Abstract
Interventional radiology provides local management of bone metastases (BM) with a palliative intent in most cases, or with a curative intent in selected patients. Its role has rapidly expanded in the last decade, offering new treatment solutions often in combination with surgery, radiation therapy and medical treatments. The aim of the present paper is to increase awareness, acceptance and adoption of interventional radiology procedures for the treatment of BM; and to present the joint position of the Italian College of Musculoskeletal Radiology and the Italian College of Interventional Radiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Epidemiology and clinical manifestations of bone metastases
Bone metastases (BM) are the most common malignant lesions of the bone, commonly involving the axial skeleton, pelvic ring and proximal extremities [1]. In the USA, it is estimated that approximately 100,000 individuals develop BM every year [2]; and the incidence is expected to increase in the future due to the improved survival of cancer patients. The incidence of BM is particularly high in patients suffering from breast, prostate or lung cancer; intermediate in those presenting with melanoma, renal or thyroid cancer; and relatively low in patients presenting with gastrointestinal tumors.
BM significantly impact patients’ clinical status due to pain, fractures, compression of nearby structures such as nerves, hypercalcemia, and often require radiation therapy (RT) and/or surgery, especially when spinal cord compression is noted. All these events are commonly known as skeletal-related events (SREs) [3]. Pain is the most common SRE and may have a nociceptive, neuropathic, or mechanical origin [4]. Fractures are the second most common SRE and have a significant impact on patients’ quality of life and survival [1, 3]. Usually three types of fractures occur in cancer patients, namely pathologic fractures (PF), impending fractures (ImF), and fractures related to bone insufficiency (BiF). PF are a direct consequence of bone weakness due to the presence of BM, which are generally lytic and located in weight-bearing bones. ImF are painful BM in weight-bearing areas, often requiring preventive consolidation to avoid PF. Lastly, BiF result from bone reabsorption due to medical therapies (e.g., hormonal therapies, steroids) and/or cytokines produced by the tumor [5,6,7].
Current treatment modalities to treat pain and prevent fractures are medical therapy (including analgesics, bisphosphonates and denosumab) and RT; both allow good but far from excellent results. As a matter of fact, it has been demonstrated that pain is not adequately treated in 56–82.3% patients [8]. Although RT is considered the best evidence-based non-interventional treatment for BM-related pain, several limitations have been observed: (a) ineffectiveness in case of radio-resistant tumors such as renal cancer or melanoma; (b) 1–2-week latency between the end of treatment and the onset of pain relief; (c) overall (complete and partial) pain relief observed in less than 60% of patients and recurrent pain in up to 50% of those who respond by 20–24 weeks after the end of treatment; (d) retreatment is not always possible if the maximal radiation dose has already been delivered; (e) no immediate bone consolidation is provided [3,4,5]. Although the recently introduced tumoricidal stereotactic body RT yields higher rates of local tumor control and pain management compared with standard external beam RT [9, 10], the risk of secondary fracture is significantly increased when the former technique is used [9]. Despite all the aforementioned drawbacks, RT still remains the most common treatment for BM, especially for patients in poor general conditions. On the other hand, bone-targeting agents interfering with tumor-mediated osteolysis, can reduce but not completely remove the risk of SREs [3].
In this setting, interventional radiology procedures may play an important primary or complementary role to manage BM [11,12,13], especially in the palliative setting where pain and fracture management is necessary.
The aim of the present paper is to increase awareness, acceptance and adoption of interventional radiology procedures for the treatment of BM; and to present the joint position of the Italian College of Musculoskeletal Radiology and the Italian College of Interventional Radiology.
Interventional procedures
Osteoplasty
Indications
Percutaneous osteoplasty refers to polymethylmethacrylate (PMMA) injection into weakened or fractured bone. Osteoplasty is shown to be effective in achieving consolidation and pain relief in patients affected by painful lytic BM at risk for PFs. Osteoplasty is also currently used to treat BiF. The best indication of osteoplasty is bones in which compressive stress is predominant, such as in the vertebral body, the acetabulum and the proximal/distal epiphyses of long bones.
In the vertebral body, despite an increased risk for epidural leakage, posterior wall disruption does not represent an absolute contraindication to vertebroplasty [14] (Fig. 1). On the other hand, in case of tumor compression of the spinal cord or in case of vertebral instability due to tumor involvement of the posterior elements of the vertebra, surgical decompression/stabilization should be considered as early as possible. In long bones, pure diaphyseal consolidation by osteoplasty should be avoided unless the intent is purely palliative in an end-stage bedridden patient. This is due to the fact that PMMA has limited resistance to torsion and bending; therefore, surgical endomedullary nailing is the preferred option to achieve effective biomechanical stabilization. Moreover, prior osteoplasty precludes endomedullary nailing, rendering surgical external fixation the only available option when fixation is required [15].
a, b Painful lytic metastasis of T12 in a 70-year*-old patient suffering from hepatocellular carcinoma; the metastasis disrupted the posterior wall of the vertebral body (white arrows). c, d Percutaneous vertebroplasty was safely performed with immediate pain relief and no cement leakage (vertebroplasty was also performed in T11 and L1 during the same interventional session due to painful pathological fractures)
Absolute contraindications to osteoplasty are local/systemic infection, known allergy to the PMMA and irreversible coagulopathy.
Technique
The procedure can be performed under fluoroscopic and/or CT-guidance often under local anesthesia/mild sedation. PMMA is injected under continuous fluoroscopic guidance through a 10–13 G trocar in its toothpaste consistency. PMMA-based cements certified for radiological use are radiopaque, which enables the operator to monitor the PMMA distribution within the bone and allows the prevention of leakages. The consolidation time of PMMA is variable (8–20 min), and its polymerization results in an exothermic reaction with temperature > 70 °C, which is, however, inadequate to achieve effective local tumor control.
Results
Osteoplasty has been proved to be effective for the symptomatic treatment of osteolytic tumors including multiple myeloma. In the spine, effective and long-lasting pain control is achieved in 60–85% cases [16]. Outside the spine, referring mainly to the pelvic ring, similar results have been reported [17]; however, careful injection is warranted, especially for acetabular tumors with cortical disruption, as intra-articular leakage may result in rapidly evolving chondrolysis [18].
For long bones, effective pain management and functional amelioration can be achieved in 89.4% and in 71.8% cases, respectively [15]; nevertheless, the rate of secondary fractures is estimated to be as high as 8–9.1% with the highest rate (40.6% at 1-year) reported in the proximal femur [15, 19, 20].
