Abstract
The changing climate is affecting the melting process of glacier ice and snow in Himalaya and may influence the hydro-geochemistry of the glacial meltwater. This paper represents the ionic composition of discharge from Bilare Banga glacier by carrying out hydro-geochemical analysis of water samples of melting season of 2017. The pH and EC were measured on-site in field, and others parameters were examined in the laboratory. The abundance of the ions observed in meltwater has been arranged in decreasing order for cations as Ca2+ > Mg2+ > Na+ > K+ and for anions as HCO3− > SO42− > Cl− > NO3−, respectively. Analysis suggests that the meltwater is mostly dominated by Ca2+ and HCO3−. It has been observed that the ionic concentration HCO3− is dominant and Cl− is the least in the catchment. Piper plot analysis suggests that the chemical composition of the glacier discharge not only has natural origin but also has some anthropogenic input. Hydro-geochemical heterogeneity reflected the carbonate-dominated features (Ca2+–HCO3−) in the catchment. The carbonate weathering was found as the regulatory factor to control the chemistry of the glacial meltwater due to the high enrichment ratio of (Ca2+ + Mg2+) against TZ+ and (Na+ + K+). In statistical approach, PCA analysis suggests that geogenic weathering dynamics in the catchment is associated with carbonate-dominant lithology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Himalayan mountain region is one of the youngest chain of mountains having the world’s highest range and huge freshwater reserves as snow and glaciers that helps in sustaining the downstream population and ecosystem (Singh et al. 2016a, b; Shekhar et al. 2017). It holds hydrological importance because of the potential reserve of freshwater as glacier, lake and river (Schild 2008). Glaciers are considered as indispensable climatic indicator as well as natural resources (Maurer et al. 2016). The component of the glaciers, such as snow and ice melting, considerably contributes to river flow at downstream, which is very crucial for the drinking, irrigation and hydropower generation (Lutz et al. 2014). In general, extreme water discharge in summer season in this region is a result of enhanced melting of snow and glaciers at higher altitude (Singh et al. 2008). It has been reported that the freshwater bodies such as glacial meltwater and glacial lake are getting contaminated under the natural as well as anthropogenic activities (Kanakiya et al. 2014; Kotadiya and Acharya 2014), generating serious problem at the global scale (Iscen et al. 2008). The responsibility of the contamination has been attributed to changing climate and global warming (Szopińska et al. 2018), but there has been no established relationship due to scarcity of database. This has led to a serious concern at regional as well as global level about fate of the glaciers and future freshwater availability. The glacial locations are ideal sections for understanding the process of water–rock interactions and the natural influence on the hydro-geochemistry as little interventions of human activities are noticed. The varying discharge of glacial meltwater influences the ionic concentration and dissolved load in its river system that influences the hydro-geochemistry and water quality. The glacier dynamics and higher meltwater discharge influence the rate of erosion and hence the process of physical and chemical weathering making it higher than the continental average. Therefore, it is very important for the prospective of changing water quality and needs to conduct research for recognizing the responsible factors for deteriorating water quality (Saleem et al. 2015; Vetrimurugan et al. 2013; Zhu et al. 2013). Hydro-geochemistry of Himalayan glacial meltwater is helpful in understanding the weathering dynamics of glacial environment (Chauhan and Hasnain 1993; Singh and Hasnain 1998a, b; Sharma et al. 2013; Singh et al. 2015; Kumar et al. 2018a, b).The knowledge of the hydro-geochemistry is vital due to the dependency of a large populations on glacial meltwater. The correct assessment of chemical characteristics of ions activity and other physical–chemical parameters is necessary for understanding the weathering reactions, anthropogenic impact on the water resources within the catchment. The characteristics of the glacial meltwater depend on the flow of water under subglacial conduits and its interaction with the rock surface (Haritashya et al. 2010; Kumar et al. 2009). It also depends on the velocity of water and interaction time with bedrocks (Sharma et al. 2013; Singh et al. 2014; Singh and Ramanathan 2015). Numerous glaciological studies have been carried out like glacier surge (Philip and Sah 2004), dynamics of glaciers (Sam et al. 2015), mapping of glacier facies as well as discharge and mass balance reconstruction (Singh et al. 2018), dissolved micronutrient nanoparticles (Kumar et al. 2018a) and sediment dynamics with respect to discharge (Kumar et al. 2018b) in the Shaune Garang basin, but there is not any documented research regarding the geochemical evidence in this basin. The paper attempted to highlight the chemical characteristics of the meltwater draining from Bilare Banga glacier of Baspa basin through the qualitative and quantitative methods. It is affected by the characteristics of the parent rocks as well as the weathering process. This is helpful in addressing the migration and transport mechanism of solute particles to the downstream locations. Therefore, the present study focused on the geochemical characterization of Bilare Banga (Shaune Garang basin) glacial meltwater is able to fill the research gap.
