Abstract
Background
Radiofrequency ablation (RFA) of vertebral body metastases (VBM) has been reported as safe and effective in retrospective studies. This single-arm prospective multicenter clinical study evaluates RFA in the treatment of painful VBM.
Methods
Fifty patients with VBM were prospectively enrolled during a 13-month period at eight US centers under an IRB-approved study. Percutaneous RFA was performed under imaging guidance with cement augmentation at the discretion of the operator. Pain, disability and quality of life were evaluated at baseline, prior to discharge, days 3, 7, 30 and 90 using the Numerical Pain Rating Scale, Oswestry Disability Index (ODI), the Functional Assessment of Cancer Therapy-General 7 (FACT-G7) and Functional Assessment of Cancer Therapy Quality-of-Life Measurement in Patients with Bone Pain (FACT-BP). Adverse events were monitored throughout this time interval.
Results
Twenty-six male and 24 female patients (mean age 61.0) underwent 69 treatments (30 thoracic and 39 lumbar). Cement augmentation was performed in 96 % of reported levels. Significant improvement in mean scores for pain, disability and cancer-specific health-related quality of life from baseline to all time intervals was seen. NRPS improved from 5.9 to 2.1 (p < 0.0001). ODI improved from 52.9 to 37.0 (p < 0.08). FACT-G7 improved form 10.9 to 16.2 (p = 0.0001). FACT-BP improved from 22.6 to 38.9 (p < 0.001). No complications related to the procedure were reported.
Conclusion
RFA with cement augmentation safely and effectively reduces pain and disability rapidly, while increasing quality of life in patients suffering from vertebral body metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The growth of malignant cells in the osseous environment has been long recognized as a complication of cancer dating to 1889 by Paget [1]. Vertebrae are the most common sites of bone metastases likely due to their highly vascularized anatomy, with an incidence of 30–70 % in patients with metastatic cancer [2–4]. Furthermore, vertebral body metastases (VBM) represent the most common etiologies of chronic pain, fractures and spinal cord compression in this patient population [5, 6].
Current standard-of-care treatments for patients with VBM include steroids, bisphosphonates, chemotherapy, radiation therapy, surgical management and ablative therapies [7, 8]. The mainstay of treatment for palliation of pain is conventional fractionated external beam radiation therapy (EBRT); however, up to 40 % of patients may demonstrate no pain relief and 65 % will have residual pain after treatment [9]. Stereotactic body radiotherapy (SBRT), a highly conformal and image-guided hypofractionated external beam radiotherapy, has been shown to provide increased rate and degree of pain relief, and local control in treatment of spinal metastatic disease, however, is less commonly utilized for metastatic disease and carries increased risk of serious adverse events, such as radiation-induced myelopathy (1–5 %) and vertebral compression fractures (11–39 %) [10]. The combination of radiation therapy and minimally invasive procedures such as radiofrequency ablation (RFA) and cement augmentation is emerging as a promising therapeutic regimen [10, 11].
Minimally invasive thermal ablative procedures, such as RFA, cryoablation and microwave ablation, have emerged as viable options in the palliative treatment of musculoskeletal metastases because of the short procedure time, minimally invasive nature and ability to be performed on an ambulatory basis [12]. However, device access to vertebral body tumors can be challenging due to the posterior location of most spinal metastases [13]. Such anatomical and technical considerations in vertebral procedures have resulted in a limited number of VBM lesions reported in studies of percutaneous ablation of painful osseous metastases [14, 15]. Retrospective studies have demonstrated that RFA with cement augmentation is an effective method for palliation of pain from VBM [16–19]. There is, however, a lack of prospective experience in the USA evaluating the effects of RFA in VBM. The purpose of this prospective multicenter study was to assess pain palliation, degree of disability and improvement in quality of life in patients with vertebral metastatic tumors following percutaneous RFA.
