Abstract
Since late September 2014, there have been approximately 10 cases in Japan where people have died from inhaling smoke from herbal blends containing a newly emerged synthetic cannabinoid. Although the drug was tentatively identified by some drug-monitoring institutions as 5-fluoro-ADB, to our knowledge, its data have not been published in any scientific context. We recently encountered an autopsy case in which 5-fluoro-ADB was involved. The deceased was a 34-year-old man who was found dead in his room. The postmortem interval was estimated at 35–40 h. The direct cause of the death was asphyxia due to aspiration of stomach contents into the trachea, which likely took place during vomiting under low-consciousness conditions provoked by inhalation of the 5-fluoro-ADB smoke. The cadaver was subjected to autopsy at our department. Femoral vein blood, right heart blood, left heart blood, urine, stomach contents, and nine solid tissues including the adipose tissue were collected and frozen until analysis. The extraction of 5-fluoro-ADB and internal standard 5-fluoro-AMB was performed using a modified QuEChERS method plus filtration through Captiva ND Lipids cartridges, followed by liquid chromatography–tandem mass spectrometry (LC–MS–MS) analysis. Because this study dealt with various kinds of human matrices, we used the standard addition method for quantitation to overcome the matrix effects. The levels of 5-fluoro-ADB in the cadaver specimens were generally low; it could not be detected from blood or urine specimens. The levels of 5-fluoro-ADB in solid tissues were 1.17–7.95 ng/g. Because the highest levels were found for the adipose tissue and heart muscle, the final extracts of the adipose tissue and/or heart muscle were concentrated 10- and 200-fold to obtain product ion mass spectra of 5-fluoro-ADB using LC–MS–MS and its mass spectrum by gas chromatography–mass spectrometry, respectively. Both spectra completely coincided with those obtained from the reference standard 5-fluoro-ADB, confirming that the target compound was 5-fluoro-ADB. The quantitative results obtained by selected reaction monitoring of LC–MS–MS showed the highest level, at 7.95 ng/g, in the adipose tissue, followed by stomach contents, brain, heart muscle, pancreas, and spleen. For the lung, liver, kidney, and skeletal muscle, levels were below the quantitation limit (about 0.5 ng/g), although very small peaks above the detection limit (about 0.1 ng/g) could be observed for all of the above solid tissues. The low levels of 5-fluoro-ADB in the solid tissues were likely as a result of only a small amount of 5-fluoro-ADB incorporated into the body via the lungs due to the short period from the beginning of smoking the herb to the fatal asphyxia resulting from aspiration of a massive amount of stomach contents into the trachea under low-consciousness conditions. In addition, we measured the content of 5-fluoro-ADB in three packages, all of which were opened, that were found under a pillow near the deceased; their levels of 5-fluoro-ADB were 49.2 mg/g, 12.2 μg/g, and 0.77 μg/g. To our knowledge, this is the first reported identification and quantitation of 5-fluoro-ADB in human specimens and herbal products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, synthetic cannabinoids [1–5] and cathinones [6–9] have become widely distributed, and are now causing social problems throughout many parts of the world. Although the traditional marijuana and hashish containing Δ9-tetrahydrocannabinol as the psychoactive compound have been associated with almost no fatalities thus far, two reports have described fatalities caused by the synthetic cannabinoids MAM-2201 [10] and NNEI [11]. We also reported a fatal case due to the combined use of two synthetic cannabinoids, AB-CHMINACA and 5-fluoro-AMB, and the N-methyl-d-aspartate receptor channel blocker diphenidine [12].
Very recently, a new and very dangerous synthetic cannabinoid has emerged; since late September 2014, there have been approximately 10 deaths in Japan caused by smoking this compound (Kikura-Hanajiri, personal communication). Although it was tentatively identified by some drug-monitoring institutions as 5-fluoro-ADB (see its structure in Fig. 1), to our knowledge, data have not been published in any scientific context. We recently encountered an autopsy case in which 5-fluoro-ADB was involved. Three silver-colored packages containing herbal blend mixtures were also found in the room of the deceased by law enforcement, and were delivered to our laboratories for analysis. In this study, we identify and quantitate 5-fluoro-ADB in various specimens collected at autopsy as well as in the illegal herbal products. To our knowledge, this is the first report to identify and quantitate the new designer drug 5-fluoro-ADB from biological and herbal blend specimens.
