Abstract
This research was conducted in the urban area of Patna region, the capital and largest city of Bihar, which is part of the Indo-Gangetic alluvium plain. This study aims to identify the sources and processes controlling groundwater’s hydrochemical evolution in the Patna region’s urban area. In this research, we evaluated the interplay between several measures of groundwater quality, the various possible causes of groundwater pollution, and the resulting health risks. Twenty groundwater samples were taken from various locations and examined to determine the water quality. The average EC of the groundwater in the investigated area was 728 ± 331.84 µS/cm, with a range of around 300–1700 µS/cm. Positive loadings were seen for total dissolved solids (TDS), electrical conductivity (EC), calcium (Ca2+), magnesium (Mg2+), sodium (Na+), chloride (Cl−), and sulphate (SO42−) in principal component analysis (PCA), demonstrating that these variables accounted for 61.78% of the total variance. In the groundwater samples, the following main cations are the most prevalent such as Na+ > Ca2+ > Mg2+ > K+, while the dominant anions are HCO3− > Cl− > SO42−. The elevated HCO3− and Na+ ions indicate that carbonate mineral dissolution might affect the study area. The result demonstrated that 90% of samples fall into the Ca-Na-HCO3 type, remaining in the mixing zone. The presence of the NaHCO3 kind of water is suggestive of shallow meteoric water, which may have originated from the river Ganga that is located nearby. The results show that a multivariate statistical analysis and graphical plots successfully identify the parameters controlling groundwater quality. In accordance with guidelines for safe drinking water, the electrical conductivity and potassium ion concentrations in the groundwater samples are 5% higher than acceptable levels. People who take large amounts of salt replacements report feeling tight in the chest, vomiting, having diarrhoea, developing hyperkalaemia, having trouble breathing, and even experiencing heart failure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is believed to have a higher level of purity when compared to other sources of water; as a result, it can satisfy the increasing demand for use in drinking, agricultural, and industrial settings caused by growing populations (Mahammad et al. 2022; Mahammad and Islam 2021). Although our planet is referred to as the “blue planet” because more than 70% of its surface is covered by water, most of the water on the planet is salty and cannot be used for the myriad of purposes that are important to both human society and continental ecosystems (Avci et al. 2018; Zhang et al. 2020). There are several potential applications for groundwater, including its usage as safe and secure drinking and household water source. Groundwater is essential to many natural and human ecosystems (Satyanarayana et al. 2017). Since groundwater is less prone than surface water to be contaminated by microorganisms, more wells have been dug globally to meet the rising need for freshwater (Bhatt et al. 2022). Under the expanding urbanization, industrialization, and population explosion, increased freshwater resources are needed worldwide (Li et al. 2017). Concerns regarding public health have persisted for some time, especially in Southeast Asia, due to the region’s rapid population expansion and the pervasive presence of inorganic pollutants. This is because many shallow aquifers in the area contain unhealthy levels of inorganic pollutants like arsenic, fluoride, nitrate, and other heavy metals and metalloids (Bhatt et al. 2022; Van Geen et al. 2019; Kumar et al. 2020). As a matter of concern to India, over the past decades, development in population and economic expansion, the groundwater-based agriculture sector has contributed approximately 46% of the gross national product and played an essential part in the country’s financial progress (CGWB 2015). Over the past few years, people in India have been increasingly affected by groundwater pollution issues and its revitalization. In general, natural and biological variables, including water-rock interactions, groundwater residence duration, and ion exchange processes, impact the chemical concentration in groundwater. The major sources of contamination in the groundwater are caused by anthropogenic influences such as industrial waste processes, mining operations, and chemical and fertilizer use in agriculture (Adimalla et al. 2020; Gaikwad et al. 2020). Some contaminants, such as fluoride, boron, and nitrate, have had far-reaching effects on groundwater in recent years, resulting in carcinogenic effects on human health (Subba Rao et al. 2017; Adimalla 2019; Kadam et al. 2019; Wagh et al. 2020). Thus, it is a significant and tough challenge for researchers to determine the origin and prevalence of contaminants and their possible health implications for efficient groundwater resource management. As a result, numerous theoretical and applied studies have been carried out in various regions to assess groundwater quality in the drinking, industry, and agriculture sector. Consequently, several new studies have been introduced to interpret the cations, anions, and heavy/trace metals in the groundwater to distinguish the natural and anthropogenic origins that alter groundwater quality (Brindha et al. 2017; Wagh et al. 2018) and their consequences on human health (Ali et al. 2019; Yasmin et al. 2019; Sarafraz et al. 2020; Chegbeleh et al. 2020; Patel et al. 2020; Jain et al. 2021). The World Health Organization’s recommended threshold (> 10 µg/l) has been linked to skin pigmentation due to elevated concentrations of inorganic contaminants. At the same time, chronic exposure can lead to heart disease and many types of cancer, including the skin, liver, lungs, and bladder (Parvez et al. 2011; Rahman et al. 2017; Zhang et al. 2019). Drinking water with high concentrations of nitric oxide (NO3) (> 45 mg/l) has been directly linked to an enhanced risk of methemoglobinemia, stomach cancer, goitre, congenital abnormalities, high blood pressure, and even hypotension (Crandall et al. 2013; Drozd et al. 2016). Furthermore, the concentration of the most contributing ions, such as Ca2+, Mg2+, Na+, K+, HCO3−, SO42−, and Cl− in surface water, can predict weathering, precipitation, sedimentation, and anthropogenic activities. These parameters are also helpful in determining whether water is appropriate for drinking and irrigation purposes. As a result, a firm and vigilant approach is required to ensure the excellent health of surface water, which could otherwise lead to waterborne diseases, which are among the leading causes of death worldwide (Uddin et al. 2021; Noori et al. 2019). However, an indexing technique has recently been widely used to evaluate the exposed population’s risk due to polluted groundwater. The risk to dermal health and ingestion has been assessed under the exposure routes utilizing the US EPA guidelines for hazardous quotient (HQ), hazardous index (HI), and chronic daily intake (CDI) (Magesh et al. 2018; Adimalla 2019; Kumar and Singh 2020). Management solutions have frequently been provided to accurately depict water quality and health hazards to minimize exposure to contaminated drinking water. Hydro-geochemical studies are required to recognize the mechanism of natural and anthropogenic processes concerned with the alteration of groundwater quality. Therefore, hydrochemical, hydrogeochemical, and health risk assessment techniques are widely used to investigate groundwater contamination’s origin. These techniques proved useful for more scientifically identifying groundwater composition and quality (Subba Rao et al. 2020; Haji et al. 2021; Bhatt et al. 2022). Because of this, maintaining sustainable use of groundwater resources and protecting them is crucial for India’s economy and public health, particularly in this region of the Bihar state. The state of Bihar is experiencing fast economic development, influencing the way of life, the state’s natural resources, and the environment. However, continued shortcomings in economic growth remain a problematic area in agriculture, which is crucial in alleviating poverty in Bihar since most of the population lives in rural areas, about 90%. Even though the state is endowed with water and land, there is a significant need to raise both the farming and irrigation intensity (Sukumaran et al. 2015). Assured water availability for drinking, agriculture, and industries are the key factors in determining the future economic scenario. In recent decades, the remarkable feature in irrigation development is the conspicuous growth in groundwater use. However, in Bihar, the groundwater meets the irrigation to only about 65% of the gross irrigated area. The major credit for the increase in groundwater use goes to a large extent to the farmer’s investment and spread of the groundwater market. The state has about 0.9 million shallow and 1700-deep tube wells (Sukumaran et al. 2015). As groundwater irrigation is under the direct control of the farmers and is receptive to precision agriculture and improved irrigation efficiency, it is safe to develop groundwater to at least 60–70% level to increase the irrigation potential (Vijay Shankar et al. 2011). Therefore, this study delivers a new perspective on groundwater vulnerability in the Patna region of the Indo-Gangetic River basin. Existing groundwater quality research is vital for improving the management of groundwater and its protection. The water quality for irrigation, drinking, and industrial purposes can be determined using hydrochemistry data. The objectives focus on assessing groundwater quality concerning being utilizable for drinking and agricultural purposes. In addition, the physicochemical parameters were assessed to evaluate the possible risk to human health. Because groundwater is the second water source utilized for drinking, household, industrial, and agricultural purposes, it is crucial in the area under investigation. Therefore, frequent monitoring and suitability appraisal of groundwater quality are essential to avoid further human health deterioration in the study area with elevated pollution levels.