Study area
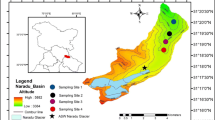
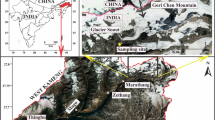
Geologically, this part of Baspa Valley comes in the group of Tethys Himalaya. A comprehensive study on the geological as well as geographical development of entire basin recommends the control of Raksham group of granite, granodiorite and pegmatite rocks (Dutta et al. 2017). Bilare Banga is the second largest glacier of Shaune Garang catchment (Philip and Sah 2004). Glacier is located in the Shaune Garang catchment joining the Baspa River in the downstream. The glacier discharge of the catchment meets to the main Shaune Garang River at altitude of 3975 m above the sea level. Location of study region is shown through the map (Fig. 1), while the geomorphic facies of Bilare Banga glacier are presented in Table 1. The altitude of glacier varies from 4250 m to 5160 m asl. The total area of catchment is 8.54 km2 having the glacier area of about 3.51 km2. The study area falls under the influence of Western disturbance as well as Indian summer monsoon (Kumar et al. 2016, 2018a, b), and the intensity of precipitation events is more in summer compared to winter (Wulf et al. 2010). The chemical characterization of meltwater has not been carried out in this region; hence, this attempt in view of the geological condition of the area becomes important to know the influence in the downstream catchment.
Materials and method
Water sampling protocol and analysis
Glacial meltwater sampling was carried out at different locations of downstream catchment of Bilare Banga stream in the melting season of 2017. Sampling bottles were cleaned by distilled water followed by glacial water, before collection of water at 12 different locations of varying altitudes. Glacial meltwater was filtered on known weight of Millipore membrane (Whatman 0.45 µm) to separate suspended sediment. The meltwater is collected from the five locations in polyethylene bottle (1/4 l) about 10–15 cm deeper to the water surface directly. The in situ measurement of pH, EC and temperature has been performed through handheld multi-parameter (HI-98129, HANNA) instrument. The atomic absorption spectroscopy, (AAS) with accuracy of 0.05 ppm for Ca, Mg, K and Na while 0.01 ppm for silicon, has been used to analyze the cations (Ca2+, Mg2+, K+ and Na+). Major anions (Cl−, NO3− and SO42−) were analyzed through ion chromatography (PERKIN ELMEWR), Dionex ICS 900, USA (accuracy up to 0.1 ppm). The concentration of HCO3 was analyzed through titration method as defined by the APHA (1998). The utmost precaution has been taken during water sampling, and it was preserved at 4 °C in the laboratory before subjected to analysis. A set of samples was used for evaluation of every calibration curve. Multivariate statistical analysis was applied to assess and understand the variations within the dataset. Correlation matrix analysis has been performed to examine the interrelationship among the measured variables within the dataset. Principal component analysis (PCA) is used for knowing sources of dissolved ionic concentration in glacial meltwater. Bartlett’s sphericity methods were tested to know the strength and efficiency of PCA which is helpful in reducing the dimensionality in data (Kumar et al. 2014; Pant et al. 2018).
Results and discussion
Hydro-geochemistry
The accuracy of the charge balance error between total cations (TZ+) and total anions (TZ−) was calculated as 5.86%, which indicates the precision and quality of the data and considered acceptable. The CBE error was calculated through following equation:
where TZ+ is total cation and TZ− is total anion.