Case history
The deceased was a 34-year-old man, who was found dead in his room in a house owned by his mother. He was lying in a prone position on his futon. There were remarkable postmortem vibices on the front chest, abdomen, and the front parts of both thighs. He was grasping a small handmade aluminum foil pipe in his right hand. Around his nostrils and mouth, there were vomit debris attached. Three opened silver-colored packages of different brands of herbal blends were found under his pillow. Medical examination at the scene found no evidence of no criminality except for the suspected drug abuse by the deceased; the cadaver was relatively fresh, and no injuries were found on the body surface. The cadaver was stored in a refrigerated morgue at 2 °C for 1 day. The autopsy was performed at our department.
At the beginning of the autopsy, the postmortem interval was estimated to be 35–40 h. External macroscopic observations noted that livor mortis was remarkably associated with vibices over wide areas of the front body surface. There was remarkable congestion of the face, and petechiae were noted on both palpebral and bulbar conjunctivae.
There were no serious injuries related to death. Internally, the trachea was filled with a large amount of stomach contents, which reached the tracheal bifurcation, thus completely occluding the airway. Such a massive aspiration of stomach contents into the trachea is usually not observed for persons with average physical strength and in a state of clear consciousness. This phenomenon is likely due to lowered consciousness together with vomiting provoked by the inhalation of synthetic cannabinoid(s). There were petechiae on the inside surface of the scalp and on the surface of both lungs and the heart. Congestion of both lungs was remarkable. These findings are consistent with asphyxia as the direct cause of death; the indirect cause appeared to be synthetic cannabinoid poisoning.
Routine analysis of blood alcohol using gas chromatography showed a low level of alcohol (0.3 mg/ml) in the blood. Immunochemical drug screening using the Triage Drugs of Abuse panel (Alere, Waltham, MA, USA) for urine specimens showed a positive result for barbiturate drugs. NAGINATA screening for conventional drugs and toxic compounds in whole blood using gas chromatography–mass spectrometry (GC–MS) [13] revealed the presence of a low level of quetiapine and a nicotine metabolite. The deceased had a history of admission to a mental health hospital because of heavy dependence on the designated drugs for about 3 months in 2013 and 2014. Upon discharge in 2014, which had occurred just several days earlier, he had been prescribed quetiapine, olanzapine, levomepromazine, biperiden, and troxipide by the psychiatrist. However, the NAGINATA screening test identified only quetiapine, and the reason for the failure to detect the other psychotropic drugs was not clear.
Materials and methods
Materials
5-Fluoro-ADB (methyl (R)-2-[1-(5-fluoropentyl)-1H-indazole-3-carboxamido]-3,3-dimethylbutanoate) was donated by Dr. R. Kikura-Hanajiri of the National Institute of Health Sciences, Tokyo, Japan. 5-Fluoro-AMB, used as an internal standard (IS) (see its structure in Fig. 1) for analysis of 5-fluoro-ADB, was purchased from Cayman Chemical (Ann Arbor, MI, USA). Other common chemicals used were of the highest purity commercially available. Plastic centrifuge tubes with caps (5-ml capacity, 6 × 1.5 cm external diameter) and stainless beads (5 mm external diameter) for crushing solid tissues were purchased from TAITEC, Saitama, Japan. The QuEChERS dispersive SPE centrifuge tubes with caps (2-ml capacity), each of which contained 25 mg of primary-secondary amine (PSA), 25 mg of end-capped octadecylsilane (C18EC), and 150 mg of magnesium sulfate, and Captiva ND Lipids cartridges (3-ml capacity) were purchased from Agilent (Santa Clara, CA, USA).
Whole blood specimens from the right and left atria and femoral vein, urine, stomach contents, and solid tissue specimens (brain, lung, heart muscle, liver, spleen, kidney, pancreas, skeletal muscle, and adipose tissue) were collected from the cadaver at autopsy and kept frozen at −80 °C until analysis; the adipose tissue specimen was obtained from the abdominal subcutaneous area.
