Abstract
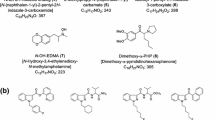
From November 2013 to May 2014, 19 newly distributed designer drugs were identified in 104 products in our ongoing survey of illegal products in Japan. The identified compounds included 8 synthetic cannabinoids, FUB-PB-22 (1), 5-fluoro-NNEI indazole analog (5-fluoro-MN-18, 2), AM-2201 indazole analog (THJ-2201, 3), XLR-12 (4), 5-fluoro-AB-PINACA (5), 5-chloro-AB-PINACA (6), AB-CHMINACA (7), and 5-fluoro-AMB (8); 5 cathinone derivatives, DL-4662 (9), α-PHP (10), 4-methoxy-α-POP (11), 4-methoxy-α-PHPP (12), and 4-fluoro-α-PHPP (13); and 6 other substances, namely, the benzofuran derivative 2-(2-ethylaminopropyl)benzofuran (2-EAPB, 14), nitracaine (15), diclofensine (16), diphenidine (17), 1-benzylpiperidine (18), and acetylfentanyl (19). To our knowledge, this is the first report on the chemical properties of compounds 9–11 and 14. A total of 33 designer drugs, including compounds 1–19, were detected in the 104 illegal products, in 60 different combination patterns. The numbers of detected compounds per product ranged from 1 to 7. In addition, several products contained three different types of compounds, such as synthetic cannabinoids, cathinone derivatives, and phenethylamine derivatives per product. It is apparent that the types of compounds emerging as illegal products are becoming more diverse, as are their combinations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since 2009, the world has seen a dramatic emergence of synthetic cannabinoids, cathinone derivatives, and other new psychoactive substances (NPS) [1–6]. In fact, 384 NPS have been reported to the United Nations Office on Drugs and Crime (UNODC) since 2009, with 97 NPS being reported in 2013 alone [7]. The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) similarly reported that 81 NPS were identified by the EU Early Warning System in 2013 [8]. As a part of our ongoing survey of designer drugs in the illegal drug market in Japan, we reported the appearance of 26 newly distributed substances in Japan in 2013 [9–11]. The detected compounds were classified into three major types of designer drugs: 10 were synthetic cannabinoids, 8 were cathinone derivatives, and 5 were phenethylamines. In addition, 3 other substances were detected, including the opioid receptor agonist MT-45 and the methylphenidate analog 3,4-dichloromethylphenidate [9–11]. In this study, we describe the identification of 19 newly distributed designer drugs, of which 8 were synthetic cannabinoids (1−8), 5 were cathinone derivatives (9−13), and 6 were other substances (14−19) (Fig. 1). In addition, we investigated the combination patterns of detected designer drugs, including compounds 1–19, in 104 illegal products purchased between November 2013 and May 2014.
Materials and methods
Samples for analyses
The analyzed samples were purchased via the Internet between November 2013 and May 2014 as 104 chemical-type or herbal-type products being sold in Japan. Among them, we show the analytical data of 14 products (A–N) for describing the identification of compounds 1–19 in this article. Each of the herbal-type products (A–D) contained about 3 g of mixed dried plants. The 7 powder-type products called ‘‘fragrance powder’’ consisted of 400 mg of a white powder (G, I–L), a mixture of a white solid and a pale brown solid (E), or a mixture of a white solid and a pale purple solid (N). The 3 liquid-type products called ‘‘liquid aroma’’ consisted of about 5 ml of brown liquid (F and M) or pale yellow liquid (H). Products M and N were analyzed by nuclear magnetic resonance (NMR) spectroscopy. A 5-ml sample of liquid product M was evaporated to dryness, and then compound 10 (182 mg) was obtained from the dried sample as a brown oil. Compounds 9 and 14 were directly analyzed as white solids from products E and N, respectively, without isolation. Compound 11 from product G was directly analyzed without isolation.
