Abstract
Studies on dissolved ion chemistry and suspended sediment concentration of meltwater draining from Chhota Shigri Glacier were carried out in the year 2008 and 2009. The cationic and anionic concentrations follow the trend: Ca2+ > Mg2+ > Na+ > K+ and HCO3 − > SO4 2− > Cl− > NO3 − > PO4 3−, respectively. The higher ratios of (Ca + Mg)/TZ+ and (Ca + Mg)/(Na + K); strong correlations between Ca-Mg, Ca-HCO3 and Mg-HCO3; and low ratio of (Na + K)/TZ+ for both the years indicate that chemical characteristics of Chhota Shigri Glacier meltwater are mostly controlled by carbonate weathering followed by silicate weathering. The C ratio shows that dissolution and dissociation of atmospheric CO2 is the major proton producer for glacier meltwater. Statistical analysis was used to identify different factors controlling dissolved ions chemistry of study area. There have been marked seasonal and diurnal variations in the total dissolved solid (TDS) and suspended sediment concentration of glacier meltwater observed during the sampling periods. TDS of meltwater is high during October (low discharge) and low during August (high discharge) in both years, implying that TDS is inversely correlated with discharge. On the other hand, suspended sediment concentration is higher during August (high flow regime) and low during October (low flow regime) during the sampling periods, generally following the discharge pattern of the glacier.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Himalaya, located along the south of the Tibetan Plateau, runs about 200–300 km from south to north and 2,400 km from west to east with an average elevation of about 6,000 m (Liu et al. 2010). There are more than 12,000 glaciers found in the Himalaya (Kaul 1999; ICIMOD 2001) covering an area of about 33,000 km2 (Rai and Gurung 2005). Many rivers of Asia such as Ganges, Indus, Brahmaputra, Huang He or Yellow River, Yangtze, Mekong and Salween are fed by the glaciers of Himalaya. Hence, Himalayan glaciers are considered among the most important glaciers in the world because they contribute significantly to the continental solute budget (Kumar et al. 2009). Discharge of water from Himalayan glaciers contributes significantly to the overall runoff of the Himalayan rivers (Immerzeel et al. 2010) and it is important for drinking water supply, irrigation and hydroelectric power generation (Singh et al. 2006).
Dissolved ions chemistry of natural water provides important information about the geology of the area, material loads into the oceans, types of rock weathering and atmospheric precipitation (Xu et al. 2010). The chemical constituents of meltwater draining from glaciers are indicative of chemical weathering taking place below the glaciers (Lorrain and Souchez 1972; Reynolds and Johnson 1972; Raiswell 1984; Hasnain et al. 1989). High rates of physical and chemical weathering shown by drainage systems of higher Himalaya (Singh and Hasnain 1998) are attributed to high monsoonal rain fall, high seismicity of relatively younger Himalaya and frequent avalanching due to steep valleys (Sarin et al. 1992; Hasnain and Chauhan 1993).
Many hydrochemical and sediment transport studies have been carried out on Himalayan glaciers (Hasnain et al. 1989; Singh et al. 1995; Hasnain and Thayyen 1996, 1999a, b; Thayyen et al. 1999; Ahmad and Hasnain 2000, 2001; Kumar et al. 2002; Singh et al. 2003, 2005; Haritashya et al. 2006; Kumar et al. 2009; Haritashya et al. 2010; Singh et al. 2012, 2013, 2014; Sharma et al. 2013). Chemical characterisation of glacier meltwater is mainly controlled by the rock–water interaction, weathering of rock minerals, meltwater runoff and atmospheric precipitation (Singh et al. 1998; Ahmad and Hasnain 1999). In general, bicarbonate is the dominant anion in the meltwater draining from glaciers, whereas carbonation is the dominant chemical mechanism controlling the major ions chemistry of glacier meltwater (Kumar et al. 2009). Sediments present in the glacier meltwater are derived from various parts of the glacier such as ablation zone, accumulation zone and lateral moraines (Singh et al. 1995). Large amount of sediment materials such as moraines, debris and boulders are present in the ablation zone of a glacier; hence, this zone contributes large amount of sediment to the meltwater (Singh et al. 2003). A very limited number of studies are available on hydrochemical and sediment transport of the Chhota Shigri Glacier and none of them are detailed in discussion. Hence, in this paper, we have carried out detailed study covering sources of dissolved ions, weathering and geochemical processes controlling meltwater chemistry, seasonal and diurnal variations of total dissolved solid (TDS) and suspended sediment concentration in the glacier meltwater.
Study area
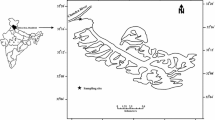
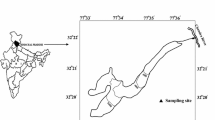
The Chhota Shigri Glacier is situated on the northern slope of Pir Panjal range of western Himalaya and falls in the Lahaul-Spiti district of Himachal Pradesh, India. Geographically, this glacier is located between 32°11′–32°17′ N and 77°29′–77°33′ E and is a valley-type glacier. The present snout of Chhota Shigri Glacier is situated at an elevation of about 4,055 m a.s.l. The shape of the snout is almost concave but some changes are observed in the shape of the snout every year. The length of this glacier is 9 km from snout to the accumulation zone near the Sara Umga Pass (4,900 m a.s.l) and its width varies from 1.5 to 3.0 km (Nijampurkar and Rao 1992). Many small suspended glaciers and four tributaries are present in the drainage basin of Chhota Shigri Glacier. The total glacierized area is about 16.3 km2 whereas Chhota Shigri Glacier area including tributaries is 15.7 km2 (Wagnon et al. 2007). This glacier is oriented towards north but a variety of orientations is shown by its different tributaries (Azam et al. 2012). The Chhota Shigri Glacier stream flows in a NW direction, and about 2.5 km downstream from snout, it meets Chandra River. The sampling site was located at 32°17′24.58′′ N and 77°31′55.21′′ E on the Chhota Shigri stream at an elevation of about 3,900 m a.s.l., 2.0 km downstream from the snout of Chhota Shigri Glacier (Fig. 1).