Extraction procedure for human specimens except adipose tissue
One gram or 1 ml of solid tissue or fluid specimens, respectively, was placed in a 5-ml plastic tube with a cap containing 4-ml acetonitrile and 1,000 ng of 5-fluoro-AMB (IS) dissolved in 10 μl acetonitrile. Tissue specimens were minced with clean surgical scissors; this step was skipped for fluid or stomach content specimens. Five stainless beads were added to the mixture, and the tube was capped, held to a bead beater-type homogenizer (Beads Crusher μT-12; TAITEC), and vigorously shaken at 3,200 rpm for 5 min. All of the suspension solution except the beads was transferred to a large test tube, and 5 ml of acetonitrile was added, and the solution was gently shaken. Six 1-ml portions were taken from the 10-ml homogenate suspension, and were prepared, with and without the addition of different amounts of 5-fluoro-ADB dissolved in 10 μl of acetonitrile, in 1.5-ml plastic centrifuge tubes with caps to construct a standard addition calibration curve, vortexed for 30 s, and centrifuged at 10,000 rpm for 2 min. The clear supernatant was decanted into the QuEChERS dispersive SPE centrifuge tube containing PSA, C18EC, and magnesium sulfate, vortexed for 30 s, and centrifuged at 10,000 rpm for 2 min. The upper acetonitrile layer was passed through a Captiva ND Lipids cartridge. A 3.5-μl aliquot of the eluate was then analyzed using liquid chromatography–tandem mass spectrometry (LC–MS–MS).
When the product ion mass spectrum via LC–MS–MS or mass spectrum via GC–MS was recorded, the final acetonitrile eluate was condensed approximately tenfold or 200-fold, respectively, by evaporation.
Extraction procedure for the adipose tissue specimen
One gram of solid adipose tissue was placed in a 5-ml plastic tube with a cap containing 4 ml of acetonitrile and 5,000 ng of IS dissolved in 10 μl of acetonitrile. The specimen was minced with clean surgical scissors. The 5-ml plastic tube containing the mixture was heated at 80 °C for 10 min, and the five stainless steel beads were added to the mixture. The tube was capped, held to the bead beater-type homogenizer, and vigorously shaken at 3,200 rpm for 5 min. Despite vigorous shaking, the liquefied fat layer and acetonitrile layer did not mix well. All of the mixture except the beads was transferred to a scaled 50-ml conical flask, and total volume of up to 50 ml was made by the addition of acetonitrile, followed by gentle shaking. At this stage, the liquid fat and acetonitrile were completely mixed. Six 1-ml portions taken from the 50-ml homogenate in acetonitrile were prepared with and without the addition of different amounts of 5-fluoro-ADB dissolved in 10 μl of acetonitrile in 1.5-ml plastic centrifuge tubes with caps, vortexed for 30 s, and centrifuged at 10,000 rpm for min. Each supernatant fraction was subjected to QuEChERS dispersive solid-phase extraction and filtered through the Captiva ND Lipids cartridge, as described above; a 3.5-μl aliquot of the eluate was analyzed using LC–MS–MS.
Extraction procedure for herbal blend specimens
For analysis of 5-fluoro-ADB in the three herbal blend products, 10 mg of each herbal debris was treated as described in a previous report [14]. Briefly, 10 mg of the plant debris was sonicated in 1.0 ml of acetonitrile for 10 min, and centrifuged at 10,000 rpm for 2 min. The supernatant layer was decanted into a test tube, followed by appropriate dilution with acetonitrile (from no dilution to 1,000 dilution; overall 100–100,000 dilution). To 1.0 ml of each diluted acetonitrile extract solution, 10 μl of acetonitrile solution, with or without an appropriate amount of reference standard 5-fluoro-ADB, was added and the mixture shaken gently to construct a standard addition calibration curve. For quantitation of 5-fluoro-ADB in herbal mixtures, IS was not used; instead, only units of peak area were used. A 3.5-μl aliquot of the final solution was injected into the LC–MS–MS instrument.
LC–MS–MS conditions
LC–MS–MS with electrospray ionization (ESI) was conducted on an Agilent 1200 LC-SL system containing a microdegasser and high-performance autosampler, which was connected to a 6460 Triple Quad LC/MS tandem MS instrument (Agilent). For LC separation, a ZORBAX Eclipse Plus C18 column (100 × 2.1 mm internal diameter, particle size 1.8 μm; Agilent) was used. The LC conditions were as follows: injection volume, 3.5 μl; flow rate, 0.25 ml/min; elution mode, gradient with 10 mM ammonium formate/0.1 % formic acid in distilled water (A) and acetonitrile (B) from 60 % A/40 % B to 100 % B over 15 min, followed by isocratic elution with the final solvent composition for 10 min. The column and autosampler were operated at room temperature.