Chemicals and reagents
FUB-PB-22, 5-fluoro-NNEI indazole analog (5-fluoro-MN-18), AM-2201 indazole analog (THJ-2201), XLR-12, 5-fluoro-AB-PINACA, 5-chloro-AB-PINACA, AB-CHMINACA, 5-fluoro-AMB, 4-methoxy-α-PHPP, 4-fluoro-α-PHPP, nitracaine, and acetylfentanyl were purchased from Cayman Chemicals (Ann Arbor, MI, USA). Diclofensine, diphenidine, and 1-benzylpiperidine were purchased from LGC (Teddington, UK), Tocris Bioscience (Bristol, UK), and Wako Pure Chemical Industries (Osaka, Japan), respectively. All other common chemicals and solvents were of analytical reagent grade or HPLC grade. As solvents for NMR analysis, methanol-d 4 (99.96 %), pyridine-d 5 (99.96 %), and dimethyl sulfoxide (DMSO)-d 6 (99.96 %) were purchased from the ISOTEC division of Sigma–Aldrich (St. Louis, MO, USA).
Preparation of sample solutions
For qualitative analyses, 10 mg of each herbal-type product was crushed into powder and extracted with 1 ml of methanol under ultrasonication for 10 min. A 2-mg portion of each powder-type product was extracted with 1 ml of methanol under ultrasonication for 10 min. A 20-µl portion of each liquid-type product was mixed with 1 ml of methanol under ultrasonication for 10 min. After centrifugation (3,000 rpm, 5 min) of each extract, the supernatant solution was passed through a centrifugal filter (Ultrafree-MC, 0.45 µm filter unit; Millipore, Bedford, MA, USA) to serve as the sample solution for the analyses. If necessary, the solution was diluted with methanol to a suitable concentration before the instrumental analyses.
Analytical conditions
Each sample solution was analyzed by ultra-performance liquid chromatography–electrospray ionization mass spectrometry (UPLC–ESI-MS) and by gas chromatography–mass spectrometry (GC–MS) in the electron ionization (EI) mode according to our previous report [12]. Three elution programs were used in the LC–MS analysis. Each analysis was carried out with a binary mobile phase consisting of solvent A (0.1 % formic acid in water) and solvent B (0.1 % formic acid in acetonitrile). Elution program (1) used for analysis of cannabinoids was as follows: 35 % B (4-min hold) and 65–75 % B (4–16 min), and up to 90 % B (16–17 min, 6-min hold) at a flow rate of 0.3 ml/min. Elution program (2) used for the analysis of cathinone derivatives and other compounds was as follows: 5–20 % B (0–20 min), and up to 80 % B (20–30 min, 5-min hold). Elution program (3) used for the analysis of compounds 5 and 6 was as follows: 45 % B (15-min hold) and up to 90 % B (15–16 min, 2-min hold), and down to 45 % B (18–19 min, 5-min hold). In this study, products A, B, and D were analyzed using program (1), products E–L were analyzed using program (2), and product C was analyzed using program (3). GC–EI-MS was performed on an Agilent 6890N GC with a 5975 mass selective detector (Agilent, Santa Clara, CA, USA) using a capillary column (HP-1MS capillary, 30 m × 0.25 mm i.d., 0.25 μm film thickness; Agilent) with helium gas as a carrier at 0.7 ml/min. The conditions were: electron energy, 70 eV; injector temperature, 220 °C; injection, splitless mode for 1.0 min; mass selective detector temperature, 280 °C; scan range, m/z 40–550. Two programs were used in the GC–MS analysis, as follows. The oven temperature program (1), 80 °C (1-min hold) and an increase at a rate of 5 °C/min to 190 °C (15-min hold) followed by an increase at 10 °C/min up to 310 °C (15-min hold), and the oven temperature program (2), 150 °C (1-min hold) and increase at a rate of 20 °C/min to 280 °C (10-min hold) followed by an increase at 5 °C/min up to 310 °C (15-min hold). Program (2) was used for the analysis of product C. Other products were analyzed using program (1).
The obtained GC mass spectra were compared to those of an EI-MS library (Mass Spectra of Designer Drugs 2013; Wiley-VCH, Weinheim, Germany). We also used our in-house EI-MS library of designer drugs obtained by our ongoing survey of illegal products and commercially available reagents for the structural elucidation.
