Abstract
The chemical compositions of various ions in meltwater of the Chhota Shigri glacier were analysed during the observation period from May to October 2010. Total 164 samples of meltwater were collected in two times a day at 8.0 a.m and 6.0 p.m. Bicarbonate and calcium were the dominant anion and cation in meltwater of the investigation area. High ratios of (Ca + Mg) versus TZ+ and (Ca + Mg) versus (Na + K) demonstrate that hydrogeochemistry of the study area was mainly governed by carbonate type weathering. The average sulphate mass fraction of meltwater was computed to be 0.31 ± 0.12, showing dominancy of bicarbonate over sulphate. This excess bicarbonate would be generated by an alternative proton supply mechanism due to biological activities in the bed rock lithology and sulphide oxidation may be about the same as carbonation as a driver of chemical weathering at Chhota Shigri glacier. In general, major ions and total dissolved solid concentrations showed decreasing trend with increasing discharge from the study area. The average daily mean dissolved load of this glacier was much lower than that of the Gangotri glacier, which may be due to low meltwater runoff and lithology of the investigation area. The cation weathering rates of study area for early, peak and late melt seasons were computed to be 694, 1631 and 481 meq m−2 a−1, respectively. The average value of chemical denudation rates of the Chhota Shigri glacier meltwater was found lower than that of the Dokriani glacier.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Large areas in the Himalayas are covered by snow and glaciers (Srinivasulu and Kulkarni 2004), showing uneven glacier distributions having more concentration in the north-western regions as compared to north-eastern regions of the Himalayan mountain series (Mehta et al. 2013). Himalayan glaciers feed to various rivers such as Ganga, Brahmaputra, Indus, Yellow River and Yangtze. Over 1.4 billion population depend on these rivers water (Immerzeel et al. 2010). The Himalayan climate is robustly controlled by the mid-latitude westerlies and South Asian monsoon in the western and eastern side, respectively (Vaux et al. 2012). In the Western Himalayan region (Indian part), meteorological parameters such as temperature and precipitation are strongly regulated by local atmospheric circulations (Mohanty and Dimri 2004) and local topography (Dimri 2004).
Hydrogeochemical characteristics of glacier meltwater have been used to understand the processes responsible for solute acquirement in the subglacial environments (Raiswell 1984; Souchez and Lemmens 1987; Tranter et al. 1993; Brown et al. 1994). The solute acquisition processes comprise the chemical weathering of various types of rocks taking place in the environment of glacier (Rainwater and Guy 1961; Collins 1978; Hasnain and Thayyen 1996). The rate and degree of chemical weathering are regulated by various factors such as geological (lithology, slope and elevation) and climatic (runoff, precipitation, temperature and evapo-transpiration) (Gaillardet et al. 1999; Millot et al. 2003; West et al. 2005; Hren et al. 2007; Wu et al. 2008). Temporal variations in the ionic concentration of meltwater are probably due to the varying intensity of chemical weathering at values of different discharge (Kumar et al. 2009).
The hydrochemistry of the glaciers situated in the region of Central Himalaya has been studied on large scale by various workers (Chauhan and Hasnain 1993; Singh et al. 1998; Hasnain and Thayyen 1999; Ahmad and Hasnain 2000, 2001; Kumar et al. 2009; Singh et al. 2012, 2014, 2015a). However, relatively few hydrochemical studies are reported on Western Himalayan glaciers (Sharma et al. 2013; Singh and Ramanathan 2015, 2017; Singh et al. 2015b, c, d) and most of them are for short period of time. Beside these probably no documented works are available on dissolved load and chemical weathering rates of the Chhota Shigri glacier meltwater. So to fill this gap, we have focused on detailed study covering major ions chemistry and relationship between dissolved ions concentrations and discharge. The key objectives of this paper are to identify the sources of solutes in the glacier meltwater, temporal variations in the major ions concentration with discharge, dissolved load and chemical weathering rates of meltwater of the study area.
Location of the study area
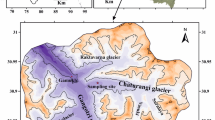
The Chhota Shigri glacier lies between 32°11′–32°17′N and 77°29′–77°33′E in the Lahaul–Spiti, Himachal Pradesh, India. A well-defined snout of this glacier is located in a narrow valley at approximately 4055 m altitude from sea level (Azam et al. 2014; Singh et al. 2015d). Mainly, two tributary glaciers are feeding to the Chhota Shigri glacier from east and west (Ramanathan 2011). The valley of this glacier is 0.5 km wide near the snout and 2–3 km wide at the accumulation zone (Sharma 2007). East side of this glacier, Bara Shigri glacier (total area 131.1 km2 and length 28 km) is situated (Dutt 1961; Berthier et al. 2007; Sangewar and Shukla 2009). The Chhota Shigri glacier expands from 6263 to 4050 m a.s.l having area including tributaries of 15.7 km2 and total length of 9 km (Wagnon et al. 2007). The sampling site for collection of meltwater samples was situated on the stream of this glacier at 32º17′24.58″N and 77°31′55.21″E (Fig. 1).
