Abstract
A couple bought “aroma liquid” and “bath salt” type drugs at a dubious drug shop. Both of them orally took the liquid type drug; although the male subject showed no symptoms, the female subject suffered shivering, convulsions, and low levels of consciousness. The woman was taken to an emergency hospital to receive intensive medical treatment, but died about 20 h after admission. The aroma liquid solution, and the antemortem blood and urine collected during medical treatment at the hospital were brought to our laboratory by the police for analysis of the causative drug(s). In addition, a sample of postmortem femoral vein blood was collected from the cadaver. After some screening tests, we finally identified PV9 (α-POP) in all specimens by gas chromatography–mass spectrometry and liquid chromatography–tandem mass spectrometry (LC–MS–MS). The concentration of PV9 was 18.3 mg/ml in the aroma liquid solution, 45.7 ng/ml in the antemortem blood, 20.3 ng/ml in the antemortem urine, and 180 ng/ml in the postmortem femoral vein blood. The concentrations in antemortem blood and urine and in postmortem blood were greatly lowered by dilution during the intensive medical treatment, including intravenous drip infusion of a large volume of solution. The probable coexistence of a β-hydroxyl metabolite was also investigated by mass chromatography and analysis of fragment ions of the product ion spectrum obtained by LC–MS–MS. To our knowledge, this is the first reported identification and quantitation of PV9 in human specimens in a fatal PV9 poisoning case.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cathinones and synthetic cannabinoids are now the most widely distributed drugs of abuse in the world [1–4]. Among various types of cathinones, α-pyrrolidinophenone derivatives have gained popularity as abused drugs, because of their ability to exert strong psychedelic effects. This is caused by the lipophilic pyrrolidinyl moiety, which provides the molecule with a higher ability to cross the blood–brain barrier [5].
Recently, we encountered a fatal case of drug poisoning, in which PV9 (α-POP) was judged as the cause of death. To our knowledge, this is the first demonstration of PV9 in human blood and urine.
Case history
According to the explanation by police, a couple bought both “aroma liquid” type and “bath salt” type drugs at a dubious drug shop. Both of them orally took the liquid type drug at a hotel; the volume of the aroma liquid ingested by the female subject (18 years of age) was not clear. Although the male subject showed no signs, the female subject showed various symptoms soon after ingestion, such as shivering, convulsions, and low levels of consciousness. She was taken to an emergency hospital, where she received intensive medical treatments including an intravenous drip infusion of a large volume of solution and gastrolavage. Despite the efforts of the medical team, she was pronounced dead about 20 h after admission. The police authority, of course, intervened in this incident. As evidence materials, the hospital gave the police the bottle of aroma liquid, and blood and urine specimens of the deceased that were collected during treatment. The police brought all of them to our laboratory for forensic analysis. In addition, we were allowed to sample the postmortem femoral vein blood from the cadaver.
Routine analysis for blood alcohol by gas chromatography showed negative results. The immunochemical drug screening kit Triage DOA for the urine specimens (Alere, Waltham, MA, USA) also showed negative results. NAGINATA screening for conventional drugs and toxic compounds in whole blood using gas chromatography–mass spectrometry (GC–MS) [6] showed a low level (semiquantitative concentration: 0.964 μg/ml) of caffeine. Because our in-house MS screening for drugs of abuse suggested no drugs, we consulted the Cayman Spectral Library [7], which strongly suggested the presence of PV9 in the solution in the aroma liquid bottle.
Materials and methods
PV9-HCl [α-POP-HCl, 1-phenyl-2-(pyrrolidin-1-yl)octan-1-one monohydrochloride] and PV8-HCl [α-PHPP-HCl, 1-phenyl-2-(1-pyrrolidinyl)-1-heptanone monohydrochloride] (for their structures, see Fig. 1) were purchased from Cayman Chemical (Ann Arbor, MI, USA); 5-ml plastic centrifuge tubes with caps from Labcon (Petaluma, CA, USA); the QuEChERS dispersive-SPE centrifuge tubes with caps, each of which contained 25 mg of primary secondary amine, 25 mg of end-capped octadecylsilane (C18EC), and 150 mg of magnesium sulfate, from Agilent (Santa Clara, CA, USA). Other common chemicals were of the highest purity commercially available.