Water draining from the Bilare Banga glacier has been found to be alkaline and may be linked to weathering of rocks (Meybeck 1987; Sharma et al. 2013), and pH varies from 6.98 to 8.23 with an average value of 7.45 ± 0.44. Electrical conductivity (μS/cm) has shown the range of 54.0–91.0 with an average value of 73.0 ± 12.9 μS/cm. Highly varying electrical conductivity (EC) indicates that the catchment’s geochemistry is controlled by meteorological parameters, interaction of water with rock that controls the hydro-chemical facies (Kumar et al. 2014, 2018b). The EC also represents the concentrations of total dissolved ions in meltwater (Shichang et al. 2000; Singh et al. 2015). Total ionic budget of meltwater shows major cation contribution of Ca2+ and Mg2+ constituting 41.07% and 28.29%, respectively, in the total cationic budget. The average Ca2+ and Mg2+ concentrations in the meltwater of Bilare Banga glacier was observed to be 288.29 ± 48.95 and 198.56 ± 32.41 μeq/l, respectively. Similarly, anionic budget of meltwater indicates that bicarbonate (HCO3−) is the major anion contributor (47.81%) of the total TZ− trailed by SO42− (42.05%), Cl− (9.26%) and NO3− (0.91%). The concentration of bicarbonate (HCO3−) in meltwater ranged from 214 to 350 μeq/l having mean of 298 ± 45.0 μeq/l followed by sulfate (SO42−) concentration varied from 200 to 356 μeq/l having mean of 262.4 ± 57.5 μeq/l. The concentrations of dissolved silica fluctuated from 14.6 to 25.8 µmol/l with mean value of 20.5 ± 4.18 µmol/l. Study shows that Ca2+ and HCO3− are dominant in the glacial meltwater of Bilare Banga. The cationic abundance in the meltwater follows decreasing order like Ca2+ > Mg2+ > Na+ > K+, and the concentration of anion follows decreasing order from HCO3− > SO42− > Cl− > NO3−. Excessive concentration of bicarbonate in meltwater is an indicative of higher weathering of silicate-dominant lithology of the area, whereas the higher Cl− ion may be endorsed to the contribution of rainwater (Singh et al. 2014). The detailed distribution of the dissolved ionic concentration in meltwater of Bilare Banga glacier is presented through Table 2.
Hydro-chemical process in glacial catchment
The evolution of the chemical species in the glacial melt has been accredited to weathering process of parental rock present in the glaciated region (Singh et al. 2012, 2014). The weathering of the chemical species in the rocks such as calcite, dolomite and dissolution or evaporation process of halite gypsum and anhydrite are the main factors in controlling the hydro-geochemistry of the glacial meltwater (Pant et al. 2018). Richness of cations of calcium, magnesium and carbonates in glacial meltwater may be credited to the presence of carbonate minerals and its weathering. However, the richness of Ca2+, Mg2+, Na+ and K+ is also due to the weathering of silicate. Low content of chlorine and sodium ions and high content of bicarbonates and calcium ions in meltwater reflect the stimulus weathering of the rocks having the richness of minerals like carbonates, silicates and sulfide (Tranter et al. 1993) as well as atmospheric CO2. Chloride (Cl−) and sulfate (SO42−) are primarily generated with the halite and sulfide oxidation in the river catchment. Sulfate minerals such as gypsum are also a main factor for controlling the concentration of Cl− and SO42− in the glacial meltwater (Mortatti and Probst 2003; Thomas et al. 2015). To detect the derivations of chemical species in water deliberately by weathering phenomena, ionic ratios of water are computed and shown through Table 3. Average ratio of HCO3−/Na2+ is 1.87 ± 0.35, which indicates that glacial meltwater is influenced by carbonate weathering. In addition, the proportion of (Ca2+ + Mg2+)/TZ+ is 0.69 ± 0.03 and (Na+ + K+)/TZ+ is 0.31 ± 0.03, which indicates the presence of silicate weathering process is lesser than the carbonate weathering (Pant et al. 2018). (Ca2+ + Mg2+) versus (Na+ + K+) ratio observed to be 2.29 ± 0.29 suggests meltwater is influenced by the minerals of calcite and dolomite. The C-ratio [(HCO3−/(HCO3− + SO42−)] explains the importance of proton-producing reactions such as carbonation and sulfide oxidation being an essential component for chemical weathering of carbonate rocks (Huang et al. 2008). C-ratio < 0.50 represents the occurrence of major reaction in the glacial meltwater such as carbonate dissolution (carbonation) as well as sulfide oxidation reaction, although values of the whole C-ratio closer to 1 indicate dominance of carbonic reaction in the region as well as atmospheric input of CO2-derived protons. We observed the C-ratio of meltwater from Bilare Banga as 0.53. The C-ratio of glaciers like Sutri Dhaka, Chhota Shigri, Dudu and Gangotri is represented through Table 4 which reflects the dominance of carbonate weathering in Chhota Shigri, Sutri Dhaka as well as Bilare Banga glacier, while Gangotri and Dudu glaciers are majorly influenced by sulfide weathering. About 85% contribution of bicarbonate in the glacial meltwater is due to higher weathering of carbonate while other 15% is contributed by the weathering of silicate (Raymahasay 1986). The similar result has been reported by Singh and Hasnain (1998a, b) for the Alaknanda basin where the dominance of carbonate weathering is 78% while silicate weathering is of 22%. The Rajkhot watershed of Higher Himalaya also showed the similar trend (Blum et al. 1998). In the same manner, the domination of carbonate weathering designates the partial role of assimilation of atmospheric CO2 in the Bilare Banga glacier meltwater. However, the strontium isotopes study would be helpful in confirming the role of atmospheric CO2 assimilation (Kumar et al. 2009) in the Himalayan region.