The climatic condition of the Chhota Shigri glacier is mainly characterized by cold winter (October–April) (Dobhal et al. 1995) and also influenced by Indian monsoon during summer from July to September and mid-latitude westerlies during winter from January to April, hence situated in the monsoon-arid transition zone (Wagnon et al. 2007; Bookhagen and Burbank 2010). Very limited meteorological studies are available on this glacier. During the study period 1987–1989, variations between minimum and maximum temperatures at equilibrium line (4600 m a.s.l) were recorded from −5.2 to 10.5 °C, whereas near the snout minimum and maximum temperatures were recorded to be 4 and 16 °C, respectively (Dobhal et al. 1995).
Geologically, this glacier drains mainly through the Central Crystallines rocks situated in the range of Pir Panjal (Western Himalaya). This Crystalline axis contains mainly gneiss and migmatites (Kumar and Dobhal 1997). Different types of rocks like muscovite-biotite schist, schistose gneiss, augen gneiss, muscovite-quartzite, porphyritic granite and granite gneiss are found between the Bara Shigri and Chhota Shigri glaciers along with light grey green colour feldspar crystal in late-stage pegmatitic veins (Kumar et al. 1987).
Methodology
Meltwater sampling of this glacier was carried out during the whole melt season (May–October 2010). Total 164 samples of meltwater were collected on two times a day at 8 a.m and 6.0 p.m in clean, narrow necked polyethylene bottles (300 ml capacity). After meltwater sampling, pH and electrical conductivity (EC) were analysed by portable electrode. Filtration of meltwater samples was carried out by using pneumatic pump, filtration setup and 0.45 µm Milipore filter papers and stored in cold room at 4 °C for analysis of various dissolved ions.
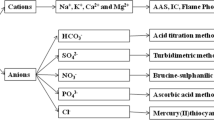
Sodium, calcium, potassium and magnesium were measured by atomic absorption spectrophotometer (AAS). Dissolved silica, nitrate, sulphate, phosphate and bicarbonate were measured by molybdosilicate, brucine-sulphanilic acid, turbidimetric, ascorbic acid and acid titration methods, respectively (APHA 2005). Mercury(II) thiocyanate method was utilized for chloride analysis in the meltwater samples (Florence and Farrar 1971). Result of analytical accuracy was better than ±5% for the analysis of various cations and anions. Piper trilinear plot was plotted with the help of water quality analysis software Aquachem. Statistical Package for Social Sciences (SPSS) was used for statistical analysis.
Results and discussion
The statistical summary of the analytical data obtained from chemical analysis of the meltwater is presented in Table 1. Errors in the charge balance were calculated by the formula:
where TZ− is total anions and TZ+ is total cations. The average value of this error is <9%, showing the quality and reliability of the analytical results. Electrical conductivity (EC) is commonly used as surrogate measure of concentrations of total dissolved ions in meltwater (Collins 1979, 1981; Kang et al. 2000). Electrical conductivity values of the meltwater ranged from 12.7 to 68.8 µs/cm (36.8 ± 15.2 µs/cm), whereas pH values of meltwater ranged between 5.9 and 7.4 (6.6 ± 0.2) showing that the nature of meltwater fluctuated from acidic to slightly alkaline. Bicarbonate was the dominant anion and its concentrations ranged from 130 to 340 µeq/l (216 ± 46.1 µeq/l), constituting 61.7% of the total anions (TZ−), whereas sulphate concentrations fluctuated in between 25.1 and 305 µeq/l (113 ± 74.9 µeq/l), which account for 32.4% of the total anions. Calcium was the major cation, and its concentrations varied from 52 to 258 µeq/l (150 ± 49.1 µeq/l), comprising 51.1% of the total cations (TZ+). After calcium, magnesium was another abundant cation and its concentrations fluctuated from 27.1 to 141 µeq/l (74.2 ± 29.1 µeq/l), constituting 25.4% of the total cations. Dissolved silica concentrations of meltwater varied from 9.2 to 49.1 µmol/l (26.0 ± 11.8 µmol/l).