Osteosynthesis
Indications
Percutaneous osteosynthesis is consistent with screw fixation to consolidate minimally/non-displaced fractures of the pelvic ring [21]. The technique can also be performed in the proximal femur to consolidate ImF (i.e., Mirels’ score ≥ 8) without significant trochanteric and cortical involvement [22, 23]. Additionally, fractures of the shoulder girdle have been also reported to be managed by this technique [24] (Fig. 2).
Whenever possible, surgery should be preferred to percutaneous osteosynthesis; since the long-term effectiveness of this technique still requires further supporting evidence. Accordingly, osteosynthesis should be strictly reserved for non-surgical cancer patients with limited life expectancy to provide rapid analgesia and mobilization without the need for suspension of systemic therapies, which is often necessary with surgical treatments. Moreover, compared to surgery, risk of bleeding and infection is also significantly reduced.
Technique
Percutaneous osteosynthesis is performed under fluoroscopic and/or CT-guidance. Due to the long procedural time (around 2 h due to challenging bone access), general anesthesia is usually preferred over local anesthesia/mild sedation.
Threaded screws are used in case of mildly displaced fractures in order to achieve maximal inter-fragment compression. Totally threaded, non-compressive screws are preferred in cases of non-displaced fractures. Commonly applied screws are self-drilling self-tapping thus allowing for accurate cut and thread and avoidance of cut bone jamming. Finally, PMMA-injectable screws providing distal holes are preferred in cases of advanced osteoporotic bone to enhance screw anchoring.
The most challenging phase of the procedure is often the perpendicular bridging of the fracture line by means of a 1.8–2 mm k-wire; after which, the screw is manually advanced over the wire by means of a screwdriver until its distal tip is safely anchored within distal healthy bone, and its head abuts the cortical bone.
Results
Osteosynthesis has been shown to be effective for the symptomatic treatment of pelvic fractures or those of the proximal femur. In a study of 64 patients undergoing pelvic osteosynthesis alone or in combination with osteoplasty, a median pain reduction of 6/10 points was noted, with only two secondary fractures observed in the proximal femur [25]. Another similar series of 33 patients reported an effective analgesic/functional amelioration in 87.1% cases at 1-month follow-up [21]; nevertheless, three major and one minor complications were reported. Moreover, although no secondary fractures were reported, unfavorable local evolution of the treated site (i.e., poor consolidation and/or screw loosening) was noted in 12.5% cases on imaging follow-up (mean 8.7 month) thus, suggesting the need for strict clinical follow-up. Additionally, in a small series of 11 osteosyntheses of the proximal femur, rapid pain control and consolidation were reported (significant and mild amelioration in pain/walking in 63.6% and 27.3% patients, respectively) at 1-month follow-up, without any secondary fracture [23].
Thermal ablation
Indications
Thermal ablation is used to destroy tumors through the direct application of heat- or cold-based energies [13, 26,27,28,29], delivered percutaneously with the exception of High-intensity focused ultrasound (HIFU). Thermal ablation can be performed with a curative or palliative intent (Fig. 3).
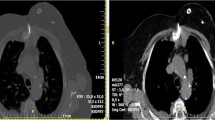
Curative treatment. a Single metastasis (white arrow) of the rib in a 45-year-old patient with a history of breast cancer. b The metastasis was FDG-avid on PET-CT (white arrow). c After cryoablation, d effective local tumor control was noted at 12-month PET-CT follow-up. Palliative treatment. e Painful (VAS 9/10) T5 FDG-avid metastasis from gastric cancer in a 68-year-old patient. f Bilateral bipolar RFA was performed with thermal monitoring at the level of the posterior wall (white arrow) and hydro-dissection of the epidural space (black arrow); g, h in the end, vertebroplasty was performed to prevent a secondary vertebral body collapse. Pain relief (5/10) was noted by the 15th postoperative day
All techniques applying heat-based energy achieve tumor destruction through coagulation necrosis. On the other hand, cryo-mediated damage is much more complex [30]. The final necrotic volume depends on the amount of energy delivered and the local tissue characteristics such as vascularization, which may be responsible for energy dissipation (i.e., heat/cold-sink effect) [26, 31].
Compared to RT, the effects of ablation are immediate and there is no limit to the number of ablative treatments that can be performed on the same tumor, which is not the case for RT as it cannot be repeated once the maximum dose of the target organ is reached. In the majority of cases, bone consolidation can be combined with ablation in the same session.
Anesthesia (deep sedation, general anesthesia, nerve block or spinal anesthesia) is usually required [32]. Although all imaging modalities can be potentially used, CT and fluoroscopy play a major role.
Absolute contraindications include coagulation impairment and infection. Antibiotic prophylaxis remains controversial although it is generally applied [32].
Techniques
Laser Lasers deliver electromagnetic energy in the infrared wavelength through small optic fibers deployed coaxially into 18G needles. Lasers do not interfere with metallic implants and are fully MRI compatible. However, only small ablation zones can be obtained since, even when multiple fibers are activated simultaneously, ablations zones rarely exceed 2–3 cm [31,32,33]; for this reason, lasers can be applied to treat only small benign bone tumors such as osteoid osteoma [34].
Radiofrequency ablation (RFA) RFA is probably the most commonly applied technique in bone ablation [31, 33, 35]. Coagulative necrosis is achieved through ionic agitation (i.e., frictional heating) induced by electric current delivered through electrodes. For this reason, RFA cannot be used if pacemakers or other implantable electric devices are present, or in case of osteoblastic BM impeding effective conduction of the electrical current, which can flow between the electrode and the grounding pads attached on the skin of the patient (unipolar system), or between two electrodes or two dipoles localized at the distal tip of the same electrode (bipolar system). Compared to the unipolar system, the bipolar electrode requires a smaller power and produces smaller but more predictable ablation zones. For these reasons, they are specifically used in the spine [36, 37]. Once heat is generated, it diffuses away from the electrode tip by contiguity. High impedance (i.e., tissue resistance in receiving the electrical current, for example, in sclerotic BM) represents the main limitation in achieving large ablation zones. Electrodes are generally cooled with cold saline to avoid tissue charring and carbonization around the tip with the aim of reducing impedance, thus enlarging the ablation zone. Nevertheless, RFA cannot achieve ablation zones that exceed 3–4 cm.