Statistical analysis
Approach of statistical analysis, such as principal component analysis, correlation as well as factor analysis, was executed to understand the interrelationship among the variables. The Bartlett’s sphericity test having χ2 (observed) = 125.76, which is substantially greater than the critical value (χ2 = 38.95 at ‘degree of freedom’ 55, p values < 0.0001, significance level 0.05), shows the importance of PCA in understanding substantial decrease in dimensionality of the data (Singh et al. 2011; Kumar et al. 2018b). The PCA helps in standardization of the data collected on different scale of measurements through the diagonalization of correlation matrix. The variables in this statistical test automatically give the auto scale to mean = 0 and variance = 1. The scree plot between eigenvalues and factors (Fig. 2) shows that the principle component (PC) values > 1 explain 83.9% of the total variance among four PCs. PC1, PC2, PC3 and PC4 are able to explain 34.34%, 19.49%, 15.90% and 14.23%, respectively, of the total variance. Statistical tools such as correlation matrix are used to find out the interrelationship between two hydro-geochemical species for investigating the grade of dependence of variables among each other (Ramanathan 2007; Vasanthavigar et al. 2013; Pant et al. 2018). Table 5 represents the correlation analysis during the study period. Some of the parameters like EC and Na+ are strongly correlated with SO42−, and similarly, a good correlation is also observed for Ca2+–HCO3−, Mg2+–H4SiO4 and Cl−–NO3−. All these water parameters show the strong and positive correlation (r > 0.6), indicating that the hydro-geochemistry of Bilare Banga glacier is primarily influenced by them. Factor analysis of the glacial meltwater shows that factor-1 with eigenvalue 3.77 describes 34.3% of the total variance considered as main factor (Table 6). On the basis of results, total variance is very high for Ca2+, SO42− and HCO3− but has moderate loadings of electrical conductivity. This result determines that the EC in the meltwater is largely influenced through the presence of sodium and chloride; however, bicarbonate also plays significant role in determining conductivity. Factor 2 describes 19.4% of total variance and demonstrates that the high loading of sodium, nitrate and chloride in the meltwater is due to ion exchange at water interface and dissolution of sodium-bearing minerals and silicate-wearing minerals. Similarly, factors 3 and 4 explain 15.9% and 14.2% of total variance, respectively, during the study period. Factor analysis further suggests a high loading of magnesium that influences the temporary hardness of the water. Thus, all these four PCs are collectively responsible toward 83.96% of total variance. Apart from this, communality of the variables and their proportion define that extracted common factor is larger than almost 0.8. Hence, factor analysis represents all variances of the dataset. The particular control of variables in each factor specifies the partial mixing of different water types. Hierarchical cluster analysis shows that glacial meltwater is divided into two principal cluster components which are presented through the dendrogram (Fig. 3). Maximum water samples lie in cluster 1, which has Ca–HCO3− and mixed Ca–Mg–SO42− water-type facies. On the other hand, cluster 2 defines the less dominant water types in the catchment.