We measured the accurate mass numbers of the target compounds by liquid chromatography–quadrupole time-of-flight mass spectrometry (LC–QTOF-MS) in the ESI mode according to our previous report [6].
NMR spectra were obtained on ECA-800 and 600 spectrometers (JEOL, Tokyo, Japan). Structural assignments were made based on interpretation of 1H NMR, 13C NMR, heteronuclear multiple quantum coherence (HMQC), heteronuclear multiple-bond correlation (HMBC), 15N HMBC, and double quantum filtered correlation spectroscopy (DQF-COSY) spectra.
Results and discussion
Identification of unknown peaks 1, 3, and 5
LC–MS and GC–MS analyses were performed to identify unknown peaks 1, 3, and 5 in product A (Fig. 2a, b, i). Based on the LC–MS and GC–MS data, the three peaks were finally identified as synthetic cannabinoids; namely, FUB-PB-22 (Fig. 2c, j), an AM-2201 indazole analog (THJ-2201) (Fig. 2e, l), and 5-fluoro-AB-PINACA (Fig. 2g, n), by direct comparison of the data to those of the purchased authentic compounds (Fig. 2d, k; Fig. 2f, m; Fig. 2h, o), respectively. Compounds 1, 3, and 5 were detected as newly distributed designer drugs in Japan. These compounds are analogs of known cannabimimetic substances, QUPIC (PB-22) [6], AM-2201, and AB-PINACA [4], respectively, the pharmacological effects for which have not been reported. In addition, FUB-PB-22 (1) and 5-fluoro-AB-PINACA (5) will be controlled as Shitei-Yakubutsu (designated substances) in Japan from July 2014.
Liquid chromatography–mass spectrometry (LC–MS) and gas chromatography–mass spectrometry (GC–MS) analyses of product A. The LC–ultraviolet photodiode array (LC–UV-PDA) chromatogram (a) and total ion chromatogram (TIC) (b) are shown. Electrospray ionization (ESI) mass and UV spectra of peaks 1 (c), 3 (e), 5 (g), the authentic FUB-PB-22 (d), AM-2201 indazole analog (THJ-2201, f), and 5-fluoro-AB-PINACA (h) are also presented. Panels (i) through (o) show TIC (i) and electron ionization (EI) mass spectra of peaks 1 (j), 3 (l), 5 (n), the authentic FUB-PB-22 (k), AM-2201 indazole analog (THJ-2201, m), and 5-fluoro-AB-PINACA (o) obtained by GC–MS analysis
Identification of unknown peaks 2 and 4
Two unknown peaks 2 and 4 were detected along with 5-fluoro-AB-PINACA (5) in the LC–MS and GC–MS chromatograms for product B (Fig. 3a, b, g). Peaks 2 and 4 were identified as the synthetic cannabinoids 5-fluoro-NNEI indazole analog (5-fluoro-MN-18) (Fig. 3c, h) and XLR-12 (Fig. 3e, j) by direct comparison of the data to those of the purchased authentic compounds (Fig. 3d, i; Fig. 3f, k), respectively. 5-Fluoro-NNEI indazole analog (2) is an analog of a known NNEI indazole analog (MN-18), which has already been detected in illegal products [11]. Although there is no pharmacological information about compound 2, a cyclopropylmethanone-type XLR-12 (4), which is an analog of XLR-11, has been reported to show affinity for the cannabinoid CB1 and CB2 receptors (K i = 10 and 0.09 nM, respectively) [13]. In addition, 5-fluoro-NNEI indazole analog (5-fluoro-MN-18, 2) and XLR-12 (4) will be controlled as designated substances in Japan from July 2014.