Materials and Methods
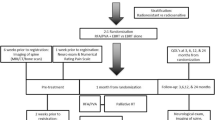
Fifty patients were enrolled in an institutional review board (IRB) approved study at eight sites in the USA between August 2013 and September 2014—patient demographics and baseline characteristics are presented in Table 1. All patients signed written informed consent, and the study was conducted in compliance with federal HIPAA regulations. Inclusion criteria were painful VBM in at least one thoracolumbar vertebra with the pain concordant to the metastatic lesion on cross-sectional imaging, aged 18 years or older and considered candidates for spinal tumor ablation by the operating physician. Exclusion criteria were VBM in cervical vertebrae and posterior tumor extension with cord compression.
Treatment Procedure
Patients completed a physical examination prior to treatment, and a review of the patients’ imaging was performed to confirm the focal pain correlated with the cross-sectional imaging and prepare a treatment plan (Figs. 1A, 2A). Ablation within each vertebral body was performed using the STAR Tumor Ablation System (DFINE, San Jose, CA), which contains a bipolar RF probe with a unique extensible electrode and articulating design, permitting percutaneous navigation throughout the vertebral body. Multiple thermocouples incorporated along the articulating segment of the RF probe provide real-time thermal profile of the ablation zone and are used to monitor the size of ablation (Figs. 1B, 2B). A procedure was considered technically successful if the index vertebra was able to be accessed using the articulating bipolar ablation instrument and create adequate overlapping ablation zones to cover the metastatic lesion per the preoperative plan prior to cement augmentation, when performed.
A Axial T1-weighted fat saturated post-contrast image at L5 level demonstrates enhancement of the metastasis (white dashed arrows), involving the posterior 1/3 of the vertebral body extending to the pedicle. B 2 weeks after ablation; axial T1-weighted fat saturated post-contrast image at L5 level demonstrates peripheral enhancement (white arrows) around the area of overlapping ablations. Central low signal intensity area is focus of multiple overlapping treatments. Pain by day 30 had decreased to 2/10 compared with 9/10 at baseline
A Axial CT image prior to probe placement. Metastasis seen as low attenuation lesion (white arrows) identified, however, may underestimate degree of lesion based on MR imaging. B Axial CT imaging during placement of the bipolar RFA probe. Posterior medial articulation is performed of the probe as it exits the outer cannula to target tumor extension more posteriorly located. The ablation zone will grow radially outwards from the insulative tip (black arrow) and thermocouples (white arrows) allow for real-time temperature measurements as the ablative zone increases. Three overlapping ablations were performed to cover a wider area. Cement augmentation was not performed in this case as there was no fracture and the vertebral body was not felt to be at immediate risk. Despite this, pain relief decreased by 30 % at discharge
Patients were sedated using conscious sedation or general anesthesia at the discretion of the operating physician. The vertebral body was accessed using a transpedicular or parapedicular approach under computed tomography (CT) or fluoroscopic guidance with a 10-gauge coaxial cannula. The RF probe was inserted within the appropriate vertebral body location(s) based on cross-sectional preoperative imaging. Thermal energy was applied to achieve desired ablation zone of 10, 20 or 30 mm in length as determined by the thermocouple (TC) temperatures on the RF electrode. When required, due to size or location of tumor, the RF probe was repositioned within the vertebra via the same access cannula by articulating the distal end to adjacent areas and overlapping ablation zones were created to complete tumor ablation. In most cases (47/50), cement augmentation (StabiliT® Vertebral Augmentation System, DFINE, San Jose, CA) was performed following ablation via the same access cannula. Small posterior lesions measuring less than 25 % of the vertebral body (n = 3 of 69) considered by the operator not at high risk of impending vertebral body fracture were excluded from vertebral augmentation post-ablation.