Hydro-geochemical facies
To perform the quantification of water type, Piper’s trilinear diagram is broadly used to understand the geochemical evolution in water system (Piper 1944, 1953). The classification of water types in the piper plot demonstrates the basis of ratio of the geochemical characteristics in water (Xiao et al. 2012; Singh et al. 2016a, b). Characterization of glacial meltwater of the study area has been carried out by plotting average data of ablation season of 2017 on Piper’s trilinear diagram (Fig. 4). The outcomes of the study on the basis of piper plot encompass two triangles at base, which designate anions (SO42− and CO32− + HCO3−) and cations (Ca2+, Mg2+ and Na+ + K+), while a diamond shape specifies dominance of anions and cations. Hydro-geochemical results helped in concluding the water type which is mainly influenced by Ca–HCO3− and mixed influence of Ca–Mg–SO42−, demonstrating the dominancy of carbonate weathering in the catchment. Ca–HCO3− water type is the predominant phase of water in any natural system. Mixed Ca–Mg–SO42− facies of water is generally formed with the interaction of pyrite and gypsum minerals present in the catchment. The gradual changes in the chemical features may be attributed to the longer interaction between minerals and flowing water. On the other hand, cation plot shows that maximum number of the water samples falls in the middle part of the trilinear plot. Some of samples fall in the bottom left corner, representing the governance of Ca+ ion in the meltwater (Singh and Ramanathan 2017). Some of the water facies show increased concentration of Na+ and K+ ions which is an indicative of stimulus effect of local sources of pollutants that would have accumulated on the glaciers surface after being trapped in the surface ice through deposition from the atmosphere (Karim and Veizer 2000; Ravikumar et al. 2013; Pant et al. 2018). The anion diagram helps in understanding that the maximum water samples fall under the lower left corner to HCO3− apex, indicating the dominancy of carbonate. The results show a complete characteristic of hydro-geochemistry, governance of alkali earth elements (69.36%) like calcium and magnesium over the alkaline (30.62%) sodium and potassium concentrations. In anion contribution of weak acid, HCO3− (~ 47.81%) is dominating over the strong acid SO42− (~ 42.05%) in the catchment.
Conclusion
The present study provides fundamental information to chemical characterization of meltwater of Bilare Banga glacier. Chemical characteristics of meltwater show that pH of water moves considerably from acidic to neutral with the presence of Ca2+ and HCO3− as dominant ions. High ratio of (Ca2+ + Mg2+) versus TZ+ and (Na+ + K+) as well as strong correlation between Ca2+–HCO3, Ca2+–Mg2+ and Mg2+–HCO3− indicates that carbonate weathering is the controlling factor of dissolved ions in glacial meltwater. The changing concentration of the ions in the river water is inversely associated with the runoff. It is further observed that the ratio of “Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−)” in the catchment is less than 0.5 which is an indicative of the control of weathering on the ionic compositions in the glacial meltwater. Piper diagram shows that Ca2+–HCO3− is most prevailing water type in this region. The C-ratio demonstrates the process of dissolution and dissociation of CO2 present in the local atmosphere which also controls the production of H+ (proton). Hierarchical cluster analysis shows that the glacial meltwater is divided into two principal cluster components and most of the water samples fall in the cluster 1, which has Ca–HCO3− and mixed Ca–Mg–SO42− water-type facies. Based on the statistical analysis, it is clear that the hydro-geochemistry in this region is mainly governed through the weathering of carbonate and silicate-dominant lithology. In addition, suspension of sulfate-bearing minerals, pyrite oxidation and precipitation are also contributing for the same.