LC–MS and GC–MS analyses of product B. Panels (a) and (b) show LC–UV-PDA chromatogram (a) and TIC (b). ESI mass and UV spectra of peaks 2 (c), 4 (e), and the authentic 5-fluoro-NNEI indazole analog (d) and XLR-12 (f) are also shown. Panels (g) through (k) show TIC (g) and EI mass spectra of peaks 2 (h), 4 (j), and the authentic 5-fluoro-NNEI indazole analog (i) and XLR-12 (k) obtained by the GC–MS analysis
Identification of unknown peak 6
An unknown peak 6 was detected along with the 5-fluoro-AB-PINACA (5) peak in the LC–MS and GC–MS chromatograms for product C (Fig. 4a–c, f). Based on the GC–MS and LC–MS data, the unknown peak 6 was finally identified as dicarboxamide derivative 5-chloro-AB-PINACA (Fig. 4d, g) by direct comparison of the data to those of the purchased authentic compound (Fig. 4e, h). This is the first report of the detection of 5-chloro-AB-PINACA (6) as a newly distributed illegal drug in Japan.
LC–MS and GC–MS analyses of product C. LC–UV-PDA chromatogram (a), TIC (b), mass chromatogram at m/z 365 (c), and ESI mass and UV spectra of peak 6 (d) and the authentic 5-chloro-AB-PINACA (e) are shown. TIC (f) and EI mass spectra of peak 6 (g) and the authentic 5-chloro-AB-PINACA (h) obtained by GC–MS analysis are also presented
Identification of unknown peaks 7 and 8
In the LC–MS and GC–MS analyses, unknown peaks 7 and 8 were detected with FUB-PB-22 (1) in product D (Fig. 5a, b, g). Peaks 7 and 8 were identified as synthetic cannabinoids AB-CHMINACA (Fig. 5c, h) and 5-fluoro-AMB (Fig. 5e, j), respectively, by direct comparison of the data to those of the purchased authentic compounds (Fig. 5d, i; Fig. 5f, k). The S-form of the dicarboxamide derivative AB-CHMINACA (N-[(1S)-1-(aminocarbonyl)-2-methylpropyl]-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide, 7) has been reported to have a potent affinity for the cannabinoid CB1 receptor (K i = 0.5 nM) [14]. Although there is no pharmacological information about 5-fluoro-AMB (methyl 2-[1-(5-fluoropentyl)-1H-indazole-3-carboxamido]-3-methylbutanoate, 8), its analog, methyl 2-[1-(4-cyanobutyl)-1H-indazole-3-carboxamido]-3,3-dimethylbutanoate, has been reported to have a potent affinity for cannabinoid CB1 receptor (K i = 0.7 nM) [14].
LC–MS and GC–MS analyses of product D. The LC–UV-PDA chromatogram (a) and TIC (b) are shown. ESI mass and UV spectra of peaks 7 (c), 8 (e), and the authentic AB-CHMINACA (d) and 5-fluoro-AMB (f) are also shown. Panels (g) through (k) present the TIC (g) and EI mass spectra of peaks 7 (h), 8 (j), and the authentic AB-CHMINACA (i) and 5-fluoro-AMB (k) obtained by GC–MS analysis
Identification of the unknown peaks 9 and 12
We detected two unknown peaks, 9 and 12, in the LC–MS and GC–MS chromatograms for product E (Fig. 6a, b, f). Peak 12 was identified as the cathinone derivative 4′-methoxy-α-pyrrolidinoheptanophenone (4-methoxy-α-PHPP) (Fig. 6d, h) by direct comparison of the data with those of the purchased authentic compound (Fig. 6e, i). 4-Methoxy-α-PHPP (12) is a para-methoxy analog of a known α-PHPP [11]. The pharmacological and toxicological properties of 12 are not known. Compound 12 was detected as a newly distributed designer drug in Japan. In the LC–MS analysis, the unknown peak 9 at 14.8 min showed a protonated molecular ion signal at m/z 266 ([M+H]+) (Fig. 6c). The accurate mass spectrum obtained by LC–QTOF-MS gave an ion peak at m/z 266.1746, suggesting that the protonated molecular formula of compound 9 was C15H24NO3 (calcd. 266.1756).
