Abstract
The rapid increase in the number of new psychoactive substances and their abuse is the most recent drug abuse issue worldwide. Although abuse of synthetic cannabinoids is highly restricted in South Korea, the rapid increase in the number of new substances is forcing the legal regulation authority to continuously improve the drug regulation act. As a result of drug screening by the National Forensic Service from 2009 to June 2013, 26 species of synthetic cannabinoids were identified in materials seized mainly by the Police Agency and the Prosecutor’s Office in South Korea. One of the most remarkable trends in synthetic cannabinoids is the increase in halogenated derivatives and new substances, including UR-144 and A-836,339 originally developed as analgesics by Abbott Laboratories. The N-pentyl fluorinated analog of UR-144 (XLR-11) has become the most frequently found synthetic cannabinoid in 2013 since its first appearance in 2012, whereas abuse of A-836,339 analogs has been little reported despite their abuse potential. Until early 2011, nicotine was the most frequently found active coingredient with synthetic cannabinoids. However, various psychoactive substances such as Δ9-tetrahydrocannabinol, α-pyrrolidinobutiophenone, α-pyrrolidinovalerothiophenone, and N,N-diallyl-5-methoxytryptamine have often been found as coingredients in herbal highs since late 2011. These coingredients should also be systematically regulated, because they can cause unexpected side effects. It is suggested that authorities in different countries share information about synthetic cannabinoids and their coingredients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The recent increase in the abuse of new psychoactive substances (NPSs) has become a remarkable worldwide trend. These NPSs are traded and abused in the forms of “legal highs” or “research chemicals” via the Internet or international mailing services. Their chemical structures are continuously being modified to avoid legal regulations [1, 2]. The NPSs are classified as synthetic cannabinoids, cathinones, phenethylamines, tryptamines, and amphetamine derivatives. Among them, synthetic cannabinoids are most frequently abused because their structure–activity relationships are well known from previous studies, which make it easy to modify their chemical structures. Synthetic cannabinoids were first identified in herbal incense in 2008 [3, 4], and their abuse is increasing continuously all over the world [5–7]. The psycho activity of synthetic cannabinoids increases as their CB1 receptor affinity increases. Most of the classical synthetic cannabinoids such as JWH-018 or JWH-073 have CB1 receptor affinity and psychotropic effects higher than Δ9-tetrahydrocannabinol (THC) [8, 9], which is the active ingredient in cannabis. CB2 selective agonists show greater analgesic effects than psychotropic effects, and have been developed for medical use [10–12]. However, some of the CB2 selective agonists such as UR-144 have been found in herbal highs intended for recreational use [13]. Classical synthetic cannabinoids such as JWH-series indole compounds and CP-47,497 homologs were synthesized based on previous studies including pharmacology [14–16]. However, recently identified synthetic cannabinoids include new substances that lack pharmacological information, and therefore pose a real risk of severe toxicity or even fatal intoxication [17, 18]. Some of the NPSs imported to South Korea are interdicted by customs, while others are seized by the Police Agency and the Prosecutor’s Office during drug trafficking. The National Forensic Service (NFS) identifies psychoactive substances seized mainly by the Police Agency and some by the Prosecutor’s Office, and this collection may reflect the regional status of NPS abuse in South Korea. Thus, we report on the trends of synthetic cannabinoids identified by the NFS from 2009 to June 2013, along with the legal regulation status in South Korea to reduce widespread use of these NPSs.