The tandem MS conditions were as follows: interface, ESI mode; polarity, positive ion mode; ion source temperature, 320 °C; ion source voltage, 500 V; quantitation, selected reaction monitoring (SRM) mode, using peak area; ion transitions, m/z 378 → 233 for 5-fluoro-ADB and m/z 364 → 233 for 5-fluoro-AMB (IS); fragmentor voltage and collision energy, 120 and 21 V for 5-fluoro-ADB, and 120 and 17 V for 5-fluoro-AMB, respectively.
Data acquisition, peak integration, and calculations were performed on an Agilent MassHunter computer workstation (Revision: Acquisition, B02.01; Qualification, B03.01; SP2 and Quantification, B04.00).
GC–MS conditions
The GC–MS instrument used was an Agilent 6850 gas chromatograph connected to a 5975 Series mass spectrometer (Agilent). GC conditions were as follows: separation column, Agilent HP-5 ms fused-silica capillary (30 m × 0.25 mm internal diameter, 0.25 μm film thickness); injector temperature, 250 °C; interface temperature, 280 °C; injection mode, splitless; injection volume, 1 μl; carrier gas (He) pressure, 151 kPa; oven temperature program, initial temperature at 60 °C (2-min hold) followed by ramp at 20 °C/min up to 300 °C. MS conditions were as follows: ion source temperature, 230 °C; ionization mode, electron ionization (EI) at 70 eV; emission current, 35 μA; detection gain, 1,118 V; identification, scan mode; scan range, m/z 50–400; scan speed, 2.86 scans/s.
Standard addition method
Although the standard addition method is frequently used for analysis with atomic absorption spectroscopy in order to overcome matrix effects [15], it is not popular in the field of forensic toxicology. We began using the standard addition method in our laboratories in a study analyzing ethylene glycol (EG) and propylene glycol (PG) in whole blood specimens collected from non-occupational and healthy subjects [16], where non-negligible concentrations of EG and PG were found in whole blood of healthy subjects. In order to measure the preexisting compounds, the standard addition method had to be employed. Since then, we have realized that the standard addition method is very useful for investigating the distribution of xenobiotics in human body fluid and solid tissue specimens, as the matrices collected from human cadavers have quite different properties. In an investigation involving measurement of postmortem distribution of α-pyrrolidinovalerophenone using LC–MS–MS in a case of fatal poisoning, we were embarrassed by the remarkably different matrix effects among different human body fluids and organs [8], and the standard addition method was very useful for overcoming the matrix effects and different recovery rates. The standard addition method seems to be the best choice in particular for comparison of concentrations in matrices with fairly different properties, and this method was employed in the current study. The details of the procedure and calculation of the results have been detailed in our previous reports [8, 17].
Matrix effects and recovery rates
In order to obtain the values of matrix effects and recovery rates for 5-fluoro-ADB in human specimens, the starting point is to measure all concentrations of a target compound in all specimens. In this experiment, the testing of the matrix effects and recovery rates of 5-fluoro-ADB in the heart muscle and adipose tissue are simply provided as examples. After measuring 5-fluoro-ADB concentrations in both matrices according to the methods previously described, we prepared two concentrations of reference standard 5-fluoro-ADB dissolved in pure acetonitrile for each matrix: one with concentrations 10 times higher and 10 times lower than those in the matrix for the heart muscle specimen; and one concentrations two times and 50 times lower than those in the matrix for the adipose tissue specimen. Then, 1 g of the heart muscle or adipose tissue was crushed in acetonitrile with addition of IS, diluted, and gently shaken according to each method as previously described. Although we usually take six 1-ml portions from 10 or 50 ml of tissue homogenate in acetonitrile for constructing a standard addition calibration curve, in this case we took four 1-ml portions from each matrix homogenate, which were subjected to the centrifugation, the QuEChERS dispersive solid-phase extraction, and the filtration through Captiva ND Lipids cartridges, also as previously described, with no additions. The final three eluates from the cartridges were combined, and 10 µl of the reference standard 5-fluoro-ADB acetonitrile solution at either tenfold or twofold higher concentration was added to 1.0 ml of the combined extract acetonitrile solution and shaken gently. A 3.5-µl aliquot was injected into the LC–MS–MS system to obtain a peak area designated as A. A 3.5-µl aliquot of the final eluate from the fourth 1.0-ml portion of the homogenate, with no addition, was also injected into the LC–MS–MS system to obtain a peak area designated as B. Finally, a 3.5-µl aliquot of the reference standard 5-fluoro-ADB acetonitrile solution at either 10- or 50-fold lower concentration was injected into the LC–MS–MS system to obtain a peak area designated as C. The matrix effect and recovery rate was calculated for both matrices, as follows: matrix effect (%) = [(A − B)/C] × 100; recovery rate (%) = [B/(A − B)] × 100.