LC–MS and GC–MS analyses of product E. The LC–UV-PDA chromatogram (a) and TIC (b) are shown, along with the ESI mass and UV spectra of peaks 9 (c), 12 (d), and the authentic 4-methoxy-α-PHPP (e). Panels (f) through (i) show TIC (f) and EI mass spectra of peaks 9 (g), 12 (h), and the authentic 4-methoxy-α-PHPP (i) obtained by GC–MS analysis
The 1H and 13C NMR data (Table 1) and the observed DQF-COSY, HMQC, HMBC, and 15N HMBC correlations shown in Fig. 7a revealed that the structure of compound 9 is 1-(3,4-dimethoxyphenyl)-2-(ethylamino)pentan-1-one (DL-4662), as shown in Fig. 1. The fragment ions at m/z 100, 137, and 165 of compound 9 in the GC–MS spectrum (Fig. 6g) further confirmed the structure. Compound 9, which is a dimethoxy-analog of a known cathinone derivative, α-ethylaminopentiophenone [11], is a novel illegal drug, and its chemical and pharmacological data have not been reported.
Identification of the unknown peaks 10 and 13
Unknown peaks 10 and 13 were detected along with a peak for known compound 25B-NBOMe [10] in the LC–MS and GC–MS chromatograms for product F (Fig. 8a, b, f). Based on the GC–MS and LC–MS data, peak 13 was finally identified as the cathinone derivative 4′- fluoro-α-pyrrolidinoheptanophenone (4-fluoro-α-PHPP) (Fig. 8d, h) by direct comparison of the data to those of the purchased authentic compound (Fig. 8e, i). In the LC–MS analysis, unknown peak 10 at 19.0 min showed a protonated molecular ion signal at m/z 246 ([M+H]+) (Fig. 8c). The accurate mass spectrum obtained by LC–QTOF-MS gave an ion peak at m/z 246.1840, suggesting that the protonated molecular formula of compound 10 was C16H24NO (calcd. 246.1858).
LC–MS and GC–MS analyses of product F. The LC–UV-PDA chromatogram (a) and TIC (b) are shown, along with ESI mass and UV spectra of peaks 10 (c), 13 (d), and the authentic 4-fluoro-α-PHPP (e). Panels (f) through (i) show TIC (f) and EI mass spectra of peaks 10 (g), 13 (h), and the authentic 4-fluoro-α-PHPP (i) obtained by GC–MS analysis
The 1H and 13C NMR spectra of compound 10 were very similar to those of a known cathinone derivative, α-POP, except for the additional C2H4 of the n-alkyl moiety (positions 5 and 6) of α-POP, as shown in Tables 2 and 3 [11]. The observed 1H and 13C NMR data (Tables 2, 3) and DQF-COSY, HMQC, HMBC, and 15N HMBC correlations (data not shown) revealed that the structure of compound 10 corresponded to α-pyrrolidinohexylphenone (α-PHP), as shown in Fig. 1. The fragment ions at m/z 77, 105, and 140 of compound 10 in the GC–MS spectrum (Fig. 8g) further confirmed the structure. Compound 10 is a desmethyl analog of MPHP, which is controlled as a designated substance in Japan, and its chemical and pharmacological data have not been reported.
Identification of unknown peak 11
Unknown peak 11 was detected in the GC–MS and LC–MS chromatograms for product G (Fig. 9a, b, d). The proposed fragmentation and the presumed structure of peak 11 obtained by GC–MS analysis are shown in Fig. 9e. The LC–MS data revealed that peak 11 gave a protonated ion signal at m/z 304 ([M+H]+) (Fig. 9c). The accurate mass spectrum obtained by LC–QTOF-MS gave an ion peak at m/z 304.2269, suggesting that the protonated molecular formula of compound 11 was C19H30NO2 (calcd. 304.2269).
The 13C NMR spectrum of compound 11 was similar to that of α-POP except for the C-1′ and C-3′ to C-5′ carbons of a phenyl group and the 4′-methoxy carbon (Table 2) [11]. The observed 1H and 13C NMR data (Tables 2, 3) and DQF-COSY, HMQC, HMBC, and 15N HMBC correlations (data not shown) suggested that the structure of compound 11 is 4′-methoxy-α-pyrrolidinooctanophenone (4-methoxy-α-POP), as shown in Fig. 1. The fragment ions at m/z 135 and 168 of compound 11 in the GC–MS spectrum corroborated the structure (Fig. 9e). Compound 11 was detected as a newly distributed designer drug, and its chemical and pharmacological data have not been reported.