MicroWave Ablation (MWA) MWA uses electromagnetic energy, which is delivered through an antenna [31, 35, 38]. Electromagnetic waves induce molecular agitation and subsequent tissue heating. Compared to RFA, MWA energy can radiate through all biological tissues and enables faster and larger (up to 5 cm when multiple antennas are activated simultaneously) ablations. The main drawbacks of MWA are: (a) oval-shaped ablations, especially with first generation antennas; (b) limited experience available with BM due to the fact that the technique is relatively new [39, 40].
Cryoablation (CA) CA destroys tumors using cold temperatures (up to -40 °C) dissipated into tissues through dedicated cryoprobes [30,31,32,33, 35]. Rapid argon decompression at the distal tip of the probe is responsible for iceball growth, while helium is used to melt the iceball (i.e., active thawing). Repeated cycles of freezing–thawing have been demonstrated to be more effective in enlarging the iceball size. For this reason, a double 10-min freeze cycle interrupted by a 10-min thaw is the classic ablation protocol. Cellular death occurs through a complex mechanism that is not completely understood, including mechanical damage to cellular membranes induced by ice crystals, osmotic changes into the tissue, endothelial injury, ischemia, and cryo-immunological effects. Compared to the other techniques of ablation, the main advantages of CA are: (a) precise control of the ablation area due to adequate visualization by common imaging modalities (especially CT or MRI), thus allowing for precise intra-operative evaluation of tumor coverage; (b) multiple probes (up to 40 with the most recent systems, each with different sizes and shapes of ablation) can be simultaneously activated with a final synergistic effect; therefore, the iceball can be shaped according to tumor morphology, and large-volume BM can be treated; (c) possibility of treating osteoblastic BM since the iceball can easily go through cortical and blastic bone; (d) since the iceball has intrinsic anesthetic properties, the procedure is less painful compared to heat-based techniques; accordingly, CA is preferred for the treatment of BM with soft tissue extension. In terms of drawbacks, CA is expensive and time-consuming; moreover, it requires enough space in the operating theatre to lodge gas bottles, unless mural systems delivering the gas are available.
HIFU HIFU is a heat-based technique that does not require any skin incision or needle insertion to destroy the tumor as it works with a focalized ultrasound beam (i.e., mechanical energy) that is generated by a transducer placed on the skin of the patient [31, 41,42,43,44]. The beam passes through tissues without damaging them, and is focused on the target lesion where the mechanical energy is converted into thermal energy. The most advanced technique of guidance is MRI, and the combined system is called MRgFUS (i.e., Magnetic Resonance-guided Focused Ultrasound Surgery). Apart from its totally “non-invasive” profile, MRgFUS has the benefit of allowing optimal MRI resolution to target BM, and allows monitoring of the temperature reached within the tumor, surrounding it as well as in interposed tissues (except bone). Nevertheless, the technique is still not considered safe for spinal ablation and accordingly only superficial or lytic BM showing cortical disruption are treated by this technique. On the other hand, since bone can absorb the ultrasound beam, it is possible to induce high periosteal temperatures to achieve effective pain management in case of painful BM. Drawbacks include limited availability of the technique, long procedural time (2–3 h), and contraindication in patients contraindicated to MRI.
Protective measures to adopt during bone ablation Thermal ablation is generally considered safe; nevertheless, the most common complications include the unintentional ablation of nearby non-target organs, particularly nerves. Therefore, careful mapping of the regional anatomy is mandatory to identify structures at risk. Protective measures should be adopted when there is less than 10 mm safety margin between the presumed ablation area and nearby non-target structure.
Several different protective measures are available and include: (a) mechanical displacement of the ablation area (i.e., gentle retraction of the cryoprobe during CA); (b) physical displacement of the non-target structure; (c) clear visualization of the ablation zone with common imaging (i.e., iceball monitoring); (d) temperature monitoring/adjustment in close proximity to the non-target structure; (e) monitoring of the functional status of the non-target structure (i.e., nerve root). In bone ablation, all these measures can be applied (Table 1) alone or in combination to avoid iatrogenic injuries, in particular for nerves and skin. CO2 or fluids are often injected to achieve physical displacement. CO2 is preferred over room air since it is much more soluble thus limiting the risk of air embolism. Simple saline (or 5% electrically inert dextrose solution in case of RFA) can be also used (at adjusted dilutions with iodine contrast medium to optimize the visualization under CT-guidance [45]) to displace the non-target organs (i.e., “salinoma”). The great advantage of fluid dissection over CO2 is that fluid temperature may allow for warming-up or cooling-down of the non-target structure during ablation.
Angioplasty balloon (5–10 mm × 20–40 mm) interposition is rarely used due to the high risk of nerve damage during balloon manipulation.
Temperature monitoring is usually achieved directly through percutaneous deployment of thermocouples or fiber-optic sensors that can be easily combined with fluid dissection, especially when the latter alone is not deemed sufficient. In case of MRgFUS ablation, non-invasive MRI-mediated temperature monitoring can be obtained.
Electrostimulation and somatosensory-evoked potentials allow for the functional monitoring of nerves during the ablation and are particularly useful since nerve roots are very sensitive outside the physiologic range (10–45 °C) [46,47,48,49,50]. Finally, when BM originating from superficial bones are treated, skin protection should be provided. Usually, fluids (including local anesthetic agents) are injected within the sub-cutaneous tissue to increase its width; additionally, sterile gloves filled with hot or cold saline can be transiently applied on the target area to mitigate skin temperature change during the ablation.
Results
Despite the relative few patients that can benefit from curative treatments, growing evidence is demonstrating the effectiveness of such therapeutic treatments in selected patients (Table 2). Interestingly, encouraging data have also been reported in patients affected by tumors that are commonly considered aggressive, such as lung cancer [51]. Accordingly, it is important to take into account each patients’ tumor biology when selecting candidates for curative ablation.
A considerable number of papers provide evidence of ablation in achieving fast and effective pain relief in patients presenting with painful BM (Table 3). The largest experience has been obtained with RFA and CA. Nevertheless, MWA and HIFU have also recently been shown to be effective. Given the “non-invasive” profile of HIFU, it is likely that it will obtain a prominent position in the future for the palliative management of BM.
Embolization
Indications
The aim of trans-arterial embolization (TAE) is to devascularize hyper-vascular BM and to preserve all non-target vessels. Accordingly, TAE should be as selective as possible.