Clinical Follow-Up
Patients were asked to complete four validated clinical instruments to measure their pain, disability and quality of life prior to treatment. Pain was assessed with the Numerical Pain Rating Scale (NPRS) that ranges from 0 (no pain) to 10 (worst possible pain). Back-related disability was measured using the Modified Oswestry Disability Index (MODI) which is a validated scale comprised of ten questions designed to assess pain intensity and activities of daily living [20]. The responses are valued and calculated to arrive at a composite index score. The ratings and corresponding categories are 0–20 (minimal disability), 21–40 (moderate disability), 41–60 (severe disability), 61–80 (crippled) and 81–100 % (bed bound). Quality of life was assessed using two cancer-specific health-related quality-of-life instruments, FACT-G7 and FACT-BP (FACIT Elmhurst, IL). These are standardized questionnaires based on the Functional Assessment of Cancer Therapy-General (FACT-G) [21]. The FACT-G7 is abbreviated to seven evaluable questions and considered a rapid version of the FACT-G which has 27 questions [22]. FACT-BP is specifically designed for use in cancer patients with bone pain and contains 15 evaluable questions. Each questionnaire provides a calculation to generate a composite score. Higher scores are interpreted as greater patient quality of life, and lower scores are interpreted as lower patient quality of life. FACT-G7 has a scale from 0 to 28 and FACT-BP 0–60.
Validated minimal clinically important differences (MCID) in NPRS (≥2 point change), MODI (>10 % change), FACT-G7 (>2–3 point change) and FACT-BP (>3–6 point change) were used to interpret pain, disability and quality-of-life score differences in terms of clinical relevance [23, 24]. Clinical success was defined as a MCID from baseline.
Follow-up assessments occurred at discharge (NPRS only), 3 days, 1 week, 1 and 3 months. Complications were recorded through the 3-month follow-up period and graded using the classification system used by the Society of Interventional Radiology (SIR) and the National Cancer Institute’s Common Toxicity Criteria (version 4.03) [25, 26].
Statistical Analysis
Patients’ baseline assessments were used as controls. Analysis of pain, disability and quality-of-life assessments was performed at each visit to assess a change from baseline, and a one-sample t test was used to compare this difference to 0. Because a minimum pain score was not required for inclusion in this study, additional analyses were performed to assess the clinical effect of this treatment on patients with baseline NPRS scores of ≥4, which was an inclusion criteria reported in previous studies of ablative therapy in painful osseous metastases. Two-sided 95 % confidence intervals were also calculated to assess statistical significance. Analysis of covariance (ANCOVA) models were constructed to explore the effect of various relevant covariates on outcomes.
Results
Study Population
Patient demographics and baseline characteristics are presented in Table 1. Over 50 % of the primary cancer types originated from breast, kidney and lung. Sixteen patients (32 %) received prior radiation therapy at differing times prior to t-RFA treatment. Thirty-four patients had a single VBM, 13 patients had two VBM, and three patients had three VBMs for a total of 69 vertebrae treated, 57 % of which were in the lumbar region of the spine. Of the thoracic VBMs, all were in the T6–T12 range except for one (T1). All patients were treated in a single session under either conscious sedation (n = 35, 70 %) or general anesthesia (n = 15, 30 %). In 77 % of the vertebrae, a unilateral approach was used (Table 2). Ablation time per vertebral body averaged 6.7 min using a mean 2.3 overlapping ablation zones. Technical success was achieved in 100 % of patients, and 66/69 lesions received vertebral body cement augmentation.
Clinical Follow-Up
Every effort was made to contact patients in the follow-up phase and included multiple attempts to reach the patient by phone. Pain, disability and quality-of-life scores were obtained in 90 % of patients at day 3, 92 % at week 1, 80 % at month 1 and 68 % at month 3. The reasons for incomplete follow-up included inability to contact six patients (12 %) death due to causes unrelated to the procedure in five patients (10 %), withdrawal of consent in four patients (8 %) and deteriorating health preventing questionnaire completion in one patient (2 %). One patient (2 %) completed the 3-month follow-up but did not provide a pain score. Forty-two patients (84 %) did not receive any additional radiation therapy during the follow-up period. Of the remaining eight patients included in the study, two (4 %) received radiation therapy after RFA and six patients (12 %) were unable to confirm any additional therapy.