Solute acquisition processes
Presence of solute in the source water is determined either from weathering of rock minerals (crustal sources) or from snow and ice-melt (atmospheric sources) (Wadham et al. 1998, 2000). The plot between (Ca + Mg) and TZ+ (Fig. 2) depicts strong relationship among the plotted points with equivalent ratios ranged from 0.39 to 0.83 (0.76 ± 0.06). Whereas, (Ca + Mg)/(Na + K) ratios varied from 0.64 to 4.78 with an average ratio of 3.32 ± 0.85. The plot between (Na + K) and TZ+ (Fig. 3) demonstrates comparatively small input of (Na + K) to the TZ+ with an average value of 0.24 ± 0.06. High (Ca + Mg)/(Na + K) and (Ca + Mg)/TZ+ ratios and small input of (Na + K) to the TZ+ show that hydrogeochemistry of the meltwater was mainly governed by carbonate type weathering with minor role from silicate type weathering. The plotted marks of glacier meltwater samples in (Ca + Mg) and SO4 (Fig. 4) scatter plot depict a good correlation between the two data set; showing that sulphate minerals dissolution may supply sulphate in the glacier meltwater (Singh et al. 2015d).
The ratio of SO4 to (SO4 + HCO3) is defined as sulphate mass fraction (SMF), which is used to describe the environments of chemical weathering. Here assuming that the other sources of SO4 is low and unit of ions concentration is in equivalent (Tranter et al. 2002). If SMF value is <0.5, it signifies the importance of carbonation (Eqs. 1, 2) (Tranter et al. 2002), whereas if the value of SMF is 0.5, it shows that meltwater is obtained from the combination of oxidation of sulphide and dissolution of carbonate (Eq. 3) (Tranter et al. 2002).
The average SMF value for meltwater of the Chhota Shigri glacier was calculated to be 0.31 ± 0.12, showing that dissolution of carbonate and sulphide oxidation (which provide SMF ratio of 0.5) cannot supply all the HCO3 − to the meltwater. Therefore, hydrolysis of the carbonate may be arising before oxidation of sulphide (Tranter et al. 1997). In case of SMF value <0.5, excess of HCO3 − over SO4 2− would be produced by the presence of an alternative mechanism for supply of proton such as microbial oxidation of organic carbon in the bed rock (Wadham et al. 2000) and sulphide oxidation may be about the same as carbonation as a driver of chemical weathering at Chhota Shigri glacier.
Even though weathering of rock is the main source of different cations and anions in the glacierized area due to the rock water interaction but atmospheric contribution may also have little input to the solute chemistry of the meltwater. There are very few studies that deal with atmospheric contribution to the chemical characterization of Western Himalayan glaciers meltwater (Sharma et al. 2013; Singh et al. 2015c, d). The plot between (Na + K) against Cl− (Fig. 5) explains that the values of sodium and potassium dominated over chloride. The Na/Cl ratios ranged from 0.9 to 22.4 (3.3 ± 2.9), whereas the ratios of K/Cl in meltwater varied from 0.6 to 17.1 (2.7 ± 2.6). These ratios were significantly higher than the sea aerosols (K/Cl = 0.0176 and Na/Cl = 0.85) displaying minor input of these ions from atmospheric precipitation to the dissolved ions budget of the investigation area as reported by earlier researchers on various aquatic environment (Pandey et al. 1999; Kumar et al. 2009; Sharma et al. 2012, 2013; Singh et al. 2012, 2014, 2015a, c, d; Priya et al. 2016).
Nitrate and phosphate concentrations in the Chhota Shigri glacier meltwater were very low with respect to other dissolved ions showing good quality of meltwater in the investigation area. Anthropogenic activities due to shepherds with lots of goat and sheep in this glacier during the observation period may be the probable source of NO3 − and PO4 3−. Atmospheric input (acidic NO3 − aerosols) (Tranter et al. 1993) may be other possible source of NO3 − in the meltwater.
Classification of hydrogeochemical facies
Classification of hydrogeochemical facies/water type of the study area can be made by plotting the values of dominant anions and cations in the Piper (1944) trilinear plot. Comparable qualities of water will fall together as groups in this diagram hence, used for determination of similarities and differences among them (Todd 2001). On the basis of Piper trilinear diagram (Fig. 6), it is clear that the percentage values of (Ca + Mg), i.e. alkaline earth metals were more than (Na + K), i.e. alkali metals. On the other hand percentage values of HCO3, i.e. (weak acid) was more than (SO4 + Cl), i.e. strong acid. Such type of trend in this diagram shows that carbonate type weathering was the main mechanism governing hydrogeochemistry of the investigation area (Singh et al. 2015d). This diagram indicates that most of the meltwater samples were Ca–HCO3 type of water with minute contribution from Ca–SO4 type of water.