TAE can be applied in case of hyper-vascular BM in order to:
-
a.
minimize the blood loss during subsequent surgery; in this scenario, TAE should be performed within 3 days from surgery to reduce the risk of tumor revascularization [52,53,54,55];
-
b.
reduce pain and spontaneous bleeding of tumors that cannot benefit from surgical or percutaneous treatment;
-
c.
reduce tumor vascularization before percutaneous ablation to limit the heat/cold-sink effect (Fig. 4).
Fig. 4 a Large (13 cm) painful right iliac metastasis from hepatocellular carcinoma in a 55-year-old patient undergoing combined single-session embolization and cryoablation; due to the metastasis, the patient was bedridden. b, c Percutaneous embolization (with glue) was performed just before cryoablation to reduce the cold-sink effect. d, e Cryoablation was accomplished by means of 10 cryoprobes producing a large iceball (*), and with concomitant hydro-dissection to protect the femoral nerve (white arrow). f 1-month MRI follow-up showed complete devascularization of the tumor (*); at the same time interval the patient was able to walk again
Technique
Many techniques and embolic agents can be used depending on indication, vessels (i.e., size, presence of arterio-venous shunts) and regional anatomy.
In the spine, special consideration to the anterior spinal artery must be made to avoid unintentional embolization resulting in spinal cord ischemia.
Embolic agents can be classified as temporary or permanent and as liquid or solid. Permanent agents are most commonly used for preoperative TAE and for palliative cases; however, temporary agents such as gelatin sponge have also shown their efficacy before surgery [56]. Liquid agents, such as N-butyl cyanoacrylate and Onyx, are more useful (sometimes in combination with coils or plugs) in the presence of arterio-venous shunts. Lastly, particles are most commonly used for bone devascularization and the choice of particles diameter (40–1200 micron) is dependent on vessel size and desired distal embolization.
Results
In a large series of 93 patients undergoing pre-surgical embolization of spinal tumors, the benefits of embolization have been demonstrated in terms of reduction of intra-operative blood loss, especially when hyper-vascular BM from renal cell carcinomas were treated, and extensive surgery (corpectomy/vertebrectomy) was performed [57].
Another series of 243 patients undergoing BM embolization reported > 50% and 97% reduction in pain and analgesic consumption, respectively. Nevertheless, post-embolization syndrome, ischemic pain at the embolization site, paresthesia, skin breakdown, and sub-cutaneous necrosis were observed in 35% patients [58].
Pre-procedure work-up, clinical and technical requirements
Normal coagulation parameters, renal function and the absence of infection should be checked. The patients’ allergy history should also be recorded. Patients’ current medical therapy with particular consideration to anticoagulants and/or antiplatelets should be carefully reviewed and adjusted before the procedure.
Anesthesiology assistance is mandatory during interventional procedure on BM: the choice of the best type of anesthesia should be performed in accordance with the anesthesiology team. Strict sterile environment is required.
After careful review of the diagnostic radiological examinations available, the interventional radiologist should accurately plan the procedure and note the following on a dedicated form: the goal of the procedure (palliative vs. curative); all the non-target structures at risk and protective measures necessary to prevent iatrogenic damage; the need for biopsy (i.e., the primary cancer is unknown; more than one primary tumors are suspected; a molecular target for directed medical therapy can be identified); and the risk of fracture directly related to the BM or secondary to the interventional procedure such as in case of ablation or embolization.
All these details should be documented and discussed with the patient during a dedicated outpatient consultation [39]; in this occasion, a written informed consent should also be obtained.
Interventional strategies
According to the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Quality Improvement guidelines for bone tumor management, the strategy of the interventional treatment can be curative or palliative (Fig. 5) [32].

(Adapted from reference [32])
Curative or palliative treatment for the management of BM.
Curative treatment can be proposed to selected oligometastatic patients presenting with limited bone disease (< 3 potentially treatable BM, each ≤ 3 cm in size). A series of prognostic factors favouring curative treatments have been identified: oligometastatic/metachronous status; the absence or limited cortical bone disruption; limited BM size (< 2 cm); long life expectancy; and good patient performance status [59]. Moreover, curative treatments may be indicated in patients with a slow-evolving disease [60], or in those presenting with a long-lasting stability of the systemic metastatic burden with one or few BM not responding to conventional systemic therapies (i.e., oligoprogression). Nevertheless, all the aforementioned conditions are generally uncommon and for this reason, only a few patients may benefit from curative treatments.
Only ablative techniques allowing tumor destruction may be used for curative treatment, which requires at least 5–10 mm safety margins to achieve effective local tumor control.
Palliative treatments may be proposed to the vast majority of patients with BM and are intended to manage one or more SREs:
-
If fractures should be prevented or treated, percutaneous osteoplasty or osteosynthesis is applied based on the predominant biomechanics of the affected bone and the features of the target BM (Fig. 6).
Fig. 6 
(Adapted from Cazzato RL et al. “Percutaneous bone and spine consolidation in cancer patients: who and how?” Magna cum Laude Education Exhibit; Radiological Society of North America; 2017)
Consolidative algorithm based on the biomechanics of the involved bone and the features of the target bone metastasis.
-
If pain management is needed and there is no risk of fracture, thermal ablation should be performed. Patients should be offered such treatment in case of focal pain (≥ 4/10 on a 0–10 visual analogic scale over the 24 h) corresponding to a focal BM on cross-sectional imaging. Ideally, ablation should target the interface between the normal bone and the BM; nevertheless, whenever possible, complete BM destruction should be achieved.
-
Tumor debulking can be achieved with ablation such as in the case of BM extending to surrounding soft tissues or spinal tumors growing quickly into the spinal canal. Nevertheless, it should be noted that local tumor control cannot be achieved if the BM has already invaded the anterior epidural space.
Despite the curative or palliative intent, when ablation and/or embolization are performed (or following RT), bone strength is impaired; and a substantial risk of secondary BiF exists. Accordingly, based on simple biomechanical consideration, interventional or surgical consolidation is mandatory to avoid a secondary BiF, which usually occurs within the first few weeks even though cases of delayed BiF have been reported [61]. Consolidation should ideally be performed during the same interventional session, or at the latest during the same hospital stay.