Geology
Geologically, the Chhota Shigri Glacier falls in the Central Crystallines of the Pir Panjal range of the Himachal Himalaya and rests on the basement rocks composed of granite. This crystalline axis is mostly made up of meso-to-ketazonal metamorphites, migmatites and gneisses (Kumar and Dobhal 1997). Geological map of the Chhota Shigri Glacier and the surrounding area is shown in Fig. 2. Rohtang gneiss is the main lithological unit of the Chhota Shigri Glacier catchment, while some chalcopyrite is found in the lateral moraines up to an altitude of 4,700 m a.s.l (Kumar et al. 1987; Katoch 1989; Hasnain et al. 1989). Older Palaeozoic granite rocks are exposed in the upper Chandra Valley around 3 km upstream of Chhota Dara and Haimanta Formation overlies these with a tectonic break, in which fine-grained biotite–schists, black slates and phyllites are exposed (Kumar and Dobhal 1997).
Geological map of Chhota Shigri Glacier including surrounding area (Source: Adapted from Kumar et al. 1987)
Granite, granite gneiss, augen gneiss, schistose gneiss, porphyritic granite, muscovite-biotite schist, and milky-white muscovite–quartzite are found between Chhota Shigri and Bara Shigri glaciers with common occurrence of late stage pegmatitic veins having light grey green colour feldspar crystals (Kumar et al. 1987). Intense heating effect is seen from brown biotite having fine-grained texture, which shows periodic reheating of the underlying granitic rocks (Rawat and Purohit 1988). Small veins of antimony having pyrites traverse through the granites to the eastern side of the Chhota Shigri Glacier, very close to the paleo-snout of Bara Shigri Glacier. Just at the contact of these veins, a muscovite–quartz-rich rock is seen, which appears different from the main granitic rock (Kumar et al. 1987).
Climatic condition
Climate is one of the most important factors which influence the dynamics of the glacier (Ramanathan 2011). The climate regime of Chhota Shigri Glacier is influenced by both the winter mid-latitude westerlies and summer Asian monsoon, hence falls in the monsoon–arid transition zone. Here, in summer (July–September), most of the precipitation occurs due to the Asian monsoon, whereas significant amount of precipitation also occurs in winter (January–April) due to mid-latitude westerlies (Wagnon et al. 2007). Very short period of meteorological data is available on Chhota Shigri Glacier. Mean temperature observed on the surface of Chhota Shigri Glacier is about 3.4, 3.2, 3.2, 0.7 and −0.76 °C during the period 18 August–8 September 1986, 2 August–5 September 1988, 17 August–11 September 1989, 2–8 October 2003 and 18 August–10 October 2010, respectively. Whereas the corresponding mean relative humidity for these periods are 71, 78, 82, 70 and 63 %, respectively (Rizvi 1987; IMD 1987; Apte et al. 1988; Kulandaivelu et al. 1989; Upadhyay et al. 1989; Sharma 2007; JNU-IFCPAR 2010). Wind flows from south (Sara Umga pass) to north (Chandra river valley) with varying speeds between 3 and 15 km h−1 (Purohit et al. 1991; Sharma 2007). The highest amount of precipitation was recorded on 15 August 1988 from the study area; otherwise, small amount of precipitation was measured (Upadhyay et al. 1989; Purohit et al. 1991). Generally, visibility is good in the morning, but deteriorates later in the day due to the presence of low stratus clouds occupying the valley (Rizvi 1987; Apte et al. 1988; Kulandaivelu et al. 1989; Upadhyay et al. 1989; Purohit et al. 1991).
Materials and methods
Chhota Shigri Glacier meltwater samples were collected during the period from 19 August to 11 October 2008 (n = 63) and 2009 (n = 118) three times a day at 0800, 1300 and 1800 hours in 300 ml prewashed polyethylene bottles following the method of Ostrem (1975). Twenty-four hour meltwater samples were collected on 20 August 2008. Electrical conductivity (EC) and pH were measured in the field immediately after the meltwater sample collection by using HACH-Sension 156 portable multiparameter meter. By using filtration setup and pneumatic pump, glacier meltwater samples were filtered through 0.45 μm Milipore membrane filter in the field. Suspended sediment collected on the filter paper was dried and weighed to determine total suspended matter. Bicarbonate was analysed by acid titration method (APHA 2005) in the field. The meltwater samples were transported to School of Environmental Sciences, Jawaharlal Nehru University, New Delhi, and preserved at 4 °C in the cold room for analysis of different chemical parameters.
Sulphate analysis was carried out by turbidimetric method (APHA 2005). The molybdosilicate method and ascorbic acid method were used to measure the concentration of dissolved silica and phosphate, respectively (APHA 2005). Chloride was analysed by mercury(II) thiocyanate method (Florence and Farrar 1971) and nitrate was determined by brucine–sulphanilic acid method (APHA 2005). Atomic absorption spectrophotometer (Electronic Corporation of India Limited, AAS4141) was used for analysis of major cations (Ca2+, Mg2+, Na+ and K+). Analysis of Na+ and K+ were carried out in the emission mode, whereas Ca2+ and Mg2+ were analysed in the absorption mode. Precision of analytical result was better than ±5 % for the measurement of dissolved ions. Plotting of Piper diagram was carried out by using Aquachem software. Correlation matrix and factor analysis of the meltwater samples were carried out using Statistical Package for Social Sciences.
Result and discussion
Dissolved ion chemistry
Results of chemical analyses of meltwater draining from Chhota Shigri Glacier are summarized in Table 1. The observed charge balance errors (TZ+ − TZ−/TZ+ + TZ− × 100) were <10 %, confirming the quality and reliability of the chemical analysis. EC value of the Chhota Shigri Glacier meltwater was 56.2 ± 24.7 μs/cm (15.4–92.8) and 42.5 ± 17.1 (11.0–59.9) in 2008 and 2009, respectively. Meltwater of study area was acidic to slightly alkaline in nature with an average value of 6.7 ± 0.3 (6.3–7.3) in 2008 and 6.5 ± 0.3 (5.5–6.9) in 2009. Calcium and magnesium were the major cations accounting for 36.1 and 32.2 % of total cations (TZ+) in 2008 and 38.5 and 36.6 % of total cations (TZ+) in 2009, respectively. The average Ca2+ concentration was recorded as 128 ± 48 μeq/l (47.4–193) in 2008 and 104 ± 29.2 μeq/l (47.5–173) in 2009, whereas average Mg2+ concentration was found to be 118 ± 49.0 μeq/l (20.0–175) in 2008 and 98.8 ± 39.9 μeq/l (20.0–148) in 2009. The abundance order of cations in both years was Ca2+ > Mg2+ > Na+ > K+. Bicarbonate was the most dominant anion accounting for 60.1 % of the total anions (TZ−) in 2008 and 66.8 % of the total anions (TZ−) in 2009, whereas sulphate was the next dominant anion after bicarbonate constituting 36.9 % of total anions (TZ−) in 2008 and 31.7 % of total anions (TZ−) in 2009. The average HCO3 − concentration was found to be 261 ± 96.4 μeq/l (70.0–400) in 2008 and 219 ± 67.3 μeq/l (110–320) in 2009, whereas average SO4 2− concentration was recorded as 160 ± 78.6 μeq/l (40.5–251) in 2008 and 104 ± 45.5 μeq/l (22.6–190) in 2009. The average dissolved silica concentration was measured as 71.8 ± 47.6 μmole/l (0.94–160) in 2008 and 33.4 ± 18.7 μmole/l (3.2–57.3) in 2009.