The aroma liquid bottle containing a small amount of pink liquid, and the antemortem whole blood and urine, all collected at the emergency hospital, were brought to our laboratory for forensic analysis. The postmortem femoral vein blood was sampled from the cadaver about 6 days after death; during the postmortem interval, the cadaver was kept refrigerated in the morgue at about 3 °C.
Extraction procedure
To 0.1 ml of whole blood or urine in a 5-ml plastic centrifuge tube, 100 ng of PV8-HCl (internal standard, IS) with or without an appropriate amount of reference standard PV9-HCl all dissolved in 10 μl of acetonitrile and 0.1 ml of distilled water were added and vortexed gently. Acetonitrile (1 ml) was added to the mixture,which was then sonicated for 5 min and centrifuged at 10,000 rpm for 2 min. The supernatant layer was decanted into the QuEChERS dispersive-SPE centrifuge tube (2 ml) containing 25 mg of primary secondary amine, 25 mg of C18EC, and 150 mg of magnesium sulfate [8], followed by vortexing for 30 s and centrifuging at 10,000 rpm for 2 min. A 3.5-μl aliquot of the upper acetonitrile layer was subjected to analysis by liquid chromatography–tandem mass spectrometry (LC–MS–MS).
The aroma liquid solution was diluted 100,000-fold with acetonitrile. To 1.0 ml of the diluted product solution, 100 ng of PV8-HCl (IS) with or without an appropriate amount of reference standard PV9-HCl dissolved in 10 μl of acetonitrile was added and vortexed gently. A 3.5-μl aliquot of the solution was directly subjected to analysis by LC–MS–MS without any extraction procedure.
GC–MS conditions
The GC–MS instrument was an Agilent 6850 gas chromatograph connected to a 5975 mass spectrometer (Agilent). GC conditions were: separation column, Agilent HP-5 ms fused-silica capillary (30 m × 0.25 mm i.d., 0.25 μm film thickness); injector temperature, 250 °C; interface temperature, 280 °C; injection mode, splitless; injection volume, 1 μl; carrier gas (He) pressure, 21.9 psi; oven temperature program, initial temperature at 60 °C (2-min hold) followed by ramp at 20 °C/min up to 325 °C. MS conditions were: ion source temperature, 230 °C; ionization mode, electron ionization (EI) at 70 eV; emission current, 35 μA; detection gain, 1,118 V; identification, scan mode; scan range, m/z 50–300; scan speed, 2.86 scans/s.
LC–MS–MS conditions
LC–MS–MS was conducted on an Agilent 1200 LC-SL system connected to a 6460 Triple Quad LC/MS tandem MS instrument (Agilent). The LC-SL system contained a microdegasser and a high-performance autosampler. LC conditions were: separation column, ZORBAX Eclipse Plus C18 column (100 × 2.1 mm i.d., particle size 1.8 μm, Agilent); injection volume, 3.5 μl; flow rate, 0.25 ml/min; elution mode, gradient with 10 mM ammonium formate/0.1 % formic acid in distilled water (A) and acetonitrile (B) from 90 % A/10 % B to 100 % B in 20 min followed by isocratic elution with 100 % B for 10 min. Column and autosampler were operated at room temperature.
Tandem MS conditions were: interface, electrospray ionization (ESI) mode; polarity, positive; ion source temperature, 320 °C; ion source voltage, 500 V; quantitation, selected reaction monitoring (SRM) mode using peak area; ion transitions: m/z 274 → 91.1 for PV9 and m/z 260 → 91.1 for PV8 (IS); collision and fragment energies, 21 and 100 V, respectively, for both compounds.
Data acquisition, peak integration, and calculations were performed with a computer workstation (Agilent Masshunter, Revision Acquision B. 02. 01, Qualification B. 03. 01SP2 and Quantification B. 04. 00).