Correlation coefficients between hydrogeochemical parameters
Numerically, correlation coefficient between hydrogeochemical parameters shows the degree to which one variable is statistically correlated with other variable (Vasanthavigar et al. 2013). Result of correlation matrix between various ions in meltwater of the study area is shown in Table 2. EC and TDS displayed significant correlation with HCO3 −, SO4 2−, H4SiO4, Ca2+, Mg2+ and Na+. Such types of good correlation imply that solute chemistry of this glacier was mainly controlled by these chemical parameters. Strong correlation exists between Ca–Mg, Ca–HCO3 and Mg–HCO3, indicating common provenance, which may be derived from carbonate weathering. Ca2+ and Mg2+ also displayed good correlation with SO4 2− indicating similar source, probably derived from the dissolution of sulphate minerals (Singh et al. 2015c, d). H4SiO4 demonstrated potential correlation with Na+ and moderate correlation with K+. On the other hand, Na+ was also moderately correlated with K+, which may be generated from weathering of silicate type minerals.
R-mode factor analysis
For the explanation of relationships among different hydrogeochemical parameters of the Chhota Shigri glacier meltwater, R-mode factor analysis is used here. Table 3 shows factor analysis results of the meltwater. Three factors were identified as a significant contribution of factors because its eigenvalue was greater than one, which explained about 76.9% of the total variance in this result. The eigenvalue and variance in the data set were 7.64 and 58.8% for factor 1, 1.23 and 9.49% for factor 2 and 1.13 and 8.66% for factor 3. Factor 1 demonstrated strong loading for Ca2+, Mg2+, HCO3 −, SO4 2−, H4SiO4, K+, Na+, TDS and EC. This factor described role of silicate and carbonate type weathering, dissolution of sulphate containing minerals and oxidation of sulphide controlling hydrochemistry of the investigation area. In the factor 2, pH and NO3 − have potential loading, showing possible role from atmospheric precipitation as well as anthropogenic input. Cl− and PO4 3− showed strong loading for factor 3, which may explain the role of atmospheric precipitation along with anthropogenic activities inside the Chhota Shigri glacier.
Temporal variations in the dominant anions and cations concentration with discharge
Temporal variations in the dominant anions and cations concentration of glacier meltwater are function of discharge. In general, dissolved ions concentrations showed decreasing trend with increasing meltwater runoff from the glacier. Daily mean meltwater runoff from this Shigri glacier for May–October, 2010 was computed to be 1.2, 3.3, 7.6, 7.9, 2.9 and 0.2 m3 s−1, respectively (Singh et al. 2016). Discharge patterns indicated that August received maximum runoff followed by July, June, September, May and October. Temporal variations in the concentrations of major anions, major cations, TDS and discharge are shown in Fig. 7a–c. Generally, concentrations of TDS and major ions of the Chhota Shigri glacier meltwater were minimum during high flow period (July and August) and maximum during low flow period (September and October). Intense melting takes place during peak melt season due to the presence of large coverage of exposed glacier ice, small snow cover area and higher temperature (Singh et al. 2005, 2006; Han et al. 2010). High discharge increased dilution of the major ions, resulting in low concentrations of major cations, major anions and TDS in the meltwater of the study area during peak flow period, whereas reduction in meltwater runoff occurred during late melt period because of low solar insolation (Singh and Hasnain 1998; Pandey et al. 1999), due to resultant of this rise in TDS and major ions concentrations of the meltwater.
Dissolved load
Dissolved load was calculated by meltwater runoff and total dissolved solid (TDS) of the study area. Daily mean dissolved load for May–October, 2010, was calculated to be 3.41, 9.49, 14.1, 13.2, 7.42 and 0.77 t day−1, respectively. The average daily mean dissolved load during the study period was computed to be 8.06 t day−1. Maximum dissolve load was monitored in July, constituting 29.2% of the total dissolved load after that August 27.3%, June 19.6%, September 15.3%, May 7.0% and October 1.6% of the total dissolved load. Even though TDS (total dissolved solid) concentration in the meltwater of investigation area was low in July and August as compared to the other months, but dissolved load was maximum due to high discharge in the month of July and August. The average daily mean dissolved load of the investigation area was too lower than the Gangotri glacier (250 t day−1) (Singh et al. 2014), may be due to low discharge and geology of the Chhota Shigri glacier.