Patients experienced a mean pain score of 5.9 on a 0–10 scale at baseline. Pain decreased to a mean of 3.7 immediately after the procedure with additional reduction to 2.1 at month 3 (Table 3; Fig. 3). On a patient-by-patient basis, pain relief was rapid with 32/49 (65 %) of the patients experiencing an MCID of ≥2 point change within 3 h of treatment. Percent of patients reporting an MCID in pain was 62, 59, 63 and 70 % at day 3, week 1, month 1 and month 3, respectively. Pain score decrease was statistically significant at each follow-up time point.
As this study did not have a minimum inclusion criteria for pain, six patients that were enrolled had a baseline NPRS < 4 prior to ablation. For this reason, a subset of 44 patients who recorded a baseline pain level of ≥4 was completed. These patients had a mean pain score of 6.4 at baseline which decreased to 4.0, 2.9 and 2.3 at discharge, month 1 and month 3, respectively. An MCID pain score of ≥2 points change was observed in 71, 68, 64, 67 and 73 % of patients at discharge, day 3, week 1, month 1 and month 3, respectively.
Of the patients who received RFA without cement augmentation, all patients (3/3) demonstrated clinical success immediately after the procedure with a mean improvement of 4.3 NPRS points. At last follow-up (mean 2.3 months) for all non-cemented patients, mean improvement was 5.0 NPRS points. Furthermore, the 16 patients (32 %) who received radiation therapy prior to RFA also experienced significant pain relief (pre-treatment: 5.5; 90 days; 2.1, p < 0.01).
Mean disability, as measured by MODI, at baseline was 52.9 %, categorized as severe disability. MODI improvement was statistically significant at each interval (Table 3). A decrease was observed at months 1 and 3 with scores of 41.2 and 37.0, respectively, representing an MCID and a categorical improvement in functional status from severe to moderate disability. On an individual patient basis, MCID in MODI was achieved in 56 % of patients at month 3.
Quality of life as measured by FACT-G7 and FACT-BP had mean scores of 11.0 and 22.6, respectively, at baseline. The mean scores significantly increased by day 3 to 15.0 for FACT-G7 and 33.0 for FACT-BP (Table 3). Improvement in both scores persisted throughout the 3-month follow-up with an MCID achieved in 68 % of patients for FACT-G7 and 79 % of patients for FACT-BP.
There were six adverse events reported in six patients during the course of this study. This included: pain outside the target vertebrae due to progression of the primary or other metastatic disease (n = 3), ruptured disk (n = 1) adjacent to the index vertebra, neuropathic pain (n = 1) and syncope (n = 1). The ruptured disk was present prior to treatment of the index vertebrae, became painful (between the month 1 and month 3 visit) and was resolved following bilateral nerve blocks. Neuropathic pain was present prior to the procedure and intermittent after the procedure (n = 1). The operating physicians deemed five of the adverse events as unrelated and one, the ruptured disk, as unlikely related to the RFA and/or vertebral augmentation procedures.
Discussion
This prospective study reports rapid onset of clinically significant pain relief, decreased disability and improved quality of life following percutaneous RFA treatment of spine metastases with and without vertebral augmentation.
Pain relief was rapid with 65 % of patients experiencing at least a two-point decrease in worst pain within 3 h of treatment. Durable pain relief was observed with 68 % of the patients experiencing >50 % reduction in pain at 3 months. Of particular importance, the 16 patients (32 %) who underwent prior radiation therapy also experienced significant pain relief. Further, patients were more mobile and demonstrated significant improvements in disability over the 3-month follow-up period. With such improvement in pain and disability, it is not surprising quality of life, assessed by cancer-specific questionnaires, improved to a clinically meaningful degree by day 3 and persisted through 3 months.
