Abstract
Microalgae as one of the key components of food chains in aquatic environments are promising biological models for investigating gamma irradiation effects on eukaryotic organisms. Understanding resistance mechanisms in these organisms as simple models might illuminate how gamma irradiation resistance improves in these algae and even more complex organisms. The present study aimed to investigate the effects of ionizing irradiation on Chlorella vulgaris as a stress-tolerant microalga and to find how the eukaryotic cells would tolerate such stressful conditions. The physiological responses and biochemical alterations of C. vulgaris were analyzed at three different time points (0, 16, and 48 h) after 600 Gy gamma irradiation. Compared to the control, gamma-irradiated algae had slower growth rate with significantly longer lag phase, less chlorophyll and protein contents at time 0, which were compensated and recovered during the next 48 h. The results also showed spontaneous H2O2 burst accompanied by a higher rate of lipid peroxidation and electrolyte leakage, a rapid increase of catalase activity and more ferric reducing antioxidant power immediately after irradiation. During a 48-h period, most alterations stabilized. Raising trends were observed in the carotenoid contents, the ratios of carbohydrates, amide I and amide II to fatty acids. Principal component analysis and hierarchical clustering suggest two possible distinct mechanisms: a “quick” one including spontaneous responses by boosting H2O2, amplifying enzymatic antioxidant systems, and increasing compatible solutes like proline and a “delayed” responsive strategy including the increase of soluble carbohydrates, carotenoids, and stress-related proteins triggered several hours after irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are several phases motivated by absorbed energy between the initial stage of absorption of ionizing radiation (IR) and the final damage of biological systems. In this process, two main forms of interaction can be defined: direct and indirect actions. Deposition of the radiation energy, directly into the targets is materialized in “direct actions,” while in “indirect actions,” diffusible intermediates produced by energy absorption of an external medium, attacks cell components (Lee et al. 2009; Esnault et al. 2010). The exposure of water to ionizing energy generally results in a range of free radicals such as ionized water molecules (H2O•+) as well as H• and •OH radicals. Subsequently, ionized molecules produce secondary reactive oxygen species (ROS) such as O2•− and H2O2 via a chain of reactions, which eventually cause oxidative stress (Lee et al. 2009). However, as it is maintained in literature, there are other factors that could be operative and bring about different effects in response to IR-induced oxidative stress. These include the radiation types, dose rates, genotype, and developmental stages, as well as variations of interindividual responses. Therefore, it is difficult to predict a typical response of photosynthetic organisms to ionizing radiation (IR), although there appear to be some common patterns (Boyer et al. 2009; Kim et al. 2009). Thus, determining cell sensitivity and resistance to radiation is a matter of inquiry which requires further detailed investigation. To some degree, it is evident that the efficient removal of harmful ROS enables the cells to defend themselves against oxidative stress, which is a crucial process in IR-resistant microorganisms (Daly 2011; Pavlopoulou et al. 2016). It has been shown that Deinococcus radiodurans and Halobacterium salinarum, for instance, due to their ability to accumulate small antioxidant molecules, could defend their proteins from oxidation and preserve the function of enzymes needed to repair and reorganize IR-damaged DNA and thereby survive (Daly 2009; Slade and Radman 2011; Robinson et al. 2011; Ishino and Narumi 2015). Matching the collected reports from D. radiodurans R1 has shown a link between desiccation resistance and the radiotolorance of D. radiodurans. These experiments have indicated that sensitive IR strains also have some degree of sensitivity to dryness. Furthermore, according to gene expression, common responses to these stresses show that they are associated with necessary proteins for repairing double-strand DNA breaks and protective responses to oxidative stress. Thus, two views exist for IR resistance in D. radiodurans: (i) there are putative genes with repairing functions or (ii) a number of antioxidant protection compounds result in conventional repair pathways (Mattimore and Battista 1996; De Groot et al. 2005; Gabani and Singh 2013). Besides, in addition to these nonphotosynthetic model organisms, selected sources of cyanobacteria and microalgae were reported to be impervious to UV, X-ray, and gamma-ray radiation, which makes them appealing objects to investigate and further reveal molecular standards of cellular radiation resistance of photosynthetic organisms (Posner and Sparrow 1964; Asato 1971; Kovacs and Keresztes 2002; Ermavitalini et al. 2017; Shabana et al. 2017).
Eukaryotic microalgae can thrive and grow under a vast range of environmental conditions in fresh and marine aquatic ecosystems and produce valuable products, such as carotenoids, pharmaceuticals, and nutraceuticals (Safi et al. 2014). Although these microorganisms play an important ecological role and are recognized as reliable indicators of environmental conditions, few studies have investigated their responses to ionizing radiation. It is worth mentioning that eukaryotic microorganism responses to ionizing radiation are more complex and while there are only a few eukaryotes found to be ionizing radiation-resistant, limited experiments have concentrated on them to consider and clarify the principles of this phenomenon (Santier et al. 1985; Joiner et al. 2001; Wang et al. 2004). For example, recently, a new microalga species, Coccomyxa actinabiotis, was isolated from a nuclear facility. Coccomyxa actinabiotis could resist against a high dose of ionizing radiation (up to 20,000 Gy), (Rivasseau et al. 2016). However, no detailed information about its resistance mechanism has been reported as yet.
Among the green eukaryotic microalgae, Chlorella vulgaris is one of the best-known examples which are able to be resilient and adapt to a range of extreme and severe conditions (Priyadarshani and Rath 2012). This unicellular microalga has the capability to mature in environments with high doses of salt or heavy metals, extreme temperatures or light intensities. This alga is also able to resist ionizing radiation from intense ultraviolet (UV) radiation to gamma rays, as well as charged particles (Malanga and Puntarulo 1995; Evseeva et al. 2010; Cheng et al. 2013). Chlorella vulgaris exposed to different levels of UV-B showed cell growth inhibition and stimulated carotenoid accumulation. Additionally, transcriptomic analysis of Chlorella grown at evaluated UV condition demonstrated various expressed genes, related to preservation and remobilization of resources of energy, protein protection, certifying lipid homeostasis of membrane, and modifying antioxidative mechanisms (Poong et al. 2018). By imposing stress on a cell, its physiological state will change, which in turn can illuminate regulatory mechanisms and system relationships of the altered cell and its cellular metabolic functions. Although there are several reports on the susceptibility or tolerance of microalgae such as C. vulgaris to UV radiation (Wong et al. 2015; Lai et al. 2018), a comprehensive morphological and physiological analysis of the resistance and response of microalgae to ionizing radiation seems to be lacking. Due to outstanding resistance of this alga to different types of stresses mentioned in the above studies, we hypothesized that C. vulgaris can tolerate high doses of gamma irradiation. We also predicted that the mechanism recruited to resist or avoid radiation-induced oxidative damage possibly would be similar to its responses to salinity, heavy metals, and UV. We were also interested to find which distinct strategic mechanisms would be used by C. vulgaris in response to gamma radiation and whether the gamma-related responses would be similar to those reported for gamma-resistant bacteria or other photosynthetic organisms.
Consequently, this study aimed to identify and account for specific responses of C. vulgaris to IR in detail, in order to obtain a clearer picture of its cellular resistance mechanism against irradiation. For this purpose, chemical and physiological responses of C. vulgaris strain UTEX 265 to acute doses of gamma rays were investigated by FT-IR analyses and examining physiological parameters. We considered C. vulgaris as a good eukaryotic model organism to examine the effect of ionizing radiation on unicellular algae. This approach allowed us to diagram the dynamics of the responses and related metabolic pathways over specific time courses. According to our knowledge, little is known about Chlorella genus IR resistance and the physiological abilities of this alga to withstand gamma irradiation have not been investigated as yet.
Materials and methods
Culture conditions
Chlorella vulgaris strain UTEX 265 was from the UTEX culture collection of algae (University of Texas, Austin, USA). The alga was cultured under sterile control conditions at 25 °C temperature, 100 μmol photons m−2 s−1 light intensity and 18:6 h (light/ dark) photoperiod in Bold’s Basal Medium (BBM) in 250-mL Erlenmeyer flasks containing 100 mL growth medium (Chia et al. 2013). The algal suspensions with equal volume and cell number were prepared after determining the growth stationary stage for the next step.
Gamma radiation
In a preliminary experiment, gamma irradiation-withstanding-ability was monitored with different gamma doses (control, 300, 600, 1200 Gy at a dose rate of 0.5 Gy s−1 at exposure times of 10, 20, and 40 min, respectively), selected according to previous related studies (Tale et al. 2018). For all doses, equal volumes (250 mL) of algal batches with the same concentration of cells (40 × 106 cells mL−1) were prepared and irradiated in a 60Co irradiator source at the Atomic Energy Organization’s Energy Agency (Tehran Atomic Energy Organization, I.R.I) with gamma. Subsequent to irradiation, irradiated and nonirradiated (control) batches were transferred to Erlenmeyer flasks with 1000 mL volumes, with equal volumes of new medium (250 mL) added and incubated as described in section 2.1. The algal growth curve was plotted at a time course of 96 h and according to the median lethal dose (LD50), using erythrosin B (erythrosin B turns dead cells red). Gamma irradiation of 600 Gy was selected to be used in the main stage of the experiment for further analysis. Therefore, a new batch of algal culture was prepared as before and was irradiated with 600 Gy at the same dose rate and then analyzed over a period of 48 h. Each treatment (irradiated at 600 Gy and nonirradiated control) was repeated at least three times (N = 3) independently. Nine flasks (1000 mL Erlenmeyer) were used for the whole procedure. For biochemical and physiological analyses, samples were taken at three time points (0, 16, and 48 h) from three flasks which were either immediately used or fixed in liquid nitrogen for subsequent analyses.