Follow-up after interventional treatments
In the majority of cases, the main goal of percutaneous treatment is pain management [8, 62]. For this reason, many authors evaluate procedural success on the basis of clinical data by applying pain scales (e.g., VAS scale, Brief Pain Inventory) or quality of life scores (e.g., McGill Quality of Life Questionnaire) [39, 40, 63,64,65]. Moreover, scores assessing the performance status of the patient such as the Karnofsky Performance Score are also used [66]. Additionally, some authors also evaluate the consumption of analgesics including opioids [40, 66] that are nevertheless limited by side effects such as constipation, sedation, and nausea. Clinical data are collected at least 1 week after treatment (to reduce the placebo effects or other confounding factors, such as the effects of analgesics administered during the procedure or pain related to the procedure itself), and thereafter, follow-up is scheduled at 1, 3 and 6 months according to the patients’ status and disease evolution.
Unless new symptoms occur, imaging follow-up is unnecessary in patients with diffuse metastatic disease who underwent palliative treatments [39, 67]. On other hand, oligometastatic patients treated with a curative intent should undergo periodic imaging follow-up to assess local tumor control (i.e., identification of residual viable or recurring tumor in the treated area). The choice of the most appropriate imaging technique is of paramount importance, and contrast-enhanced techniques (in particular MRI or PET-CT) represent the best choice. MRI is able to depict even the smallest focus of active disease [68, 69]. To avoid confounding variables, such as inflammation, the first follow-up study should be carried out 4 or even 8–12 weeks after the treatment [40, 59, 70]. Finally, it is important to notice that RECIST criteria cannot be used in the setting of BM as RECIST has only been used for soft tissue metastases; moreover, BM is often considered “non-measurable” [68].
Possible interactions with other treatments
The application of interventional radiology for the management of BM is relatively recent. This may explain why no comparative studies are available between interventional treatments and RT, which still remains the widely accepted gold standard for the treatment of SREs, particularly pain [71]. In the few studies describing the combined use of RT and percutaneous treatments [63, 66, 72], authors emphasized the achievement of better results in terms of pain relief when the combined approach was applied as compared to a single treatment modality, without significant increase in morbidity. The exact mechanisms through which the combined treatment relieves pain remain largely unknown, although it has been advocated that tumor microenvironment perturbation resulting from ablation may enhance the effect of RT [73].
The development of immunotherapy has resulted in new and revolutionary advances. Researchers started from the observation of rare cases of abscopal effects after RT [74]. This refers to the remission of tumors outside the radiation field. It has been postulated that this phenomenon is immune mediated and is mainly observed in patients undergoing RT and immunotherapy. Indeed, focal treatments such as RT may trigger and potentiate the effects of immunotherapeutic agents; and it has been postulated that similar to RT, thermal ablation can also stimulate a systemic immune-mediated antitumor response [75,76,77,78].
Medical therapy for BM disease relies on agents (bisphosphonates or denosumab) avoiding bone loss [3, 7]. To our knowledge, no side effects have been described in patients receiving such agents and interventional treatments.
Surgery can be the definitive solution in oligometastatic patients with slowly evolving disease and long life expectancy. However, there are different limitations to its use since surgical interventions carry a significant risk of infection and bleeding coupled to a long recovery time [67, 71]; as a result of which, a long delay to systemic therapies is expected. All these aspects can negatively affect patients’ quality of life and may significantly impact their survival.
Joint position of the Italian College of Musculoskeletal Radiology and the Italian College of Interventional Radiology
-
1.
Due to the complex clinical scenario regarding BM, we suggest that the most adapted therapeutic strategy should be chosen in consensus by a dedicated multidisciplinary tumor board including medical oncologists, radiation oncologists, surgeons and interventional radiologists. The final therapeutic decision should take into consideration the oncologic and clinical status of patients as well as their expectations in order to offer an individualized treatment strategy.
-
2.
In the multidisciplinary tumor board, interventional techniques may be considered as the sole therapeutic option or as part of a more complex therapeutic strategy integrating different interventional and non-interventional treatments.
-
3.
Interventional treatments have several different advantages including the synergic effect with all the other non-interventional treatments, no need for significant interruption of systemic therapies, reduced morbidity and in-hospital stay, and patients’ fast recovery. All these aspects may play a crucial role for the selection of the interventional treatment by the tumor board.
-
4.
The interventional treatment should be selected with a clear intent, which can be curative in few selected patients needing local tumor control; or palliative in order to prevent or treat SREs (i.e., pain and fractures).
-
5.
Once the tumor board has selected an interventional treatment, the interventional radiologist should see the patient during a dedicated consultation to explain the risks and the benefits of the procedure.
-
6.
Due to technical and anatomic specifications, interventional treatments should be performed by trained interventional radiologists (or by young interventional radiologists under the supervision of a senior). The physician performing the procedure is responsible for the selection of the best imaging modality and the most suitable device/technique.
-
7.
Interventional procedures on BM are usually painful, and the interventional radiologist performing the procedure may not be able to provide alone the best intra-operative anesthesia and postoperative analgesia; as a consequence, dedicated anesthesiology teams should be involved. Effective analgesic treatment should also be provided in the postoperative phase, which is generally within the first 24 h where more pain is expected.
-
8.
Based on the aim of the procedure, dedicated clinical and/or radiologic follow-up should be scheduled and directly performed by the interventional team.
-
9.
Given the paucity of clinical studies comparing all the different therapeutic options available for the treatment of BM, interventional radiologists themselves and the tumor board should promote clinical trials comparing all the different interventional and non-interventional techniques.