RFA has been the most widely studied local ablative therapy of skeletal metastases, with two prospective clinical trials [15, 27]. While these studies have reported significant reduction in pain following RFA, they include relatively small percentages of vertebral metastases (14.5 and 9.3 %). The largest cryoablation trial (Callstrom Cancer 2013) of the treatment of skeletal metastases was equally effective in demonstrating pain reduction, but only one patient (1 %) of the total subset had a vertebral body metastasis. These studies are inherently limited with respect to generalizations about feasibility and safety of RFA adjacent to the spinal cord and the effect on disability and quality of life. One prospective study that did include a large percentage of vertebral lesions (n = 34/53, 64 %) reported RFA followed by cement augmentation of osseous metastases resulted in rapid pain relief but limited clinical follow-up to pain at 24 h [18].
The current observational study represents the first prospective clinical evaluation dedicated to evaluating pain, disability and quality of life following RFA treatment of spinal metastatic lesions. Although there is no comparison arm in this study, the results compare favorably to those reported in a meta-analysis of 16 randomized trials studies of external beam radiation therapy (EBRT), the standard of care for treatment of skeletal metastases [9]. The overall response rate was 60 % in patients undergoing EBRT as single or multi-fraction. The current study demonstrated a clinical success rate of 70 % with a 65 % clinical success rate achieved 3 h post-procedure at discharge. This rate of pain relief is of particular importance when considering the comparative duration of therapy. Whereas RFA and VA are performed in a single outpatient session, radiation therapy is often performed in multiple sessions over a 2-week period. And while 8 Gy in one fraction has been reported to provide similar palliative relief to multi-fraction EBRT, the likelihood of re-treatment was 2.5-fold higher in single-fraction RT arm patients (p < 0.00001) [9]. The multi-fraction course of therapy increases physical demand on the patient with debilitating pain. Most importantly, the accelerated rate at which pain relief was achieved in this study as compared to the 4–6 weeks commonly required to achieve full palliative effect of conventional fractionated EBRT alone can be important to patients with stage IV cancer and limited life expectancy [28, 29].
While this study demonstrates clinical utility of RFA in treatment of VBM, we believe the adjunctive utility of RFA with conventional RT is an important consideration. The 16 patients (32 %) who underwent prior radiation therapy also experienced significant pain relief (pre = 5.5, day 90 = 2.1, p < 0.01) demonstrating the added value of spine RFA in patients with inadequate pain relief following radiation therapy or who have reached their dose tolerance limits. While distinguishing effects of RT from RFA may be difficult, it is notable that this cohort who received RT had decreased NPRS from 5.5 to 3.3 at just 3 days. This is more consistent with the immediate effect of pain relief with ablation, as opposed to the effect of RT. Di Staso reported reduction in both time to pain relief and recurrence rate when combining RFA and EBRT, raising the potential for improved palliation, reducing the recurrence rate and increasing duration of treatment effect by combining these therapies [11]. A number of recent studies have reported increased fracture rates following the use of stereotactic body radiation therapy (SBRT) for spinal metastatic lesions and suggest that VA in conjunction with RT and/or RFA may allow for lower fracture rate and perhaps an overall cost in palliative treatment of vertebral metastases; however, further detailed investigation is warranted [10]. The operator’s decision to perform vertebral augmentation in this study was based on spine and tumor-specific predictive factors for vertebral fracture progression proposed in the Spinal Instability Neoplastic Score (SINS) [30], specifically type and extent of vertebral involvement. This resulted in augmentation of vertebrae with osteolytic or mixed lesions and either collapse or tumor involvement of more than 25–50 % of the vertebral body. Only three of 69 lesions were not considered to put the vertebra at risks of further instability and were not augmented post-ablation.