In this study, we attempted to assess the different mechanisms controlling the hydrochemical evolution of groundwater and to identify the potential sources in the urban area of the Patna region. An integrated approach using conventional graphical plots such as Piper plot, Sulin diagram, cluster analysis, and multivariate statistical methods has been used to address the objectives. Because of the lack of historical data in this area, this study plays a vital role in contributing to the groundwater quality database and in the sustainable use and management of a regional aquifer in the urban area of the Patna region. Therefore, the study was carried out with the main objectives (1) to assess whether groundwater is suitable for use in agriculture and as drinking water, (2) to detect the source of physicochemical parameters that provide insight into the quality of groundwater, and (3) to examine the potential risk due to exposure of selected physicochemical parameters to human health. In short, the study’s results will recommend scientific knowledge about the origin and evolution of groundwater contaminants in the area under investigation. The research’s secondary goal is to assist government agencies, water planners, and resource managers in improving groundwater management.
Study area
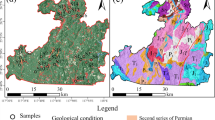
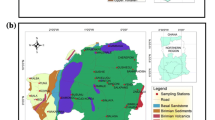
The study focuses on Patna’s urban area, the largest city and the capital of Bihar (Fig. 1). Patna is situated on the south bank of the Ganga, 47 m above mean sea level, between latitudes 25°13′N and 25°45′N, as well as longitudes 84°43′E and 86°44′E, and it has a land area of 3172 km2. It is a part of the Indo-Gangetic alluvium plain. The climate is subtropical, with hot summers having an average temperature of 32.40 °C and relatively cold winters with an average temperature of ~ 17.2 °C. The location has extreme summer and winter temperatures (CGWB: Ground Water Data Book 2013). The yearly average rainfall is 1076 mm, with July and August receiving highly significant precipitation. The rainy season typically lasts from around the middle of June to the middle of September. The residential sector occupies about 47.5% of the land, while agriculture occupies 17.66%. The other distributions are public and semi-public 10.18%, followed by transportation (5.90%) and commercial use (4.46%) of the total area (Zafar et al. 2022). The study area is a part of the Ganga basin and has a relatively flat topography. The natural solid levee structure or upland that runs all along the southern bank of the Ganga acts as a natural barrier, directing many of the streams that flow from the south along a parallel path until they eventually join the Ganga further east of the district line. Hence, the pollution by these channels is not affecting the water quality in the Patna region. The area comprises alluvial sediments deposited unconformably over the Archaean basement during the Precambrian period (Sandhu et al. 2011; USGS 2022). A Quaternary alluvial formation composed of clay, silt, and sand, and occasionally gravel forms the area’s subsoil. The groundwater potential across the entire district is good to exceptional. The upper clay zone is semi-pervious because small boulders (nodules of CaCO3) and fine sand are present in some areas, and groundwater exists under phreatic conditions. More coarse-grained sand and gravel can be found in the aquifers located farther underground (CGWB: Ground Water Information Booklet 2013).
Materials and methods
Water sample collection
The water sampling from 20 locations in Patna urban area was collected, and chemical analysis was conducted per the American Public Health Association (APHA) guidelines. We used systematic random sampling to gather groundwater samples at a consistent distance of fewer than 3 km between each sampling site. Groundwater samples were taken during the study period of 2019–2020 in the urban area of Patna from the boreholes, domestic or pumping well. In the study region, the boreholes used to collect the samples ranged in depth from 250 to 300 feet, and they were positioned at a total of 20 separate locations. A global positioning system (GPS) was used to map the study area’s groundwater sampling locations. Before water samples were collected, each sampling bottle was meticulously cleaned and dried, and then bottles were rinsed using a water sample taken at the time of collection. Water samples were taken in 1-l acid-washed, high-density polyethylene (HDPE) bottles with an internal stopper after 15 to 20 min of pumping. These bottles were then filtered through 0.45-µm Millipore membrane filter paper. After collecting the samples, the sampling location underwent the necessary labelling procedures. A global positioning system (GPS) was utilized to precisely locate each sampling site’s latitude, longitude, and altitude, and the source as samples were taken (Model: Garmin GPS).
Chemical analysis procedure
An icebox was used to transfer the samples from the field to the laboratory. During the chemical analysis of water samples, the procedures outlined by APHA (2017) were utilized, and the results obtained were compared to the quality standards outlined by BIS and WHO. A portable field kit was utilized at each sampling location to take pH, electrical conductivity (EC) readings, and total alkalinity (TA). When measuring the cations and anions, a detection limit of 0.05 ppm was used for the cations, and 0.1 ppm was used for the anions to determine the ions present in the water samples; a UV–Vis spectrophotometer was used. The acid titration method was utilized to analyse the bicarbonate. Ion chromatography was used to perform analyses on chloride and sulphate. Analyses of sodium, potassium, calcium, and magnesium followed the standard procedures outlined in IS-1500:2012 (Cotruvo 2017). The following equation was utilized to determine the groundwater samples normalized charged balance index (NCBI) water quality.
In this equation, TZ+ represents the total amount of cations, while TZ− represents the whole sum of anions.
Several indicators were used in the process to assess groundwater quality. The collected data and the suggested range by WHO and BIS were compared. The formulae in Table 6 were used to calculate the irrigation quality metrics electrical conductivity (EC), magnesium hazard (MH), potential salinity (PS), soluble sodium percentage (Na%), and Kelley’s ratio (KR). The graphical representations of Piper (1944), Sulin (1946), Doneen (1964), and the US salinity diagram (USSL 1954) were used to analyse the correlation of the analytical data to identify the qualities of groundwater and its suitability for different uses. The principal component analysis (PCA), cluster analysis (CA), and Pearson correlation analysis were performed using the statistical application XLSTAT.