Materials and chemicals
We used 140 species of seized materials containing synthetic cannabinoids, which were composed of dried leaves (n = 97), powder (n = 11), tablets (n = 13), and ashes in smoking apparatus (n = 19). Although the materials were separated in different packages and had different evidence numbers, they were considered one sample if their appearance, logos, and identified ingredients were the same. The materials were seized mainly by the National Police Agency and some by the Prosecutor’s Office of South Korea from 2009 to June 2013 and were sent to the NFS for forensic identification. All solvents used were of analytical grade. JWH-018, JWH-122, JWH-210, and AM-2201 reference materials were obtained from TRC (Toronto, ON, Canada). MAM-2201, RCS-4, UR-144, A-836,339, and the CP-47,497-C8 homolog were obtained from Cayman Chemicals (Ann Arbor, MI, USA). JWH-019, JWH-073, and JWH-250 were purchased from Lipomed (Arlesheim, Switzerland). Standard stock solutions of the reference materials (100 mg/l in methanol) were used to identify the synthetic cannabinoids in the seized materials, and were stored at −20 °C when not in use.
Drug identification of the seized materials
Preliminary drug screening on the seized articles was carried out using gas chromatography–electron ionization mass spectrometry (GC–EI-MS). Each analyte was identified through comparison of mass spectra and retention times with those of commercial standards and MS libraries. The GC–MS analysis was performed on an Agilent 5975C mass selective detector equipped with an Agilent 7890A gas chromatograph (Santa Clara, CA, USA) and an HP-5MS capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm). The injector was operated in the splitless mode, and the injection volume was set to 0.2–1.0 μl. Inlet temperature and helium flow rate were set to 260 °C and 1.0 ml/min, respectively. Oven temperature was initially 80 °C for 3 min, increased to 290 °C at a heating rate of 20 °C/min, and held at that temperature for 16 min. The source and transfer line temperatures were set to 260 and 280 °C, respectively. The MS was operated in the scan mode, and the acquisition range was set to m/z 40–550. Sample preparation procedures are described below.
Dried leaves
About 0.1 g of dried leaves was extracted in 1 ml of methanol under ultrasonication for 10 min or shaking for 30 min. After extraction, the extracts were centrifuged at 3,000 rpm for 5 min, and the methanol extracts were diluted further with methanol to a suitable concentration, if necessary, before GC–MS analysis.
Powder
About 0.01 g of powder was dissolved in 1 ml of methanol. The extracts were centrifuged at 3,000 rpm for 5 min, and the supernatant was diluted with methanol to a suitable concentration, if necessary, before GC–MS analysis.
Tablets
Each tablet sample was crushed to powder with a mortar and pestle and then extracted using the same process described for powder samples.
Ashes in smoking apparatus
Ashes remaining in various types of smoking apparatus were extracted with an appropriate volume of methanol, and diluents of the extracts were used as samples for GC–MS analysis. In cases of unreported compounds, additional structural elucidations were performed on unknown compounds by nuclear magnetic resonance and liquid chromatography–time-of-flight–mass spectrometry [13, 19]. When necessary, the synthetic cannabinoids in the samples were quantified using the GC–MS method developed in the previous study [20].
Results and discussion
Synthetic cannabinoids identified by the NFS from 2009 to June 2013
Synthetic cannabinoids were identified mainly in the form of dried leaves, and others were identified in tablets, powders, or ashes in smoking apparatus. Powder is generally used to prepare homemade herbal highs by mixing or spraying solutions on commercially available herbs. Since a herbal incense containing JWH-018 was first identified in Korea, 26 species of synthetic cannabinoids have been identified by the NFS in 140 samples related to 88 cases investigated by the Police Agency and the Prosecutor’s Office. The seized dried leaf materials contained one to three species of synthetic cannabinoids and some of them also contained active coingredients (Table 1). Until early 2011, the major coingredients of herbal highs were nicotine and caffeine, which were considered natural ingredients of the base herbs used for the preparation of homemade herbal highs. However, since N,N-diallyl-5-methoxytryptamine (5-MeO-DALT), THC, and phenazepam were first identified in material seized in late 2011, various psychoactive coingredients have been identified in seized materials. In particular, since 2013, new psychoactive substances such as α-pyrrolidinovalerothiophenone (α-PVT) and α-pyrrolidinobutiophenone (α-PBP) have often been found in herbal highs seized in South Korea, and there was even a herbal incense product containing only α-PVT without synthetic cannabinoids. THC and oxycodone were also found in ashes of smoking apparatus, which means that they were abused together with the synthetic cannabinoids. Various NPSs such as phenethylamines, tryptamines, and amphetamines were identified as coingredients in tablets, whereas synthetic cannabinoids were only found in small amounts. Administration of several of these drugs could induce a drug interaction or synergistic effect, which could result in severe intoxication. However, there is insufficient toxicological information about the toxicological effect that may be caused by multidrug administration of these new psychoactive substances.