Algal growth
In order to demonstrate algal ability to recover from gamma irradiation conditions, the growth curve of algae was plotted by calculating cell concentration. The cell concentration was determined using a hemocytometer over a 96-h period. Besides, the specific growth rate (μ) was determined by following equation (Fogg and Thake, 1987): μ = (lnN2 − lnN1)/(t2 − t1), where, N1 and N2 are cell concentration at time t1 (time point 0) and t2 (time point 48 h).
Photosynthetic pigments
Şükran et al. (1998) method was modified and employed to measure algal pigments including chlorophylls and carotenoids. Algal suspensions (10 mL) were centrifuged for 10 min at 5900×g. After disruption of cell walls with pellet pestle and repeating freeze/thaw cycles, the pellets were homogenized in 20 mL chilled acetone 80% (v/v) in darkness and the mixtures were centrifuged as before. The supernatant was collected and the absorption was read at 663, 646, and 470 nm. The content of pigments was calculated using the Wellburn and Lichtenthaler (1984) formulas as mg per mL, divided by the cell concentration and expressed as pg per cell.
Electrolyte leakage
In order to determine cell membrane stability under gamma irradiation, Liu’s method was employed to determine the electrolyte leakage (EL) (Bajji et al. 2002). Ten milliliter of algal suspension was centrifuged at 2400×g for 10 min, washed twice with 10 mL of distilled water, resuspended in 10 mL of distilled water, and incubated in water at 25 °C in darkness for 24 h. After that, a conductivity meter (Jenway, Model 4510) was used to measure initial electrolyte leakage (EC1). Later, the samples were heated at 100 °C for 20 min and the secondary electrolyte leakage (EC2) was measured. The following equation was applied for EL calculation: (EL) (%) = (EC1/EC2) × 100.
MDA content
Malondialdehyde (MDA) content was used as a marker to assay the rate of lipid peroxidation (Xiong et al. 2014). The pellet obtained from 10 mL of algal culture was homogenized in 5 mL trichloroacetic acid (TCA) solution 10% (w/v), and the mixture was centrifuged at 9300×g for 15 min. For each 1 mL aliquot of the supernatant, 1 mL of 0.5% (w/v) thiobarbituric acid (TBA) solved in 20% (w/v) TCA was added and the mixture was heated at 96 °C for 30 min and then cooled in an ice bath. The absorbance of the supernatant was read at 532 and 600 nm. The extinction coefficient of 155/(mM cm) was used for calculating MDA concentration.
Proline content
Free proline content was determined based on the method proposed by Bates et al. (1973). Pellets from 10 mL algal culture were extracted in 5 mL of 3% sulfosalicylic acid and centrifuged for 15 min at 5900×g. Two milliliter of supernatant was added to 2 mL ninhydrin and 2 mL of glacial acetic acid. The mixture was then heated for 60 min at 100 °C and then cooled quickly in an ice bath. Subsequently, 4 mL of toluene was added into the solution and was vortexed for 30 s. The organic phase containing proline was used for absorbance reading at 520 nm. By reference to L-proline (Sigma, USA) the proline content was calculated.
Glycine betaine content
Glycine betaine was quantified as previously described by Sairam et al. (2002). Harvested cells from 10 mL algal culture were washed with distilled water and then centrifuged. The pellets added with an equal volume of 2 N H2SO4 and 0.8 mL chilled in KI–I2 reagent (1.75 g I2 and 2 g KI in 10 mL deionized water) were briefly vortexed and incubated in an ice bath for 16 h. One milliliter of the homogenate was carefully mixed with 1 mL of 1,2-dichloroethane and was incubated for 3 h at room temperature. The absorbance of the organic phase was read at 365 nm. Glycine betaine (GB) concentration was estimated through a curve prepared from known amounts of standard glycine betaine (Merck, Germany). The results were expressed as μg GB per cell.
Soluble carbohydrates
The soluble sugars were measured according to the Kochert (1978) method. For this 100 mL of algal suspension was used for each measurement and was repeated three times at every time point. The cultures were centrifuged for 10 min at 5900×g and the resulting pellets were dried overnight at 70 °C. Dry algal powder (0.1 g) was homogenized in 70% ethanol (v/v). One milliliter of the extract was adjusted to a volume of 2 mL by adding deionized water and then it was mixed with 1 mL of 5% phenol solution (w/v) (Biobasic, Canada). The optical density of the samples were measured by spectrophotometer after adding 5 mL of 80% sulfuric acid at 480 nm for rhamnose, 485 nm for glucose, and 490 nm for mannose. The sugars are quantified through their standard curves and results are stated as μg carbohydrate per cell.
H2O2 assay
H2O2 concentration was defined based on Alexieva et al. (2001). Pellets from 10 mL of algal culture were suspended in 2 mL of 0.1% (w/v) chilled TCA in an ice bath. The mixture was centrifuged at 16,000×g for 15 min. Afterwards, 1 mL of the supernatant was mixed with 1 mL of 10 mM potassium phosphate buffer (pH 7.0) and 2 mL of 1 M KI. The mixture absorbance was recorded at 390 nm. The concentration of H2O2 was estimated from a standard curve and results expressed as nmol of H2O2 per cell.
Enzyme assay
Pellets from 10 mL algal culture were homogenized with 50 mM potassium phosphate extraction buffer on ice. Cell disruption was performed using a pellet pestle and freeze/thaw cycles were repeated. The disruption was checked under a light microscope. The homogeneous mixture was centrifuged for 20 min at 15,700×g and 4 °C. The supernatant was used as an enzyme extract to measure the activity of antioxidant enzymes.
SOD activity
The activity of superoxide dismutase (SOD) was examined by determining its capacity to hinder the photochemical reduction of nitro blue tetrazolium according to Beauchamp and Fridovich (1971). Phosphate buffer (50 mM) (pH 7.8) containing 2 μM riboflavin, 75 μM nitro blue tetrazolium (NBT), 0.1 mM Na-EDTA, 13 mM methionine, and 50 μL of enzyme extract was considered as the 1 mL reaction mixture. The resulting mixture was exposed to a 5000-lx fluorescent lamp for 15 min, and then its absorbance was read at 560 nm. The enzyme-free reaction mixture was used as a control. The amount of the enzyme which caused 50% of the NBT’s inhibitory restraint considered as one unit of SOD.
CAT activity
The reaction mixture (1 mL) contained 50 mM phosphate buffer (pH 7.0), 30 mM H2O2 and 100 μL of enzyme extract. Hydrogen peroxide decomposition and absorption reduction were monitored for 60 s at 240 nm. The enzyme activity was calculated using EC = 39.4 Mm−1 cm−1 and expressed in terms of μM H2O2 mg−1 protein s−1 (Aebi 1984).
APX activity
The method of Nakano and Asada (1981) was used to calculate ascorbate peroxidase activity. One milliliter of the reaction mix included 50 mM phosphate buffer (pH 7), 0.1 mM EDTA, 0.3 mM ascorbate, 0.1 mM H2O2 and 100 μl of enzyme extract. The enzyme activity was read at 290 nm in 60 s and was expressed using EC = 2.8 mM−1 cm−1 and in ascorbate μM per mg protein per unit of time.
FRAP value
Ferric reducing antioxidant power (FRAP) assay is a useful method to measure the total antioxidant activity of a biological system (Benzie and Strain 1999). In short, 300 mM acetate buffer (pH 3.6), 10 mM TPTZ (2,4,6-tripyridyl-s-striazine) (Sigma, Canada) in 40 mM HCl and 20 mM FeCl3·6H2O were mixed in the ratio at 10:1:1 to give the working FRAP reagent. Two thousand eight hundred fifty microliter of this reagent was added to 150 μL algal extract (the extract was prepared according to part 2.11.) and kept in darkness at 37 °C for 30 min. The absorbance was read at 593 nm and the results expressed as μmol cell−1.
Total protein
Bradford method was used for total soluble protein content determination using crystalline bovine serum albumin (BSA) as a standard reference (Bradford 1976). Accordingly, 1 mL of potassium phosphate extraction buffer was used to homogenize the pellets from 10 mL of algal culture in order to extract proteins. Results are expressed as μg protein per 106 cells.
Na+ and K+ contents
The pellets harvested from algal culture were dried overnight at 70 °C. 3% (w/v) aqueous sulfosalicylic acid was used to digest algal pellets in order to obtain powdered algal material. After 4 °C overnight incubation, the extract was centrifuged for 15 min at 1200×g. A flame photometer (model: P378; Portlab; Italy) was used to determine the Na+ and K+ concentration according to Skoog et al. (1996).
FT-IR spectroscopy
One milliliter of algal samples was centrifuged at 100×g for 5 min. The supernatant was removed and the pellets resuspended in 100 μl Milli-Q water. Each sample was then adjusted to a cell number of 50 × 106 cells mL−1 with Milli-Q water. One hundred microliter of each sample was transferred onto glass and allowed to dry at 40 °C overnight. The FT-IR spectra were collected using a Nicolet Magna-550 spectrometer instrument in KBr pellets. The spectral range of 400–4000 cm−1 was collected. Each algal sample was analyzed in triplicate in its relative time course. The spectra were baseline corrected and matched using Essential FTIR Spectroscopy software. The full spectral information was exported as a CSV (Comma delimited) file and imported to GraphPad Prism Software (v6) for additional analysis. The maximum band intensity of wavenumber ranges of carbohydrate (1200–900 cm−1), nucleic acid (1270–1200 cm−1), Amide I (1640–1670 cm−1) and Amide II (1530–1570 cm−1) were taken from the data and normalized by that of fatty acid (2900–2980 cm−1). Finally, the data were graphed as the ratio of carbohydrate, nucleic acid, amide I and amide II to fatty acid.