Health risk assessment
The geochemical parameters (pH, EC, and TDS) have been shown to have no direct impact on consumers’ health by research carried out by WHO publications and literature surveys (Snousy et al. 2021). The rate of increasing parameters may result from decreased aesthetic values of the water and chalky or mineral-like taste, depending on the environment the water has travelled through, which causes the dissolved minerals to affect the flavour of the water. Therefore, in this paper, the identified ions (Ca2+, Mg2+, Na+, K+, HCO−, SO2−, and Cl−) were discussed regarding health consequences. However, the primary factor in the elevation of the geochemical parameter is also responsible for the deterioration of water and has a significant and direct impact on human health (Ravindra et al. 2019; Prasad et al. 2021).
Results and discussion
General hydrochemistry of groundwater
This research was carried out in the Patna area. The descriptive data of the physicochemical parameters, the maximum, minimum, mean, and standard deviation for the groundwater samples, are outlined in Table 1. The pH of groundwater samples varies from acidic to alkaline, with pH values of 7.13 to 8.10 and an average value of 7.39 ± 0.23. The higher pH of groundwater is not suitable for drinking. This may be due to the dissolution of alkaline cations, bicarbonates from the minerals, or dissolved hydroxide (Zaghlool 2020). Also, the tendency of an alkaline chemical reaction within the groundwater system can be deduced from the pH levels of all the groundwater samples collected in the research region. It falls within the recommended drinking range of 6.5 to 8.5. Thus, it is safe to consume (Shakeri et al. 2022). According to one study (Sukumaran et al. 2015), the pH value of groundwater in the Patna region was observed to range from 6.59 to 7.69; however, another study (Zafar et al. 2022) reported that the range of pH varied between 6.5 and 8.5. Electrical conductivity varies widely, ranging from 300 to 1700 µS/cm and an average of 728 ± 331.84 µS/cm. A high electrical conductivity in groundwater indicates a high ionic strength, which might have been imparted by the soil and agricultural activities in the region under study (Kumar et al. 2015). TDS varies from 279.66 to 1126 mg/l, with a mean value of 462.70 ± 229.44 mg/l. According to the studies, groundwater that falls into the freshwater class and has TDS values below 1000 mg/l can be consumed and used for irrigation (Davis and De Weist 1966). The high value of EC and TDS at some sampling locations is due to increased dissolved salts and defective drainage systems (Zafar et al. 2022). The cationic concentration such as Ca2+, Mg2+, Na+, and K+ range from 44.11 to 121.14 mg/l, 16.45 to 64.01 mg/l, 46.13 to 134.53 mg/l, and 4.8 to 46.83 mg/l, respectively, while the mean concentrations of HCO3−, Cl−, and SO2− were noted to be 307.15 ± 41.43 mg/l, 50.53 ± 45.20 mg/l, and 35.23 ± 28.87 mg/l, respectively. It may be deduced from the fact that the standard deviation of SO42− and K+ is nearly equal to its mean value that the concentrations of these ions randomly fluctuate in the groundwater throughout the Patna region. In the groundwater samples, the following main cations are the most prevalent such as Na+ > Ca2+ > Mg2+ > K+, while the dominant anions are HCO3− > Cl− > SO42−. The elevated HCO3− and Na+ ions indicate that silicate mineral dissolution might affect the study area (Ali et al. 2018). The percentage contribution of the cations in the Patna region was 39.96% of Na+, 38.02% of Ca2+, 16.64% of Mg2+, and 5.38% of K+, respectively. Anions contribute 78.17% of HCO3−, 12.86% of Cl−, and 8.97% of SO42− in the total anionic budget. Results indicate that the contribution of Na+ is about 39.96% of the total cationic budget and 78.17% of HCO3− in the Patna region’s total anionic groundwater budget produced by the dissolution of silicate and cal-silicate minerals (Holland 1978). The high Na+ concentration suggests that it has received a greater contribution from sodium-bearing sources. Still, the higher Mg2+ content compared to Ca2+ could result from ferromagnesium minerals, an ion exchange between Na+ and Ca2+, or the precipitation of CaCO3 (Subba Rao 2018). In addition, the potassium feldspar minerals are more resistant to chemical weathering and the fixing of potassium ions on clay products, which results in the K+ concentration having the lowest content among the concentrations of cations. A combination of Ca2+, Mg2+, and HCO3 causes the hardness of the groundwater, and their high concentrations are responsible for hardness in the Patna region. The presence of carbonate-containing minerals in the area under study is indicated by high HCO3− values. The presence of degraded organic matter, which can potentially contribute to the existence of HCO3− in groundwater, is also indicated by high HCO3− values (Rina et al. 2012). The high evaporation rate may be attributable to a high concentration of Cl in the groundwater. The presence of SO42− in the groundwater may be due to the dissolution of gypsum or anhydrite minerals. According to Snousy et al. (2021) and Ledesma-Ruiz et al. (2015), a higher concentration of SO42− indicates that its concentration levels in groundwater randomly vary. The result demonstrates that the geochemistry of the researched area is not consistent over the entire region. Additionally, NO3− in the groundwater may be contributed by fertilizers, agricultural or municipal waste, or sewage leaching.
The mechanism controlling the geochemistry of the groundwater
The weathering of rocks, precipitation, natural and anthropogenic activities, and climatic conditions are the primary contributors of dissolved ions to groundwater. In most cases, the chemical weathering that occurs because of the interaction of water and bedrock determines the makeup of groundwater’s constituent elements. A scatter plot is used to illustrate the interaction that exists between the physical parameters and the chemical parameters. The residence time of water with many subsurface environments determines the influence of various rock–water interaction processes on groundwater’s geochemistry. A scatter plot of Ca2+/Na+ versus HCO3−/Na+ and Ca2+/Na+ versus Mg2+/Na+ was utilized to differentiate between these processes. This suggests that carbonate dissolution and silicate weathering significantly influence groundwater’s chemical composition formation (Fig. 2a and b). It can also be explained by the weathering of silicate minerals, which causes these minerals to react with carbonic acid and release bicarbonates (Rajmohan and Elango 2004). The aquifer contains silicate minerals such as quartz, quartzite, muscovite, biotite, and feldspar (GSI 2011). As a result, silicate weathering possibly contributes to the primary cations in groundwater, such as sodium, potassium, calcium, and magnesium. The ratio of Ca2+ to Mg2+ was investigated to understand better the impact of calcite and dolomite dissolution in groundwater. The Ca2+/Mg2+ ratio being more prominent than one indicates that the predominant source of these ions in the study was calcite dissolution (Singh et al. 2017; Rina et al. 2012).