Chemical denudation rates
Proglacial streams at high altitude are most active driving force for weathering and erosion (Singh and Hasnain 2002). Evaluation of chemical denudation rates includes contribution of the portion of different elements delivered to the water stream in dissolved state from rock weathering in the catchment (Chakrapani et al. 2009). Geochemical denudation rates in the glacierized area are accelerated by the huge supply of mechanically abraded fresh debris from the glacier (Yde et al. 2005). The cation weathering rates were computed from discharge, major cations concentrations and catchment area of this glacier. The cation weathering rates of the meltwater of study area for early (May–June), peak (July–August) and late melt (September–October) seasons were calculated to be 694, 1631 and 481 meq m−2 a−1, respectively. Although major cations concentrations were minimum during peak melt season but due to the presence of high discharge during this period, cation denudation rates were high, whereas during late melt season even though major cations concentrations were high but due to the presence of relatively low discharge during this period, cation denudation rates were minimum in the study area. The chemical weathering rates were calculated from meltwater runoff, dissolved solute concentrations contributed from chemical weathering and catchment area of this glacier. The chemical weathering rates of the meltwater of study area were computed to be 32.8, 60.0 and 25.2 t km−2 year−1 for early, peak and late melt seasons, respectively. Its value was higher during the peak melt period as compared to early and late melt period, may be due to the presence of high discharge from this glacier during the peak melt period. The average chemical weathering rates of the investigation area were lower than the Dokriani glacier (321 t km−2 year−1). This may be due to the presence of high southwest monsoonal rainfall and supraglacial moraines in the Garhwal Himalayan glacier such as Dokriani glacier (Hasnain and Thayyen 1999). Average cation weathering rates of the study area and its comparison with various glaciers are given in Table 4. This table indicates that cation denudation rate of the investigation area was higher than the Longyearbreen, Kuannersuit and Haut Glacier d’Arolla glaciers and lower than the Gornergletscher and Dokriani glaciers.
Conclusions
The present study has focused on chemical characterization of meltwater of the Chhota Shigri glacier, aiming to know about the solute chemistry, weathering and hydrogeochemical processes regulating hydrochemistry and seasonal change in the major ions concentration with discharge. The pH values of meltwater ranged between 5.9 and 7.4 (6.6 ± 0.2) showing that nature of meltwater is fluctuating from acidic to slightly alkaline. The major anions and cations composition in µeq/l are HCO3 − > SO4 2− > Cl− > NO3 − > PO4 3− and Ca2+ > Mg2+ > Na+ > K+, respectively. The predominance of calcium and magnesium to the TZ+ is described by high ratios of (Ca + Mg) to total cations and (Ca + Mg) to (Na + K), showing dominancy of carbonate type weathering as dominant source of solutes in the Chhota Shigri glacier meltwater. Low ratio of sulphate mass fraction (SMF) of meltwater indicates that dissolution of carbonate and oxidation of sulphide cannot supply all the HCO3 − to glacier meltwater (Tranter et al. 1997). Here, excess of HCO3 − over SO4 2− would be generated by microbial oxidation of organic carbon (another proton supply mechanism) in the bed rock of Chhota Shigri glacier (Wadham et al. 2000). The Piper trilinear diagram indicates that Ca–HCO3 is the major hydrogeochemical facies with small contribution from Ca–SO4 type of hydrogeochemical facies. Multivariate statistical methods were used for the identification of solute sources and weathering processes regulating solute acquisition in meltwater of this glacier.
Concentrations of TDS and major ions show inverse relationship with meltwater runoff, meaning their concentrations are minimum during peak flow period (July–August) because of more runoff resulting in dilution of various ions. Hence, major ions and TDS concentrations of meltwater are minimum during peak melt season, whereas their concentrations are maximum during end of melt season (September–October) because of less runoff and low dilution of major ions in the meltwater. The cation weathering rates of meltwater are high during peak melt season because of more runoff and low during late melt period due to low runoff from the investigation area.
References