Sources of dissolved ions and mechanism controlling meltwater chemistry
The chemical characteristics of meltwater draining from the glacier in terms of dissolved ions can be explained on the basis of chemical weathering of various rock-forming minerals present in the glacier basin (Singh et al. 1998; Kumar et al. 2009). Carbonate and silicate weathering, sulphide oxidation and sulphate minerals dissolution are the major dissolved ions-producing processes in the glacier catchment (Bhatt et al. 2000; Ahmad and Hasnain 2001; Singh et al. 2012, 2014). The (Ca + Mg) vs TZ+ scatter plot (Fig. 3) shows that all sampling points fall above the 1:1 line and have good correlation among the sampling points with average equivalent ratios of 0.69 ± 0.03 in 2008 and 0.74 ± 0.06 in 2009 (Table 1). Whereas Na + K/TZ+ ratios were 0.31 ± 0.03 in 2008 and 0.26 ± 0.06 in 2009 (Fig. 4, Table 1). (Ca + Mg)/(Na + K) ratios were high, i.e. 2.31 ± 0.44 in 2008 and 2.98 ± 0.76 in 2009. Higher contribution of (Ca + Mg) to the total cations (TZ+) and low ratios of (Na + K)/TZ+ for both years suggests that major source of dissolved ions in the meltwater of Chhota Shigri Glacier is carbonate weathering with relatively small contribution from silicate weathering, as reported by earlier workers (Sharma 2007; Ramanathan et al. 2009; Singh 2011). Main source of Na+, K+ and dissolved silica in the meltwater of study area are the weathering of silicate minerals such as feldspar, quartz, etc. The (Ca + Mg) vs (HCO3 + SO4) scatter plot (Fig. 5) indicates that all sampling points fall above 1:1 line and shows good correlation throughout the range of sampling points. This shows that excess of (HCO3 + SO4) over (Ca + Mg) is balanced by the alkalis (Na + K) from weathering of silicate minerals (Sharma et al. 2012; Khadka and Ramanathan 2012).
Natural waters draining only carbonates are characterized by high ratios of Ca/Na close to 50, Mg/Na close to 10 and HCO3/Na close to 120 (Stallard 1980; Meybeck 1986; Negrel et al. 1993), whereas waters draining silicate-dominant lithology show low ratios of Ca/Na, i.e. 0.35 ± 0.15; Mg/Na, i.e. 0.24 ± 0.12; and HCO3/Na, i.e. 2 ± 1 (Gaillardet et al. 1999). The equivalent ratios of Ca/Na = 2.26 ± 0.32 in 2008 and 2.73 ± 0.68 in 2009, Mg/Na = 2.04 ± 0.36 in 2008 and 2.50 ± 0.78 in 2009, and HCO3/Na = 4.64 ± 0.71 in 2008 and 5.65 ± 0.86 in 2009 in the Chhota Shigri Glacier meltwater are higher than those of meltwater draining silicate lithology and lower than that draining carbonate rocks. These ratios show that the dissolved ions chemistry of meltwater draining from Chhota Shigri Glacier is influenced by weathering of carbonate and silicate minerals.
Most of the base cations are derived from acid hydrolysis (Raiswell et al. 1980). For acid hydrolysis, aqueous protons are required, derived from two main sources (Raiswell 1984; Tranter et al. 1993; Brown et al. 1996; Hasnain and Thayyen 1999a). The first possibility for the production of aqueous proton is the dissociation of dissolved atmospheric CO2 (Eq. 1) whereas second possible source is pyrite oxidation (Eq. 4).
The general reaction of silicate minerals weathering with H2CO3 can be written as (Pandey et al. 1999; Anshumali and Ramanathan 2007)
The relative importance of two major proton delivery mechanisms, i.e. carbonation and sulphide oxidation, can be evaluated by (HCO3 / HCO3 + SO4) ratio, known as the C ratio (Brown et al. 1996). If C ratio is 1, it shows the significance of carbonic acid weathering where dissolution and dissociation of atmospheric CO2 are mainly producing protons (Eqs 1 and 2). Whereas C ratio of 0.5 signifies coupled carbonate dissolution and sulphide oxidation reactions and protons are derived from the oxidation of sulphide (Eq. 4). The average C ratio for the meltwater draining from the Chhota Shigri Glacier was 0.64 ± 0.07 in 2008 and 0.69 ± 0.08 in 2009, which shows that dominance of carbonate dissolution and dissociation and dissolution of atmospheric CO2 are the major proton producer (Brown et al. 1996; Hasnain and Thayyen 1999a; Singh and Hasnain 2002).
Water classification
Hydrogeochemical evolution including identification of water types of meltwater can be evaluated by plotting the concentrations of major anions (HCO3 −, SO4 2− and Cl−) and cations (Ca2+, Mg2+, Na+ and K+) in the Piper trilinear diagram (Piper 1944). This diagram consists of one lower left and one lower right side triangular diagrams and a central diamond-shaped diagram. The relative composition of major anions and cations are plotted in the two triangular diagrams, while the central diamond-shaped diagram represents overall characteristics of meltwater combining the composition of major anions and cations. The Piper plot (Fig. 6) showed that majority of meltwater samples were CaHCO3-type water with small contribution from MgHCO3 type of water in 2008, whereas in 2009 a majority of meltwater samples were also CaHCO3-type water but with considerable number of MgHCO3-type water also being represented. The Piper plot pattern indicates that alkaline earth metals (Ca + Mg) exceed alkalis (Na + K) and weak acid (HCO3 −) exceeds strong acid (SO4 + Cl). Such a combination suggests that the major source of dissolved ions in Chhota Shigri Glacier meltwater is carbonate weathering.