Statistical analysis
Each experiment was repeated at least three times independently. One-way analysis of variance (ANOVA) was conducted to analyze the records and compare mean differences, using the SPSS package (Ver.19). Additionally, the post hoc Duncan test was run to locate possible mean differences. Principle component analysis (PCA) was performed according to the growth data, FT-IR results and physiological parameters under control and gamma irradiation for three time courses (1, 16, and 48 h after irradiation). Spreadsheets of multi-data were generated in Excel, scaled in BioStatFlow software (v.2.9), and then transferred into the ClustVis online tool for PCA analysis and hierarchical clustering analysis (HCA) (Metsalu and Vilo 2015). To cluster treatments, Heatmap function with row wise scaling and correlation-based clustering were used.
Results
Primary selection of irradiation dosage and time courses
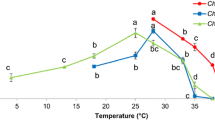
In order to monitor the ability of C. vulgaris to withstand gamma irradiation the algae were exposed to different intensities of gamma radiation (Fig. 1a). Comparison of growth kinetics between irradiated microalgae with unirradiated control samples during 96 h of irradiation indicated that severe (1200 Gy) gamma irradiation induced an extended lag phase in the growth curve and high cell mortality after 36 h (Fig. 1a). The algal cells treated with milder doses of irradiation (300 and 600 Gy) showed sigmoidal growth curves with short lag phases and the mortality was induced after 36 h of gamma exposure for algae treated with 300 Gy irradiation and 48 h for the algae treated with 600 Gy irradiation. Based on these preliminary results, in terms of growth kinetics, the cells treated with 600 Gy did not behave much differently from those receiving 300 Gy gamma energy. Therefore, due to the ability of C. vulgaris to withstand gamma irradiation up to 600 Gy, this dosage was selected for further analysis in the main experiment. Three time points of sampling for chemical and physiological analysis were selected through the initial growth curve for the main experiment: (1) just after irradiation (0 h), (2) the middle point of logarithmic phase (16 h after irradiation), and (3) at the endpoint of stationary phase of growth (48 h after irradiation).
a Preliminary assessment of different doses of gamma irradiation (control, 300, 600, 1200 Gy) on growth curves of C. vulgaris for determining gamma irradiation-withstand-ability. b Effect of 600-Gy gamma irradiation on the cell viability of C. vulgaris at different time courses after irradiation. The values are means of at least three replicates ± standard deviation (SD). Different letters indicate significant differences (P < 0.05)
Gamma irradiation effect on growth, physiology, and chemical compounds
Growth determination
General responses of algae to stress conditions provoke considerable physiological injury, which is usually followed by morphological and anatomical abnormal changes. According to the results, during 48 h after 600 Gy gamma irradiation, all growth parameters including cell number, growth rate, and viability were significantly different from those of control. The results showed that compared to the control, gamma-irradiated algae had a slower growth rate with a significantly longer lag growth phase (Fig. 1a).
The growth analysis showed a significant decrease in the growth rate (μ) of the algae after 48 h of being subjected to gamma radiation. Based on the results, compared to the control (μ = 0.99 day−1), the specific growth rate of the algae exposed to gamma irradiation (μ = 0.48 day−1) reduced about 51% during 48 h after irradiation. The data also indicated that the viability of the gamma irradiated algae decreased compared to that of non-irradiated control (Fig. 1b). In this regard, algal mortality (about 30%) was mostly observed during 16 h after gamma irradiation, which reduced to about 21% after 48 h of irradiation. No significant mortality was observed in gamma-free control samples for 48 h.
Pigment accumulation
The contents of chlorophyll a and b, as well as total chlorophyll, were reported as pg cell−1 to monitor the pigments in individual algal cells (Fig. 2a–c). The results showed all the pigments did not significantly change during 48 h in the control algae while the gamma-irradiated algae had lower contents of total chlorophylls a and b compared to unirradiated controls immediately (0 h) and 16 h after irradiation. However, after 48 h, the contents of chlorophylls in irradiated algae were approximately equal to that of the control group. The carotenoid content of gamma-irradiated algae was about similar to that of control immediately after irradiation, however, at 16 and 48 h after irradiation, the carotenoids of irradiated algae significantly increased in a time-dependent manner compared to the control (Fig. 2d). The maximum carotenoid content was observed 48 h after gamma irradiation, which was about 1.3 times more than that of the control.
The effects of gamma irradiation on a chlorophyll a, b chlorophyll b, and c total chlorophyll and d total carotenoids of C. vulgaris at different time courses after irradiation. The values are means of at least three replicates ± standard deviation (SD). Different letters indicate significant differences (P < 0.05)
Proline, GB, and SCs
A rapid accumulation of proline content occurred immediately after irradiation (Fig. 3a), which was twice that of the control. Although a decreasing trend in proline contents of radiated samples was observed after 16 and 48 h, the contents were still higher than those of the control samples. There was no significant difference in the proline content of the control group in none of the studied time courses.
The effects of gamma irradiation on a proline, b glycine betains, c glucose, d mannose, and e rhamnose of C. vulgaris at different time courses after irradiation. The values are means of at least three replicates ± standard deviation (SD). Different letters indicate significant differences (P < 0.05)
During 48 h after irradiation, glycine betaine (GB) slightly increased in both the irradiated and control algae (Fig. 3b). The content of GB in the gamma-irradiated algae was significantly lower (1.25-fold) than the control at all three time points (0, 16 h, and 48 h). The highest amount of GB was observed at 48 h in the control samples, which was about 1.2 times more than that of the irradiated algae.
The contents of soluble carbohydrates (SCs) showed a general increasing trend in the irradiated algae at the beginning (0 h) and during 48 h after gamma irradiation, whereas there were no significant differences in contents of SCs in the control samples taken at this period (Fig. 3c–e). The maximum contents of glucose, rhamnose and mannose were recorded in the algae at 48 h after irradiation, which were 1.26, 3.2, and 1.47 times more than those of the control, respectively.
Lipid peroxidation
The two prominent markers of stress-induced membrane damage, MDA and EL contents increased immediately after gamma irradiation, which were 1.5 and 1.3 times higher than those of the control, respectively (Fig. 4). However, during 48 h after irradiation, the levels of MDA and EL reduced to the same level of those in the control. The results also illustrated no statistical difference in MDA or EL of the control group at all studied time courses.
ROS induction and FRAP power
The results indicated that H2O2, as a common ROS that has been related to PCD within living cells during abiotic stresses, accumulated immediately after gamma irradiation about twice more than the control. The H2O2 production trend subsequently decreased over the 48 h, but it was still 1.7 and 1.3 more than the control at 16 h and 48 h of irradiation, respectively (Fig. 5a). During the experiment, the value of H2O2 content in the gamma-free control algae did not significantly change at the time points.
The FRAP, as a potential antioxidant capacity indicator, showed an immediate increase of about 30% in the irradiated algae compared to the control (Fig. 5b). Henceforth, although the FRAP level slightly decreased in the irradiated samples at the next time intervals, it was significantly higher than that of the control during the whole period of 48 h. The FRAP level of nonirradiated control remained at the same level during the experiment.
Protein content
A rapid decrease (60%) of protein took place in the gamma-irradiated algae immediately after radiation (Fig. 6a). This reduction was reduced to some extent and reached about 34% at 16 h after irradiation. Interestingly, the level of protein completely recovered in the irradiated algae 48 h after irradiation and reached the same level of that of the unirradiated control. Again, no significant differences were found in the protein content during the whole period of the experiment.
Enzyme activity
Gamma irradiation effect on antioxidant enzymes activities is shown in Fig. 7. Compared to the control, the catalase (CAT) activity per mg total protein significantly increased up to 61% immediately after gamma irradiated and remained at more or less the same level at 16-h sampling time. Although at 48 h after irradiation, the CAT activity in the irradiated algae slightly reduced, it was significantly higher than that of the non-irradiated control. No significant difference was observed in the CAT activity among the unirradiated samples taken from different time-points of the experiment (Fig. 6b).
As shown in Fig. 6 c and d, SOD and ascorbate peroxidase (APX) activities, in comparison to the control, were decreased in gamma-irradiated algae immediately after gamma irradiation, and remained at the same level during the 48-h period of the experiment. A mean reduction of 39% and 25% in the activity of APX and SOD in the irradiated algae compared to the unirradiated control was observed, respectively, during the experiment. The activity of APX and SOD also remained relatively unchanged in the control algal samples during the whole period of the experiment.
Ratio of K+/Na+
Immediately after irradiation, the ratio of K+/Na+ in irradiated samples increased by up to 48% in comparison to the controls (Fig. 7a). This ratio of K+/Na+ remained relatively unchanged in the gamma-treated algae at 16 h and 24 h after gamma irradiation. On the other hand, no significant difference was observed in the ratio of K+/Na+ of the controls taken at different time intervals of the experiment. Furthermore, the K+ content of the irradiated samples increased over the three time intervals with the highest content after 48 h, while the non-irradiated samples showed no changes at all (Fig. 7b).
FT-IR analysis
FT-IR analysis was performed to measure possible significant changes in metabolic plasticity of C. vulgaris in response to gamma radiation. The EMSC2-normalized FT-IR spectra confirmed that the metabolic fingerprint of C. vulgaris varied significantly over the studied time courses after irradiation (Fig. 8). There was a general increase in the ratios of carbohydrate, amide I and amide II to fatty acid in both the control and gamma-irradiated algae during a 48 h time course (Fig. 8a–c). Immediately and at 48 h after gamma irradiated treatment, these ratios in the irradiated group of algae had similar levels to that of the control. However, at 16 h after irradiation, these ratios in the irradiated algae were lower than those in the control.