Standard addition method
The standard addition method [9] was employed to quantitate PV9 in the aroma liquid solution, and whole blood and urine specimens. The method can overcome matrix effects and recovery rate differences. In addition, the method requires no blank human specimens, which avoids the ethical problem of collecting human specimens containing no drugs. However, a disadvantage of this method is the need to construct a calibration curve with at least six plot points to obtain a single concentration value. The minus concentration where the straight standard addition calibration curve intersected with the horizontal x-axis of target compound concentration showed the existing concentration of the target compound in a specimen [9].
Matrix effects and recovery rates
Although the standard addition method can overcome the matrix effects together with differences in recovery rate, we were interested in the matrix effects and recovery rates for the present extraction procedure in combination with LC–MS–MS analysis. To determine the matrix effects and recovery rates for PV9 in body fluids, we first measured all concentrations of PV9 in the matrices by the standard addition method. According to each concentration of PV9 in a matrix, we prepared reference standard PV9 solution dissolved in acetonitrile at two concentrations; one with a concentration ten times lower than that in the matrix, and another one with a concentration ten times higher than that in the matrix. Then, two sets of the same extraction procedure were again conducted for each matrix; we obtained a couple of the final QuEChERS dispersive-SPE centrifuge tubes after centrifugation for a matrix. To the upper acetonitrile layer of one of the two final centrifuge tubes, 10 μl of the PV9 acetonitrile solution at the high concentration was added. The centrifuge tube with addition of reference standard PV9 was capped, shaken vigorously, and centrifuged; a 3.5-μl aliquot of the upper acetonitrile layer was injected into the LC–MS–MS system to obtain a peak area designated as A. A 3.5-μl aliquot of the upper acetonitrile layer of the counterpart final QuEChERS dispersive-SPE centrifuge tube without any addition was also injected into the LC–MS–MS system to obtain a peak area designated as B. A 3.5-μl aliquot of the PV9 acetonitrile solution without any extraction, the concentration of which was ten times lower than that in the corresponding matrix, was finally injected into the LC–MS–MS system to obtain a peak area designated as C. The matrix effect and recovery rate could be calculated as follows. Matrix effect (%) = [(A − B)/C] × 100. Recovery rate (%) = [B/(A − B)] × 100.
Results and discussion
Identification of PV9 by GC–MS and LC–MS–MS
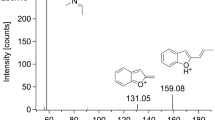
According to the screening result using the Cayman Spectral Library, we carefully recorded the EI mass spectra of the aroma liquid product solution and the reference standard PV9 after dilution with acetonitrile by GC–MS, as shown in Fig. 2. The peaks of m/z 168 appeared as the base peaks for both the product solution and reference standard. The small peaks appearing below m/z 120 for the aroma liquid solution also coincided with those for the reference standard PV9.
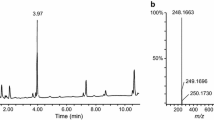
Figure 3 shows product ion mass spectra obtained from reference standard PV9, the aroma liquid product solution, and the extract of postmortem femoral vein whole blood measured by LC–MS–MS. The three spectra completely coincided.
The recorded GC–MS and LC–MS–MS data show that the present compound detected from the aroma liquid product and whole blood of the deceased can be identified as PV9. To our knowledge, this is the first demonstration of PV9 in human specimens, although Uchiyama et al. [10] first reported identification of PV9 in a brown powder product.
Validation for quantitative analysis of PV9
Figure 4 shows the SRM chromatograms of reference standard PV9, PV9 extracted from the femoral vein blood, and PV8 spiked into whole blood as IS. Sharp peaks appeared at 12.8 min for PV9 and 11.7 min for PV8 (IS). All chromatograms showed almost no impurity peaks with low backgrounds. To confirm the absence of PV8 in the specimens, the SRM chromatogram with tracing at m/z 91.1 using precursor ion at m/z 260 without spiking PV8 was recorded. As shown in the bottom panel of Fig. 4, a very small peak appeared, but it was negligible; we interpreted the very small peak as the carryover of IS.