Results and discussion
Identification of 5-fluoro-ADB using LC–MS–MS and GC–MS
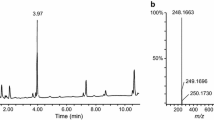
Because the preliminary results showed that the adipose tissue and heart muscle contained the highest concentrations of the target compound, we took the final acetonitrile extract solutions from the two solid tissues, concentrated 10- and 200-fold for LC–MS–MS and GC–MS analysis, respectively. Figure 2 shows the product ion mass spectra of LC–MS–MS obtained from the reference standard 5-fluoro-ADB, heart muscle extract, and adipose tissue extract. The spectra obtained from both tissues coincided well with that of the reference standard 5-fluoro-ADB, with no impurity peaks. The base peak appeared at m/z 233, which was used for quantitative analysis.
Product ion mass spectra of the extracts of the heart muscle and adipose tissue specimens collected from the deceased cadaver in comparison with that of the reference standard 5-fluoro-ADB, recorded by using liquid chromatography–tandem mass spectrometry (LC–MS–MS) together with the probable fragmentation mode
Figure 3 shows the mass spectra of GC–MS obtained from the reference standard 5-fluoro-ADB and from the adipose tissue extract. The spectrum obtained from the adipose tissue also coincided with that of the reference standard 5-fluoro-ADB. Therefore, we were able to conclude that 5-fluoro-ADB was identified in the adipose tissue and heart muscle of the cadaver.
Validation of the method
Figure 4 shows an example of the SRM chromatograms for the target compound and IS extracted from the heart muscle and adipose tissue. The target compound 5-fluoro-ADB and IS 5-fluoro-AMB appeared at retention times of 9.58 and 8.78 min, respectively, under our analytical conditions. The bottom panel of Fig. 3 shows the absence of 5-fluoro-AMB in the adipose tissue extract, indicating that 5-fluoro-AMB was able to be used as IS in this study. It should be noted that the backgrounds were generally very low, and there were no impurity peak interfering with the target or IS peaks.
Table 1 shows standard addition calibration equations for 5-fluoro-ADB in the specimens in which the test compound could be quantitated. The correlation coefficient values obtained for all six specimens were greater than 0.999. The detection limit (signal-to-noise ratio ≥3) for the compound using this method was around 0.1 ng/ml or g. The lower quantitation limit (signal-to-noise ratio ≥10) was around 0.5 ng/ml or g.
Because we employed the standard addition method for quantitation, without the use of blank specimens, it was impossible to present the usual accuracy and precision data. Instead, as shown in Table 2, we repeated intraday and interday determinations of 5-fluoro-ADB in the heart muscle and adipose tissue specimens as examples. The repeatability, expressed as relative standard deviations, was not greater than 7.26 %.
Although the standard addition method can overcome matrix effects and low recovery rates, the matrix effects for 5-fluoro-ADB in various matrices under the present extraction conditions are of interest. In this study, we used acetonitrile deproteinization plus QuEChERS dispersive solid-phase extraction plus filtration through a Captiva ND Lipids cartridge coupled to an LC–MS–MS analysis system. The matrix effects for 5-fluoro-ADB were 87.7 ± 2.98 and 98.8 ± 1.20 % (n = 3 in each) for the heart muscle and adipose tissue specimens, respectively. The recovery rates of the test compound were also excellent, at 91.8 ± 1.43 and 101 ± 1.12 % (n = 3 in each) for the heart muscle and adipose tissue specimens, respectively. The final treatment with the Captiva ND Lipids cartridge appears to be very useful in preventing the depressive matrix effects likely caused by ionized phospholipids.