Changes in the ratios of a carbohydrate:fatty acid, b amideI:fatty acid, c amide II:fatty acid, and d nucleic acid:fatty acid of C. vulgaris under gamma irradiation in comparison to unirradiated control samples. Each column is the mean (± SD) of three replicates of FTIR spectrum. Different letters indicate significant differences (P < 0.05)
Compared to the control, the gamma-irradiated algae had a relatively lower ratio of nucleic acids to fatty acids immediately and during a 48-h period after irradiation (Fig. 8d) although the ratio significantly increased at the final time interval in the experimental sample.
PCA and HCA analysis
Figure 9 a and b indicate the load plots of principal components 1 and 2 derived from growth analysis, FT-IR analysis and related physiological parameters in gamma-irradiated or nonirradiated C. vulgaris. The results from PCA of the irradiated and control indicated that the principal component 1 (PC1) explained 59.8 and 76.3% of the whole variation, respectively. Principal component 2 (PC2) explained 33.9 and 14.1% of the entire variation under gamma-irradiated and control conditions, respectively. Therefore, PC1 and PC2 cumulatively captured 93.7 and 90.4% of the whole dissimilarities under irradiated or control conditions, respectively. Based on the PCA individually acquired from gamma and control samples, the examined parameters fell into 5 different groups (Fig. 9a, b, circled). It was shown that after gamma irradiation, parameters including FRAP, proline, H2O2, CAT, K+/Na2+ ratio, MDA, EL, and soluble sugars were separated in two different groups; group I at the left-down and group II at right-up the corner of the biplot. These parameters were observed to increase under gamma irradiation. Between the antioxidant enzymes, CAT activity was located in group I and pulled to the left-down of the plot and thus was more sensitive than APX and SOD in response to gamma irradiation. SOD and APX with specific growth rate, along with glycine betaine, nucleic acid/fatty acid ratio, viability, and cell number pulled into the right-down corner of the plot and significantly decreased under gamma irradiation (group V in Fig. 9b). Compared to the control, the amount of total carotenoids was pulled into the right-upper side of the biplot and increased under gamma irradiated condition (Fig. 9b)
The heat maps obtained from hierarchical clustering analysis (HCA) under control or gamma-irradiated condition are shown in Fig. 10a and b, respectively. The heat maps from HCA indicated that under control or gamma-radiated conditions, all the parameters could be differently clustered into four groups. Under gamma irradiation, CAT, SOD, and APX activity, FRAP potency, proline, number of cells, viability, and H2O2 content, as well as MDA and EL clustered into a separate group (group I in Fig. 10b). These parameters reached their peaks immediately after irradiation and gradually declined during a testing period of 48 h. Three parameters including the specific growth rate, K+/Na2+ ratio and mortality grouped into a separate cluster (cluster II). These parameters reached their peaks at 16 h after irradiation. The parameters including protein, soluble carbohydrates, chlorophylls, total carotenoids, glycine betaine, the ratios of carbohydrate, amide I and amide II to fatty acid clustered into group III. These parameters often showed to be gradually increasing after irradiation and maximized at 48 h after gamma irradiation. Group IV only contained one parameter, nucleic acid to fatty acid ratio, which was high at two time-points (0 h and 48 h) and low at 16 h after irradiation.
Discussion
There is high complexity in response to IR in different photosynthetic organisms (Esnault et al. 2010; Cheng et al. 2013; Ermavitalini et al. 2017). The different reactions to IR mainly originate from individual responses and individual phenotypic plasticity (Esnault et al. 2010). In order to understand plant responses to oxidative stresses, inquiry of IR effects will give an attractive pattern such that a comparison between IR stress and other stresses possibly will lead to an innovative outlook to oxidative stress (Kovacs and Keresztes 2002).
The majority of experiments exploring the effects and responses of ionizing radiation have been performed in bacteria or prokaryotic blue-green algae. Properties found in cyanobacteria like Arthrospira platensis made them as appropriate microorganisms to gamma stress. Previous reports showed that gamma irradiation at low doses (1 kGy), could stimulate A. platensis growth with small modifications in the morphology. It was shown that gamma irradiation boosted carotenoid accumulation along with stimulated carbohydrates and protein production. In addition to the antioxidant system, radiation resistance of A. platensis was attributed to a transforming DNA repair system including DNA polymerase and nucleotide ligasein the cyanobacterium Anacystis nidulans cells (Kovacs and Keresztes 2002; Abomohra et al. 2016).
In contrast to prokaryotic algae, little information exists about the effects and defensive response of eukaryotic microalgae against gamma radiation. Eukaryotic microalgae, due to being responsive to extreme seasonal fluctuations, are highly suitable biological indicators of environmental changes. We utilized C. vulgaris, a unicellular chlorophyte, as a model system for biochemical and physiological studies on the gamma-stress response. The main goal of this study was to consider the mechanism of defiance at the physiological level and recovery in C. vulgaris and to clarify the responses of this alga to IR conditions.
It was also previously demonstrated that UV-B irradiation at 5 Wm−2, an extreme UV dose, significantly decreased the growth of C. vulgaris (Ganapathy et al. 2017). It was also shown that Chlorella pyrenoidosa treated with different doses of thorium (Th), a natural radioactive element, tolerated ionizing radiation and started to grow 24 h after treatment with respect to Th (Peng et al. 2017); however, no data were presented about the radiation dose of the alga tolerance in that study. In the current study, the growth of irradiated and non-irradiated control was monitored during a period of 96 h. in order to investigate the ability of C. vulgaris when recovering from increasing doses of 300, 600, and 1200 Gy gamma irradiation. Based on the analysis of the results, the algae irradiated at doses of 300 and 600 Gy were mostly sustainable, but at the same time had less recovery power than the control. The results suggested that gamma irradiation doses higher than 1 kGy remarkably reduced the viability of C. vulgaris. Similarly, Cheng et al. (2016) showed that a Chlorella sp. mutant could tolerate gamma rays up to 900 Gy. Choi et al. (2015) also explained that gamma radiation more than 1 kGy was lethal for the green alga, Zygnema sp. and reduced its viability. Similarly, Badri et al. (2015) suggested that Arthrospira sp. PCC8005 irradiated with 800 Gy gamma rays will show an early death phase. On the other hand, although the majority of the algae have a restricted ability to resist high doses of ionizing radiation, there have been reports of the green microalgae with a high degree of radio-resistance (6–20 kGy) (Farhi et al. 2008; Choi et al. 2015; Rivasseau et al. 2016). Overall results of the present study suggested C. vulgaris is a “relatively radio-resistant” microorganism. Based on these results, the dose of 600 Gy was selected for studying the physiological responses of Chlorella to gamma irradiation in the main stage of the experiment.
Physiological and biochemical parameters were analyzed under 600 Gy gamma irradiation and compared with control conditions. Photosynthetic pigments allow the algae and plants to absorb sunlight energy, so chlorophyll content is a crucial factor affecting the performance of photosynthesis and the physiological status of the algae. Diminished biosynthesis of chlorophyll and carotenoids are frequently explained as symptoms of stress and toxicity (Poskuta et al. 1996; Prasad et al. 2004). Therefore, as a visible symptom, the chlorophyll content could be employed to monitor the stress-induced injuries as well as evaluation of the degree of tolerance of C. vulgaris to gamma stress. According to the results, at 48 h after gamma treatment, no significant differences were seen in Chl a, Chl b, and total Chl contents as the photosynthetic pigments between gamma-irradiated and control samples. The high content of chlorophyll after exposure to gamma radiation showed the ability of the C. vulgaris to maintain its chlorophylls or the photosynthetic reaction center complexes under radiation stress. In contrast, Ganapathy et al. (2017) demonstrated that UV-B radiation at 5 Wm−2 markedly reduced the content of chlorophyll a and b in C. vulgaris. Additionally, Lamaia et al. 2005 and Peng et al. 2017 reported that the content of chlorophylls in C. pyrenoidosa had reduced under the induction of heavy metals, such as lead and cadmium. Enhanced pigment degradation and, or damage might be mediated by stress-induced ROS accumulations (Kovacs and Keresztes 2002; Aghaie et al. 2018; Anaraki et al. 2018). Gomes et al. (2017) also reported a negative correlation between photosynthetic performance and ROS production in Chlamydomonas reinhardtii after exposure to gamma radiation. The reduction of chlorophylls under such conditions, at least partially, might also be associated with enhancement of compatible solutes like proline synthesis due to the fact that chlorophylls and proline are both synthesized from a shared substrate (Aspinall and Paleg 1981; Bahreininejad et al. 2013). Ultrastructural studies in plants also have shown that dilations between thylakoid membranes and loss of grana stacking were induced by gamma irradiation at exposures above 0.2 kGy (Kovacs and Keresztes 2002). According to the present study, although a little reduction in the contents of algal chlorophylls took place immediately after initial gamma shock, the contents were found to recover as much as the level of the control after 48 h of irradiation. Farhi et al. (2008) confirmed that green microalgae irradiated with elevated doses of gamma rays showed the photosynthesis recovery 5 days after irradiation, which can be attributed to the amount of photosynthetic pigments recuperation. Peng et al. (2017), also argued that Chlorella sp. treated with radioactive elements, recovered its chlorophyll content with the increase of exposure time, up to 2 days after irradiation.