In addition, Ca2+ + Mg2+ was plotted against HCO3− + SO42− (Fig. 2c) to identify the parental rock responsible for the ion exchange process in the groundwater (Maurya et al. 2020). The points will move away from the equiline or to the right if there is an ion exchange. Conversely, the points will move to the left if there is a reverse ion exchange. The scatter plot for most samples falls near the equiline, suggesting the involvement of silicate and carbonate weathering as the primary geochemical process in groundwater (Su et al. 2019). However, plots shifting towards the left indicate the presence of a reverse ion exchange process in contributing Ca2+ and Mg2+ to the cationic budget (Subba Rao and Chaudhary 2019). The possible source of sodium concentration in groundwater is due to the dissolution of rock salts and weathering of sodium-bearing minerals in the groundwater (Kumar et al. 2009). Also, most of the dots in the scatter plot of Ca2+ + Mg2+ vs Cl− (Fig. 2d) were situated to the left of the equiline, suggesting that the research area may have a mechanism for reverse ion exchange and carbonate weathering. The Na+ against Cl− plots were used to assess groundwater’s predominant silicate weathering and ion-exchange processes (Fig. 3a). The influence of ion exchange can be seen above the equiline. Most samples discovered to be above 1:1 line were found to have been influenced by silicate weathering, whereas samples found to be below the equiline were found to have been affected by evaporation. The Na+/Cl− ratio of about 1 shows that halite dissolution is the primary process controlling the release of Na+ in groundwater (Meybeck 1987), whereas the Na+/Cl− value greater than one suggests that silicate weathering is the leading process for controlling the release of Na+ in groundwater. The range of the Na+/Cl− value in the study area is between 0.44 and 2.44, suggesting evaporation’s effect on the groundwater quality. Most of the samples lie above the 1:1 equiline in the scatter plot of Na+ + K+ against Cl− (Fig. 3b), showing an abundance of cations, which may be associated with high alkalinity and the formation of alkali carbonates and sulphates in the study region.
Hydrogeochemical facies
A functional Piper diagram for separating pertinent analytical data for understanding the water’s sources of dissolved elements has been analysed (Piper 1944). The hydrochemical properties of the groundwater samples were examined and shown using a Piper diagram (Fig. 4). The hydrochemical facies are determined by solution kinetics, rock-water interactions, the geology of that region, sources of contamination, and the flow pattern of the aquifers (Liu et al. 2019, 2021). It gives us the ability to characterize the quality of the water, which can vary depending on its chemical composition (Srinivasamoorthy et al. 2014). The assumption that most natural water contains cations and anions in a condition of chemical equilibrium led to the development of this approach. It is generally believed that Ca, Mg, and Na make up most of the earth’s abundant cations. The three most frequent types of anions are bicarbonate (HCO3−), sulphate (SO2−), and chloride (Cl−). Bicarbonate is considered a “weak acid”, while sulphate and chloride are “strong acids”. Piper (1944) recommended sketching a diamond that summarizes two triangles that correlate with cations and anions. The cations are denoted by the triangle on the left, while the triangle on the right indicates the anions. The axis for calcium is found at the base of the cation triangle, magnesium is located on the left side, and sodium and potassium can be found on the right. In anion, the base represents chloride, the left represents carbonate plus bicarbonate, and the right represents sulphate. According to the Piper diagram, most groundwater samples have no dominant cation type; most water samples in the anion plot field have bicarbonate and few in the no prevalent anion types of water. Water samples from the Sultanganj, Bakarganj, Lodipur, Kurji, Digha, Nishad tola, Danapur Cantt, Saguna More, Khagaul, Fulwari, Anisabad, Mithapur, Kankarbagh, Pulse Hospital, and Earth hospital comes in the no dominant cation types of water. The plot revealed that Ca2+-Mg2+-HCO3− type water had dominated the study area’s groundwater samples, indicating temporary hardness contributed due to sufficient freshwater recharge (Handa 1969; Herojeet et al. 2016). The mechanism responsible for the geochemical evolution of groundwater is depicted as six distinct types of water: The result demonstrated that 90% of samples fall into the type IV (Ca-Na-HCO3) and the rest falling in the mixing zone. The result shows that groundwater is of a Ca-Na-HCO3 type at sites where groundwater is saline in North Patna Sahib, Lodipur, Digha, Nishad tola, Saguna More, Mithapur, Kankarbagh, Pulse hospital, and Earth hospital.
In addition to the Piper plot, Sulin diagram was utilized to identify the groundwater source, which may be either marine or meteoric. The Sulin diagram (Fig. 5) is made up of two squares that are identical in size. The square in the upper right corner depicts the origin of marine water and includes components such as MgCl2 for older marine water and CaCl2 for recent marine water. The square in the lower left corner depicts the origin of meteoric water, which is indicated to be NaHCO3 for shallow meteoric water and Na2SO4 for deep meteoric water. After projecting the groundwater samples on the Sulin diagram, the two squares are dissected into two triangles to complete the analysis. Three distinct water forms can be identified: Na2SO4, MgCl2, and NaHCO3. Mostly, the water samples (85%) were of a shallow meteoric origin (NaHCO3); two of the water samples (10%) were of a recent marine origin (MgCl2), and one of the water samples was discovered to be of a deep meteoric origin (Na2SO4). The NaHCO3 kind of water is indicative of shallow meteoric water, which may originate from the neighbouring river Ganga and irrigation water that makes its way into the groundwater of the Patna region. The Na2SO4 water type is associated with deep meteoric water percolation. The bicarbonate ions gradually decline with depth because of carbonate precipitation, sulphate solubility with water temperature enrichment, and intensive fertilizer application (Snousy et al. 2021). MgCl2 water type reflects the evaporation and dissolving of salts from marine deposits inside Quaternary aquifer materials, possibly by mixing deeper aquifers with groundwater.
Multivariate statistical analysis
Normalization and standardization of dataset
In addition, the normalization and standardization of the dataset have been done to explore the multivariate statistical analysis. In normalization, data has been scaled at standard scale without distorting the differences in the values of the dataset, whereas standardization assumes that the dataset is in Gaussian distribution and measures the variable at different scales, making all the variables equally contribute to the analysis. The mean is 0, and SD is 1 for all values after rescaling the normalized data. Z-score is the best way for standardization. The results of the normality test (Table 2) show all water quality parameters (pH, EC, TDS, Ca2+, Mg2+, Na+, K+, HCO3−, SO2−, and Cl−) with significance values (p values). This indicates that most of the data do not follow a normal distribution; hence, Spearman’s correlation analysis may be utilized to ascertain the degree to which the data are correlated with one another (Ghasemi and Zahediasl 2012; Gevera et al. 2020). After that, the data on the water were analysed with principal component analysis (PCA) and Spearman’s ranked order correlation to find the most important factors in groundwater as well as the strength of the relationships between them.
Principal components analysis
For the suitability of the dataset to be used for PCA, Bartlett’s sphericity test was considered, which locates the correlation matrix. In addition, Kaiser–Meyer–Olkin (KMO) was conducted, determining whether adequate sampling exists. Liu et al. (2003) reported that for PCA to be performed, the KMO sampling adequacy for the set of variables must be larger than 0.5, and for a component to be considered significant, its loading value must be greater than 0.5. In our dataset, the total sampling adequacy of the KMO was found to be 0.70, indicative of the suitability for PCA. The Bartlett sphericity test showed that the observed value was more significant than the critical value. As a result of statistical analysis, the dataset is of good quality, and PCA could be used (Aguirre et al. 2019; Ustaoğlu and Tepe 2019; Long and Luo 2020; Kumar et al. 2018, 2019; Kumar et al. 2022a, b).