Ahmad S, Hasnain SI (2000) Meltwater characteristics of Garhwal Himalayan glaciers. J Geol Soc India 56:431–439
Ahmad S, Hasnain SI (2001) Chemical characteristics of stream draining from Dudu glacier: an Alpine meltwater stream in Ganga Headwater Garhwal Himalaya. J China Univ Geosci 12(1):75–83
APHA (2005) Standard methods for examination of water and wastewater. American Public Health Association, Washington
Azam MF, Wagnon P, Vincent C, Ramanathan AL, Linda A, Singh VB (2014) Reconstruction of the annual mass balance of Chhota Shigri glacier (Western Himalaya, India) since 1969. Ann Glaciol 55(66):69–80
Berthier E, Arnaud Y, Kumar R, Ahmad S, Wagnon P, Chevallier P (2007) Remote sensing estimates of glacier mass balances in the Himachal Pradesh (Western Himalaya, India). Remote Sens Environ 108(3):327–338
Bookhagen B, Burbank DW (2010) Toward a complete Himalayan hydrological budget: spatiotemporal distribution of snowmelt and rainfall and their impact on river discharge. J Geophys Res 115(F3):F03019. doi:10.1029/2009JF001426
Brown GH, Sharp MJ, Tranter M, Gurnell AM, Nienow PW (1994) The impact of post-mixing chemical reactions on the major ion chemistry of bulk meltwaters draining the Haut Glacier d’Arolla, Valais, Switzerland. Hydrol Proc 8:465–480
Chakrapani GJ, Saini RK, Yadav SK (2009) Chemical weathering rates in the Alaknanda–Bhagirathi river basins in Himalayas, India. J Asian Earth Sci 34:347–362
Chauhan DS, Hasnain SI (1993) Chemical characteristics, solute and suspended sediment loads in the meltwaters draining Satopanth and Bhagirath Kharak glaciers, Ganga Basin, India. In: Snow and glacier hydrology (Proceedings of the Kathmandu Symposium, November 1992), IAHS Publication Number 218, pp 403–410
Collins DN (1978) Hydrology of an alpine glacier as indicated by the chemical composition of meltwater. Zeitschrift fur Gletscherked Glazialgeologie 13:219–238
Collins DN (1979) Hydrochemistry of meltwaters draining from an Alpine glacier. Arct Alp Res 11(3):307–324
Collins DN (1981) Seasonal variation of solute concentration in melt waters draining from an Alpine glacier. Ann Glaciol 2:11–16
Dimri AP (2004) Impact of horizontal model resolution and orography on the simulation of a western disturbance and its associated precipitation. Meteorol Appl 11(2):115–127
Dobhal DP, Kumar S, Mundepi AK (1995) Morphology and glacier dynamics studies in monsoon–arid transition zone: an example from Chhota Shigri glacier, Himachal Himalaya, India. Curr Sci 68(9):936–944
Dutt GN (1961) The Bara Shigri glacier, Kangra District, East Punjab, India. J Glaciol 3(30):1007–1015
Florence TM, Farrar YJ (1971) Spectrophotometric determination of chloride at the parts-per-billion level by the mercury(II) thiocyanate method. Anal Chim Acta 54:373–377
Gaillardet J, Dupre B, Louvat P, Allegre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30
Han H, Liu S, Wang J, Wang Q, Xie C (2010) Glacial runoff characteristics of the Koxkar Glacier, Tuomuer-Khan Tengri Mountain Ranges, China. Environ Earth Sci 61:665–674
Hasnain SI, Thayyen R (1996) Sediment transport and solute variation in meltwater of Dokriani glacier (Bamak), Garhwal Himalaya. J Geol Soc India 47:731–739
Hasnain SI, Thayyen RJ (1999) Controls of major-ion chemistry of the Dokriani glacier meltwaters, Ganga basin Garhwal Himalaya. J Glaciol 45(149):87–92
Hren MT, Chamberlain CP, Hilley GE, Blisniuk PM, Bookhagen B (2007) Major ion chemistry of the Yarlung Tsangpo-Brahmaputra river: chemical weathering, erosion, and CO2 consumption in the southern Tibetan plateau and eastern syntaxis of the Himalaya. Geochim Cosmochim Acta 71:2907–2935
Immerzeel WW, van Beek LPH, Bierkens MFP (2010) Climate change will affect the Asian water towers. Science 328:1382–1385
Kang SC, Qin DH, Yao TD, Ren JW (2000) A study on precipitation chemistry northern slope of Mt. Xixiabangma. Acta Scientiae Circumstantiae 20:574–578
Kumar S, Dobhal DP (1997) Climatic effects and bedrock control on rapid fluctuations of Chhota Shigri glacier, northwest Himalaya, India. J Glaciol 43(145):467–472
Kumar S, Rai H, Purohit KK, Rawat BRS, Mundepi AK (1987) Multi disciplinary glacier expedition to Chhota Shigri glacier. Department of Science and Technology, Government of India, New Delhi, Technical Report Number 1, pp 1–29
Kumar K, Miral MS, Joshi S, Pant N, Joshi V, Joshi LM (2009) Solute dynamics of meltwater of Gangotri glacier, Garhwal Himalaya, India. Environ Geol 58:1151–1159
Mehta M, Dobhal DP, Srivastava D (2013) Influence of debris cover on terminus retreat and mass changes of Chorabari Glacier, Garhwal region, central Himalaya, India. J Glaciol 59(217):961–971