Atmospheric and anthropogenic impact on meltwater chemistry
There have been various studies on the contribution of atmospheric sources to the chemical composition of river water (Meybeck 1983; Biksham and Subramanian 1988; Sarin et al. 1989; Pandey et al. 1999; Jha et al. 2009; Kumar et al. 2009; Sharma et al. 2012; Singh et al. 2012). The importance of atmospheric inputs can be evaluated by considering the elements to chloride ratio because of abundant concentration of chloride in the seawater and relatively low concentration in most of the rocks (Dudeja et al. 2011). The (Na + K) vs Cl scatter plot (Fig. 7) indicates that Na+ and K+ concentrations are much higher than Cl− in the study area for both years. The average ratios of Na/Cl and K/Cl are 7.23 ± 6.18 and 6.36 ± 5.73 in 2008 and 21.9 ± 15.9 and 16.0 ± 11.1 in 2009, respectively; much higher than for marine aerosols (i.e. Na/Cl = 0.85 and K/Cl = 0.0176) indicating relatively minor contribution from atmospheric precipitation to the chemical composition of Chhota Shigri Glacier meltwater (Pandey et al. 1999; Kumar et al. 2009; Dudeja et al. 2011; Sharma et al. 2012; Singh et al. 2012)
Trace amount of NO3 − and PO4 3− was reported from the meltwater of Chhota Shigri Glacier, which indicates the palatability of the meltwater. Tourists and shepherds with lots of sheep and goats arrive in the study area during the sampling period. Hence, anthropogenic activities may be one of the possible sources of NO3 − and PO4 3− in the study area, with additional inputs of NO3 − from acidic NO3 − aerosols (Tranter et al. 1993) (Eq. 6).
Effective CO2 pressure
The effective CO2 pressure (pCO2) of meltwater draining from the glacier can be used to characterize the various hydrological weathering environments (Sharp 1991; Wadham et al. 1998). Open and closed system weathering conditions are kinetic phenomena, which depend on those factors affecting the weathering rates (i.e. rock minerals availability and reactivity) and the rate of extent of CO2 transfer to the glacier meltwater (Raiswell 1984; Raiswell and Thomas 1984; Thomas and Raiswell 1984). The average pCO2 values (10−2.5 atm in 2008 and 10−2.3 atm in 2009) for Chhota Shigri Glacier meltwater were higher than the atmospheric pCO2 (10−3.5 atm) showing disequilibrium with respect to atmosphere. The higher values of pCO2 of meltwater are due to open system weathering, low temperature, high turbulence and dominant carbonate weathering process in the study area (Ahmad and Hasnain 2001; Singh et al. 2012). The pCO2 values for the study area are close to the earlier reported value for Gangotri and Chhota Shigri glaciers (Singh et al. 2012; Sharma et al. 2013).
Statistical analysis
Correlation matrix and factor analysis are one of the important statistical tools widely used in statistical analysis of hydrochemical data. Statistical analysis gives better information than graphical analysis about sources of dissolved ions in the study area as it considers a finite number of variables (Srivastava and Ramanathan 2008).
Correlation matrix
Sources of dissolved ions in glacier meltwater can be evaluated on the basis of association among the dissolved ions (Singh et al. 1998; Ahmad and Hasnain 2001). The correlation matrix is a bivariate method, which shows how much one variable predicts the other variable (Kumar et al. 2007). The correlation matrix of meltwater samples of Chhota Shigri Glacier for both years is shown in Tables 2 and 3. EC was strongly correlated with TDS (r 2 = 0.990 in 2008 and r 2 = 0.974 in 2009) showing that as the concentrations of all dissolved ions/constituents increase, electrical conductivity also increases (Rajmohan and Elango 2004; Srivastava and Ramanathan 2008). Good correlation was observed between Ca2+ and Mg2+ (r 2 = 0.951 in 2008 and r 2 = 0.963 in 2009), Ca2+ and HCO3 − (r 2 = 0.972 in 2008 and r 2 = 0.866 in 2009) and Mg2+ and HCO3 − (r 2 = 0.955 in 2008 and 0.894 in 2009) showing common source, which may be carbonate weathering. Na+ displayed strong correlation with K+ (r 2 = 0.946 in 2008 and r 2 = 0.925 in 2009), while H4SiO4 showed good correlation with Na+ (r 2 = 0.836 in 2008 and r 2 = 0.776 in 2009) and K+ (r 2 = 0.805 in 2008 and r 2 = 0.730 in 2009) indicating similar source, i.e. silicate weathering. Ca2+ showed strong correlation with SO4 2− (r 2 = 0.968 in 2008 and r 2 = 0.888 in 2009), whereas Mg2+ also showed good correlation with SO4 2− (r 2 = 0.954 in 2008 and r 2 = 0.888 in 2009). These associations indicate that probably pyrite oxidation and sulphate mineral dissolution are the source of these ions in the study area.
Factor analysis
The aim of factor analysis is to explain the observed relationship among the variables in terms of simpler relations (Jha et al. 2009). Factor analysis is a multivariate, exploratory, statistical tool that can be used to explain the variability in a dataset (Singh and Hasnain 2002; Boruvka et al. 2005). Here, R-mode factor analysis was used because it has numerous positive features, which can be used for the interpretation of hydrogeochemical data (Lawrence and Upchurch 1992). Results of factor analysis of Chhota Shigri Glacier meltwater are given in Table 4. If the eigenvalue is greater than 1, it is a significant contributing factor (Briz-Kishore and Murali 1992; Singh et al. 2012). Two factors were extracted showing eigenvalue >1, which control the dissolved ions chemistry of Chhota Shigri Glacier meltwater in 2008. These two factors explained about 80.8 % of the total variability. Factor 1 explained about 71.9 % of variance in dataset, showing strong loading of EC, H4SiO4, HCO3 −, SO4 2−, Cl−, Ca2+, Mg2+, Na+, K+ and TDS. This factor shows contribution from carbonate and silicate minerals weathering, sulphide oxidation, sulphate minerals dissolution, evaporates dissolution and atmospheric precipitation. Factor 2 represents 8.9 % of the variance in the dataset, having positive loading for NO3 −. This factor may be attributed to atmospheric deposition along with anthropogenic activities taking place in the basin area.