According to the findings in Table 3, there are only two significant main components (eigenvalues greater than 1) that are relevant to groundwater datasets. These two components account for 67.95% of the total variance, as shown in Fig. 6. According to Table 3, the PC1 in the datasets is the essential variable since it accounts for 61.78% of the total variance and positive loading with EC, TDS, Ca2+, Mg2+, Na+, Cl−, and SO2−. The interactions between water and rock could have formed these groupings inside the aquifer matrix. Some studies indicate that Cl− and SO42− ions are frequently associated with sedimentary rocks (Battistel et al. 2016). Some studies expressed that high amounts of chloride and sulphate in groundwater are common signs of contamination (Srivastava and Ramanathan 2008). In general, the cations sodium (Na+), magnesium (Mg2+), and calcium (Ca2+) found in the groundwater are natural mechanisms derived from the leaching of rocks and present in leachate from landfills (Selvam et al. 2017; Mthembu et al. 2020). Therefore, the primary factors responsible for the most significant variance in electrical conductance and dissolved solids are the aquifer erosion matrix and the input from ions. PC2 accounts for 13.24% of the total variation and exhibits positive loading with EC, TDS, Ca2+, Mg2+, and Cl− while displaying negative loading with pH, Na+, K+, SO42−, and HCO3−. This component signifies the crucial impact that pH plays in the dissolution of minerals because of the weathering of the aquifer matrix. In addition, the ions Ca2+, Na+, and SO42− may originate from fertilizers. The pH shows a negative loading in PC1 and PC2, representing chemical components due to the water environment under geologic features, which satisfies the previously published scientific results (Bu et al. 2010). K+ loading, on the other hand, may suggest the presence of agricultural water and waste effluent infiltration.
Cluster analysis
Another multivariate statistical methodology that can be used for assessing hydrochemical data is called hierarchical cluster analysis (HCA). This method has been effectively employed in several investigations of the hydrogeochemistry of groundwater (Singh and Kumar 2017; Atanacković et al. 2013). According to Lang et al. (2006), HCA categorizes and quantifies the degree to which different water samples are alike by considering a wide range of chemical indicators in the water. This method distinguishes three primary groups of groundwater quality characteristics and generates a dendrogram (also known as a tree diagram), which may be seen in Fig. 7. Cluster 1 consists of the elements electrical conductivity (EC), TDS, and HCO—all of which can be caused by the contact of water and rock and leads to the dissolution of aquifer matrix, particularly the weathering of carbonates when alkaline conditions are present. It is possible that the weathering of carbonates is the source of the low acidity and high alkalinity found in maximum locations. The primary contributors to a high HCO3− concentration in groundwater are the dissolution of CaCO3 and redox reactions, and as HCO3− is produced in the groundwater, the pH level rises. Ions Ca2+, Na+, Cl−, SO42−, and Mg2+ make up cluster 2, suggesting the possibility that these ions in groundwater originated naturally due to interactions between water and rock. However, they may also reflect the influence of residential sewage and agricultural runoff contamination. Calcium and sulphate ions could be produced using calcium sulphate (CaSO4) fertilizers. Cluster 3 has a high concentration of potassium (K+) and pH, which leads one to believe that the leaching of potassium from feldspar rocks in the aquifer medium may have been caused by the decomposition of biogenic vegetation as well as the leaching of fertilizers from the soil horizon (Mbissik et al. 2021).
Correlation matrix analysis
The correlation matrix and the correlation coefficient could identify the interrelationships between the various indicators used to assess water quality. Using a linear correlation, we found that every pair of elements in the groundwater dataset was significantly correlated (p < 0.01) and significant level (p < 0.05) and determined by comparing the data to a standard correlation formula. Table 4 illustrates the correlation coefficients matrix calculated for the various groundwater quality measures examined. The correlation matrix revealed inter-parameter correlations that agreed with the findings of the PCA and HCA. The result shows that the EC was positively correlated with Na+ (r = 0.77) and an almost linear correlation with TDS (r = 0.96), representing a confidence level of 99%. There is a strong relationship between TDS and Na+ (r = 0.73), suggesting that increased concentrations of TDS and Na+ ions in the groundwater emerged because of weathering of rocks, causing an increased salinity and ionic strength. In addition, Ca2+ has a positive association with Mg2+ (r = 0.34), a moderately positive correlation with Cl− (r = 0.77), and a significant positive correlation with SO42− (r = 0.84). Also, the relationship between Mg2+ and Cl− is positive (r = 0.70), whereas the correlation between Mg2+ and SO42− is relatively weak (r = 0.36). These connections reflect the lithologic influence of the dissolution of aquifer materials and the interactions between water and rock. In addition, the ionic alterations and secondary salts found in groundwater may have been caused by the penetration of wastewater, such as sewage from homes and water from farms (Ledesma-Ruiz et al. 2015).
Water quality assessment
Impact of water quality on human health
The results from the physicochemical analysis of the groundwater are described in Table 5. The standards of water quality of WHO (2011) and BIS (2012) drinking water were compared with the groundwater of the study area. The pH value of groundwater varies from 7.13 to 8.10. Based on the World Health Organization and the Bureau of Indian Standards, all the sampling locations lie under the acceptable limit. The pH of drinking water rarely impacts human health, and there is no suggested guideline value for pH based on health considerations. Discomfort in the digestive tract and prolonged contact with high pH values (higher than 11) can trigger irritation to the respiratory tract, skin, and eyes and cause the hair fibres to swell with a pH of 10–12.5. In addition, redness and irritation of the eyes can develop when the pH is below 4, and irreversible and significant damage to the epithelium can occur when the pH is below 2.5 (WHO 2011). During the investigation, the pH of the groundwater was found to be the highest at the sampling location of Saguna More, and it was found to be the lowest in South Patna Sahib. Except for Saguna More and South Patna Sahib, the pH of all the sampling locations was roughly equivalent to one another. Therefore, the pH values in the areas under study did not exceed the permissible limit; yet these values fell within the range of basic or alkaline conditions. TDS refers to various ranges of minerals or salt sand correlated with EC that signify wide dissolved ionic components and directly impact human health (Rao et al. 2012; Adimalla and Venkatayogi 2017). Drinking water with high amounts of TDS and EC is often detrimental to people with kidney and heart disorders. Furthermore, suppose it includes poisonous salts and ions. In that case, it may induce laxative or constipation issues, heart disease, arteriosclerotic heart disease, cardiovascular illness, gallbladder inflammation, and gallstones; affect those with kidney ailments; cause cancer; and even lead to death (Sasikaran et al. 2012; Alam et al. 2021). The EC values in the sampling locations ranged from 300 to 1700 µS/cm. Following the categorization provided by Davis and Dewiest (1966), only 3 (15%) sampling sites out of 20 have an electrical conductivity (EC) of less than 500 µS/cm, which means that they are comparable to freshwater. The remaining 17 (85%) samples out of 20 falls into the marginal water type category, which has an EC of between 500 and 1500 µS/cm. The remaining 3 sampling sites have an EC of more than 1000 µS/cm, unfit for drinking (Davis and Dewiest 1966; WHO 2011). These findings make it abundantly evident that the water in the study locations was significantly ionized and possessed a greater ionic concentration activity because of an excessive quantity of soluble solids (Azrina et al. 2011; Rahmanian et al. 2015). The TDS content of the groundwater samples ranged anywhere from 279.66 to 1126 ppm in the all-sampling site in the Patna region. Ninety-five percent of the samples had a total dissolved solids (TDS) concentration of less than 1000 mg/l, indicating that the water poses no danger when consumed, while 5% of the groundwater had a TDS concentration of more than 1000 mg/l, indicating that it should not be consumed. However, an excess intake of Ca2+ impacts individuals predisposed to hypercalcemia and milk-alkali syndrome. People with kidney function deficiencies are more likely to experience diarrhoea and hypermagnesemia if they consume excessive Mg2+ (WHO 2011). An earlier study found that heart disease and acute myocardial infection were more likely to occur when there was a high concentration of soluble Ca2+ and Mg2+ and a high total hardness (Meyers 1975). In addition, being in contact with hard water increases a risk factor for the aetiology of atopic eczema, a form of dermatitis (Thomas and Sach 2000). Table 4 demonstrates that 100% of groundwater samples for the value of Na+ and 85% of the groundwater samples for the value of K+ are within the WHO-prescribed standards, indicating that they are safe for everyday use. It has been determined that the content of HCO− in the water is under the limit as prescribed by the World Health Organization (WHO), making it safe for human consumption. As a result, HCO− contributes to the health problems arising from the increased pH and TDS discussed before. The ingestion of drinking water with high concentrations of SO42− has a significant adverse effect on health, such as a decline in the gastrointestinal retention of food. Also, the laxative effect causes a significant increase in stool mass, which increases the risk of dehydration brought on by diarrhoea. A high concentration of SO42− can enhance the presence of a perceptible taste, and the swallowing of undesired metals due to the deterioration of distribution systems can occur when there is a significant level of oxidation in the water (WHO 2004). It is possible that high levels of Cl− could lead to congestive heart failure. The higher EC raises the corrosivity of the water, which in turn increases the uptake of undesirable metals (WHO 2004, 2009). As a result of the test, it was determined that the concentration of SO42− and Cl− was acceptable for use by water consumers because it fell below the permissible limit.