Algae are also known to have different types of carotenoids and many of them retain antioxidant and radical scavenging operations. According to our results, a significant and time-dependent increase of total carotenoids was observed in the gamma irradiated algae. The results were in accordance with several other reports. For example, in a previous study, A. platensis also increase its carotenoids in response to gamma irradiation (Kojima et al. 2002, 2011; Ko et al. 2012; Abomohra et al. 2016; Liu et al. 2016). Kovács and Keresztes (2002) stated that pigment protection against damage was done through carotenoids, when triplet chlorophyll was saturated by excitation energy (Ali et al. 2015). Similarly, C. vulgaris exposed to UV-B radiation and C. zofingiensis coped with high light intensity, reacted to store more secondary carotenoids compared to the controls while their carotenoids content was less affected than total chlorophyll (Choi et al. 2015; Ganapathy et al. 2017). More accumulation of carotenoids induced by gamma radiation probably shows a vital role in response to the ionizing stress in the algae. It was noted that the carotenoids not only protect chlorophylls but also help stabilize plasma membrane against membrane lipid peroxidation, which normally occurs because of stress-induced ROS (Malanga and Puntarulo 1995). This might also be found from our results, because further accumulated carotenoids in gamma-irradiated C. vulgaris at 48 h after irradiation negatively correlated with the amount of H2O2, the rate of lipid peroxidation (MDA content) and the percentage of EL. A low hydrogen peroxide (H2O2) as an endogenous ROS, which results in low lipid peroxidation rate, could also strongly be associated with high antioxidant activity triggered after algal exposure to gamma radiation. This was further supported in our study by observing a strong FRAP triggered immediately in irradiated microalgae, which remained relatively constant or at least higher than the control during a 48-h period. It was concluded that in addition to the activities of antioxidant enzymes, thus reducing antioxidant power might be associated with the presence of nonenzymatic antioxidant compounds, such as carotenoids or phytic acid which significantly increased upon irradiation (Alothman et al. 2009). Besides, in confirmation with these results, it was inferred by FTIR spectra that the relative abundance of amide (II and I) increased in gamma-irradiated algae, especially at 48 h after irradiation. The side chains of amide compounds found to have a key role in further quenching of stress-related ROS (Kaur et al. 2015). It was also demonstrated that regulated antioxidants with amide groups in their structure, such as melatonin, could help the cells to recover their redox state through ROS scavenging. These compounds were shown to stimulate the activities of some antioxidant enzymes. Thus, it appeared that a higher amount of amid-bearing compounds was responsible for alleviating the detrimental effects of gamma by gifting a scavenging power to irradiated C. vulgaris.
In response to various stress conditions, a selection of compatible solutes with low molecular weight was identified to accrue in seedlings (Serraj and Sinclair 2002). Metabolites such as proline, GB, polyols, trehalose, and sucrose showed to improve stress tolerance. These metabolites maintain cell turgor, alleviate membrane stability, protect proteins, avert membrane leakage, play a role as a redox buffer (Hare and Cress 1997), and maintain ROS concentration within normal ranges (Hayat et al. 2012). Important roles such as providing carbon, nitrogen, and energy sources in stressed cells have been also identified for metabolites such as proline (Delauney and Verma 1993; Kuznetsov and Shevyakova 1999). The present study showed that C. vulgaris had a higher ability to accumulate intracellular proline in response to gamma irradiation stress than the control. These results seem compatible with Lin and Wu (2014), who observed that soil algae and cyanobacteria demonstrated a high proficiency in gathering more intracellular proline contents as a response to oxidative stress. Al-Enezi and Al-Khayri (2012) also found a high proline accumulation in Phoenix dactylifera induced by X-ray radiation, which resulted in overcoming the stress. Proline was maximized in the algae at the initial 16 h after irradiation, and although from this point up to the end of the experiment it slightly declined, it was significantly higher than that of the control. So, it seems that the proline accumulation may be a rapid and spontaneous defense mechanism against gamma irradiation stress in C. vulgaris. Declined proline accumulation after passing more time of irradiation might be explained based on the fact that the algal cell recovered from the tension after initial gamma irradiation shock. It was shown that proline might interact with enzymes to sustain enzyme structure and activity. In fact, this solute has been reported to reduce in vitro enzyme denaturation caused due to heat, NaCl stress, and gamma radiation (Borzouei et al. 2010, 2013; Chandrashekar et al. 2013; Kebeish et al. 2015). Interestingly, proline performed as a molecular stabilizer for maintaining integrity and stability of protein structure or improving several enzymes activities, upon exposure to different stress conditions (Cuin and Shabala 2007; Mishra et al. 2008).
Similar to proline, the accumulation of intracellular, soluble carbohydrates (SCs) such as glucose, mannose, and rhamnose, have revealed a vital role in improving harmful effects of stress over reducing osmotic potential, storage of carbon, and scavengers of free radicals (Hasegawa et al. 2000). It was shown that sulfated heteropolysaccharides compounds consisting of galactose, xylose, glucose, etc. found in green algae could interact with ROS in ionizing radiation stress (Ngo and Kim 2013; Usov and Zelinsky 2013). In our study, following gamma irradiation, more algal carbohydrates (SCs) were produced over time such that the highest amount of carbohydrate was recorded, especially for glucose at the final stage of the experiment (48 h after irradiation). Therefore, in contrast to proline, SC production might be a delayed defense strategy against gamma radiation in C. vulgaris. Our results were in accordance with Abomohra et al. (2016) who showed that gamma radiation up to 1.5 kGy increased total carbohydrates in A. platensis after 15 days of irradiation. Farhi et al. (2008) and Choi et al. (2014) also determined the radio-resistance feature in green microalgae for gamma irradiation (up to 6 kGy) with a decreased ability for carbohydrate production due to increasing gamma irradiation. The need for more carbohydrates (as a source for ATP production) under ionizing stress may be illuminated by repairing mechanisms which need energy to function (Abomohra et al. 2016). Rivasseau et al. (2010) affirmed that under irradiation conditions, a high level of carbohydrate concentration constantly holds, implying that the carbohydrate energy supply pathways are affected but persist. Consequently, one can consider that sugar compounds are consumed as cellular energy sources and expanded additionally under gamma irradiation (Abomohra et al. 2016). Additionally, the carbohydrate/fatty acid ratio did change significantly in the C. vulgaris in response to gamma irradiation compared to the unirradiated control. A higher carbohydrate accumulation 48 h after irradiation could be attributed to more production of soluble sugars which could help the cells to improve the tolerance to stress by acting as scavengers of ROS.
Other compounds such as glycine betaine (GB) are utilized to maintain the osmolality of cells (Sakamoto and Murata 2002; Ashraf and Foolad 2007; Hussain Wani et al. 2013). Several marine algae have been described to accumulate GB as an osmolyte-induce stabilization compound (Mishra et al. 2008). This osmolyte is mainly accumulated in chloroplasts and especially has important roles in protecting the thylakoid membrane of chloroplasts. Thus, it might affect photosynthesis plus regulating K+ efflux (Cuin and Shabala 2007). In this study, the amount of glycine betaine was significantly lower in the gamma-irradiated algae compared to the unirradiated samples. Meanwhile, a mild and gradual increase of GB was observed over time after gamma irradiation in C. vulgaris. Therefore, this delay in accumulation of GB after irradiation might be due to the fact that C. vulgaris may not respond to gamma irradiation initially by GB production or at least that GB synthesis may be a delayed defense mechanism against ionizing radiation stress.
Protein accumulation in response to abiotic stresses has been reported in various studies (Pareek et al. 1997; Ashraf and Harris 2004; Parvaiz and Satyawati 2008; Won et al. 2015). In our study, the content of total protein significantly declined in irradiated C. vulgaris immediately after irradiation started; however, more proteins accumulated in the algae during the next period of the experiment, which was as much as that of the control at 48 h after irradiation. The initial decline in algal protein at time point zero after irradiation might be related to direct or ROS-induced indirect effects of ionizing radiations which degrade biological molecules such as lipids, nucleic acid, and protein. It was claimed that just 20% of DNA destruction is directly related to radiation, and the remaining 80% is indirectly damaged by ROS (Ghosal et al. 2005). In this context, Kojima et al. (2011) suggested that gamma irradiation provokes ATP production, which in turn induces the production of ROS. There are also studies evidencing that gamma radiation decrease the total protein and carbohydrate content due to metabolic activities and hydrolyzing enzyme activities (Maity et al. 2009).
Osmotic adjustments may play a role in protein accumulation under stress conditions (Parvaiz and Satyawati 2008). The newly stress-related polypeptides could be produced as a result of stressful conditions (Pareek et al. 1997). Farhi et al. (2008) described that even low doses of irradiation improved free amino acid concentration. The increase in protein content was attached to the amino acid rise, which played an important role in the DNA repair mechanism (Maity et al. 2009; Rivasseau et al. 2010; Won et al. 2015). There are several reports of gamma-induced proteins. For instance, a purified peptide from marine C. ellipsoidea showed antioxidative potency (Ivanova et al. 2010). Furthermore, a protein found in Arthrospira fusiformis, was noticed to promote antioxidant protection systems in individual cells exposed to low doses of gamma irradiation (Ivanova et al. 2010); and a de novo synthesized class of heat shock proteins (HSPs) helped to preserve against the stresses like radiation (Tammam et al. 2005; Abo-Shady et al. 2008; Rivasseau et al. 2010). Yoon et al. (2013) also found higher protein content in mutant Spirogyra varians brought by gamma irradiation. Accordingly, 18 new expressed proteins, predicted to be involved in photosynthesis, carbohydrate biosynthesis and energy metabolism, were identified in their study.