References
Foster R, Stavas JM (2014) Bone and soft tissue ablation. Semin Interv Radiol 31(2):167–179
Criteria For Palliation of Bone Metastases—Clinical Applications IAEA (International Atomic Energy Agency), Vienna, 2007 ISBN 92–0–104507–7
Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J on behalf of the ESMO Guidelines Working Group (2014) Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 25(Suppl 3):iii124–37
Johnstone C, Lutz ST (2014) External beam radiotherapy and bone metastases. Ann Palliat Med 3(2):114–122
Decroisette C, Monnet I, Berard H, Quere G, Le Caer H, Bota S et al (2011) Epidemiology and treatment costs of bone metastases from lung cancer: a French prospective, observational, multicenter study (GFPC 0601). J Thorac Oncol 6(3):576–582
Yang Y, Ma Y, Sheng J, Huang Y, Zhao Y, Fang W et al (2016) A multicenter, retrospective epidemiologic survey of the clinical features and management of bone metastatic disease in China. Chin J Cancer 35:40
Kommalapati A, Tella SH, Esquivel MA, Correa R (2017) Evaluation and management of skeletal disease in cancer care. Crit Rev Oncol/Hematol 120:217–226
Kelekis A, Cornelis F, Tutton S, Filippiadis D (2017) Metastatic Osseous Pain Control: Bone Ablation and Cementoplasty. Semin Interv Radiol 34(4):328–336
Husain ZA, Sahgal A, De Salles A, Funaro M, Glover J, Hayashi M et al (2017) Stereotactic body radiotherapy for de novo spinal metastases: systematic review. J Neurosurg Spine 27(3):295–302
Greco C, Pares O, Pimentel N, Moser E, Louro V, Morales X et al (2015) Spinal metastases: From conventional fractionated radiotherapy to single-dose SBRT. Rep Pract Oncol Radiother 20(6):454–463
Barile A, Arrigoni F, Bruno F, Palumbo P, Floridi C, Cazzato RL et al (2018) Present role and future perspectives of interventional radiology in the treatment of painful bone lesions. Future Oncol. https://doi.org/10.2217/fon-2017-0657
Arrigoni F, Bruno F, Zugaro L, Splendiani A, Di Cesare E, Barile A et al (2018) Role of interventional radiology in the management of musculoskeletal soft-tissue lesions. Radiol Med. https://doi.org/10.1007/s11547-018-0893-4
Arrigoni F, Bruno F, Zugaro L, Natella R, Cappabianca S, Russo U et al (2018) Developments in the management of bone metastases with interventional radiology. Acta Biomed 89(1-S):166–174
Saliou G, el Kocheida M, Lehmann P, Depriester C, Paradot G, Le Gars D et al (2010) Percutaneous vertebroplasty for pain management in malignant fractures of the spine with epidural involvement. Radiology 254(3):882–890
Cazzato RL, Buy X, Eker O, Fabre T, Palussiere J (2014) Percutaneous long bone cementoplasty of the limbs: experience with fifty-one non-surgical patients. Eur Radiol 24(12):3059–3068
Tsoumakidou G, Too CW, Koch G, Caudrelier J, Cazzato RL, Garnon J et al (2017) CIRSE guidelines on percutaneous vertebral augmentation. Cardiovasc Interv Radiol 40(3):331–342
Anselmetti GC, Manca A, Ortega C, Grignani G, Debernardi F, Regge D (2008) Treatment of extraspinal painful bone metastases with percutaneous cementoplasty: a prospective study of 50 patients. Cardiovasc Interv Radiol 31(6):1165–1173
Leclair A, Gangi A, Lacaze F, Javier RM, Bonidan O, Kempf JF et al (2000) Rapid chondrolysis after an intra-articular leak of bone cement in treatment of a benign acetabular subchondral cyst: an unusual complication of percutaneous injection of acrylic cement. Skeletal Radiol 29(5):275–278
Cazzato RL, Palussière J, Buy X, Denaro V, Tonini G, Grasso F et al (2015) Percutaneous long bone cementoplasty for palliation of malignant lesions of the limbs: a systematic review. Cardiovasc Interv Radiol 38(6):1563–1572
Deschamps F, Farouil G, Hakime A, Barah A, Guiu B, Teriitehau C et al (2012) Cementoplasty of metastases of the proximal femur: is it a safe palliative option? J Vasc Interv Radiol 23(10):1311–1316
Cazzato RL, Koch G, Buy X, Ramamurthy N, Tsoumakidou G, Caudrelier J et al (2016) Percutaneous image-guided screw fixation of bone lesions in cancer patients: double-centre analysis of outcomes including local evolution of the treated focus. Cardiovasc Interv Radiol 39(10):1455–1463
Mirels H (1989) Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res 249:256–264
Cazzato RL, Garnon J, Tsoumakidou G, Koch G, Palussière J, Gangi A et al (2017) Percutaneous image-guided screws mediated osteosynthesis of impending and pathological/insufficiency fractures of the femoral neck in non-surgical cancer patients. Eur J Radiol 90:1–5
Garnon J, Koch G, Ramamurthy N, Caudrelier J, Rao P, Tsoumakidou G et al (2016) Percutaneous CT and fluoroscopy-guided screw fixation of pathological fractures in the shoulder girdle: technical report of 3 cases. Cardiovasc Interv Radiol 39(9):1332–1338
Deschamps F, de Baere T, Hakime A, Pearson E, Farouil G, Teriitehau C et al (2016) Percutaneous osteosynthesis in the pelvis in cancer patients. Eur Radiol 26(6):1631–1639
Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW et al (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology 273(1):241–260
Barile A, Arrigoni F, Zugaro L, Zappia M, Cazzato RL, Garnon J et al (2017) Minimally invasive treatments of painful bone lesions: state of the art. Med Oncol 34(4):53
Arrigoni F, Barile A, Zugaro L, Fascetti E, Zappia M, Brunese L et al (2018) CT-guided radiofrequency ablation of spinal osteoblastoma: treatment and long-term follow-up. Int J Hyperthermia 34(3):321–327
Silvestri E, Barile A, Albano D, Messina C, Orlandi D, Corazza A et al (2018) Interventional therapeutic procedures in the musculoskeletal system: an Italian Survey by the Italian college of musculoskeletal radiology. Radiol Med 123(4):314–321
Cazzato RL, Garnon J, Ramamurthy N, Koch G, Tsoumakidou G, Caudrelier J et al (2016) Percutaneous image-guided cryoablation: current applications and results in the oncologic field. Med Oncol 33(12):140
Ringe K, Panzica M, von Falck C (2016) Thermoablation of bone tumors. RöFo 188(6):539–550
Gangi A, Tsoumakidou G, Buy X, Quoix E (2010) Quality improvement guidelines for bone tumour management. Cardiovasc Interv Radiol 33(4):706–713