Identification of unknown peak 14
We detected unknown peak 14 in the LC–MS and GC–MS chromatograms for product H (Fig. 10a, b, d). In LC–MS analysis, unknown peak 14 at 15.5 min showed a protonated molecular ion signal at m/z 204 ([M+H]+) (Fig. 10c). The accurate mass spectrum obtained by LC–QTOF-MS gave an ion peak at m/z 204.1377, suggesting that the protonated molecular formula of compound 14 was C13H18NO (calcd. 204.1388).
The structure of compound 14 was elucidated by NMR analysis (Fig. 7b; Table 4). The 1H and 13C NMR spectra of compound 14 suggested the existence of 17 protons and 13 carbons (Table 4). Interpretation of DQF-COSY, HMQC, and HMBC spectra of compound 14 revealed the presence of a 2-propylbenzofuran moiety (positions 2 to 7a and position 1′ to 3′, Fig. 7b). The remaining C2H6N unit was presumed to be an N-ethylamine group. In addition, 15N HMBC correlations between NH and each of H-1′, H-3′, and H-2″, and an HMBC correlation between a methine (position 2′) and a methylene (position 1″) were observed (Fig. 7b). These results revealed that a 2-propylbenzofuran moiety was connected at position 2′ to the N-ethylamine group at nitrogen (Fig. 7b). Therefore, the structure of compound 14 was clarified as a benzofuran derivative, 2-(2-ethylaminopropyl)benzofuran (2-EAPB), as shown in Fig. 1. Compound 14 was detected as a newly distributed designer drug, and its pharmacological data have not been reported. However, the benzofuran derivatives 5-APB [5-(2-aminopropyl)benzofuran] and 6-APB [6-(2-aminopropyl)benzofuran] have been reported as potent triple monoamine reuptake inhibitors for dopamine, norepinephrine, and serotonin in vitro [15]. In addition, it has been reported that the 2-ethylaminopropyl regioisomer of 14, 5-EAPB [5-(2-ethylaminopropyl)benzofuran], is offered for sale as an alternative to other benzofurans in the UK [16].
Identification of the unknown peaks 15–19
GC–MS and LC–MS analyses were performed to identify the five unknown peaks 15, 16, 17, and 18 and 19 in products I (Supplementary material, Fig. S1a, b, e), J (Fig. S2a–d, g), K (Fig. S3a–d, i), and L (Fig. S4a, b, e), respectively. Based on the GC–MS and LC–MS data, the five peaks were finally identified as nitracaine (Fig. S1c, f), diclofensine (Fig. S2e, h), diphenidine (Fig. S3e, j), 1-benzylpiperidine (Fig. S3g, l), and acetylfentanyl (Fig. S4c, f), respectively, by direct comparison of the data with those of the purchased authentic compounds (Fig. S1d, g; Fig. S2f, i; Fig. S3f, k; Fig. S3h, m; Fig. S4d, g). Researchers in Ireland reported that nitracaine (15), which is the nitro analog of the local anesthetic dimethocaine, emerged in December 2013 as a new psychoactive substance on an Internet website selling “research chemicals” [17]. Diclofensine (16) has been reported as an inhibitor of monoamine uptake [18], while diphenidine (17) has been reported as an N-methyl-d-aspartate (NMDA) channel blocker [19]. The opioid receptor agonist acetylfentanyl (19) will be controlled as a designated substance in Japan from July 2014.
Combination patterns of detected compounds in illegal products
As described in our previous reports [3, 20], our survey of illegal products distributed in Japan revealed that the average number of synthetic compounds detected per illegal product was 2.6 in 2010–2012. In some cases, synthetic compounds of different types, such as synthetic cannabinoids and cathinone derivatives, were present in the same product [3]. In this study, 34 compounds, including compounds 1–19, were detected in the products, and the detected compounds were categorized into three types: synthetic cannabinoids (10 compounds), cathinone derivatives (13 compounds), and other classes of designer drugs (10 compounds) (Table 5). Table 6 provides a list of the different combination patterns of detected compounds in 104 illegal products purchased between November 2013 and May 2014. Sixty combination patterns were detected, with the number of compounds per product ranging from one to seven: one detected compound (10 patterns), two (23 patterns), three (14 patterns), four (4 patterns), five (6 patterns), six (2 patterns), and seven (1 pattern), as shown in Table 6.