Two factors were also identified having eigenvalue >1, which controls the meltwater chemistry of the study area in 2009. The total variance explained by these two factors is 77.4 %. Factor 1 has strong positive loading for EC, pH, H4SiO4, HCO3 −, SO4 2−, Ca2+, Mg2+, Na+, K+ and TDS and accounts for 69 % of the variance in the dataset. This factor explains contribution from weathering of carbonate and silicate minerals associated with sulphate minerals dissolution and sulphide oxidation. Factor 2 accounts for 8.4 % of variance in the dataset and indicates strong loading of NO3 −. This factor may be responsible for contribution from atmospheric deposition and anthropogenic activities.
Seasonal and diurnal variations of the total dissolved solid
Generally, meltwater draining from glacier shows seasonal and diurnal variation of the dissolved ions concentration. Different weathering intensities of glacier meltwater at different values of runoff are responsible for variation in the dissolved ions concentration with time (Kumar et al. 2009). Daily mean TDS concentration for August, September and October 2008 was measured to be 15.7 ± 2.28, 47.9 ± 3.1 and 50.6 ± 4.88 mg l−1, respectively, whereas daily mean TDS concentration for August, September and October 2009 was found to be 13.8 ± 1.67, 31.0 ± 6.11 and 32.1 ± 1.68 mg l−1, respectively. Hence, TDS shows high values during October (low flow period) and low during August (high flow regime) in both years. Such a trend is observed because of low supply of solar energy during October reducing the discharge and dilution of the solute concentration; hence, TDS value of Chhota Shigri Glacier meltwater is high in October. On the other hand, during August, high incoming solar radiation increases the discharge and dilution of solute concentration (Singh and Hasnain 1998; Singh et al. 2006), resulting low TDS value of glacier meltwater during August.
A distinct diurnal variation in the TDS of meltwater draining from Chhota Shigri Glacier was observed. High TDS was observed in the morning which decreased in the evening and again showed increasing trend in night (Fig. 8). The discharge from Chhota Shigri Glacier shows peak runoff in evening between 1400 and 1700 hours and reaching minimum runoff in the morning at around 0700 hours (Sharma 2007; JNU-IFCPAR 2009). Thus, TDS shows inverse relationship with discharge, i.e. TDS is low during high discharge and high during the low discharge. The observed diurnal variation in the TDS of meltwater could be explained by the model of Rainwater and Guy (1961), Collins (1983) and Kumar et al. (2009). According to this model, low supply of solar radiation in the morning reduces the discharge and dilution of subglacial meltwater. At pressure melting, the subglacial meltwater produced has higher dissolved ions concentration because of longer contact with solute-rich base and higher residence time. On the other hand, englacial channels become active due to increase in solar insolation in the afternoon. As a resultant of this, huge amount of subglacial meltwater is available for runoff and after certain lag of time; discharge is maximum in evening causing dilution of TDS of glacier meltwater.
Suspended sediment characteristics
Suspended sediment concentration in the meltwater draining from Himalayan glaciers is highly variable because of the influences of climate, sediment sources variability, tectonic setting, debris entrainment processes and types of rock and weathering (Kumar et al. 2002). Daily mean suspended sediment concentration for August, September and October 2008 was found to be 376 ± 229, 40.1 ± 17.8 and 12.0 ± 7.24 mg l−1, respectively, whereas daily mean suspended sediment concentration for August, September and October 2009 was observed to be 368 ± 225, 34.5 ± 29.9 and 8.85 ± 3.16 mg l−1, respectively. Suspended sediment concentration is high during August (high flow period) and low during October (low flow period) in both years. Such variation in the suspended sediment concentration is attributed to climate and development of the drainage network of the basin (Pandey et al. 1999; Singh et al. 2003). High discharge (due to high incoming solar radiation), increased stream area cross section and more availability of glacial debris are the major factors behind the high suspended sediment concentration in August (Singh and Hasnain 1998; Pandey et al. 1999; Haritashya et al. 2006). Whereas suspended sediment concentration is much lower in October because of low discharge (due to low supply of incoming solar radiation), sediment evacuation by the drainage system in the preceding months and remaining small amount of subglacial sediment or morainic material flushed out from the valley walls of the glacier in the later part of the ablation season (Ostrem 1975; Collins 1990; Singh and Hasnain 1998).
Diurnal variations in the suspended sediment concentration of Chhota Shigri Glacier meltwater are given in Fig. 9. Suspended sediment concentration shows lower concentration in the morning and increase in the evening. It reaches to its maximum concentration at 1600 hours, then again follows decreasing trend at night. Such types of trend in diurnal variations in suspended sediment concentration can be explained by variation in meltwater runoff from glacier. In case of Chhota Shigri Glacier, minimum discharge is observed in the morning at around 0700 hours, whereas maximum discharge is observed in evening at 1400–1700 hours (Sharma 2007; JNU-IFCPAR 2009). The results indicate that suspended sediment concentration is directly correlated with discharge, i.e. suspended sediment concentration is low during low discharge and high during high discharge. In the morning, low temperature and low solar radiation are responsible for low discharge; hence, suspended sediment concentration is low. Whereas from morning to evening, addition of meltwater from subglacial channels leads to increased discharge from glacier; hence, suspended sediment concentration is high in evening (Singh et al. 1998).
Conclusion
The hydrogeochemical study of meltwater draining from Chhota Shigri Glacier indicates that meltwater is slightly acidic in nature. Calcium and magnesium are the major cations, whereas bicarbonate is the major anion followed by sulphate for both years. High contribution of (Ca + Mg) to total cations (TZ+), high ratio of (Ca + Mg)/(Na + K), good correlation between calcium and magnesium, calcium and bicarbonate, magnesium and bicarbonate, and low (Na + K)/TZ+ ratio suggest that major source of dissolved ions in glacier meltwater is carbonate weathering followed by silicate weathering. Piper plot shows that alkaline earth metals (Ca + Mg) dominated over alkalis (Na + K), while weak acid (HCO3 −) exceeds strong acids (SO4 + Cl). This further confirms that dissolved ions chemistry of study area is mostly controlled by carbonate weathering.