Detoxification enzyme system, CAT, SOD, APX, GPX (glutathione peroxidase), and PrxR (peroxiredoxin oxidoreductases) were demonstrated to be more active in the course of abiotic stress in distinctive plants (Esnault et al. 2010; Anaraki et al. 2018). Previously, Malanga and Puntarulo (1995) described that UV-B radiation exposed C. vulgaris showed an increase in antioxidant activity. Additionally, a low dose of radiation efficiently stimulated antioxidative enzymes implied in ROS scavenging (Xue and Hartikainen 2000; Chaudhary and Agrawal 2014). To overwhelm oxidative stress, H2O2 usually raises from superoxide due to SOD enzyme activity and further condenses to H2O by CAT and some peroxidases studied in plants (Ozkur et al. 2009; Kadkhodaie et al. 2013). Therefore, ROS detoxification could be initiated through the function of SOD and completed by cooperative action of other antioxidant enzymes (Alscher et al. 2002). However, the results from this study indicated a prompt rise of CAT activity triggered in C. vulgaris by gamma radiation, which remained high during the experiment. Though, the activity of SOD and APX significantly reduced after gamma irradiation stress in comparison to that of the gamma-free controls. The results reveal that CAT activity in comparison to the other antioxidant enzymes may act independently in C. vulgaris to gamma irradiation that could also be used as a reliable screening tool for assaying irradiation tolerance. It is possible that SOD activity changed earlier than the time-course considered in our study, and its activity was triggered immediately (for example, a few seconds or minutes) after gamma irradiation. This could be evident by the fact that a burst of H2O2 took place in Chlorella immediately after exposure to gamma radiation. The findings are very controversial in this regard, based on several studies which confirmed that CAT or APX activity decreased/remained at similar levels after stress initiation (Kachout et al. 2013). On the other hand, it was reported that both SOD and CAT were sensitive in microalgae and coped with UV-B radiation (Al-Rashed et al. 2016), which is strongly confirmed by another experiment in which D. salina was exposed to low and high doses of UV-B (Zhang et al. 2017). According to these studies, SOD activity had an upward trend initially and then declined to a lower state.
The concentration of cytosolic K+ is at least 7000-fold more than the external K+ level, which creates a considerable chemical gradient across the plasma membrane (Leigh and Jones 1984). In this regard, the ratio of K+/Na+ was examined and supposed as an index to display and decipher algal potency to distinguish between Na+ and K+ under normal or stressful conditions (Flowers 2004; Cuin and Shabala 2005). In our study, the ratio of K+/Na+ in algal cells increased over 600 Gy gamma irradiation compared to the control and fixed at similar levels during 48 h after irradiation. It is evidenced that stress-induced ROS could activate K+ efflux through proprietary potassium channels, resulting in the stimulation of proteases and endonucleases, and promoting programed cell death (Cuin and Shabala 2005; Britto et al. 2010). However, it is believed that in mild-stress conditions/or resistant plants, K+ efflux has a critical character as a ‘metabolic switch’ in stimulating catabolic activities and saving energy for recovery requirements. In the majority of cases, stress-induced K+ efflux, in response to H2O2, lasts at least 30–40 min (Marschner 2012) and, consequently, was followed by Ca2+ influx with no-release of other substances, such as proteins or sugars (Shabala 2009). This could be evidenced in our study due to the fact that compared to the control, the net amount of K+ in irradiated cells was less immediately after gamma irradiation and then became higher at 16 h or 48 h after irradiation.
Initial K+ efflux was shown to trigger the accumulation of soluble carbohydrates and amino acids like proline, a fact that is consistent with our results. We can tentatively hypothesize that cytosolic K+ decline, at the very beginning of gamma exposure, inhibited reactions with high energy demand and stimulated the energy released from catabolic processes. This could be a vital step in plant or algal cell adjustment and recovery from any stress factor. It has previously been suggested that plants under stress, stop processes related to growth and use their energy to conflict with stress-induced damages (Demidchik et al. 2014).
Various computational approaches, for instance, principal component analysis (PCA) and hierarchical clustering have been proposed, as a multivariate analysis (HCA) tool to cluster biological samples or examined parameters (Liu et al. 2010; Löw et al. 2012; Kim et al. 2013). Previously, plant genotypes were clustered by these methods according to their physiological measurements in order to label sensitive or tolerant cultivars to environmental stresses (Chunthaburee et al. 2015; El-Hendawy et al. 2017). We used these statistical methods successfully in order to show the dissimilarities in responses of the photosynthetic eukaryote, C. vulgaris, in different time courses (0, 16, and 48 h) after gamma irradiation. Combined PCA and HCA illustrated that concerning time, two distinct strategic mechanisms were used to resist against gamma irradiation in C. vulgaris. At the beginning of the gamma irradiation, the algae spontaneously changed the parameters including H2O2, antioxidant enzyme activity, FRAP potency, proline, and other related ones through the first responsive strategies named as “quick.” Especially in this context, a higher activity of CAT and more accumulation of proline helped C. vulgaris to resist rapidly to ionization stress, seemingly via triggering other delayed responsive strategies, further scavenging of reactive oxygen species and stabilizing of stress-related proteins and DNA. On the other hand, as time passed, a wide range of “delayed” responsive strategies was triggered in C. vulgaris to encounter the irradiation stress. It was suggested that due to these groups of parameters, more production of soluble carbohydrates, carotenoids, and proteins started several hours after irradiation, which helped the algae preserve the integrity of photosynthetic pigments and recover from ionization stress. Multivariate analysis might be effectively used to screen other effects of abiotic stresses on green microalgae.
Conclusion
The present study demonstrated that C. vulgaris, as a relevant biological model, could be used to evaluate the effects of ionizing irradiations in eukaryotic cells coping with natural or industrial conditions with high acute toxicity of gamma irradiation. The results from univariate and multivariate analyses revealed two distinct strategic mechanisms employed by the microalga while encountering ionizing irradiation: “quick” responsive strategies were recruited immediately at the beginning of irradiation. These strategies are known to be boosting H2O2, increasing antioxidant enzyme activity, and further accumulating of compatible solutes such as proline. On the other hand, “delayed” responsive strategies such as the increase of soluble carbohydrates, carotenoids, and stress-related proteins may be used by the algae with an extended triggering time after radiation. Our study provides a rare insight into how eukaryotic cells could tolerate a high dose of gamma toxicity. Further investigations should also be directed toward a full understanding of responsive mechanisms of gamma tolerance in the algae at various genomics, proteomics and metabolomics levels. Further research and findings may contribute to discovering gene or protein candidates with the possible potential of reducing the toxicity effect of irradiation in eukaryotic cells.
References
Abo-Shady AM, El-Naggar AH, El-Sheekh MM, Abomohra AE-F (2008) Impact of UV-B radiation on antioxidant enzymes and protein electrophoretic pattern of the green alga Chlorococcum sp. Ann Microbiol 58:195–201
Abomohra AE-F, El-Shouny W, Sharaf M, Abo-Eleneen M (2016) Effect of gamma radiation on growth and metabolic activities of Arthrospira platensis. Braz Arch Biol Techn 59:34–50
Aebi H (1984) Catalase in vitro. In: Keating C (ed) Methods in enzymology. Elsevier, Amsterdam, pp 121–126
Aghaie P, Tafreshi SAH, Ebrahimi MA, Haerinasab M (2018) Tolerance evaluation and clustering of fourteen tomato cultivars grown under mild and severe drought conditions. Sci Hortic 232:1–12
Al-Enezi NA, Al-Khayri JM (2012) Alterations of DNA, ions and photosynthetic pigments content in date palm seedlings induced by X-irradiation. Int J Agric Biol 14:329–336
Al-Rashed SA, Ibrahim MM, El-Gaaly GA, Al-Shehri S, Mostafa A (2016) Evaluation of radical scavenging system in two microalgae in response to interactive stresses of UV-B radiation and nitrogen starvation. Saudi J Biol Sci 23:706–712
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Ali H, Ghori Z, Sheikh S, Gul A (2015) Effects of gamma radiation on crop production. In: Hakeem K (ed) Crop production and global environmental issues. Springer, Cham, pp 27-78
Alothman M, Bhat R, Karim A (2009) Effects of radiation processing on phytochemicals and antioxidants in plant produce. Trends Food Sci Tech 20:201–212