References
American Public Health Association (APHA) (1998) Standard methods for the examination of water and waste water, 20th edn. APHA, AWWA, WPCF, Washington
Blum JD, Gazis CA, Jacobson AD, Chamberlain CP (1998) Carbonate versus silicate weathering in the Raikhot watershed within the High Himalayan Crystalline series. Geology 26(5):411–414
Chauhan DS, Hasnain SI (1993) Chemical characteristics, solute and suspended sediments load in meltwater draining Satopanth and Bhagirathi Kharak glaciers, Ganga basin, India. In: Young GJ (ed) Snow and glacier hydrology, vol 218. IAHS Press, Wallingford, pp 2–10
Dutta S, Mujtaba SAI, Saini HS, Chunchekar R, Kumar P (2017) Geomorphic evolution of glacier-fed Baspa Valley, NW Himalaya: record of Late Quaternary climate change, monsoon dynamics and glacial fluctuations. In: Pant NC, Ravindra R, Srivastava D, Thompson LG (eds) The Himalayan Cryosphere: past and present, vol 462. Geological Society, London. https://doi.org/10.1144/SP462.5
Haritashya UK, Kumar A, Singh P (2010) Particle size characteristics of suspended sediment transported in meltwater from the Gangotri Glacier, central Himalaya—an indicator of subglacial sediment evacuation. Geomorphology 122(1–2):140–152. https://doi.org/10.1016/j.geomorph.2010.06.006
Huang X, Sillanpää M, Duo B, Gjessing ET (2008) Water quality in the Tibetan Plateau: metal contents of four selected rivers. Environ Pollut 156(2):270–277
Iscen CF, Emiroglu O, Ilhan S, Arslan N, Yilmaz V, Ahiska S (2008) Application of multivariate statistical techniques in the assessment of surface water quality in Uluabat Lake, Turkey. Environ Monit Assess 144(1–3):269–276
Kanakiya RS, Singh SK, Sharma JN (2014) Determining the water quality index of an urban water body Dal Lake, Kashmir, India. IOSR J Environ Sci Toxicol Food Technol 08(12):64–71. https://doi.org/10.9790/2402-081236471
Karim A, Veizer J (2000) Weathering processes in the Indus River basin: implications from riverine carbon, sulfur, oxygen, and strontium isotopes. Chem Geol 170:153–177
Kotadiya NG, Acharya CA (2014) An assessment of lake water quality index of Manipu Lake of district Ahmedabad, Gujarat. Int J Sci Res 03(4):448–450
Kumar K, Miral MS, Joshi S, Pant N, Joshi V, Joshi LM (2009) Solute dynamics of melt water of Gangotri glacier, Garhwal Himalaya, India. Environ Geol 58:1151–1159. https://doi.org/10.1007/s00254-008-1592-6
Kumar A, Verma A, Dobhal DP, Mehta M, Kesarwani K (2014) Climatic control on extreme sediment transfer from Dokriani Glacier during monsoon, Garhwal Himalaya (India). J Earth Syst Sci 123:109–120
Kumar R, Singh S, Kumar R, Singh A, Bhardwaj A, Sam L, Randhawa SS, Gupta A (2016) Development of a glaciohydrological model for discharge and mass balance reconstruction. J Water Resour Manag. https://doi.org/10.1007/s11269-016-1364-0
Kumar R, Kumar R, Singh A, Sinha RK, Kumari A (2018a) Nanoparticles in glacial melt water. Mater Today Proc 5(3P1):9161–9166. https://doi.org/10.1016/j.matpr.2017.10.037
Kumar R, Kumar R, Singh S, Singh A, Bhardwaj A, Kumari A, Randhawa SS, Saha A (2018b) Dynamics of suspended sediment load with respect to summer discharge and temperatures in Shaune Garang glacierized catchment. Acta Geophys, Western Himalaya. https://doi.org/10.1007/s11600-018-0184-4
Lutz AF, Immerzeel WW, Shrestha AB, Bierkens MFP (2014) Consistent increase in High Asia’s runoff due to increasing glacier melt and precipitation. Nat Clim Change 4:587–592. https://doi.org/10.1038/nclimate2237
Maurer JM, Rupper SB, Schaefer JM (2016) Quantifying ice loss in the eastern Himalayas since 1974 using declassified spy satellite imagery. Cryosphere 10:2203–2215. https://doi.org/10.5194/tc-10-2203-2016
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Mortatti J, Probst JL (2003) Silicate rock weathering and atmospheric/soil CO2 uptake in the Amazon basin estimated from river water geochemistry: seasonal and spatial variations. Chem Geol 197:177–196