Trace amount of NO3 − and PO4 3− was reported from the study area, indicating palatability of meltwater. Correlation matrix and factor analysis results for both years indicate that dissolved ions chemistry of Chhota Shigri Glacier meltwater is regulated by carbonate and silicate weathering, oxidation of sulphide, dissolution of sulphate minerals, atmospheric precipitation and anthropogenic activities. Distinct seasonal and diurnal variation in TDS and suspended sediment concentration of meltwater were observed. TDS is inversely correlated with discharge, whereas suspended sediment concentration is directly correlated with discharge.
References
Ahmad S, Hasnain SI (1999) Hydro-Geochemical characteristics of meltwater draining from Bagni glacier, Dhauliganga basin, Garhwal Himalayas, Uttar Pradesh. J Appl Hydrol 12(2&3):53–60
Ahmad S, Hasnain SI (2000) Meltwater characteristics of Garhwal Himalayan glaciers. J Geol Soc India 56:431–439
Ahmad S, Hasnain SI (2001) Chemical characteristics of stream draining from Dudu glacier: an Alpine meltwater stream in Ganga Headwater, Garhwal Himalaya. J China Univ Geosci 12(1):75–83
Anshumali, Ramanathan AL (2007) Seasonal variation in the major ion chemistry of Pandoh Lake, Mamdi District, Himachal Pradesh, India. Appl Geochem 22:1736–1747
APHA (2005) Standard methods for examination of water and wastewater, 21st edn. American Public Health Association, Washington, DC
Apte NT, Sharma JK, Rajput RK (1988) Meteorological report of Chhota Shigri glacier expedition 1987. Multi disciplinary glacier expedition to Chhota Shigri, Department of Science and Technology, Government of India, New Delhi, Technical Report Number 2, pp 217–231
Azam MF, Wagnon P, Ramanathan AL, Vincent C, Sharma P, Arnaud Y, Linda A, Pottakkal JG, Chevallier P, Singh VB, Berthier E (2012) From balance to imbalance: a shift in the dynamic behaviour of Chhota Shigri Glacier (western Himalaya, India). J Glaciol 58(208):315–324
Bhatt MP, Masuzawa T, Yamamoto M, Sakai A, Fujita K (2000) Seasonal changes in dissolved chemical composition and flux of meltwater draining from Lirung glacier in the Nepal Himalayas. Proceedings of a workshop on debris-covered glaciers held at Seattle, Washington, USA. IAHS Publication Number 264, pp 277–288
Biksham G, Subramanian V (1988) Nature of solute transport in the Godavari basin, India. J Hydrol 103:375–392
Boruvka L, Vecek O, Jehlicka J (2005) Principal component analysis as a tool to indicate the origin of potentially toxic elements in soil. Geoderma 128:289–300
Briz-Kishore BH, Murali G (1992) Factor analysis for revealing hydrochemical characteristics of watershed. Environ Geol 19:3–9
Brown GH, Tranter M, Sharp M (1996) Subglacial chemical erosion—seasonal variations in solute provenance, Haut Glacier d’Arolla, Switzerland. Ann Glaciol 22:25–31
Collins DN (1983) Solute yield from a glacierised high mountain basin. In: Webb BW (ed) Symposium on dissolved loads of rivers and surface water quality/quality relationship. IAHS Publication Number 141, pp 41–50
Collins DN (1990) Seasonal and annual variations of suspended sediment transport in meltwaters draining from an Alpine glacier. Proceeding of Two Lausanne Symposium, IAHS Publication Number 193, pp 439–446
Dudeja D, Bartarya SK, Biyani AK (2011) Hydrochemical and water quality assessment of ground water in Doon Valley of Outer Himalaya, Uttarakhand, India. Environ Monit Assess 181:183–204
Florence TM, Farrar YJ (1971) Spectrophotometric determination of chloride at the parts-per-billion level by the mercury(II) thiocyanate method. Anal Chim Acta 54:373–377
Gaillardet J, Dupre B, Louvat P, Allegre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30
Haritashya UK, Singh P, Kumar N, Gupta RP (2006) Suspended sediment from the Gangotri Glacier: quantification, variability and associations with discharge and air temperature. J Hydrol 321:116–130
Haritashya UK, Kumar N, Singh P (2010) Particle size characteristics of suspended transported in meltwater from Gangotri Glacier, Central Himalaya—an indicator of subglacial sediment evacuation. Geomorphology 122:140–152
Hasnain SI, Chauhan DS (1993) Sediment transfer in the glaciofluvial environment—a Himalayan perspective. Environ Geol 22(3):205–211
Hasnain SI, Thayyen RJ (1996) Sediment transport and solute variation in meltwaters of Dokriani Glacier (Bamak), Garhwal Himalaya. J Geol Soc India 47:731–739
Hasnain SI, Thayyen RJ (1999a) Controls of major-ion chemistry of the Dokriani glacier meltwaters, Ganga basin, Garhwal Himalaya. J Glaciol 45(149):87–92
Hasnain SI, Thayyen RJ (1999b) Discharge and suspended sediment concentration of meltwaters, draining from the Dokriani glacier, Garhwal Himalaya, India. J Hydrol 218:191–198
Hasnain SI, Subramanian V, Dhanpal K (1989) Chemical characteristics and suspended sediment load of meltwaters from a Himalayan Glacier in India. J Hydrol 106:99–108
ICIMOD (2001) Inventory of glaciers, glacial lakes and glacial lake outburst floods, monitoring and early warning system in the Hindu Kush-Himalayan region, Nepal. (UNEP/RC-AP)/ICIMOD, Kathmandu
IMD (1987) Meteorological Report of Chhota Shigri glacier expedition 1986 (part II). Multi disciplinary glacier expedition to Chhota Shigri, Department of Science and Technology, Government of India, New Delhi, Technical Report Number 1, pp 153–172
Immerzeel WW, van Beeke LPH, Bierkens MFP (2010) Climate change will affect the Asian water towers. Science 328:1382–1385
Jha PK, Tiwari J, Singh UK, Kumar M, Subramanian V (2009) Chemical weathering and associated CO2 consumption in the Godavari river basin, India. Chem Geol 264(1–4):364–374
JNU-IFCPAR (2009) Mass balance, energy balance and hydrological balance of Chhota Shigri glacier, Himachal Pradesh. Technical field report submitted to IFCPAR. Jawaharlal Nehru University, New Delhi