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Anaraki ZE, Tafreshi SAH, Shariati M (2018) Transient silencing of heat shock proteins showed remarkable roles for HSP70 during adaptation to stress in plants. Environ Exp Bot 155:142–157
Asato Y (1971) Photorecovery of gamma irradiated cultures of blue-green alga, Anacystis nidulans. Radiat Bot 11:313–316
Ashraf M, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Ashraf M, Harris P (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Aspinall D, Paleg LG (1981) Physiology and biochemistry of drought resistance in plants. Academic Press, Sydney
Badri H, Monsieurs P, Coninx I, Wattiez R, Leys N (2015) Molecular investigation of the radiation resistance of edible cyanobacterium Arthrospira sp. PCC 8005. Microbiologyopen 4:187–207
Bahreininejad B, Razmjou J, Mirza M (2013) Influence of water stress on morpho-physiological and phytochemical traits in Thymus daenensis. Int J Plant Prod 7:155–166
Bajji M, Kinet J-M, Lutts S (2002) The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul 36:61–70
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Benzie IF, Strain J (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In: Parcker L (ed) Methods in enzymology. Academic Press, London, pp 15–27
Borzouei A, Kafi M, Khazaei H, Naseriyan B, Majdabadi A (2010) Effects of gamma radiation on germination and physiological aspects of wheat (Triticum aestivum L.) seedlings. Pak J Bot 42:2281–2290
Borzouei A, Kafi M, Sayahi R, Rabiei E, Amin PS (2013) Biochemical response of two wheat cultivars (Triticum aestivum L.) to gamma radiation. Pak J Bot 45:473–477
Boyer C, Vichot L, Fromm M, Losset Y, Tatin-Froux F, Guétat P, Badot P-M (2009) Tritium in plants: a review of current knowledge. Environ Exp Bot 67:34–51
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Britto DT, Ebrahimi-Ardebili S, Hamam AM, Coskun D, Kronzucker HJ (2010) 42K analysis of sodium-induced potassium efflux in barley: mechanism and relevance to salt tolerance. New Phytol 186:373–384
Chandrashekar K, Somashekarappa H, Souframanien J (2013) Effect of gamma irradiation on germination, growth, and biochemical parameters of Terminalia arjuna Roxb. Radiat Prot Environ 36:38–44
Chaudhary N, Agrawal SB (2014) Role of gamma radiation in changing phytotoxic effect of elevated level of ozone in Trifolium alexandrinum L. (Clover). Atmos Pollut Res 5:104–112
Cheng J, Huang Y, Feng J, Sun J, Zhou J, Cen K (2013) Mutate Chlorella sp. by nuclear irradiation to fix high concentrations of CO2. Bioresour Technol 136:496–501
Cheng J, Lu H, Huang Y, Li K, Huang R, Zhou J, Cen K (2016) Enhancing growth rate and lipid yield of Chlorella with nuclear irradiation under high salt and CO2 stress. Bioresour Technol 203:220–227
Chia MA, Lombardi AT, Melao MDGG (2013) Growth and biochemical composition of Chlorella vulgaris in different growth media. Ann Acad Bras Cienc 85:1427–1438
Choi J-i, Yoon M, Joe M, Park H, Lee SG, Han SJ, Lee PC (2014) Development of microalga Scenedesmus dimorphus mutant with higher lipid content by radiation breeding. Bioprocess Biosyst Eng 37:2437–2444
Choi J-i, Yoon M, Lim S, Kim GH, Park H (2015) Effect of gamma irradiation on physiological and proteomic changes of Arctic Zygnema sp. (Chlorophyta, Zygnematales). Phycologia 54:333–341
Chunthaburee S, Sanitchon J, Pattanagul W, Theerakulpisut P (2015) Effects of salt stress after late booting stage on yield and antioxidant capacity in pigmented rice grains and alleviation of the salt-induced yield reduction by exogenous spermidine. Plant Prod Sci 18:32–42
Cuin TA, Shabala S (2005) Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant Cell Physiol 46:1924–1933
Cuin TA, Shabala S (2007) Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ 30:875–885
Daly MJ (2009) A new perspective on radiation resistance based on Deinococcus radiodurans. Nat Rev Microbiol 7:237–245
Daly MJ (2011) Deinococcus radiodurans: revising the molecular basis for radiation effects on cells. In: Horikoshi K (ed) Extremophiles handbook. Springer, Tokyo, pp 1117–1133
De Groot A, Chapon V, Servant P, Christen R, Fischer-Le Saux M, Sommer S, Heulin T (2005) Deinococcus deserti sp. nov., a gamma-radiation-tolerant bacterium isolated from the Sahara Desert. Int J Syst Evol Micr 55:2441–2446
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmoregulation in plants. Plant J 4:215–223
Demidchik V, Straltsova D, Medvedev SS, Pozhvanov GA, Sokolik A, Yurin V (2014) Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J Exp Bot 65:1259–1270
El-Hendawy SE, Hassan WM, Al-Suhaibani NA, Schmidhalter U (2017) Spectral assessment of drought tolerance indices and grain yield in advanced spring wheat lines grown under full and limited water irrigation. Agric Water Manag 182:1–12
Ermavitalini D, Yuliansari N, Prasetyo EN, Saputro TB (2017) Efect of gamma 60co irradiation on the growth, lipid content and fatty acid composition of Botryococcus sp. Microalgae Biosaintifika 9:58–65
Esnault M-A, Legue F, Chenal C (2010) Ionizing radiation: advances in plant response. Environ Exp Bot 68:231–237
Evseeva T, Geras’Kin S, Majstrenko T, Brown J, Belykh E (2010) Comparative estimation of 232Th and stable Ce (III) toxicity and detoxification pathways in freshwater alga Chlorella vulgaris. Chemosphere 81:1320–1327
Farhi E, Rivasseau C, Gromova M, Compagnon E, Marzloff V, Ollivier J, Boisson A-M, Bligny R, Natali F, Russo D (2008) Spectroscopic investigation of ionizing-radiation tolerance of a Chlorophyceae green micro-alga. J Phys-Condens Mat 20:104–216
Flowers T (2004) Improving crop salt tolerance. J Exp Bot 55:307–319
Fogg GE, Thake B (1987) Algal cultures and phytoplankton ecology. Univ of Wisconsin Press, Madison
Gabani P, Singh OV (2013) Radiation-resistant extremophiles and their potential in biotechnology and therapeutics. Appl Microbiol Biot 97:993–1004
Ganapathy K, Chidambaram K, Janarthanan R, Ramasamy R (2017) Effect of UV-B radiation on growth, photosynthetic activity and metabolic activities of Chlorella vulgaris. J Microbiol Biotechnol 6:53–60
Ghosal D, Omelchenko MV, Gaidamakova EK, Matrosova VY, Vasilenko A, Venkateswaran A, Zhai M, Kostandarithes HM, Brim H, Makarova KS (2005) How radiation kills cells: survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress. FEMS Microbiol Rev 29:361–375
Gomes T, Xie L, Brede D, Lind O-C, Solhaug KA, Salbu B, Tollefsen KE (2017) Sensitivity of the green algae Chlamydomonas reinhardtii to gamma radiation: photosynthetic performance and ROS formation. Aquat Toxicol 183:1–10
Hare P, Cress W (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21:79–102
Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol 51:463–499
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7:1456–1466
Hussain Wani S, Brajendra Singh N, Haribhushan A, Iqbal Mir J (2013) Compatible solute engineering in plants for abiotic stress tolerance-role of glycine betaine. Curr Genet 14:157–165
Ishino Y, Narumi I (2015) DNA repair in hyperthermophilic and hyperradioresistant microorganisms. Curr Opin Microbiol 25:103–112
Ivanova KG, Stankova KG, Nikolov VN, Georgieva RT, Minkova KM, Gigova LG, Rupova IT, Boteva RN (2010) The biliprotein C-phycocyanin modulates the early radiation response: a pilot study. Mutat Res-Gen Tox En 695:40–45
Joiner MC, Marples B, Lambin P, Short SC, Turesson I (2001) Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol 49:379–389
Kachout SS, Hamza KJ, Bouraoui NK, Leclerc JC, Ouerghi Z (2013) Salt-induced changes in antioxidative enzyme activities in shoot tissues of two Atriplex varieties. Not Bot Horti Agrobot Cluj Napoca 41:115–121
Kadkhodaie A, Razmjoo J, Zahedi M (2013) Peroxidase, ascorbate peroxidase and catalase activities in drought sensitive, intermediate and resistance sesame (Sesamum indicum L.) genotypes. Int J Plant Prod 4:3012–3021
Kaur H, Mukherjee S, Baluska F, Bhatla SC (2015) Regulatory roles of serotonin and melatonin in abiotic stress tolerance in plants. Plant Signal Behav 10:e1049788
Kebeish R, Deef H, El-Bialy N (2015) Effect of gamma radiation on growth, oxidative stress, antioxidant system, and alliin producing gene transcripts in Allium sativum. Int J Res Stud Biosci 3:161–174
Kim J-H, Lee MH, Moon YR, Kim J-S, Wi SG, Kim TH, Chung BY (2009) Characterization of metabolic disturbances closely linked to the delayed senescence of Arabidopsis leaves after γ irradiation. Environ Exp Bot 67:363–371
Kim JK, Park S-Y, Lim S-H, Yeo Y, Cho HS, Ha S-H (2013) Comparative metabolic profiling of pigmented rice (Oryza sativa L.) cultivars reveals primary metabolites are correlated with secondary metabolites. J Cereal Sci 57:14–20
Ko S-C, Kim D, Jeon Y-J (2012) Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem Toxicol 50:2294–2302
Kochert G (1978) Carbohydrate determination by the phenol-sulfuric acid method. In: Basic exercises in immunochemistry. Springer, Berlin, pp 171–173
Kojima S, Ishida H, Takahashi M, Yamaoka K (2002) Elevation of glutathione induced by low-dose gamma rays and its involvement in increased natural killer activity. Radiat Res 157:275–280
Kojima S, Takai E, Tsukimoto M (2011) ATP released from low-dose gamma ray-irradiated cells activates intracellular antioxidant systems via purine receptors. Anti-Aging Medicine 8:108–113