Postmortem distribution of 5-fluoro-ADB in the various cadaver specimens
Table 3 shows the postmortem distribution of 5-fluoro-ADB in the body fluids, stomach contents, and nine solid tissues, including the adipose tissue of the cadaver. No concentrations of 5-fluoro-ADB were detected in any blood or urine specimens. Although 5-fluoro-ADB concentrations in solid tissues were generally much lower than that of AB-CHMINACA, as published in a previous report [12], the highest concentrations were consistently observed in the adipose tissue. This high concentration of synthetic cannabinoids in adipose tissue is due to the high lipophilicity of the compounds, and also likely due to the very low levels or absence of metabolizing enzymes in the adipocytes.
The low levels of 5-fluoro-ADB in all solid tissue specimens could be explained by the fact that only a small amount of 5-fluoro-ADB in the herbal smoke was inhaled in a very short period of time, as this potent synthetic cannabinoid likely exerted its powerful action on consciousness and provoked vomiting symptom very shortly after the victim inhaled the drug smoke. In this case, the direct cause of his death was judged as asphyxia due to aspiration of a massive amount of stomach content vomit into the trachea. If the reduction in consciousness and vomiting are characteristic effects of 5-fluoro-ADB, the low levels of 5-fluoro-ADB in the victim’s specimens are not surprising.
Identification and quantitation of 5-fluoro-ADB in herbal blend products
Three silver-colored packages of herbal mixtures were found near the deceased. The brand names, handwritten with a marker pen, were “GM sapphire”, “AP 31,” and “AL 37,” For each of the herbal blends, 10 mg was extracted as described in a previous report [14], and subjected to measurements of mass spectra using LC–MS–MS and GC–MS after appropriate dilution of the extract solutions. Spectra for all of these products were in complete agreement with those shown in Figs. 2 and 3, indicating that all mixtures contained 5-fluoro-ADB. We then quantitated the concentration of the compound in each of the three products; the results were 49.2 ± 2.46 mg/g, 12.2 ± 0.21 μg/g, and 766 ± 13.7 ng/g for “GM sapphire”, “AP 31,” and “AL 37,” respectively.
Conclusions
To our knowledge, this is the first scientific description of the identification and quantitation of 5-fluoro-ADB in postmortem human specimens and herbal products. This compound is one of the most dangerous synthetic cannabinoids ever known. Inhalation of the smoke from this compound is thought to have been responsible for about 10 deaths, and the product has very recently become regulated as a designated substance in Japan. These cases involving deaths likely caused by this compound have become identified only since late September 2014. Characteristic symptoms after inhalation included rapid loss of consciousness and cardiopulmonary arrest. Everyone should be alert to the life-threatening effects induced by 5-fluoro-ADB.
References
Zuba D, Byrska B (2013) Analysis of the prevalence and coexistence of synthetic cannabinoids in “herbal high” products in Poland. Forensic Toxicol 31:21–30
Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y (2013) Changes in the prevalence of synthetic cannabinoids and cathinone derivatives in Japan until early 2012. Forensic Toxicol 31:44–53
Chung H, Choi H, Heo S, Kim E, Lee J (2014) Synthetic cannabinoids abused in South Korea: drug identification by the National Forensic Service from 2009 to June 2013. Forensic Toxicol 32:82–88
Uchiyama N, Shimokawa Y, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (2014) Two new synthetic cannabinoids, AM-2201 benzimidazole analog (FUBIMINA) and (4-methylpiperazin-1-yl)(1-pentyl-1H-indol-3-yl)methanone (MEPIRAPIM), and three phenethylamine derivatives, 25H-NBOMe 3,4,5-trimethoxybenzyl analog, 25B-NBOMe, and 2C-N-NBOMe, identified in illegal products. Forensic Toxicol 32:105–115