JNU-IFCPAR (2010) Mass balance, energy balance and hydrological balance of Chhota Shigri glacier, Himachal Pradesh. Technical field report submitted to IFCPAR. Jawaharlal Nehru University, New Delhi
Katoch KC (1989) Study of moraines with special reference to metallic minerals in Chhota Shigri glacier in Lahaul and Spiti District, Himachal Pradesh. Department of Science and Technology, Government of India, New Delhi, Technical Report Number 3, pp 299–301
Kaul MK (1999) Inventory of Himalayan Glaciers. Special Publication Number 34, Geological Survey of India
Khadka UR, Ramanathan AL (2012) Major ion composition and seasonal variation in the Lesser Himalayan lake: case of Begnas Lake of Pokhara Valley, Nepal. Arab J Geosci. doi:10.1007/s12517-012-0677-4
Kulandaivelu E, Ray B, Sharma JK (1989) Meteorological report of Chhota Shigri glacier expedition1988. Multi disciplinary glacier expedition to Chhota Shigri, Department of Science and Technology, Government of India, New Delhi, Technical Report Number 3, pp 37–57
Kumar S, Dobhal DP (1997) Climatic effects and bedrock control on rapid fluctuations of Chhota Shigri glacier, northwest Himalaya, India. J Glaciol 43(145):467–472
Kumar S, Rai H, Purohit KK, Rawat BRS, Mundepi AK (1987) Multi disciplinary glacier expedition to Chhota Shigri glacier. Department of Science and Technology, Government of India, New Delhi, Technical Report Number 1, pp 1–29
Kumar K, Miral MS, Joshi V, Panda YS (2002) Discharge and suspended sediment in the meltwater of Gangotri Glacier, Garhwal Himalaya, India. Hydro Sci J 47(4):611–619
Kumar M, Kumari K, Ramanathan AL (2007) A comparative evaluation of ground water suitability for irrigation and drinking purposes in two intensively cultivated district of Punjab, India. Environ Geol 53:553–574
Kumar K, Miral MS, Joshi S, Pant N, Joshi V, Joshi LM (2009) Solute dynamics of meltwater of Gangotri glacier, Garhwal Himalaya, India. Enviro Geol 58:1151–1159
Lawrence FW, Upchurch SB (1992) Identification of recharge areas using geochemical factor analysis. Ground Water 20:680–687
Liu Y, Geng Z, Hou S (2010) Spatial and seasonal variation of major ions in Himalayan snow and ice: a source consideration. J Asian Earth Sci 37:195–205
Lorrain RD, Souchez RA (1972) Sorption as a factor in the transport of major cations by meltwater from an Alpine glacier. Quat Res 2(2):253–256
Meybeck M (1983) Atmospheric inputs and river transport of dissolved substances. In: Webb BW (ed) Dissolved loads of rivers and surface water quality/quantity relationships. IAHS Publication Number 141, pp 173–191
Meybeck M (1986) Compositiondes ruisseau non pollues de France. Sci Géo Bul 39:3–77
Negrel P, Allegre CJ, Dupre B, Lewin E (1993) Erosion sources determined by determined by inversion of major and trace element ratios in river water: the Congo basin case. Earth Planet Sci Lett 120:59–76
Nijampurkar VN, Rao DK (1992) Accumulation and flow rates of ice on Chhota Shigri glacier, central Himalaya, using radioactive and stable isotopes. J Glaciol 38(128):43–50
Ostrem G (1975) Sediment transport in glacial meltwater stream. In: Jopling AV, McDonald BC (ed) Glacio-fluvial and glacio-lacustrine sedimentation. society of economic palaeontologists and mineralogists. Special Publication Number 23, pp 101–122
Pandey SK, Singh AK, Hasnain SI (1999) Weathering and geochemical processes controlling solute acquisition in Ganga Headwater-Bhagirathi River, Garhwal Himalaya, India. Aquat Geochem 5(4):357–379
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–923
Purohit MK, Rajput RK, Roy B, Sharma JK (1991) Meteorological report of Chhota Shigri glacier expedition 1989. Department of Science and Technology, Government of India, New Delhi, Technical Report Number 4, pp 60–74
Rai SC, Gurung A (2005) Raising awareness of the impacts of climate changes. Mount Res Develop 25(4):316–320
Rainwater FH, Guy HP (1961) Some observations on the hydrochemistry and sedimentation of the Chamberlain Glacier Area Alaska. U.S. Geological Survey Professional Paper 414- c, pp cl–c14
Raiswell R (1984) Chemical models of solute acquisition in glacial meltwaters. J Glaciol 30(104):49–57
Raiswell R, Thomas AG (1984) Solute acquisition in glacial meltwaters, I. Fjallsjokull (south-east Iceland): bulk meltwaters with closed system characteristics. J Glaciol 30(104):35–43
Raiswell R, Brimblecombe P, Dent D, Liss PS (1980) Environmental chemistry. Edward Arnold, London
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ Geol 46:47–61
Ramanathan AL (2011) Status report on Chhota Shigri Glacier (Himachal Pradesh). Department of Science and Technology, Ministry of Science and Technology, New Delhi, Himalayan Glaciology Technical Report Number 1, pp 88
Ramanathan AL, Singh VB, Jose PG, Sharma P, Linda A, Kumar M (2009) Hydrogeochemistry of Chhota Shigri Glacier, Lahaul-Spiti Valley, Himachal Pradesh. Proceedings of National Symposium on Climate Change and Water Resources in India. National Institute of Hydrology, Roorkee, pp 48–49
Rawat BS, Purohit KK (1988) Geology of the area around Chhota Shigri Glacier, Lahaul Spiti District, Himachal Pradesh. Department of Science and Technology, Government of India, New Delhi, Technical Report Number 2, pp 152–157
Reynolds RC, Johnson NM (1972) Chemical weathering in the temperate glacial environment of the Northern Cascade Mountains. Geochim Cosmochim Acta 36:537–554
Rizvi SRH (1987) Report of meteorological studies carried out during Chhota Shigri Glacier expedition (August–September 1986). Multi disciplinary glacier expedition to Chhota Shigri, Department of Science and Technology, Government of India, New Delhi, Technical Report Number 1, pp 139–151