Kovacs E, Keresztes A (2002) Effect of gamma and UV-B/C radiation on plant cells. Micron 33:199–210
Kuznetsov VV, Shevyakova N (1999) Proline under stress: biological role, metabolism, and regulation. Russ J Plant Physiol 46:274–287
Lai J, Lim P, Wong C, Phang S, Beardall J (2018) Photosynthetic response and DNA mutation of tropical, temperate and polar Chlorella under short-term UVR stress. Polar Sci 20:35–44
Lamaia C, Kruatrachuea M, Pokethitiyooka P, Upathamb ES, Soonthornsarathoola V (2005) Toxicity and accumulation of lead and cadmium in the filamentous green alga Cladophora fracta (OF Muller ex Vahl) Kutzing: a laboratory study. Sci Asia 31:121–127
Lee MH, Moon YR, Chung BY, Kim J-S, Lee K-S, Cho J-Y, Kim J-H (2009) Practical use of chemical probes for reactive oxygen species produced in biological systems by γ-irradiation. Radiat Phys Chem 78:323–327
Leigh RA, Jones RW (1984) A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol 97:1–13
Lin CS, Wu JT (2014) Tolerance of soil algae and cyanobacteria to drought stress. J Phycol 50:131–139
Liu J, Mao X, Zhou W, Guarnieri MT (2016) Simultaneous production of triacylglycerol and high-value carotenoids by the astaxanthin-producing oleaginous green microalga Chlorella zofingiensis. Bioresour Technol 214:319–327
Liu Z-y, Shi J-j, Zhang L-w, Huang J-f (2010) Discrimination of rice panicles by hyperspectral reflectance data based on principal component analysis and support vector classification. J Zhejiang Univ Sci B 11:71–78
Löw M, Deckmyn G, de Beeck MO, Blumenröther MC, Oßwald W, Alexou M, Jehnes S, Haberer K, Rennenberg H, Herbinger K (2012) Multivariate analysis of physiological parameters reveals a consistent O3 response pattern in leaves of adult European beech (Fagus sylvatica). New Phytol 196:162–172
Maity JP, Chakraborty S, Kar S, Panja S, Jean J-S, Samal AC, Chakraborty A, Santra SC (2009) Effects of gamma irradiation on edible seed protein, amino acids and genomic DNA during sterilization. Food Chem 114:1237–1244
Malanga G, Puntarulo S (1995) Oxidative stress and antioxidant content in Chlorella vulgaris after exposure to ultraviolet-B radiation. Physiol Plant 94:672–679
Marschner H (2012) Marschner’s mineral nutrition of higher plants. Elsevier, Amsterdam
Mattimore V, Battista JR (1996) Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J Bacteriol 178:633–637
Metsalu T, Vilo J (2015) ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res 43:566–570
Mishra A, Mandoli A, Jha B (2008) Physiological characterization and stress-induced metabolic responses of Dunaliella salina isolated from salt pan. J Ind Microbiol Biot 35:1093–1101
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Ngo D-H, Kim S-K (2013) Sulfated polysaccharides as bioactive agents from marine algae. Int J Biol Macromol 62:70–75
Ozkur O, Ozdemir F, Bor M, Turkan I (2009) Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovata Desf. to drought. Environ Exp Bot 66:487–492
Pareek A, Singla S, Grover A (1997) Salt responsive proteins/genes in crop plants. Science Pub:365–391
Parvaiz A, Satyawati S (2008) Salt stress and phyto-biochemical responses of plants-a review. Plant Soil Environ 54:89–99
Pavlopoulou A, Savva GD, Louka M, Bagos PG, Vorgias CE, Michalopoulos I, Georgakilas AG (2016) Unraveling the mechanisms of extreme radioresistance in prokaryotes: lessons from nature. Mutat Res-Rev Mutat 767:92–107
Peng C, Ma Y, Ding Y, He X, Zhang P, Lan T, Wang D, Zhang Z, Zhang Z (2017) Influence of speciation of thorium on toxic effects to green algae Chlorella pyrenoidosa. Int J Mol Sci 18:795–805
Poong SW, Lim PE, Phang SM, Wong CY, Pai TW, Chen CM, Yang CH, Liu CC (2018) Transcriptome sequencing of an Antarctic microalga, Chlorella sp. (Trebouxiophyceae, Chlorophyta) subjected to short-term ultraviolet radiation stress. J Appl Phycol 30:87–99
Poskuta J, Parys E, Romanowska E (1996) Toxicity of lead to photosynthesis, accumulation of chlorophyll, respiration and growth of Chlorella pyrenoidosa. Protective role of dark respiration. Acta Physiol Plant 18:165–171
Posner HB, Sparrow AH (1964) Survival of Chlorella and Chlamydomonas after acute and chronic gamma radiation. Radiation Bot 4:253–257
Prasad S, Ram P, Uma S (2004) Effect of waterlogging duration on chlorophyll content, nitrate reductase activity, soluble sugars and grain yield of maize. Ann Plant Physiol 18:1–5
Priyadarshani I, Rath B (2012) Commercial and industrial applications of micro algae–a review. J Algal Biomass Utln 3:89–100
Rivasseau C, Farhi E, Compagnon E, de Gouvion Saint Cyr D, Van Lis R, Falconet D, Kuntz M, Atteia A, Couté A (2016) Coccomyxa actinabiotis sp. nov.(Trebouxiophyceae, Chlorophyta), a new green microalga living in the spent fuel cooling pool of a nuclear reactor. J Phycol 52:689–703
Rivasseau C, Farhi E, Gromova M, Ollivier J, Bligny R (2010) Resistance to irradiation of micro-algae growing in the storage pools of a nuclear reactor investigated by NMR and neutron spectroscopies. Spectroscopy 24:381–385
Robinson CK, Webb K, Kaur A, Jaruga P, Dizdaroglu M, Baliga NS, Place A, DiRuggiero J (2011) A major role for nonenzymatic antioxidant processes in the radioresistance of Halobacterium salinarum. J Bacteriol 193:1653–1662
Safi C, Zebib B, Merah O, Pontalier P-Y, Vaca-Garcia C (2014) Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sust Energ Rev 35:265–278
Sairam RK, Rao KV, Srivastava G (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163:1037–1046
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25:163–171
Santier S, Gilet R, Malaise E (1985) Induced radiation resistance during low-dose-rate γ irradiation in plateau-phase Chlorella cells. Radiat Res 104:224–233
Serraj R, Sinclair T (2002) Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Environ 25:333–341
Shabala S (2009) Salinity and programmed cell death: unravelling mechanisms for ion specific signalling. J Exp Bot 60:709–712
Shabana EF, Gabr MA, Moussa HR, El-Shaer EA, Ismaiel MM (2017) Biochemical composition and antioxidant activities of Arthrospira (Spirulina) platensis in response to gamma irradiation. Food Chem 214:550–555
Skoog DA, West DM, Holler FJ, Crouch S (1996) Fundamentals of analytical chemistry. J Chem Educ 40:614–625
Slade D, Radman M (2011) Oxidative stress resistance in Deinococcus radiodurans. Microbiol Mol Biol Rev 75:133–191
Şükran D, Güneş T, Sivaci R (1998) Spectrophotometric determination of chlorophyll-A, B and total carotenoid contents of some algae species using different solvents. Turk J Bot 22:13–18
Tale MP, devi Singh R, Kapadnis BP, Ghosh SB (2018) Effect of gamma irradiation on lipid accumulation and expression of regulatory genes involved in lipid biosynthesis in Chlorella sp. J Appl Phycol 30:277–286
Tammam A, Allam M, Osman M (2005) Mutagenesis of Dunaliella salina. Int J Agric Biol 3:477–481
Usov A, Zelinsky N (2013) Chemical structures of algal polysaccharides. In: Domínguez H (ed) Functional ingredients from algae for foods and nutraceuticals. Woodhead publishing, Cambrige, pp 23–86
Wang S-B, Chen F, Sommerfeld M, Hu Q (2004) Proteomic analysis of molecular response to oxidative stress by the green alga Haematococcus pluvialis (Chlorophyceae). Planta 220:17–29
Wellburn A-R, Lichtenthaler H (1984) Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. In: Sybesma C (ed) Advances in photosynthesis research. Martinus Nijhof Publishers, Amsterdam, pp 9–12
Won E-J, Han J, Lee Y, Kumar KS, Shin K-H, Lee S-J, Park HG, Lee J-S (2015) In vivo effects of UV radiation on multiple endpoints and expression profiles of DNA repair and heat shock protein (Hsp) genes in the cycloid copepod Paracyclopina nana. Aquat Toxicol 165:1–8
Wong C-Y, Teoh M-L, Phang S-M, Lim P-E, Beardall J (2015) Interactive effects of temperature and UV radiation on photosynthesis of Chlorella strains from polar, temperate and tropical environments: differential impacts on damage and repair. PLoS One 10:0139469
Xiong B, Zhang W, Chen L, Lin KF, Guo MJ, Wang WL, Cui XH, Bi HS, Wang B (2014) Effects of Pb (II) exposure on Chlorella protothecoides and Chlorella vulgaris growth, malondialdehyde, and photosynthesis-related gene transcription. Environ Toxicol 29:1346–1354
Xue T, Hartikainen H (2000) Association of antioxidative enzymes with the synergistic effect of selenium and UV irradiation in enhancing plant growth. Agr Food Sci 9:177–186
Yoon M, Choi J-i, Kim GH, Kim D-H, Park D-H (2013) Proteomic analysis of Spirogyra varians mutant with high starch content and growth rate induced by gamma irradiation. Bioprocess Biosyst Eng 36:765–774
Zhang X, Tang X, Wang M, Zhang W, Zhou B, Wang Y (2017) ROS and calcium signaling mediated pathways involved in stress responses of the marine microalgae Dunaliella salina to enhanced UV-B radiation. J Photochem Photobiol B 173:360–367
Acknowledgments
The authors would like to express thanks to Shahed University of Tehran for providing the facilities necessary to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toghyani, M.A., Karimi, F., Hosseini Tafreshi, S.A. et al. Two distinct time dependent strategic mechanisms used by Chlorella vulgaris in response to gamma radiation. J Appl Phycol 32, 1677–1695 (2020). https://doi.org/10.1007/s10811-020-02106-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02106-3