Gangi A, Buy X (2010) Percutaneous bone tumor management. Semin Interv Radiol 27(2):124–136
Tsoumakidou G, Thénint MA, Garnon J, Buy X, Steib JP, Gangi A (2016) Percutaneous image-guided laser photocoagulation of spinal osteoid osteoma: a single-institution series. Radiology 278(3):936–943
Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT Jr, Brace CL (2014) Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation—what should you use and why? RadioGraphics 34(5):1344–1362
Cazzato RL, Garnon J, Caudrelier J, Rao PP, Koch G, Gangi A (2018) Low-power bipolar radiofrequency ablation and vertebral augmentation for the palliative treatment of spinal malignancies. Int J Hyperth 18:1–7
Cazzato RL, Garnon J, Caudrelier J, Rao PP, Koch G, Gangi A (2018) Percutaneous radiofrequency ablation of painful spinal metastasis: a systematic literature assessment of analgesia and safety. Int J Hyperthermia 17:1–10
Carrafiello G, Laganà D, Mangini M, Fontana F, Dionigi G, Boni L et al (2008) Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg 6:S65–S69
Cazzato RL, Buy X, Grasso RF, Luppi G, Faiella E, Quattrocchi CC et al (2015) Interventional radiologist’s perspective on the management of bone metastatic disease. Eur J Surg Oncol 41(8):967–974
Pusceddu C, Sotgia B, Fele RM, Melis L (2013) Treatment of bone metastases with microwave thermal ablation. J Vasc Interv Radiol 24(2):229–233
Napoli A, Anzidei M, Marincola BC, Brachetti G, Noce V, Boni F et al (2013) MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics 33(6):1555–1568
Masciocchi C, Conchiglia A, Gregori LM, Arrigoni F, Zugaro L, Barile A (2014) Critical role of HIFU in musculoskeletal interventions. Radiol Med 119(7):470–475
Masciocchi C, Arrigoni F, La Marra A, Mariani S, Zugaro L, Barile A (2016) Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br J Radiol 89:201503
Arrigoni F, Barile A, Zugaro L, Splendiani A, Di Cesare E, Caranci F et al (2017) Intra-articular benign bone lesions treated with magnetic resonance-guided focused ultrasound (MRgFUS): imaging follow-up and clinical results. Med Oncol 34(4):55
Campbell C, Lubner MG, Hinshaw JL, Muñoz del Rio A, Brace CL (2012) Contrast media-doped hydrodissection during thermal ablation: optimizing contrast media concentration for improved visibility on CT images. AJR Am J Roentgenol 199(3):677–682
Tsoumakidou G, Garnon J, Ramamurthy N, Buy X, Gangi A (2013) Interest of electrostimulation of peripheral motor nerves during percutaneous thermal ablation. Cardiovasc Interv Radiol. 36(6):1624–1628
Haveman J, Sminia P, Wondergem J, van der Zee J, Hulshof MC (2005) Effects of hyperthermia on the central nervous system: what was learnt from animal studies? Int J Hyperth 21(5):473–487
Haveman J, Van Der Zee J, Wondergem J, Hoogeveen JF, Hulshof MC (2004) Effects of hyperthermia on the peripheral nervous system: a review. Int J Hyperth 20(4):371–391
Gage AA, Baust J (1998) Mechanisms of tissue injury in cryosurgery. Cryobiology 37(3):171–186
Hoffmann NE, Bischof JC (2002) The cryobiology of cryosurgical injury. Urology 60(2 Suppl 1):40–49
Ma Y, Wallace AN, Waqar SN, Morgensztern D, Madaelil TP, Tomasian A et al (2018) Percutaneous Image-guided ablation in the treatment of osseous metastases from non-small cell lung cancer. Cardiovasc Interv Radiol 41(5):726–733
Barton PP, Waneck RE, Karnel FJ, Ritschl P, Kramer J, Lechner GL (1996) Embolization of bone metastases. J Vasc Interv Radiol 7:81–88
Kickuth R, Waldherr C, Hoppe H, Bonel HM, Ludwig K, Beck M et al (2008) Interventional management of hypervascular osseous metastasis: role of embolotherapy before orthopedic tumor resection and bone stabilization. AJR Am J Roentgenol 191:W240–W247
Lee VN, Nithyananth M, Cherian VM, Amritanand R, Venkatesh K, Sundararaj GD et al (2008) Preoperative embolisation in benign bone tumour excision. J Orthop Surg 16:80–83
Kato S, Hozumi T, Takaki Y, Yamakawa K, Goto T, Kondo T (2013) Optimal schedule of preoperative embolization for spinal metastasis surgery. Spine (Phila Pa 1976). 15:38(22):1964–1969
Shimohira M, Nagai K, Hashizume T, Nakagawa M, Ozawa Y, Sakurai K et al (2016) Preoperative transarterial embolization using gelatin sponge for hypervascular bone and soft tissue tumors in the pelvis or extremities. Acta Radiol 57(4):457–462
Robial N, Charles YP, Bogorin I, Godet J, Beaujeux R, Boujan F et al (2012) Is preoperative embolization a prerequisite for spinal metastases surgical management? Orthop Traumatol Surg Res 98(5):536–542
Rossi G, Mavrogenis AF, Rimondi E, Braccaioli L, Calabrò T, Ruggieri P (2011) Selective embolization with N-butyl cyanoacrylate for metastatic bone disease. J Vasc Interv Radiol 22(4):462–470
Deschamps F, Farouil G, Ternes N, Gaudin A, Hakime A, Tselikas L et al (2014) Thermal ablation techniques: a curative treatment of bone metastases in selected patients? Eur Radiol 24(8):1971–1980
Cazzato RL, Bonichon F, Buy X, Godbert Y, de Figuereido BH, Pointillart V et al (2015) Over ten years of single-institution experience in percutaneous image-guided treatment of bone metastases from differentiated thyroid cancer. Eur J Surg Oncol 41(9):1247–1255
Tsoumakidou G, Borensztein M, Zini C, Garnon J, Gangi A (2014) Postablation insufficiency fracture of the iliac crest: management by percutaneous screw fixation. Cardiovasc Interv Radiol 37(4):1126–1128
Kurup AN, Callstrom MR (2017) Expanding role of percutaneous ablative and consolidative treatments for musculoskeletal tumours. Clin Radiol 72(8):645–656
Di Staso M, Gravina GL, Zugaro L, Bonfili P, Gregori L, Franzese P et al (2015) Treatment of solitary painful osseous metastases with radiotherapy, cryoablation or combined therapy: propensity matching analysis in 175 patients. PLoS ONE 10(6):e0129021
Rosenthal D, Callstrom MR (2012) Critical review and state of the art in interventional oncology: benign and metastatic disease involving bone. Radiology 262(3):765–780
Dupuy DE, Liu D, Hartfeil D, Hanna L, Blume JD, Ahrar K et al (2010) Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer 116(4):989–997