Uchiyama N, Shimokawa Y, Kawamura M, Kikura-Hanajiri R, Hakamatsuka T (2014) Chemical analysis of a benzofuran derivative, 2-(2-ethylaminopropyl)benzofuran (2-EAPB), eight synthetic cannabinoids, five cathinone derivatives, and five other designer drugs newly detected in illegal products. Forensic Toxicol 32:266–281
Namera A, Urabe S, Saito T, Torikoshi-Hatano A, Shiraishi H, Arima Y, Nagao M (2013) A fatal case of 3,4-methylenedioxypyrovalerone poisoning: coexistence of α-pyrrolidinobutiophenone and α-pyrrolidinovalerophenone in blood and/or hair. Forensic Toxicol 31:338–343
Namera A, Konuma K, Kawamura M, Saito T, Nakamoto A, Yahata M, Ohta S, Miyazaki S, Shiraishi H, Nagao M (2014) Time-course profile of urinary excretion of intravenously administered α-pyrrolidinovalerophenone and α-pyrrolidinobutiophenone in a human. Forensic Toxicol 32:68–74
Hasegawa K, Suzuki O, Wurita A, Minakata K, Yamagishi I, Nozawa H, Gonmori K, Watanabe K (2014) Postmortem distribution of α-pyrrolidinovalerophenone and its metabolite in body fluids and solid tissues in a fatal poisoning case measured by LC–MS–MS with the standard addition method. Forensic Toxicol 32:225–234
Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Suzuki O, Watanabe K (2014) Identification and quantitation of a new cathinone designer drug PV9 in an “aroma liquid” product, antemortem whole blood and urine specimens, and a postmortem whole blood specimen in its fatal poisoning case. Forensic Toxicol 32:243–250
Saito T, Namera A, Miura N, Ohta S, Miyazaki S, Osawa M, Inokuchi S (2013) A fatal case of MAM-2201 poisoning. Forensic Toxicol 31:333–337
Sasaki C, Saito T, Shinozuka T, Irie W, Murakami C, Maeda K, Nakamaru N, Oishi M, Nakamura S, Kurihara K (2014) A case of death caused by abuse of a synthetic cannabinoid N-1-naphthalenyl-1-pentyl-1H-indole-3-carboxamide. Forensic Toxicol. doi:10.1007/s11419-014-0246-5
Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Watanabe K, Suzuki O (2014) Postmortem distribution of AB-CHMINACA, 5-fluoro-AMB and diphenidine in body fluids and solid tissues in a fatal poisoning case: usefulness of the adipose tissue for detection of the drugs in unchanged forms. Forensic Toxicol. doi:10.1007/s11419-014-0245-6
Kudo K, Ishida T, Hikiji W, Hayashida M, Uekusa K, Usumoto Y, Tsuji A, Ikeda N (2009) Construction of calibration-locking databases for rapid and reliable drug screening by gas chromatography-mass spectrometry. Forensic Toxicol 27:21–31
Wurita A, Hasegawa K, Minakata K, Watanabe K, Suzuki O (2014) A large amount of new designer drug diphenidine coexisting with a synthetic cannabinoid 5-fluoro-AB-PINACA found in a dubious herbal product. Forensic Toxicol 32:331–337
Bonilla E (1978) Flameless atomic absorption spectrophotometric determination of manganese in rat brain and other tissues. Clin Chem 24:471–474
Wurita A, Suzuki O, Hasegawa K, Gonmori K, Minakata K, Yamagishi I, Nozawa H, Watanabe K (2013) Sensitive determination of ethylene glycol, propylene glycol and diethylene glycol in human whole blood by isotope dilution gas chromatography–mass spectrometry, and the presence of appreciable amounts of the glycols in blood of healthy subjects. Forensic Toxicol 31:272–280
Wurita A, Hasegawa K, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Suzuki O, Watanabe K (2014) Postmortem distribution of α-pyrrolidinobutiophenone in body fluids and solid tissues of a human cadaver. Legal Med 16:241–246
Acknowledgments
The authors are very grateful to Dr. R. Kikura-Hanajiri of the National Institute of Health Science, Tokyo, Japan, for providing us with the reference standard of 5-fluoro-ADB.
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
K. Hasegawa and A. Wurita contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hasegawa, K., Wurita, A., Minakata, K. et al. Identification and quantitation of 5-fluoro-ADB, one of the most dangerous synthetic cannabinoids, in the stomach contents and solid tissues of a human cadaver and in some herbal products. Forensic Toxicol 33, 112–121 (2015). https://doi.org/10.1007/s11419-014-0259-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-014-0259-0