Selected reaction monitoring chromatograms for the reference standard PV9 and the extract of postmortem femoral vein whole blood with ion transition from m/z 274 to 91.1, and for the extracts of the same whole blood spiked or not spiked with IS using ion transition from m/z 260 to 91.1 recorded by LC–MS–MS
Table 1 shows the standard addition calibration equations and their correlation coefficients for PV9 in the aroma liquid product and antemortem and postmortem body fluids of the deceased. All of them showed satisfactory linearity with correlation coefficients greater than 0.998. By extensive dilution of some specimens, the detection limit (signal-to-noise ratio = 3) of PV9 by this method was estimated to be around 0.05 ng/ml.
Because we employed the standard addition method for quantitation without the use of blank specimens, it was impossible to present the usual accuracy and precision data. Instead, as shown in Table 2, we repeated intraday and interday determinations of PV9 in postmortem femoral vein blood and antemortem urine specimens as an example. The repeatability expressed as relative standard deviations was not greater than 12.8 %.
Although the standard addition method can overcome matrix effects and low recovery rates, we were interested in determining the matrix effects according to the kind of specimen. In our extraction procedures, we used deproteinization with acetonitrile plus QuEChERS dispersive solid-phase extraction (SPE) for fluidal human specimens. As shown in Table 3, both antemortem urine and postmortem femoral vein blood showed only a slight and almost no depressive matrix effects, respectively, while the antemortem blood specimens showed a 31.1 % depressive effect. After compensation calculation, the recovery rates for the three specimens were not lower than 70.9 %.
Concentrations of PV9 in aroma liquid solution, and antemortem and postmortem body fluids
Table 4 shows the concentrations of PV9 in its free base form in the aroma liquid solution, antemortem whole blood, antemortem urine, and postmortem femoral vein whole blood. The product solution contained as much as 18.3 mg/ml of PV9. Other specimens contained PV9 at concentrations lower than those expected in spite of the judgement that the victim had died of PV9 poisoning. This seems reasonable given that the woman had received intensive medical care including an intravenous drip infusion of a large volume of solution, which would have greatly lowered the concentrations of PV9 in blood and urine.
Search for a major metabolite of PV9
For most synthetic cathinones, reduction of the β-ketone moiety is a major metabolic pathway [4, 11]. Therefore, we searched for the coexistence of the β-hydroxyl metabolite of PV9 (OH-PV9). If such a metabolite were to coexist, its protonated molecular ion should appear at m/z 276. Thus, we conducted mass chromatography by LC–single stage MS with ions at m/z 276 and 274 (protonated molecular ion of precursor PV9). As shown in Fig. 5a, a peak at m/z 276 appeared at a retention time of 13.2 min; the protonated molecular ion of PV9 appeared at a retention time of 12.8 min as expected. According to the comparison of the peak areas, the area of the probable metabolite was about six times less than that of the precursor PV9. The product ion mass spectrum corresponding to the peak at 13.2 min was recorded as shown in Fig. 5b. The protonated molecular ion of OH-PV9 was very small; however, a peak was observed at m/z 258 that most probably formed by subtraction of a water molecule from the protonated molecule of OH-PV9. Such a subtraction peak also appeared in the product ion mass spectrum of the β-hydroxyl metabolite of α-pyrrolidinovalerophenone (α-PVP) [11]. These data strongly suggest the coexistence of OH-PV9 in postmortem femoral vein whole blood, although its conclusive identification was not possible due to unavailability of a reference standard of OH-PV9.
Trial to search for a β-hydroxyl metabolite of PV9 in the extract of femoral vein whole blood. a Mass chromatograms obtained by liquid chromatography-single stage mass spectrometry with ion at m/z 276 and 274. b Product ion mass spectrum corresponding to the peak appearing at 13.2 min recorded by LC–MS–MS, and the probable fragment mode of the compound
Conclusions
To our knowledge, this is the first report to describe the identification of PV9 in human specimens, and the first demonstration of a fatal PV9 poisoning case. Among cathinone derivatives, human fatal poisoning cases have been reported for 3,4-methylenedioxypyrovalerone [12–14] and α-PVP [15]. Based on these reports together with the present study, it appears that drugs based on α-pyrrolidinophenone derivatives are especially dangerous to human life. Increased strict control is suggested for this group of cathinone drugs.