Di Staso M, Zugaro L, Gravina GL, Bonfili P, Marampon F, Di Nicola L et al (2011) A feasibility study of percutaneous Radiofrequency Ablation followed by Radiotherapy in the management of painful osteolytic bone metastases. Eur Radiol 21(9):2004–2010
Kurup AN, Callstrom MR (2013) Ablation of musculoskeletal metastases, pain palliation, fracture risk reduction, and oligometastatic disease. Tech Vasc Interv Radiol 16(4):253–261
Lecouvet FE, Talbot JN, Messiou C, Bourguet P, Liu Y, de Souza NM et al (2014) Monitoring the response of bone metastases to treatment with Magnetic Resonance Imaging and nuclear medicine techniques: a review and position statement by the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer 50(15):2519–2531
Barile A, Regis G, Masi R, Maggiori M, Gallo A, Faletti C et al (2007) Musculoskeletal tumours: preliminary experience with perfusion MRI. Radiol Medica. 112(4):550–561
Wallace AN, Greenwood TJ, Jennings JW (2015) Use of imaging in the management of metastatic spine disease with percutaneous ablation and vertebral augmentation. Am J Roentgenol 205(2):434–441
Kurup AN, Morris JM, Callstrom MR (2017) Ablation of Musculoskeletal Metastases. Am J Roentgenol 209(4):713–721
Greenwood TJ, Wallace A, Friedman MV, Hillen TJ, Robinson CG, Jennings JW (2015) Combined ablation and radiation therapy of spinal metastases: a novel multimodality treatment approach. Pain Physician 18(6):573–581
Di Staso M, Zugaro L, Gravina GL, Bonfili P, Marampon F, Di Nicola L et al (2011) Can radiotherapy be combined with radiofrequency ablation in the management of symptomatic osteolytic skeletal metastasis? Clin Oncol (R Coll Radiol) 23(1):65–66
Walle T, Martinez Monge R, Cerwenka A, Ajona D, Melero I, Lecanda F (2018) Radiation effects on antitumor immune responses: current perspectives and challenges. Ther Adv Med Oncol 10:1758834017742575
Takaki H, Cornélis F, Kako Y, Kobayashi K, Kamikonya N, Yamakado K (2017) Thermal ablation and immunomodulation: from preclinical experiments to clinical trials. Diagnostic Interv Imaging 98(9):651–659
Silvestrini MT, Ingham ES, Mahakian LM, Kheirolomoom A, Liu Y, Fite BZ et al (2017) Priming is key to effective incorporation of image-guided thermal ablation into immunotherapy protocols. JCI Insight 2(6):1–16
Takahashi Y, Matsutani N, Nakayama T, Dejima H, Uehara H, Kawamura M (2017) Immunological effect of local ablation combined with immunotherapy on solid malignancies. Chin J Cancer 36(1):49
Mauri G, Orsi F, Sconfienza LM (2016) Systemic effects of local tumor ablation: oncogenesis and antitumor induced immunity. Radiology 279(1):322–323
Bang HJ, Littrup PJ, Currier BP, Goodrich DJ, Aoun HD, Klein LC et al (2012) Percutaneous cryoablation of metastatic lesions from non small-cell lung carcinoma: initial survival, local control, and cost observations. J Vasc Interv Radiol 23(6):761–769
Bang HJ, Littrup PJ, Goodrich DJ, Currier BP, Aoun HD, Heilbrun LK et al (2012) Percutaneous cryoablation of metastatic renal cell carcinoma for local tumor control: feasibility, outcomes, and estimated cost-effectiveness for palliation. J Vasc Interv Radiol 23(6):770–777
McMenomy BP, Kurup AN, Johnson GB, Carter RE, McWilliams RR, Markovic SN et al (2013) Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission. J Vasc Interv Radiol 24(2):207–213
Welch BT, Callstrom MR, Morris JM, Kurup AN, Schmit GD, Weisbrod AJ et al (2014) Feasibility and oncologic control after percutaneous image guided ablation of metastatic renal cell carcinoma. J Urol 192(2):357–363
Tomasian A, Wallace A, Northrup B, Hillen TJ, Jennings JW (2016) Spine cryoablation: pain palliation and local tumor control for vertebral metastases. AJNR Am J Neuroradiol 37(1):189–195
Erie AJ, Morris JM, Welch BT, Kurup AN, Weisbrod AJ, Atwell TD et al (2017) Retrospective review of percutaneous image-guided ablation of oligometastatic prostate cancer: a single-institution experience. J Vasc Interv Radiol 28(7):987–992
Wallace AN, Tomasian A, Vaswani D, Vyhmeister R, Chang RO, Jennings JW (2016) Radiographic local control of spinal metastases with percutaneous radiofrequency ablation and vertebral augmentation. AJNR Am J Neuroradiol 37(4):759–765
Goetz MP, Callstrom MR, Charboneau JW, Farrell MA, Maus TP, Welch TJ et al (2004) Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol 22(2):300–306
Wallace AN, Greenwood TJ, Jennings JW (2015) Radiofrequency ablation and vertebral augmentation for palliation of painful spinal metastases. J Neurooncol 124(1):111–118
Bagla S, Sayed D, Smirniotopoulos J, Brower J, Neal Rutledge J, Dick B et al (2016) Multicenter prospective clinical series evaluating radiofrequency ablation in the treatment of painful spine metastases. Cardiovasc Interv Radiol 39(9):1289–1297
Callstrom MR, Dupuy DE, Solomon SB, Beres RA, Littrup PJ, Davis KW et al (2013) Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer 119(5):1033–1041
Prologo JD, Passalacqua M, Patel I, Bohnert N, Corn DJ (2014) Image-guided cryoablation for the treatment of painful musculoskeletal metastatic disease: a single-center experience. Skelet Radiol. 43(11):1551–1559
Liberman B, Gianfelice D, Inbar Y, Beck A, Rabin T, Shabshin N et al (2009) Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol 16(1):140–146
Napoli A, Anzidei M, Marincola BC, Brachetti G, Ciolina F, Cartocci G et al (2013) Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound. Invest Radiol 48(6):351–358
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Not applicable.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Cazzato, R.L., Arrigoni, F., Boatta, E. et al. Percutaneous management of bone metastases: state of the art, interventional strategies and joint position statement of the Italian College of MSK Radiology (ICoMSKR) and the Italian College of Interventional Radiology (ICIR). Radiol med 124, 34–49 (2019). https://doi.org/10.1007/s11547-018-0938-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-018-0938-8