Pant RR, Zhang F, Rehman FU, Wang G, Ming Y, Zeng C, Tang H (2018) Spatiotemporal variations of hydro-geochemistry and its controlling factors in the Gandaki River Basin, Central Himalaya Nepal. Sci Total Environ 622–623(2018):770–782. https://doi.org/10.1016/j.scitotenv.2017.12.063
Philip G, Sah MP (2004) Mapping repeated surges and retread of glaciers using IRS-1C/1D data: a case study of Shaune Garang glacier, northwestern Himalaya. Int J Appl Earth Obs Geoinf 6(2):127–141. https://doi.org/10.1016/j.jag.2004.09.002
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Trans Am Geophy Union 25:914–928. https://doi.org/10.1029/TR025i006p00914
Piper AM (1953) A graphic procedure in the geochemical interpretation of water analysis. Ground water note 12. U.S. Dept. of the Interior, Geological Survey, Water Resources Division, Ground Water Branch, Washington, p 63
Ramanathan AL (2007) Seasonal variation in the major ion chemistry of Pandoh Lake, Mandi district, Himachal Pradesh, India. Appl Geochem 22:1736–1747
Ravikumar P, Somashekar RK, Mehmood MA (2013) Water quality index to determine the surface water quality of Sankey tank and Mallathahalli Lake, Bangalore urban district, Karnataka, India. Appl Water Sci 3:247–261
Raymahasay BC (1986) Geochemistry of bicarbonate in the river water. J Geol Soc India 27:114–118
Saleem M, Jeelani G, Shah RF (2015) Hydro geochemistry of Dal Lake and the potential for present, future management by using facies, ionic ratios, and statistical analysis. Environ Earth Sci 74(4):3301–3313. https://doi.org/10.1007/s12665-015-4361-3
Sam L, Bhardwaj A, Singh S, Kumar R (2015) Remote sensing in glacier velocity estimation and a novel approach for debris covered glaciers. Prog Phys Geogr. https://doi.org/10.1177/0309133315593894
Schild A (2008) The case of the Hindu Kush–Himalayas: ICIMOD’s position on climate change and mountain systems. Mt Res Dev. https://doi.org/10.1659/mrd.mp009
Sharma P, Ramanathan AL, Pottakkal JG (2013) Study of solute sources and evolution of hydro-geochemical processes of the Chhota Shigri Glacier meltwaters, Himachal Himalaya, India. Hydrol Sci J 58(5):1128–1143. https://doi.org/10.1080/02626667.2013.802092
Shekhar M, Bhardwaj A, Singh S, Ranhotra PS, Bhattacharyya A, Pal AK, Roy I, Torres JM, Zorzano MP (2017) Himalayan glaciers experienced significant mass loss during later phases of little ice age. Sci Rep 7:10305. https://doi.org/10.1038/s41598-017-09212-2
Shichang K, Dahe Q, Tandong Y (2000) A study on precipitation chemistry in the late summer in the northern slope of Mt. Xiaxabangma. Acta Sci Circumst 20(5):574–578
Singh AK, Hasnain SI (1998a) Major ion chemistry and weathering control in a high altitude basin: Alaknanda River, Garhwal Himalaya, India. Hydrol Sci 43(6):825–843
Singh AK, Hasnain SI (1998b) Major ion chemistry and weathering control in a high altitude basin: Alaknanda River, Garhwal Himalaya, India. Hydrol Sci J 43(6):825–843. https://doi.org/10.1080/02626669809492181
Singh VB, Ramanathan AL (2015) Assessment of solute and suspended sediment acquisition processes in the Bara Shigri glacier meltwater (Western Himalaya, India). Environ Earth Sci 74:2009–2018
Singh AK, Mondal GC, Kumar S, Singh TB, Tewari BK, Sinha A (2008) Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environ Geol 54(4):745–758. https://doi.org/10.1007/s00254-007-0860-1
Singh CK, Shashtri S, Mukherjee S (2011) Integrating multivariate statistical analysis with GIS for geochemical assessment of groundwater quality in Shiwaliks of Punjab, India. Environ Earth Sci 62:1387–1405
Singh VB, Ramanathan AL, Jose PG, Sharma P, Linda A, Azam MF, Chatterjee C (2012) Chemical characterisation of meltwater draining from Gangotri Glacier, Garhwal Himalaya, India. J Earth Syst Sci 121(3):625–636
Singh VB, Ramanathan AL, Jose PG, Kumar M (2014) Seasonal variation of the solute and suspended sediment load in Gangotri glacier meltwater, central Himalaya, India. J Asian Earth Sci 79:224–234
Singh VB, Ramanathan AL, Sharma P, Pottakkal JG (2015) Dissolved ion chemistry and suspended sediment characteristics of melt water draining from Chhota Shigri glacier, Western Himalaya, India. Arab J Geosci 8:281–293