References
Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y (2013) Changes in the prevalence of synthetic cannabinoids and cathinone derivatives in Japan until early 2012. Forensic Toxicol 31:44–53
Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (2013) Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products. Forensic Toxicol 31:223–240
Swortwood MJ, Boland DM, DeCaprio AP (2013) Determination of 32 cathinone derivatives and other designer drugs in serum by comprehensive LC–QQQ–MS/MS analysis. Anal Bioanal Chem 405:1383–1397
Shima N, Katagi M, Kamata H, Matsuta S, Nakanishi K, Zaitsu K, Kamata T, Nishioka H, Miki A, Tatsuno M, Sato T, Tsuchihashi H, Suzuki K (2013) Urinary excretion and metabolism of the newly encountered designer drug 3,4-dimethylmethcathinone in humans. Forensic Toxicol 31:101–112
Zaitsu K, Katagi M, Tatsuno M, Tsuchihashi H, Ishii A (2014) Recently abused synthetic cathinones, α-pyrrolidinophenone derivatives: a review of their pharmacology, acute toxicity, and metabolism. Forensic Toxicol 32:1–8
Kudo K, Ishida T, Hikiji W, Hayashida M, Uekusa K, Usumoto Y, Tsuji A, Ikeda N (2009) Construction of calibration-locking databases for rapid and reliable drug screening by gas chromatography-mass spectrometry. Forensic Toxicol 27:21–31
Cayman Chemical (2014) Cayman spectral library. https://www.caymanchem.com/app/template/SpectralLibrary.vm. Accessed Jan 2014
Usui K, Hayashizaki Y, Hashiyada M, Funayama M (2012) Rapid drug extraction from human whole blood using a modified QuEChERS extraction method. Legal Med 14:286–296
Wurita A, Suzuki O, Hasegawa K, Gonmori K, Minakata K, Yamagishi I, Nozawa H, Watanabe K (2013) Sensitive determination of ethylene glycol, propylene glycol and diethylene glycol in human whole blood by isotope dilution gas chromatography–mass spectrometry, and the presence of appreciable amounts of the glycols in blood of healthy subjects. Forensic Toxicol 31:272–280
Uchiyama N, Matsuda S, Kawamura M, Shimokawa Y, Kikura-Hanajiri R, Aritake K, Urade Y, Goda Y (2014) Characterization of four new designer drugs, 5-chloro-NNEI, NNEI indazole analog, α-PHPP and α-POP, with 11 newly distributed designer drugs in illegal products. Forensic Sci Int 243:1–13
Shima N, Katagi M, Kawata H, Matsuta S, Sasaki K, Kamata T, Nishioka H, Miki A, Tatsuno M, Zaitsu K, Ishii A, Sato T, Tsuchihashi H, Suzuki K (2014) Metabolism of the newly encountered designer drug α-pyrrolidinovalerophenone in humans: identification and quantitation of urinary metabolites. Forensic Toxicol 32:59–67
Marinetti LJ, Antonides HM (2013) Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of results. J Anal Toxicol 37:135–146
Wyman JF, Lavins ES, Engelhart D, Armstrong EJ, Snell KD, Boggs PD, Taylor SM, Norris RN, Miller FP (2013) Postmortem tissue distribution of MDPV following lethal intoxication by “bath salts”. J Anal Toxicol 37:182–185
Namera A, Urabe S, Saito T, Torikoshi-Hatano A, Shiraishi H, Arima Y, Nagao M (2013) A fatal case of 3,4-methylenedioxypyrovalerone poisoning: coexistence of α-pyrrolidinobutiophenone and α-pyrrolidinovalerophenone in blood and/or hair. Forensic Toxicol 31:338–343
Saito T, Namera A, Osawa M, Aoki H, Inokuchi S (2013) SPME–GC–MS analysis of α-pyrrolidinovalerophenone in blood in a fatal poisoning case. Forensic Toxicol 31:328–332
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hasegawa, K., Wurita, A., Minakata, K. et al. Identification and quantitation of a new cathinone designer drug PV9 in an “aroma liquid” product, antemortem whole blood and urine specimens, and a postmortem whole blood specimen in a fatal poisoning case. Forensic Toxicol 32, 243–250 (2014). https://doi.org/10.1007/s11419-014-0230-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-014-0230-0