Irrigation water quality
Monitoring groundwater quality to improve agricultural yields and maintain soil fertility is vital. There is a possibility that the quality of the irrigation water will influence the growth of the plants and decrease productivity. The low-quality water has a detrimental effect on the heavily clayed soil. Still, it is acceptable for irrigation of sandy and porous soil, both of which allow chemicals to permeate more profoundly into the ground. Sand and porous soil are ideal for chemical application (Gaury et al. 2018). Electrical conductivity (EC), soluble sodium percentage (Na%), sodium adsorption ratio (SAR), magnesium hazard (MH), permeability index (PI), Kelley’s ratio (KR), and potential salinity (PS) are essential factors to be considered in determining the quality of irrigation water (Snousy et al. 2021). Based on the standard formulae, our results are analysed and compared with the prescribed ranges (Table 6).
Based on EC in groundwater, we could classify it into one of four distinct categories: excellent, good, permissible, or dubious. An indicator of natural water’s suitability for agricultural use is the amount of sodium in the water (Wilcox 1955). Sodium combined with carbonate produces alkaline soils, whereas combined with chloride, it produces salty soils. No or very little plant development will be supported by either type of sodium-enriched soil (Todd 1980). In the area under investigation, the sodium percentage (Na%) ranged from 30.74 to 58.03, showing that the groundwater samples were good for irrigation purposes. Considering this, 20% of the samples fall into the excellent to a good category, while only 80% are in the acceptable category (Fig. 8). As a result, all the groundwater samples in the study area fall into the good or the permitted category. This finding shows that groundwater use in these areas is appropriate for agricultural purposes.
The presence of an excessive amount of sodium in water has the unintended consequence of altering the characteristics of the soil and decreasing its permeability. Therefore, determining sodium concentration in the water is necessary before using it for irrigation. The SAR is a metric expressing the proportion of sodium ions adsorbed during an exchange reaction with soil. The SAR is utilized to conclude the sodicity of irrigation water. The SAR is the standard method for describing the sodicity risk posed by water. Figure 9 shows a plot of the analytical data from the US Salinity Laboratory (1954), in which the EC is taken as a salinity hazard and the SAR is taken as an alkalinity hazard. This plot illustrates that 60% of the groundwater samples belong to the category of C2S1, which implies water with a high level of salinity and a low level of alkalinity. The result shows that water is suitable for irrigating most soil types and crops because there is a low risk of exchange; a medium salinity and a medium sodium threat are indicated by 40% of the samples, which fall into the C3S1 class. The SAR value of the water samples was less than 10, making it relatively low. The diagram demonstrates that the water samples belong to the classifications C2S1 and C3S1, which respectively imply a low sodium hazard and a medium to high salinity hazard, as well as the fact that the water is suitable for use in irrigation. In addition, the diagram demonstrates that the water is suitable for use in industrial processes. In most water bodies, Ca2+ and Mg2+ remain in a state of balance, which is generally considered to be the case. Mg2+ is detrimental to the structure of the soil, particularly in environments where sodium predominates, and the water is excessively salty. Ca2+ does not function in the same manner in the soil system. Exchanging Na+ in irrigated soils is typically the cause of high Mg2+ content in the soil. In a state of balance, an increase in the amount of Mg2+ present in the water will negatively impact the quality of the soil, making it alkaline and leading to decreased crop yields. It is dangerous to have a magnesium ratio higher than 50, and it is not appropriate for irrigation systems. As a result, the crop yield would suffer as a direct consequence of the increased acidity of the soil. The research area has magnesium hazard levels ranging from 20.36 to 46.31, with an average value of 30.36 ± 7.71. As a result, 100% of groundwater (Table 6) in the sampling locations has magnesium levels below the hazard level of 50, which is fit for irrigation purposes in the study area.
In addition, 95% of the groundwater sample’s potential salinity (PS) value is less than the prescribed limit, indicating appropriateness for irrigation, while the remaining 5% is unsuitable. An indicator of salt concentration, Kelley’s ratio compared to Ca2+ and Mg2+ used to assess irrigation water (Kelley 1940; Paliwal 1967). Based on Kelley’s ratio, 85% of the water samples are suited for irrigation purposes in the study region, whereas 15% are inappropriate for such usage. The permeability index was also computed to understand the irrigation potential of groundwater. Based on the permeability index (PI), Doneen (1964) and Raghunath (1987) devised a standard for determining water suitability for irrigation. The groundwater samples of the study area fall into class 1 (Fig. 10) according to the permeability index and the PI values, making them suitable for irrigation.
