BE (1993) Methyl jasmonate-induced rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures. Plant Cell Rep 12:706–709. https://doi.org/10.1007/BF00233424
Naik PM, Al-Khayri JM (2016) Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plants. Abiotic Biot Stress Plants Recent Adv Future Perspect. https://doi.org/10.5772/61442
Narayani M, Srivastava S (2017) Elicitation: a stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem Rev 16:1227–1252. https://doi.org/10.1007/s11101-017-9534-0
Nourozi E, Hosseini B, Hassani A (2014) A reliable and efficient protocol for induction of hairy roots in Agastache foeniculum. Biologia (Bratisl) 69:870–879. https://doi.org/10.2478/s11756-014-0382-8
Overvoorde P, Fukaki H, Beeckman T (2010) Auxin control of root development. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a001537
Park CH, AyeThwe A, Kim SJ, Park JS, Arasu MV, Al-Dhabi NA, Park NI, Park SU (2016) Effect of auxins on anthocyanin accumulation in hairy root cultures of tartary buckwheat cultivar Hokkai T1O. Nat Prod Commun 11:1283–1286
Pavlova OA, Matveyeva TV, Lutova LA (2014) rol-Genes of Agrobacterium rhizogenes. Russ J Genet Appl Res 4:137–145. https://doi.org/10.1134/S2079059714020063
Petersen M, Abdullah Y, Benner J, Eberle D, Gehlen K, Hücherig S, Janiak V, Kim KH, Sander M, Weitzel C, Wolters S (2009) Evolution of rosmarinic acid biosynthesis. Phytochemistry 70:1663–1679. https://doi.org/10.1016/j.phytochem.2009.05.010
Petersen M, Simmonds MSJ (2003) Rosmarinic acid. Phytochemistry 62:121–125. https://doi.org/10.1016/s0031-9422(02)00513-7
Pilaisangsuree V, Somboon T, Tonglairoum P, Keawracha P, Wongsa Th, Kongbangkerd A, Limmongkon A (2018) Enhancement of stilbene compounds and anti-inflammatory activity of methyl jasmonate and cyclodextrin elicited peanut hairy root culture. Plant Cell Tissue Organ Cult 132:165–179. https://doi.org/10.1007/s11240-017-1321-5
Pistelli L, Giovannini A, Ruffoni B, Bertoli A, Pistelli L (2010) Hairy root cultures for secondary metabolites production. Adv Exp Med Biol 698:167–184. https://doi.org/10.1007/978-1-4419-7347-4_13
Ruan J, Zhou Y, Zhou M, Yan J, Khurshid M, Weng W, Cheng J, Zhang K (2019) Jasmonic acid signaling pathway in plants. Int J Mol Sci 20:2479. https://doi.org/10.3390/ijms20102479
Rychlik W (2007) OLIGO 7 primer analysis software. Methods Mol Biol 402:35–60. https://doi.org/10.1007/978-1-59745-528-2_2
Sharifi S, Sattari TN, Zebarjadi A, Majd A, Ghasempour H (2014) The influence of Agrobacterium rhizogenes on induction of hairy roots and ß-carboline alkaloids production in Tribulus terrestris L. Physiol Mol Biol Plant 20:69–80. https://doi.org/10.1007/s12298-013-0208-0
Singh A, Dwivedi P (2018) Methyl-jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: a review. J Pharmacogn Phytochem 7:750–757
Sircar D, Cardoso HG, Mukherjee C, Mitra A, Arnholdt-Schmitt B (2012) Alternative oxidase (AOX) and phenolic metabolism in methyl jasmonate-treated hairy root cultures of Daucus carota L. J Plant Physiol 169:657–663. https://doi.org/10.1016/j.jplph.2011.11.019
Soto ML, Falqué E, Domínguez H (2015) Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics 2:259–276. https://doi.org/10.3390/cosmetics2030259
Xiao Y, Gao S, Di P, Chen J, Chen W, Zhang L (2009) Methyl jasmonate dramatically enhances the accumulation of phenolic acids in Salvia miltiorrhiza hairy root cultures. Physiol Plant 137:1–9. https://doi.org/10.1111/j.1399-3054.2009.01257.x
Xing B, Yang D, Liu L, Han R, Sun Y, Liang Z (2018) Phenolic acid production is more effectively enhanced than tanshinone production by methyl jasmonate in Salvia miltiorrhiza hairy roots. Plant Cell Tissue Organ Cult 134:119–129. https://doi.org/10.1007/s11240-018-1405-x
Yang YK, Lee SY, Park WT, Il PN, Park SU (2010) Exogenous auxins and polyamines enhance growth and rosmarinic acid production in hairy root cultures of “Nepeta cataria” L. Plant Omics 3:190
Yousefian Sh, Lohrasebi T, Farhadpour M, Haghbeen K (2020) Production of phenolic acids in hairy root cultures of medicinal plant Mentha spicata L. in response to elicitors. Mol Biol Res Commun 9:23–34. https://doi.org/10.22099/mbrc.2020.36031.1475
Zgórka G, Głowniak K (2001) Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J Pharm Biomed Anal 26:79–87. https://doi.org/10.1016/s0731-7085(01)00354-5
Zhang S, Yan Y, Wang B, Liang Z, Liu Y, Liu F, Qi Z (2014) Selective responses of enzymes in the two parallel pathways of rosmarinic acid biosynthetic pathway to elicitors in Salvia miltiorrhiza hairy root cultures. J Biosci Bioeng 117:645–651. https://doi.org/10.1016/j.jbiosc.2013.10.013
Ziaková A, Brandšteterová E (2003) Validation of HPLC determination of phenolic acids present in some Lamiaceae family plants. J Liq Chromatogr Relat Technol 26:443–453. https://doi.org/10.1081/JLC-120017181
Acknowledgements
This work was supported by research funds from National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran [Grant Number I 661-970301].
Author information
Authors and Affiliations
Contributions
TL designed the project and supervised the experiments. ShY performed the overall experiments and prepared the manuscript. MF performed HPLC analyses. MF and ShY analyzed the data related to phenolic acids accumulation levels. All authors read and approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that this article has no conflicts of interest.
Additional information
Communicated by Sergio J. Ochatt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yousefian, S., Lohrasebi, T., Farhadpour, M. et al. Effect of methyl jasmonate on phenolic acids accumulation and the expression profile of their biosynthesis-related genes in Mentha spicata hairy root cultures. Plant Cell Tiss Organ Cult 142, 285–297 (2020). https://doi.org/10.1007/s11240-020-01856-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01856-9