Recently, ‘tumor extravasation’ was reported in patients undergoing balloon kyphoplasty [32]. The authors have suggested that local targeted ablative therapy prior to augmentation may mitigate risk with respect to ‘tumor extravasation’. This may occur secondary to tumor extension close to or beyond the posterior vertebral body wall with resultant transvenous or direct extension into the epidural space. RFA has been shown to be safe and effective in the treatment of posteriorly located lesions, which would be at highest risk of tumor extravasation given their proximity to the posterior wall and basivertebral venous plexus [17]. The lack of cement-related complications in this study and the ability to target posterior lesions with articulating RFA probes suggest that RFA allows for targeted treatment of metastatic vertebral body lesions prior to cement injection, and may reduce the risk of extravasation.
Given the proximity to the spinal cord and central nerve roots, the risk of neurological complications is of particular concern with RFA of vertebral metastases. We report no episodes of thermal or compressive neurological injury, including the spinal cord. Previous studies have reported a 4 % incidence of nerve injury; however, it is unclear whether this was related to the vertebral body treatments or other sites [15]. It is the opinion of the authors that concurrent use of thermocouples incorporated along the length of the device to measure lethal temperatures at the margin of the ablation zone was critical to the overall safety profile of the procedure.
Registry data can be limited with respect to patient compliance in follow-up, particularly involving patients with stage IV metastatic disease and debilitating pain. However, this study does have its merits in evaluating ablative therapy of skeletal metastasis, in that it is prospective multicenter (n = 8) enrolled a relatively large number of patients (n = 50) and had good compliance in stage IV cancer patients at 3 months (34 % lost to follow-up). Additionally, being a multicenter study, patients were enrolled from both academic and private-practice operating physicians, provides assurance that the procedure and clinical results are reproducible. In comparison, two highly cited prospective studies evaluating ablative therapy in skeletal metastases reported on 55 and 43 patients at four and nine centers with 42 and 40 % lost to follow-up, respectively [15, 27].
Other limitations of this study include lack of follow-up beyond 3 months, imaging evaluation to assess local control of disease, lack of follow-up physical examination and the ability to differentiate palliative relief due to ablation versus augmentation. Another limitation was the lack of consistent recording of medication usage before and after treatment and therefore could not be evaluated fully to assess for impact on opioid usage. While longer-term follow-up and tumor control may be of less importance in the patient with advanced spine metastases or shorter life expectancy, these are of value for future prospective investigation. While cement augmentation was performed in a majority of patients and may limit attribution of pain relief to RFA alone, three subjects underwent ablation only. It is notable that these three patients all experienced clinical success with pain relief at discharge and at last follow-up. Although a small sample, this would suggest that RFA alone would have a palliative effect. While this limits drawing direct causality of RFA alone with pain relief, augmentation in patients with VBM may be associated with a lower rate of subsequent or re-fracture depending on the severity of the vertebral compromise and radiation therapy administered [10]. Determining fracture or instability of the vertebral body in the setting of metastatic disease is also a subjective limitation as many criteria may be considered including endplate deformity, tumor type, size and location in the vertebra and percent of vertebral body involvement [31]. One prospective pilot study that addressed the palliative effect of RFA alone compared RFA (n = 8) to RFA combined with cement augmentation (n = 8) in breast cancer patients with painful spinal metastases in the absence of vertebral fractures [28, 29]. The authors concluded cement delivery in the necrosis cavity had no significant additional effect on reduction in pain or improvement in quality of life, supporting a direct relationship between tumor RFA and pain palliation due to destruction of adjacent nociceptors and reduced production of nerve stimulating cytokines [18].
There is increasing evidence that optimizing patient outcomes results in improved quality of life and that pain management is an essential part of comprehensive oncologic management [32]. The National Comprehensive Cancer Network (NCCN) guidelines version 2.2014 for Adult Cancer Pain added RFA as a treatment modality for control of local bone pain [33]. This prospective multicenter clinical series found that radiofrequency ablation with or without vertebral augmentation is a safe, effective and durable treatment for patients with painful metastatic vertebral body tumors that concomitantly improved disability and quality of life for these patients.