Conclusions
Groundwater is the primary source of drinking water for the majority of Patna’s metropolitan population, who rely instead on it. Therefore, the groundwater quality in the rural areas surrounding Patna has been examined in terms of whether it is suitable for consumption, and the sources of various water quality metrics found to be most prevalent have also been determined. The fact that groundwater is also used for irrigation means that a high ionic concentration may have negatively impacted the soil and the crops grown in this area. This research also highlights the significance of conducting systematic groundwater quality monitoring, emphasizing ions as the target of such monitoring. In the groundwater, the following main cations are the most prevalent such as Na+ > Ca2+ > Mg2+ > K+, while the dominant anions are HCO3− > Cl− > SO42−. The elevated HCO3− and Na+ ions in the study area indicate that silicate mineral dissolution might be the reason. The percentage contribution of the cations in the Patna region was 39.96% of Na+, 38.02% of Ca2+, 16.64% of Mg2+, and 5.38% of K+, respectively. Anions contribute 78.17% of HCO3−, 12.86% of Cl−, and 8.97% of SO42− in the total anionic budget. Results indicate that the contribution of Na + is about 39.96% of the total cationic budget, and HCO3− contributes 78.17% of the Patna region’s total anionic groundwater budget. This is an indication of the high dissolution of silicate and calcium-silicate minerals. For evaluating the effects of silicate, carbonate weathering, and evaporative groundwater dissolution, a scatter plot was used between Ca2+/Na+ and HCO3−/Na+. The scatter plot indicates that the most significant factor affecting groundwater quality is the silicate weathering at most sampling sites. In contrast, the weathering of carbonate minerals is only significant at a few sampling sites. The ratio of Ca2+ to Mg2+ indicates that the predominant source of these ions in the study was calcite dissolution. The range of the Na+/Cl− value in the study area is between 0.44 and 2.44, suggesting evaporation’s effect on the groundwater quality. Most of the samples lie above the 1:1 equiline in the scatter plot of Na+ + K+ against Cl−, showing an abundance of cations, which may be associated with high alkalinity and the formation of alkali carbonates and sulphates in the study region. According to the Piper diagram, most groundwater samples have no dominant cation type, most water samples in the anion plot field have bicarbonate, and few are in the non-prevalent anion types of water. The result demonstrated that 90% of samples fall into the type IV (Ca-Na-HCO3), the rest falling in the mixed zone. The Sulin diagram demonstrated that most of the water samples (85%) had a shallow meteoric origin (NaHCO3, Na2SO4). The presence of the NaHCO3 kind of water is suggestive of shallow meteoric water, which may have originated from the nearby river Ganga. In addition, irrigation water from the Patna region also contributes to the groundwater in that area. This study also concludes that nearly all groundwater exhibits rock dominance. This finding suggests that water and rock frequently interact through percolation, and several samples lean more toward evaporation. The US Salinity Laboratory graphic shows that C2S1 predominates in the groundwater samples, which are helpful for irrigation. Based on the drinking water standards, 5% of electrical conductivity and 5% of potassium ions in the groundwater samples have a higher concentration than the desirable drinking water quality. Case studies of toxicity resulting from high doses of salt substitutes have described chest tightness, nausea and vomiting, diarrhoea, hyperkalaemia, shortness of breath, and heart failure (WHO 2009). Hence, water treatment action is recommended to reduce health hazards.
Data availability
Data is available on reasonable request from the corresponding author.
References
Adimalla N (2019) Groundwater quality for drinking and irrigation purposes and potential health risks assessment: a case study from semi-arid region of South India. Expo Health 11(2):109–123. https://doi.org/10.1007/s12403-018-0288-8
Adimalla N, Venkatayogi S (2017) Mechanism of fluoride enrichment in groundwater of hard rock aquifers in Medak, Telangana state, South India. Environ Earth Sci 76:45. https://doi.org/10.1007/s12665-016-6362-2
Adimalla N, Dhakate R, Kasarla A, Taloor AK (2020) Appraisal of groundwater quality for drinking and irrigation purposes in Central Telangana India. Groundw Sustain Dev 10:100334. https://doi.org/10.1016/j.gsd.2020.100334
Aguirre BP, Masachessi G, Ferreyra LJ et al (2019) Searching variables to assess recreational water quality: the presence of infectious human enterovirus and its correlation with the main variables of water pollution by multivariate statistical approach in Córdoba, Argentina. Environ Sci Pollut Res 26:6586–6601. https://doi.org/10.1007/s11356-019-04124-2
Alam I, Ur Rehman J, Nazir S, Nazeer A, Akram M, Batool Z, Ullah H, Hameed A, Hussain A, Hussain A, Tahir MB (2021) Health risk assessment in different age-group due to nitrate, fluoride, nitrite and geochemical parameters in drinking water in Ahmadpur East, Punjab, Pakistan. Hum Ecol Risk Assess 27:1747–1763. https://doi.org/10.1080/10807039.2021.1902264
Ali S, Shekhar S, Bhattacharya P, Verma G, Chandrasekhar T, Chandrashekhar AK (2018) Elevated fluoride in groundwater of Siwani Block, Western Haryana, India: a potential concern for sustainable water supplies for drinking and irrigation. Groundw Sustain Dev 7:410–420. https://doi.org/10.1016/j.gsd.2018.05.008
Ali W, Aslam MW, Junaid M, Ali K, Guo Y, Rasool A, Zhang H (2019) Elucidating various geochemical mechanisms drive fluoride contamination in unconfined aquifers along the major rivers in Sindh and Punjab. Pakistan Environ Pollut 249:535–549. https://doi.org/10.1016/j.envpol.2019.03.043
APHA (2017) Standard methods for the examination of water and wastewater (23rd ed). Washington DC, USA
Atanacković N, Dragišić V, Stojković J, Papić P, Živanović V (2013) Hydrochemical characteristics of mine waters from abandoned mining sites; in Serbia and their impact on surface water quality. Environ Sci Pollut Res 20(11):7615–7626. https://doi.org/10.1007/s11356-013-1959-4
Avci H, Dokuz UE, Avci AS (2018) Hydrochemistry and groundwater quality in a semiarid calcareous area: an evaluation of major ion chemistry using a stoichiometric approach. Environ Monit Assess 190:641. https://doi.org/10.1007/s10661-018-7021-8
Azrina HE, Khoo MA, Idris IA, Razman MR (2011) Major inorganic elements in tap water samples in Peninsular Malaysia. Malays J Nutr 17(2):271–276
Battistel M, Hurwitz S, Evans WC, Barbieri M (2016) The chemistry and isotopic composition of waters in the lowenthalpy geothermal system of Cimino-Vico Volcanic District, Italy. J Volcanol Geotherm Res 328:22–229. https://doi.org/10.1016/j.jvolgeores.2016.11.005
Bhatt AG, Kumar A, Singh SK (2022) Hydro-geochemical evolution of groundwater and associated human health risk in River Sone subbasin of Middle-Gangetic floodplain, Bihar, India. Arab J Geosci 15:405. https://doi.org/10.1007/s12517-021-09269-4