Sarin MM, Krishnaswami S, Dilli K, Somayajulu BLK, Moore WS (1989) Major ion chemistry of Ganga-Brahmaputra river system: weathering processes and fluxes of the Bay of Bengal. Geochim Cosmochim Acta 53:997–1009
Sarin MM, Krishnaswami S, Trivedi JR, Sharma KK (1992) Major ion chemistry of the Ganga source waters: weathering in the high altitude Himalaya. Proc Indian Acad Sci (J Earth Syst Sci) 101(1):89–98
Sharma P (2007) Mass balance and chemical characteristics of Chhota Shigri Glacier-B, Lahaul-Spiti Valley, Himachal Pradesh. Ph.D. Thesis, Jawaharlal Nehru University, New Delhi
Sharma A, Singh AK, Kumar K (2012) Environmental geochemistry and quality assessment of surface and subsurface water of Mahi River basin, western India. Environ Earth Sci 65:1231–1250
Sharma P, Ramanathan AL, Pottakkal JG (2013) Study of solute sources and evolution of hydrogeochemical processes of the Chhota Shigri Glacier meltwaters, Himachal Pradesh, India. Hydro Sci J 58(5):1128–1143
Sharp MJ (1991) Hydrological inferences from meltwater quality data: the unfulfilled potential. Proceeding of the BHS 3rd National Hydrological Symposium, Southhampton, pp 5.1–5.8
Singh VB (2011) Hydro-meteorological and hydro-geochemical characteristics of Chhota Shigri glacier, Lahaul-Spiti Valley, Himachal Pradesh, India. M.Phil Dissertation, Jawaharlal Nehru University, New Delhi
Singh AK, Hasnain SI (1998) Major ion chemistry and weathering control in a high altitude basin: Alaknanda River, Garhwal Himalaya, India. Hydro Sci J 43(6):825–843
Singh AK, Hasnain SI (2002) Aspects of weathering and solute acquisition processes controlling chemistry of sub-Alpine proglacial streams of Garhwal Himalaya, India. Hydrol Proc 16:835–849
Singh P, Ramasastri KS, Singh UK, Gergan JT, Dobhal DP (1995) Hydrological characteristic of Dokriani Glacier in the Garhwal Himalayas. Hydrol Sci J 40(2):243–257
Singh AK, Pandey SK, Panda S (1998) Dissolved and sediment load characteristics of Kafni glacier meltwater, Pindar Valley, Kumaon Himalaya. J Geol Soc India 52:305–312
Singh P, Ramasastri KS, Kumar N, Bhatnagar NK (2003) Suspended sediment transport from the Dokriani Glacier in the Garhwal Himalayas. Nord Hydrol 34:221–244
Singh P, Haritashya UK, Ramasastri KS, Kumar N (2005) Diurnal variations in discharge and suspended sediment concentration, including runoff-delaying characteristics of the Gangotri Glacier in the Garhwal Himalayas. Hydrol Proc 19:1445–1457
Singh P, Haritashya UK, Kumar N, Singh Y (2006) Hydrological characteristics of the Gangotri Glacier, central Himalayas, India. J Hydrol 327:55–67
Singh VB, Ramanathan AL, Pottakkal JG, Sharma P, Linda A, Azam MF, Chatterjee C (2012) Chemical characterisation of meltwater draining from Gangotri Glacier, Garhwal Himalaya, India. J Earth Syst Sci 121(3):625–636
Singh VB, Ramanathan AL, Pottakkal JG, Linda A, Sharma P (2013) Temporal variation in the major ion chemistry of Chhota Shigri glacier meltwater, Lahaul-Spiti Valley, Himachal Pradesh, India. Natl Acad Sci Lett 36(3):335–342
Singh VB, Ramanathan AL, Pottakkal JG, Kumar M (2014) Seasonal variation of the solute and suspended sediment load in Gangotri glacier meltwater, central Himalaya, India. J Asian Earth Sci 79:224–234. doi:10.1016/j.jseaes.2013.09.010
Srivastava SK, Ramanathan AL (2008) Geochemical assessment of groundwater quality in vicinity of Bhalswa landfill, Delhi, India, using graphical and multivariate statistical methods. Environ Geol 53(7):1509–1528
Stallard RF (1980) Major elements geochemistry of the Amazon River system. PhD Thesis, WHOI-80–29, MIT/ Woods Hole Oceanographic Institution, USA
Thayyen RJ, Gergan JT, Dobhal DP (1999) Particle size characteristics of suspended sediments and subglacial hydrology of Dokriani Glacier, Garhwal Himalaya, India. Hydro Sci J 44(1):47–61
Thomas AG, Raiswell R (1984) Solute acquisition in glacial meltwaters, II Glacier d'Argentiere (French Alps): bulk meltwaters with open system characteristics. J Glaciol 30(104):44–48
Tranter M, Brown GH, Raiswell R, Sharp MJ, Gurnell AM (1993) A conceptual model of solute acquisition by Alpine glacier meltwaters. J Glaciol 39(133):573–581
Upadhyay DS, Sharma JK, Ray B, Purohit MK, Rajput RK (1989) Meteorological studies at Chhota Shigri glacier. National meet on Himalayan Glaciology, Department of science and Technology, pp 83–92
Wadham JL, Hodson AJ, Tranter M, Dowdeswell JA (1998) The hydrochemistry of meltwater draining a polythermal-based, high Arctic glacier, south Svalbard: I. The ablation season. Hydrol Proc 12:1825–1849
Wagnon P, Linda A, Arnaud Y, Kumar R, Sharma P, Vincent C, Pottakkal JG, Berthier E, Ramanathan AL, Hasnain SI, Chevallier P (2007) Four years of mass balance on Chhota Shigri glacier (Himachal Pradesh, India), a new benchmark glacier in the western Himalaya, India. J Glaciol 53(183):603–611
Xu H, Hou Z, An Z, Liu X, Dong J (2010) Major ion chemistry of waters in Lake Qinghai catchments, NE Qinghai-Tibet plateau, China. Quat Inter 212:35–43
Acknowledgments
The authors are grateful to the Department of Science and Technology (DST), Government of India and IFCPAR/CEFIPRA (Indo-French) for funding the research project on Chhota Shigri Glacier. We are also thankful to School of Environmental Sciences, Jawaharlal Nehru University, New Delhi, India for providing facilities for sample analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, V.B., Ramanathan, A., Sharma, P. et al. Dissolved ion chemistry and suspended sediment characteristics of meltwater draining from Chhota Shigri Glacier, western Himalaya, India. Arab J Geosci 8, 281–293 (2015). https://doi.org/10.1007/s12517-013-1176-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-013-1176-y