References
Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101.
Boland PJ, Lane JM, Sundaresan N. Metastatic disease of the spine. Clin Orthop Relat Res. 1982;169:95–102.
Harrington KD. Metastatic disease of the spine. J Bone Joint Surg Am. 1986;68:1110–5.
Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine (Phila Pa 1976). 1990;15:1–4.
Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509–24.
Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative’s Neuro-Oncology Disease Site Group. J Clin Oncol. 2005;23:2028–37.
Harel R, Angelov L. Spine metastases: current treatments and future directions. Eur J Cancer. 2010;46:2696–707.
Gangi A, Sabharwal T, Irani FG, Buy X, Morales JP, Adam A. Quality assurance guidelines for percutaneous vertebroplasty standards of practice committee of the society of interventional radiology. Cardiovasc Interv Radiol. 2006;29(2):173–8.
Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423–36.
Sahgal A, Whyne CM, Ma L, Larson DA, Fehlings MG. Vertebral compression fracture after stereotactic body radiotherapy for spinal metastases. Lancet Oncol. 2013;14:e310–20.
Di Staso M, Zugaro L, Gravina GL, Bonfili P, Marampon F, Di Nicola L, Conchiglia A, Ventura L, Franzese P, Gallucci M, Masciocchi C, Tombolini V. A feasibility study of percutaneous radiofrequency ablation followed by radiotherapy in the management of painful osteolytic bone metastases. Eur Radiol. 2011;21:2004–10.
Kurup AN, Callstrom MR. Image-guided percutaneous ablation of bone and soft tissue tumors. Semin Interv Radiol. 2010;27:276–84.
Algra PR, Heimans JJ, Valk J, Nauta JJ, Lachniet M, Van Kooten B. Do metastases in vertebrae begin in the body or the pedicles? Imaging study in 45 patients. AJR Am J Roentgenol. 1992;158:1275–9.
Callstrom MR, Dupuy DE, Solomon SB, Beres RA, Littrup PJ, Davis KW, Paz-Fumagalli R, Hoffman C, Atwell TD, Charboneau JW, Schmit GD, Goetz MP, Rubin J, Brown KJ, Novotny PJ, Sloan JA. Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer. 2013;119:1033–41.
Dupuy DE, Liu D, Hartfeil D, Hanna L, Blume JD, Ahrar K, Lopez R, Safran H, DiPetrillo T. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer. 2010;116:989–97.
Anchala PR, Irving WD, Hillen TJ, Friedman MV, Georgy BA, Coldwell DM, Tran ND, Vrionis FD, Brook A, Jennings JW. Treatment of metastatic spinal lesions with a navigational bipolar radiofrequency ablation device: a multicenter retrospective study. Pain Phys. 2014;17:317–27.
Hillen TJ, Anchala P, Friedman MV, Jennings JW. Treatment of metastatic posterior vertebral body osseous tumors by using a targeted bipolar radiofrequency ablation device: technical note. Radiology. 2014;273:261–7.
Lane MD, Le HB, Lee S, Young C, Heran MK, Badii M, Clarkson PW, Munk PL. Combination radiofrequency ablation and cementoplasty for palliative treatment of painful neoplastic bone metastasis: experience with 53 treated lesions in 36 patients. Skelet Radiol. 2011;40:25–32.
Wallace AN, Greenwood TJ, Jennings JW. Radiofrequency ablation and vertebral augmentation for palliation of painful spinal metastases. J Neurooncol. 2015;124(1):111–8. doi:10.1007/s11060-015-1813-2 Epub 2015 May 29.
Fritz JM, Irrgang JJ. A comparison of a modified Oswestry low back pain disability questionnaire and the Quebec back pain disability scale. Phys Ther. 2001;81:776–88.
Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9.