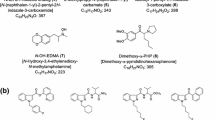
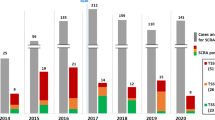
The synthetic cannabinoids identified by the NFS were classified into ten basic categories (Table 2), which were categorized into two classes according to their literature base and year of first appearance. Class 1 included classical synthetic cannabinoids, naphthoylindoles, phenylacetylinoles, benzoylindoles, and CP-47,497 homologs, which are generally produced based on previous scientific literature including studies by Huffman et al. [14–16]. In contrast, class 2 compounds are those with new basic moieties such as cyclopropylindoles, aminocarbonylindazoles, adamantylindoles, adamantylindazoles, quinolinylindoles, and cyclopropylthiazoles, which include research chemicals developed as pharmaceuticals. Class 1 synthetic cannabinoids including the JWH-series naphthoylindoles prevailed until early 2012, but class 2 analogs replaced them in late 2012 (Fig. 1). Similar phenomena were observed with the increase in fluorinated analogs. Fluorinated analogs have increased continuously since AM-2201 was first identified in 2011, and fluorinated modifications of existing compounds have become a general synthetic cannabinoid trend (Fig. 2). It may be because halogenation of the N-pentyl side chain increases CB1 affinity of synthetic cannabinoids [21]. Another reason may be to avoid legal regulation in the countries where only compounds with designated structures are controlled. The evolution of synthetic cannabinoids forces government regulatory authorities to legislate systemized regulation of possible analogs. The characteristic patterns since 2012 also include the appearance of CB2 selective agonists developed as analgesics in herbal highs. Their representatives are UR-144, A-836,339, and their analogs, which were developed by Abbott Laboratories as analgesics but are less psychoactive than other synthetic cannabinoids [22–24]. The low CB1 agonist activity makes it difficult to classify them as narcotics, and UR-144 and its N-pentyl fluorinated derivative XLR-11 have become one of the most frequently encountered synthetic cannabinoid types in herbal highs. A-836,339 was first identified along with XLR-11 in a herbal incense product seized by the Police Agency of South Korea in 2012 (Fig. 3); Uemura et al. [25] have also reported the identification of A-836,339 in a dubious product very recently. Unlike UR-144 and its analog, A-836,339 analogs have not been indentified in herbal highs as yet. However, A-836,339 has quite different basic moieties from other synthetic cannabinoids, and its analogs may be abused in the near future to avoid recent worldwide drug regulation of synthetic cannabinoids.