Singh S, Kumar R, Bhardwaj A, Sam L, Shekhar M, Singh A, Kumar R, Gupta A (2016a) Changing climate and glacio-hydrology in Indian Himalayan Region: a review. Wiley Interdiscip Rev Clim Change 7(3):393–410. https://doi.org/10.1002/wcc.39
Singh VB, Ramanathan AL, Mandal A (2016b) Hydrogeochemistry of high-altitude lake: a case study of the Chandra Tal, Western Himalaya, India. Arab J Geosci 9(4):308. https://doi.org/10.1007/s12517-016-2358-1
Singh VB, Ramanathan AL (2017) Hydrogeochemistry of the Chhota Shigri glacier meltwater, Chandra basin, Himachal Pradesh, India: solute acquisition processes, dissolved load and chemical weathering rates. Environ Earth Sci 76(5):223
Singh S, Kumar R, Bhardwaj A, Kumar R, Singh A (2018) Changing climate and glacio-hydrology: a case study of Shaune Garang basin, Himachal Pradesh. Int J Hydrol Sci Technol. https://doi.org/10.1504/IJHST.2018.10010353
Szopińska M, Szumińska D, Bialik RJ, Chmiel S, Plenzler J, Polkowska Z (2018) Impact of a newly-formed periglacial environment and other factors on fresh water chemistry at the western shore of Admiralty Bay in the summer of 2016 (King George Island, Maritime Antarctica). Sci Total Environ 613–614(2018):619–634. https://doi.org/10.1016/j.scitotenv.2017.09.060
Thomas J, Joseph S, Thrivikramji KP (2015) Hydro-chemical variations of a tropical mountain river system in a rain shadow region of the southern Western Ghats, Kerala, India. Appl Geochem 63:456–471
Tranter M, Brown GH, Raiswell R, Sharp MJ, Gurnell AM (1993) A conceptual model of solute acquisition by Alpine glacier meltwaters. J Glaciol 39(133):573–581
Vasanthavigar M, Srinivasamoorthy K, Prasanna MV (2013) Identification of groundwater contamination zones and its sources by using multivariate statistical approach in Thirumanimuthar sub basin, Tamil Nadu, India. Environ Earth Sci 68:1783–1795
Vetrimurugan E, Elango L, Rajmohan N (2013) Sources of contaminants and groundwater quality in the coastal part of a river delta. Int J Environ Sci Technol 10(3):473–486. https://doi.org/10.1007/s13762-012-0138-3
Wulf H, Bookhagen B, Scherler D (2010) Seasonal precipitation gradients and their impact on fluvial sediment flux in the Northwest Himalaya. Geomorphology 118:13–21
Xiao J, Jin ZD, Zhang F, Wang J (2012) Solute geochemistry and its sources of the ground waters in the Qinghai Lake catchment, NW China. J Asian Earth Sci 52:21–30. https://doi.org/10.1016/j.jseaes.2012.02.006
Zhu B, Yu J, Qin X, Rioual P, Jiang F, Liu Z, MuY LH, Ren X, Xiong H (2013) Identification of rock weathering and environmental control in arid catchments (northern Xinjiang) of Central Asia. J Asian Earth Sci 66:277–294. https://doi.org/10.1016/j.jseaes.2013.02.005
Acknowledgement
The support of the Ministry of Earth Sciences (MoES/PAMC/H&C/61/2015-PCII, dated March 03, 2016) through the project on Shaune Garang glacier sanctioned to Dr. Rajesh Kumar (the PI of the project) is thankfully acknowledged which has been helpful in building the glaciological human resource whose support is vital in the sampling of the meltwater as well as in the analysis. The support of USAID through a project “Contribution to High Asia Runoff from Ice and Snow” (CHARIS) under the collaboration of University of Colorado, Boulder, USA, is also acknowledged. The USAID support through CHARIS project helped not only in the research work but also in producing three PhD theses of students working under me (Dr. Rajesh Kumar). The critical review of the paper and suggestions provided by the anonymous reviewers and editor has been vital in improving the quality of the paper, and authors are grateful for the suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R., Kumar, R., Singh, A. et al. Hydro-geochemical analysis of meltwater draining from Bilare Banga glacier, Western Himalaya. Acta Geophys. 67, 651–660 (2019). https://doi.org/10.1007/s11600-019-00262-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11600-019-00262-w