Yanez B, Pearman T, Lis CG, Beaumont JL, Cella D. The FACT-G7: a rapid version of the functional assessment of cancer therapy-general (FACT-G) for monitoring symptoms and concerns in oncology practice and research. Ann Oncol. 2013;24:1073–8.
Broom R, Du H, Clemons M, Eton D, Dranitsaris G, Simmons C, Ooi W, Cella D. Switching breast cancer patients with progressive bone metastases to third-generation bisphosphonates: measuring impact using the Functional Assessment of Cancer Therapy-Bone Pain. J Pain Symptom Manag. 2009;38:244–57.
Pearman T, Yanez B, Peipert J, Wortman K, Beaumont J, Cella D. Ambulatory cancer and US general population reference values and cutoff scores for the functional assessment of cancer therapy. Cancer. 2014;120:2902–9.
Services Usdohah. National Cancer Institute, National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE) 2009; (v4.03: June 14, 2010).
Callstrom MR, York JD, Gaba RC, Gemmete JJ, Gervais DA, Millward SF, Brown DB, Dupuy D, Goldberg SN, Kundu S, Rose SC, Thomas JJ, Cardella JF. Research reporting standards for image-guided ablation of bone and soft tissue tumors. J Vasc Interv Radiol. 2009;20(12):1527–40.
Goetz MP, Callstrom MR, Charboneau JW, Farrell MA, Maus TP, Welch TJ, Wong GY, Sloan JA, Novotny PJ, Petersen IA, Beres RA, Regge D, Capanna R, Saker MB, Grönemeyer DH, Gevargez A, Ahrar K, Choti MA, de Baere TJ, Rubin J. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22:300–6.
Proschek D, Kurth A, Proschek P, Vogl TJ, Mack MG. Prospective pilot-study of combined bipolar radiofrequency ablation and application of bone cement in bone metastases. Anticancer Res. 2009;29(7):2787–92.
Johnstone C, Lutz S. External beam radiotherapy and bone metastases. Ann Palliat Med. 2014;3:114–22.
Fisher CG, DiPaola CP, Ryken TC, Bilsky MH, Shaffrey CI, Berven SH, Harrop JS, Fehlings MG, Boriani S, Chou D, Schmidt MH, Polly DW, Biagini R, Burch S, Dekutoski MB, Ganju A, Gerszten PC, Gokaslan ZL, Groff MW, Liebsch NJ, Mendel E, Okuno SH, Patel S, Rhines LD, Rose PS, Sciubba DM, Sundaresan N, Tomita K, Varga PP, Vialle LR, Vrionis FD, Yamada Y, Fourney DR. A novel classification system for spinal instability in neoplastic disease. Spine. 2010;35(22):1221–9.
Cruz JP, Sahgal A, Whyne C, Fehlings MG, Smith R. Tumor extravasation following a cement augmentation procedure for vertebral compression fracture in metastatic spinal disease. J Neurosurg Spine. 2014;21:372–7.
Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–42.
NCCN Guidelines Adult Cancer Pain ver. 22014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Sandeep Bagla reports other from DFINE Inc. during the conduct of the study. Dawood Sayed reports personal fees from DFINE Inc. outside the submitted work. Jayson Brower reports other from DFINE Inc. outside the submitted work. J. Neal Rutledge reports non-financial support from DFINE Inc. during the conduct of the study; personal fees from DFINE Inc. outside the submitted work. James Carlisle reports grants from DFINE Inc. during the conduct of the study. Ilya Lekht reports grants from null during the conduct of the study. Bassem Georgy reports other from DFINE Inc. during the conduct of the study and other from DFINE Inc. outside the submitted work.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Bagla, S., Sayed, D., Smirniotopoulos, J. et al. Multicenter Prospective Clinical Series Evaluating Radiofrequency Ablation in the Treatment of Painful Spine Metastases. Cardiovasc Intervent Radiol 39, 1289–1297 (2016). https://doi.org/10.1007/s00270-016-1400-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-016-1400-8