Number of seizures in which synthetic cannabinoids were detected by the National Forensic Service from 2009 to June 2013. Class 1 includes the classical synthetic cannabinoids such as naphthoylindole, phenylacetylinole, benzoylindole, and CP-47,497 derivatives; class 2 includes new substances with cyclopropylindole, cyclopropylthiazole, aminocarbonylindazole, adamantylindole, adamantylindazole, and quinolinylindole structures
Legal regulation of new psychoactive substances in South Korea
The numbers of chemical species and abuse cases of NPSs have increased rapidly in South Korea since 2010, and the Korean Food and Drug Administration (KFDA) added analogs of major NPSs to the list of narcotics controlled by law in February 2011 based on the Canadian analog system [26] and included analogs of JWH-018 and JWH-073, JWH-250, CP-47,497, methcathinone, and phencyclidine. The present narcotics control act regulates the analogs of classical cannabinoids, such as naphthoylindoles, phenylacetylindoles, and CP-47,497 homologs. However, some of the recent synthetic cannabinoids with new basic moieties are not included in the list, and this will force the legal regulatory authority to prepare a more detailed and expanded analog list for regulation. The rapid increase in the range of NPSs also forces the monitoring of NPSs that have abuse potential. Thus, the KFDA also applied the temporary drug designation act in June 2011 to reduce the interval required to legislate the drug regulation act. Since methylenedioxypyrovalerone (MDPV) was first placed on the list on November 2011, 40 species of NPSs have been added to the temporary drug designation list until June 2013, and most of them are being added to the drug regulation act afterward. As a result, regulations on analogs and the temporary drug designation act have contributed to the suppression of expansion of NPSs in South Korea, which was reflected in the decreased number of synthetic cannabinoid compounds identified by the NFS in 2012 (Fig. 4). However, the number increased again in 2013, and the number of seized cases has increased continuously (Figs. 1, 2). The results suggest that trade and abuse of NPSs cannot be reduced only by regional regulation, and it is impossible to block all legal highs sold via the Internet and international mailing services. Thus, international cooperation and information sharing are necessary to efficiently regulate the NPSs without the balloon effect.
Conclusions
The number of species and the amounts of NPSs are increasing rapidly every year, and new substances with no toxicological information are causing unexpected side effects or even fatal intoxication of drug abusers. The evolution of synthetic cannabinoids has been much faster than for other NPSs, and some reports are now available on the side effects and intoxication induced by synthetic cannabinoids. The most efficient ways to regulate NPSs may include a rapid legislation system and extensive regulations of the analogs of possible abuse. The regulatory system should be improved to cope with the continuous and endless evolution of NPSs worldwide.
References
EMCDDA (2011) Annual report on the state of the drugs problem in Europe. European Monitoring Centre for Drugs and Drug Addiction, Lisbon
Hillebrand J, Olszewski D, Sedefov R (2010) Legal highs on the internet. Subst Use Misuse 45:330–340
Uchiyama N, Kikura-Hanajiri R, Kawamura M, Goda Y (2009) Identification of a cannabimimetic indole as a designer drug in a herbal product. Forensic Toxicol 27:61–66
Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Fereirós N (2009) ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom 44:832–837
UNODC (2012) World drug report 2012. United Nations Office on Drugs and Crime, Vienna
Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y (2013) Changes in the prevalence of synthetic cannabinoids and cathinone derivatives in Japan until early 2012. Forensic Toxicol 31:44–53
Zuba D, Byrska B (2013) Analysis of the prevalence and coexistence of synthetic cannabinoids in “herbal high” products in Poland. Forensic Toxicol 31:21–30
Mauler F, Mittendorf J, Horváth E, De Vry J (2002) Characterization of the diarylether sulfonylester (−)-(R)-3-(2-hydroxymethylindanyl-4-oxy)phenyl-4,4,4-trifluoro-1-sulfonate (BAY 38-7271) as a potent cannabinoid receptor agonist with neuroprotective properties. J Pharmacol Exp Ther 302:359–368