The combination patterns were classified broadly into seven groups (A–G) based on the types of compounds, as shown in Table 6. Group A consisted of a mixture of only synthetic cannabinoid(s) with 17 patterns, group B was a mixture of only cathinone derivative(s) with 11 patterns, group C was a mixture of other compound(s) with 4 patterns, group D was a mixture of synthetic cannabinoid(s) and cathinone derivative(s) with 21 patterns, group E was a mixture of a synthetic cannabinoid and another compound with 1 pattern, group F was a mixture of cathinone derivative(s) and another compound with 2 patterns, and group G was a mixture of synthetic cannabinoid(s), cathinone derivative(s), and other compound(s) with 4 patterns. As in the case of group G-(1), the product contained three different types of compounds with different pharmacological effects: the synthetic cannabinoid 5-fluoro-NNEI indazole analog (2), the cathinone derivative α-POP, and the NMDA channel blocker diclofensine (16). On the other hand, in the case of group G-(2), the product contained the synthetic cannabinoid FUB-PB-22 (1), the two cathinone derivatives 4-methyl-α-ethylaminopentiophenone and 3,4-dimethoxy-α-PVP, and the phenethylamine derivative 25I-NBOMe, which is a 5-HT2A receptor agonist with hallucinogenic effects. Considering the results, it is apparent that the types of emerging compounds are becoming more diverse, as are their combinations in illegal products.
Conclusions
Nineteen newly distributed designer drugs, including eight synthetic cannabinoids (1−8), five cathinone derivatives (9–13), and six other substances—2-EAPB (14), nitracaine (15), diclofensine (16), diphenidine (17), 1-benzylpiperidine (18), and acetylfentanyl (19)—were identified in illegal products that are available in Japan. Most of the detected compounds (1–14 and 19) appeared as alternatives to controlled substances such as narcotics and designated substances in Japan. It is apparent that the types of designer drugs and their combinations in illegal products seem to be increasing in diversity. Thus, serious side effects from these combinations are possible, although it is hard to predict them. The emergence of designer drugs that are neither synthetic cannabinoids nor cathinone derivatives also seems to be increasing. Therefore, continuous monitoring and rapid identification of newly distributed designer drugs, combined with global information sharing, will be needed to supress illegal drug abuse.
References
Uchiyama N, Kikura-Hanajiri R, Kawahara N, Goda Y (2009) Identification of a cannabimimetic indole as a designer drug in a herbal product. Forensic Toxicol 27:61–66
Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y (2012) Identification of two new-type synthetic cannabinoids, N-(1-adamantyl)-1-pentyl-1H-indole-3-carboxamide (APICA) and N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide (APINACA), and detection of five synthetic cannabinoids, AM-1220, AM-2233, AM-1241, CB-13 (CRA-13), and AM-1248, as designer drugs in illegal products. Forensic Toxicol 30:114–125
Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y (2013) Changes in the prevalence of synthetic cannabinoids and cathinone derivatives in Japan until early 2012. Forensic Toxicol 31:44–53
Uchiyama N, Matsuda S, Wakana D, Kikura-Hanajiri R, Goda Y (2013) New cannabimimetic indazole derivatives, N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (AB-PINACA) and N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (AB-FUBINACA), identified as designer drugs. Forensic Toxicol 31:93–100
Takahashi K, Uchiyama N, Fukiwake T, Hasegawa T, Saijou M, Motoki Y, Kikura-Hanajiri R, Goda Y (2013) Identification and quantitation of JWH-213, a cannabimimetic indole, as a designer drug in a herbal product. Forensic Toxicol 31:145–150
Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (2013) Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products. Forensic Toxicol 31:223–240