Metcalf RC (1986) The cationic denudation rate of an Alpine glacier catchment. Zeitschrift fur Gletscherked Glazialgeologie 22(1):19–32
Millot R, Gaillardet J, Dupre B, Allegre CJ (2003) Northern latitude chemical weathering rates: clues from the Mackenzie River Basin, Canada. Geochim Cosmochim Acta 67(7):1305–1329
Mohanty UC, Dimri AP (2004) Location specific prediction of probability of occurrence and quantity of precipitation over Western Himalayas. Weather Forecast 19(3):520–533
Pandey SK, Singh AK, Hasnain SI (1999) Weathering and geochemical processes controlling solute acquisition in Ganga Headwater–Bhagirathi River, Garhwal Himalaya, India. Aquat Geochem 5(4):357–379
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–923
Priya N, Thayyen RJ, Ramanathan AL, Singh VB (2016) Hydrochemistry and dissolved solute load of meltwater in a catchment of cold-arid trans-Himalayan region of Ladakh over an entire melting period. Hydrol Res. doi:10.2166/nh.2016.156
Rainwater FH, Guy HP (1961) Some observations on the hydrochemistry and sedimentation of the Chamberlain glacier area Alaska. United States Geological Survey Professional Paper 414-c, pp cl–c14
Raiswell R (1984) Chemical models of solute acquisition in glacial meltwaters. J Glaciol 30(104):49–57
Ramanathan AL (2011) Status report on Chhota Shigri glacier (Himachal Pradesh). Himalayan Glaciology Technical Report Number 1, Department of Science and Technology, Ministry of Science and Technology, New Delhi
Sangewar CV, Shukla SP (2009) Inventory of the Himalayan glaciers. Special Publication Number 34, Geological Survey of India
Sharma P (2007) Mass balance and chemical characteristics of Chhota Shigri Glacier-B, Lahaul-Spiti Valley, Himachal Pradesh. Ph.D. Thesis, Jawaharlal Nehru University, New Delhi
Sharma A, Singh AK, Kumar K (2012) Environmental geochemistry and quality assessment of surface and subsurface water of Mahi River Basin, Western India. Environ Earth Sci 65:1231–1250
Sharma P, Ramanathan AL, Pottakkal JG (2013) Study of solute sources and evolution of hydrogeochemical processes of the Chhota Shigri glacier meltwaters, Himachal Pradesh, India. Hydrol Sci J 58(5):1128–1143
Sharp M, Brown GH, Tranter M, Wills IC, Hubbard B (1995) Comments on the use of chemically based mixing models in glacier hydrology. J Glaciol 41(138):241–246
Singh AK, Hasnain SI (1998) Major ion chemistry and weathering control in a high altitude basin: alaknanda river, Garhwal Himalaya, India. Hydrol Sci J 43(6):825–843
Singh AK, Hasnain SI (2002) Aspects of weathering and solute acquisition processes controlling chemistry of sub-Alpine proglacial streams of Garhwal Himalaya, India. Hydrol Proc 16:835–849
Singh VB, Ramanathan AL (2015) Assessment of solute and suspended sediment acquisition processes in the Bara Shigri glacier meltwater (Western Himalaya, India). Environ Earth Sci 74:2009–2018
Singh VB, Ramanathan AL (2017) Characterization of hydrogeochemical processes controlling major ion chemistry of the Batal glacier meltwater, Chandra Basin, Himachal Pradesh, India. Proc Natl Acad Sci India Sect A Phys Sci 87(1):145–153
Singh AK, Pandey SK, Panda S (1998) Dissolved and sediment load characteristics of Kafni glacier meltwater, Pindar valley, Kumaon Himalaya. J Geol Soc India 52:305–312
Singh P, Haritashya UK, Ramasastri KS, Kumar N (2005) Diurnal variations in discharge and suspended sediment concentration, including runoff-delaying characteristics of the Gangotri glacier in the Garhwal Himalayas. Hydrol Proc 19:1445–1457
Singh P, Haritashya UK, Kumar N, Singh Y (2006) Hydrological characteristics of the Gangotri glacier, central Himalayas, India. J Hydrol 327:55–67
Singh VB, Ramanathan AL, Pottakkal JG, Sharma P, Linda A, Azam MF, Chatterjee C (2012) Chemical characterisation of meltwater draining from Gangotri Glacier, Garhwal Himalaya, India. J Earth Syst Sci 121(3):625–636
Singh VB, Ramanathan AL, Pottakkal JG, Kumar M (2014) Seasonal variation of the solute and suspended sediment load in Gangotri glacier meltwater, central Himalaya, India. J Asian Earth Sci 79:224–234
Singh VB, Ramanathan AL, Pottakkal JG, Kumar M (2015a) Hydrogeochemistry of meltwater of the Chaturangi glacier, Garhwal Himalaya, India. Proc Natl Acad Sci India Sect A Phys Sci 85(1):187–195
Singh VB, Ramanathan AL, Kuriakose T (2015b) Hydrogeochemical assessment of meltwater quality using major ion chemistry: a case study of Bara Shigri glacier, Western Himalaya, India. Natl Acad Sci Lett 38(2):147–151