EMCDDA (2009) Thematic papers: understanding the “spice” phenomenon. European Monitoring Centre for Drugs and Drug Addiction, Lisbon
Malan TP Jr, Ibrahim MM, Lai J, Vanderah TW, Makriyannis A, Porreca F (2003) CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr Opin Pharmacol 3:62–67
Clayton N, Marshall FH, Bountra C, O’Shaughnessy CT (2002) CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain 96:253–260
Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, Malan TP Jr (2003) Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology 99:955–960
Choi H, Heo S, Kim E, Hwang BY, Lee C, Lee J (2013) Identification of (1-pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone and its 5-pentyl fluorinated analog in herbal incense seized for drug trafficking. Forensic Toxicol 31:86–92
Aung MM, Griffin G, Huffman JW, Wu MJ, Keel C, Yang B, Showalter VM, Abood ME, Martin BR (2000) Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend 60:133–140
Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, Cassidy MP, Wiley JL, Martin BR (2005) 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg Med Chem Lett 15:4110–4113
Weissman A, Milne GM, Melvin LS Jr (1982) Cannabimimetic activity from CP-47,497, a derivative of 3-phenylcyclohexanol. J Pharmacol Exp Ther 223:516–523
Harris CR, Brown A (2013) Synthetic cannabinoid intoxication: a case series and review. J Emerg Med 44:360–366
Patton AL, Chimalakonda KC, Moran CL, McCain KR, Radominska-Pandya A, James LP, Kokes C, Moran JH (2013) K2 toxicity: fatal case of psychiatric complications following AM2201 exposure. J Forensic Sci. doi:10.1111/1556-4029.12216
Park Y, Lee C, Lee H, Pyo J, Jo J, Lee J, Choi H, Kim S, Hong RS, Park Y, Hwang BY, Choe S, Jung JH (2013) Identification of a new synthetic cannabinoid in a herbal mixture: 1-butyl-3-(2-methoxybenzoyl)indole. Forensic Toxicol 31:187–196
Choi H, Heo S, Choe S, Yang W, Park Y, Kim E, Chung H, Lee J (2013) Simultaneous analysis of synthetic cannabinoids in the materials seized during drug trafficking using GC–MS. Anal Bioanal Chem 405:3937–3944
Nakajima J, Takahashi M, Nonaka R, Seto T, Suzuki J, Yoshida M, Kanai C, Hamano T (2011) Identification and quantitation of a benzoylindole (2-methoxyphenyl)(1-pentyl-1H-indole-3-yl)methanone and a naphthoylindole 1-(5-fluoropentyl-1H-indol-3-yl)-(naphthalene-1-yl)methanone (AM-2201) found in illegal products obtained via the Internet and their cannabimimetic effects evaluated by in vitro [35S]GTPγS binding assays. Forensic Toxicol 29:132–141
Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD (2010) Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB2 cannabinoid receptor activity. J Med Chem 53:295–315
Pace JM, Tietje K, Dart MJ, Meyer MD (2006) 3-Cycloalkylcarbonyl indoles as cannabinoid receptor ligands. Published in 2006-06-29, assigned to Abbott Laboratories
Yao BB, Hsieh G, Daza AV, Fan Y, Grayson GK, Garrison TR, El Kouhen O, Hooker BA, Pai M, Wensink EJ, Salyers AK, Chandran P, Zhu CZ, Zhong C, Ryther K, Gallagher ME, Chin CL, Tovcimak AE, Hradil VP, Fox GB, Dart MJ, Honore P, Meyer MD (2009) Characterization of a cannabinoid CB2 receptor-selective agonist, A-836339 [2,2,3,3-tetramethyl-cyclopropanecarboxylic acid [3-(2-methoxy-ethyl)-4,5-dimethyl-3H-thiazol-(2Z)-ylidene]-amide], using in vitro pharmacological assays, in vivo pain models, and pharmacological magnetic resonance imaging. J Pharmacol Exp Ther 328:141–151
Uemura N, Fukaya H, Kanai C, Yoshida M, Nakajima J, Takahashi M, Suzuki J, Moriyasu T, Nakae D (2013) Identification of a synthetic cannabinoid A-836339 as a novel compound found in a product. Forensic Toxicol. doi:10.1007/s11419-013-0201-x
Shin MH (2012) Improvement of drug designation system–introduction of emergency procedure and analogue system (in Korean). J FDC Regulatory Sci 7:13–19
Acknowledgments
This study was supported by funding from the National R & D Program of the Ministry of Education, Science, and Technology (2012-0009836) and the National Forensic Service of Korea.
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, H., Choi, H., Heo, S. et al. Synthetic cannabinoids abused in South Korea: drug identifications by the National Forensic Service from 2009 to June 2013. Forensic Toxicol 32, 82–88 (2014). https://doi.org/10.1007/s11419-013-0213-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-013-0213-6