UNODC (2014) 2014 Global synthetic drugs assessment. Amphetamine-type stimulants and new psychoactive substances, May 2014. http://www.unodc.org/documents/scientific/2014_Global_Synthetic_Drugs_Assessment_web.pdf. Accessed May 2014
EMCDDA (2014) European drug report 2014: trends and developments, May 2014. http://www.emcdda.europa.eu/attachements.cfm/att_228272_EN_TDAT14001ENN.pdf. Accessed May 2014
Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (2014) Identification of two new-type designer drugs, a piperazine derivative MT-45 (I-C6) and a synthetic peptide Noopept (GVS-111), with a synthetic cannabinoid A-834735, a cathinone derivative 4-methoxy-α-PVP and a phenethylamine derivative 4-methylbuphedrine from illegal products. Forensic Toxicol 32:9–18
Uchiyama N, Shimokawa Y, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (2014) Two new synthetic cannabinoids, AM-2201 benzimidazole analog (FUBIMINA) and (4-methylpiperazin-1-yl)(1-pentyl-1H-indol-3-yl)methanone (MEPIRAPIM), and three phenethylamine derivatives, 25H-NBOMe 3,4,5-trimethoxybenzyl analog, 25B-NBOMe, and 2C-N-NBOMe, identified in illegal products. Forensic Toxicol 32:105–117
Uchiyama N, Matsuda S, Kawamura M, Shimokawa Y, Kikura-Hanajiri R, Aritake K, Urade Y, Goda Y (2014) Characterization of four new designer drugs, 5-chloro-NNEI, NNEI indazole analog, α-PHPP and α-POP, with 11 newly distributed designer drugs in illegal products. Forensic Sci Int 243:1–13
Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y (2013) URB-754: a new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Sci Int 227:21–32
Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD (2010) Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB2 cannabinoid receptor activity. J Med Chem 53:295–315
Buchler IP, Hayes MJ, Hegde SG, Hockerman SL, Jones DE, Kortum SW, Rico JG, Tenbrink RE, Wu KK (2009) Indazole derivatives as CB1 receptor modulators and their preparation and use in treatment of diseases. Patent: WO/2009/106980 September, 2009
Iversen L, Gibbons S, Treble R, Sedtola V, Huang X-P, Rolth BL (2013) Neurochemical profiles of some novel psychoactive substances. Eur J Pharmacol 700:147–151
Advisory Council on the Misuse of Drugs (ACMD) (2013) Benzofurans: a review of the evidence of use and harm. ACMD, London. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/261783/Benzofuran_compounds_report.pdf. Accessed May 2014
Power JD, Scott KR, Gardner EA, Curran McAteer BM, O’Brien JE, Brehon M, Talbot B, Kavanagh PV (2014) The syntheses, characterization and in vitro metabolism of nitracaine, methoxypiperamide and mephtetramine. Drug Test Anal. doi:10.1002/dta.1616
Nakachi N, Kiuchi Y, Inagaki M, Inazu M, Yamazaki Y, Oguchi K (1995) Effects of various dopamine uptake inhibitors on striatal extracellular dopamine levels and behaviors in rats. Eur J Pharmacol 281:195–203
Berger ML, Schweifer A, Rebernik P, Hammerschmidt F (2009) NMDA receptor affinities of 1,2-diphenylethylamine and 1-(1,2-diphenylethyl)piperidine enantiomers and of related compounds. Bioorg Med Chem 17:3456–3462
Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y (2014) Changes in the prevalence of new psychoactive substances before and after the introduction of the generic scheduling of synthetic cannabinoids in Japan. Drug Test Anal. doi:10.1002/dta.1584
Acknowledgments
Part of this work was supported by a Health and Labor Sciences Research Grant from the Ministry of Health, Labour, and Welfare, Japan.
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Uchiyama, N., Shimokawa, Y., Kawamura, M. et al. Chemical analysis of a benzofuran derivative, 2-(2-ethylaminopropyl)benzofuran (2-EAPB), eight synthetic cannabinoids, five cathinone derivatives, and five other designer drugs newly detected in illegal products. Forensic Toxicol 32, 266–281 (2014). https://doi.org/10.1007/s11419-014-0238-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-014-0238-5