Singh VB, Ramanathan AL, Sharma P (2015c) Major ion chemistry and assessment of weathering processes of the Patsio glacier meltwater, Western Himalaya, India. Environ Earth Sci 73:387–397
Singh VB, Ramanathan AL, Sharma P, Pottakkal JG (2015d) Dissolved ion chemistry and suspended sediment characteristics of meltwater draining from Chhota Shigri glacier, Western Himalaya, India. Arab J Geosci 8:281–293
Singh VB, Ramanathan AL, Pottakkal JG (2016) Glacial runoff and transport of suspended sediment from the Chhota Shigri glacier, Western Himalaya, India. Environ Earth Sci 75:695
Souchez RA, Lemmens MM (1987) Solutes. In: Gurnell AM, Clark MJ (eds) Glacio-fluvial sediment transfer. Wiley, Chichester, pp 285–303
Srinivasulu J, Kulkarni AV (2004) Estimation of spectral reflectance of snow from IRS-1D LISS-III sensor over the Himalayan terrain. Proc Indian Acad Sci (Earth Planet Sci) 113(1):117–128
Todd DK (2001) Groundwater hydrology. Wiley, Hoboken
Tranter M, Brown GH, Raiswell R, Sharp MJ, Gurnell AM (1993) A conceptual model of solute acquisition by Alpine glacier meltwaters. J Glaciol 39(133):573–581
Tranter M, Sharp MJ, Brown GH, Wills IC, Hubbard BP, Nielsen MK, Smart CC, Gordon S, Tulley M, Lamb HR (1997) Variability in the chemical composition of in situ subglacial meltwater. Hydrol Proc 11:59–77
Tranter M, Sharp MJ, Lamb HR, Brown GH, Hubbard BP, Willis IC (2002) Geochemical weathering at the bed of Haut Glacier d’Arolla, Switzerland––a new model. Hydrol Proc 16:959–993
Vasanthavigar M, Srinivasamoorthy K, Prasanna MV (2013) Identification of groundwater contamination zones and its sources by using multivariate statistical approach in Thirumanimuthar sub-basin, Tamil Nadu, India. Environ Earth Sci 68:1783–1795
Vaux JH, Balk D, Cook ER, Gleick P, Lau WK, Levy M, Malone EL, Mcdonald R, Shindell D, Thompson LG, Wescoat JL, Williams MW (2012) Himalayan glaciers: climate change, water resources and water security. National Academies Press, National Academy of Sciences, Washington
Wadham JL, Hodson AJ, Tranter M, Dowdeswell JA (1998) The hydrochemistry of meltwater draining a polythermal-based, high Arctic glacier, south Svalbard: I. The ablation season. Hydrol Proc 12:1825–1849
Wadham JL, Tranter M, Dowdeswell JA (2000) Hydrochemistry of meltwaters draining a polythermal-based, high-Arctic glacier, south Svalbard: II. Winter and early spring. Hydrol Proc 14:1767–1786
Wagnon P, Linda A, Arnaud Y, Kumar R, Sharma P, Vincent C, Pottakkal JG, Berthier E, Ramanathan AL, Hasnain SI, Chevallier P (2007) Four years of mass balance on Chhota Shigri glacier (Himachal Pradesh, India), a new benchmark glacier in the Western Himalaya, India. J Glaciol 53(183):603–611
West AJ, Galy A, Bickle MJ (2005) Tectonic and climatic controls on silicate weathering. Earth Planet Sci Lett 235(1–2):211–228
Wu WH, Xu SJ, Yang JD, Yin HW (2008) Silicate weathering and CO2 consumption deduced from the seven Chinese rivers originating in the Qinghai–Tibet Plateau. Chem Geol 249:307–320
Yde JC, Knudsen NT, Ole BN (2005) Glacier hydrochemistry, solute provenance, and chemical denudation at a surge-type glacier in Kuannersuit Kuussuat, Disko Island, West Greenland. J Hydrol 300:172–187
Yde JC, Riger-Kusk M, Christiansen HH, Knudsen NT, Humlum O (2008) Hydrochemical characteristics of bulk meltwater from an entire ablation season, Longyearbreen, Svalbard. J Glaciol 54(185):259–272
Acknowledgements
We thank to Department of Science and Technology (DST), Govt. of India for financial support for this research work. Dr. P. Sharma, Dr. J.G. Pottakkal and Dr. A. Linda are acknowledged for collection of few meltwater samples during the study period. Virendra Bahadur Singh is grateful to Science and Engineering Research Board (SERB), Department of Science and Technology, Govt. of India for granting National Postdoctoral Fellowship (Reference No. PDF/2016/000286) to him. The authors are also thankful for partial funding given to us by SAC, CIFIPRA, INDICE, CHARIS, GLACINDIA and IRD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article is part of a Topical Collection in Environmental Earth Sciences on “Water in Central Asia”, guest edited by Daniel Karthe, Iskandar Abdullaev, Bazartseren Boldgiv, Dietrich Borchardt, Sergey Chalov, Jerker Jarsjö, Lanhai Li and Jeff Nittrouer.
Rights and permissions
About this article
Cite this article
Singh, V.B., Ramanathan, A. Hydrogeochemistry of the Chhota Shigri glacier meltwater, Chandra basin, Himachal Pradesh, India: solute acquisition processes, dissolved load and chemical weathering rates. Environ Earth Sci 76, 223 (2017). https://doi.org/10.1007/s12665-017-6465-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6465-4