BIS, (Bureau of Indian Standard) (2012) Indian standard, drinking water–specification (Second Revision): IS 10500, BIS, Delhi
Brindha K, Pavelic P, Sotoukee T, Douangsavanh S, Elango L (2017) Geochemical characteristics and groundwater quality in the Vientiane plain, Laos. Expo Health 9(2):89–104. https://doi.org/10.1007/s12403-016-0224-8
Bu H, Tan X, Li S (2010) Zhang Q (2009) Water quality assessment of the Jinshui River (China) using multivariate statistical techniques. Environ Earth Sci 60:1631–1639. https://doi.org/10.1007/s12665-009-0297-9
Central Ground Water Board (CGWB) (2013) Ground water information booklet
Central Ground Water Board (CGWB) (2015) Pilot project on aquifer mapping in Maner-Khagaul Area, Patna District Bihar, Ministry of Water Resources, River Development & Ganga Rejuvenation Government of India
CGWB (2013) Master plan for artificial recharge to groundwater in India. Central Groundwater Board, Ministry of Water Resources, Government of India
Chegbeleh LP, Aklika DK, Akurugu BA (2020) Hydrochemical characterization and suitability assessment of groundwater quality in the Saboba and Chereponi Districts, Ghana. Hydrology 7(3):53. https://doi.org/10.3390/hydrology7030053
Cotruvo JA (2017) WHO guidelines for drinking water quality: first addendum to the fourth edition. https://doi.org/10.5942/jawwa.2017.109.0087
Crandall C, Katz BG, Berndt MP (2013) Estimating nitrate concentrations in groundwater at selected wells and springs in the surficial aquifer system and Upper Floridan aquifer, Dougherty Plain and Marianna Lowlands, Georgia, Florida, and Alabama, 2002–50: U.S. Geol. Surv. Investig. Rep. 2013–5150, 65, http://pubs.usgs.gov/sir/2013/5150/
Davis SN, De Weist RJM (1966) Hydrogeology. John Wiley and Sons, New York, p 463
Doneen LD (1964) Notes on water quality in agriculture. Water Science and Engineering, University of California, Davis
Drozd VM, Branovan I, Shiglik N, Lushchyk ML, Platonova TY, Pashkevich VI, Kudelsky AV, Shimanskaya I, Danilova LI, Biko J, Reiners C (2016) Effect of nitrates in drinking water on the prevalence of thyroid cancer and other thyroid diseases: a literature review and post-Chernobyl research experience in Belarus. Cytol Genet 50(6):372–376. https://doi.org/10.3103/S0095452716060074
Gaikwad S, Gaikwad S, Meshram D, Wagh V, Kandekar A, Kadam A (2020) Geochemical mobility of ions in groundwater from the tropical western coast of Maharashtra, India: implication to groundwater quality. Environ Dev Sustain 22(3):2591–2624. https://doi.org/10.1007/s10668-019-00312-9
Gaury PK, Meena NK, Mahajan A (2018) Hydrochemistry and water quality of Rewalsar Lake of Lesser Himalaya, Himachal Pradesh, India. Environ Monit Assess 190(2):1–22
Gevera PK, Cave M, Dowling K, Gikuma-Njuru P, Mouri H (2020) Naturally occurring potentially harmful elements in groundwater in Makueni County, South-Eastern Kenya: effects on drinking water quality and agriculture. Geosciences 10(2):62. https://doi.org/10.3390/geosciences10020062
Ghasemi A, Zahediasl S (2012) Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab 10:486–489
Haji M, Karuppannan S, Qin D, Shube H, Kawo NS (2021) Potential human health risks due to groundwater fluoride contamination: a case study using multi-techniques approaches (GWQI, FPI, GIS, HHRA) in Bilate River Basin of Southern Main Ethiopian Rift, Ethiopia. Arch Environ Contam Toxicol 80:277–293. https://doi.org/10.1007/s00244-020-00802-2
Handa BK (1969) Description and classification of media for hydro-geochemical investigations. In: Symposium on ground water studies in arid and semiarid regions, Roorkee
Herojeet R, Rishi MS, Lata R, Sharma R (2016) Application of environmetrics statistical models and water quality index for groundwater quality characterization of alluvial aquifer of Nalagarh Valley, Himachal Pradesh, India. Sustain Water Resour Manag 2:39–53. https://doi.org/10.1007/s40899-015-0039-y
Holland HD (1978) The chemistry of the atmosphere and oceans. Wiley, New York, p 351
Jain CK, Sharma SK, Singh S (2021) Assessment of groundwater quality and determination of hydrochemical evolution of groundwater in Shillong, Meghalaya (India). SN Appl Sci 3:33. https://doi.org/10.1007/s42452-020-03993-4
Kadam A, Wagh V, Umrikar B, Sankhua R (2019) An implication of boron and fluoride contamination and its exposure risk in groundwater resources in semi-arid region. Environ Dev Sustain Western India. https://doi.org/10.1007/s10668-019-00527-w
Kelley WP (1940) Permissible composition and concentration of irrigation waters. Proc Am Soc Civil Eng 66:607–613
Kumar A, Singh CK (2020) Arsenic enrichment in groundwater and associated health risk in Bari doab region of Indus basin, Punjab. India Environ Poll 256:113324. https://doi.org/10.1016/j.envpol.2019.113324
Kumar SK, Rammohan V, Sahayam JD, Jeevanandam M (2009) Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environ Monit Assess 159:341. https://doi.org/10.1007/s10661-008-0633-7
Kumar SK, Logeshkumaran A, Magesh NS, Godson PS, Chandrasekar N (2015) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5:335–343. https://doi.org/10.1007/s13201-014-0196-4
Kumar R, Kumar R, Singh A, Singh S, Bhardwaj A, Kumari A, Sinha RK (2018) Hydro-geochemical analysis of meltwater draining from Bilare Banga glacier, Western Himalaya. Acta Geophysica 67:651–660. https://doi.org/10.1007/s11600-019-00262-w
Kumar R, Kumar R, Singh S, Singh A, Bhardwaj A, Chaudhary H (2019) Hydro-geochemical characteristics of glacial meltwater from Naradu Glacier catchment, Western Himalaya. Environ Earth Sci 78:683. https://doi.org/10.1007/s12665-019-8687-0
Kumar A, Roy SS, Singh CK (2020) Geochemistry and associated human health risk through potential harmful elements (PHEs) in groundwater of the Indus basin. India Environ Earth Sci 79(4):86. https://doi.org/10.1007/s12665-020-8818-7
Kumar R, Kumar R, Bhardwaj A, Singh A, Singh S, Kumari A, Sinha RK (2022a) Multivariate statistical analysis and geospatial approach for evaluating hydro-geochemical characteristics of meltwater from Shaune Garang Glacier, Himachal Pradesh, Indis. Acta Geophysica 1:1. https://doi.org/10.1007/s11600-022-00844-1
Kumar R, Kumar R, Singh S, Arif M, Kumar P, Kumari A (2022b) Chemometric approach to evaluate the chemical behavior of rainwater at high altitude in Shaune Garang catchment, Western Himalaya. Sci Rep 12:12774. https://doi.org/10.1038/s41598-022-15422-0
Lang YC, Liu CQ, Zhao ZQ, Li SL, Han GL (2006) Geochemistry of surface and ground water in Guiyang, China: water/rock interaction and pollution in a karst hydrological system. Appl Geochem 21(6):887–903. https://doi.org/10.1016/j.apgeochem.2006.03.005
Ledesma-Ruiz R, Pastén-Zapata E, Parra R, Harter T et al (2015) Investigation of the geochemical evolution of groundwater under agricultural land: a case study in northeastern Mexico. J Hydro 521:410–423. https://doi.org/10.1016/j.jhydrol.2014.12.026
Li P, Feng W, Xue C, Tian R, Wang S (2017) Spatiotemporal variability of contaminants in lake water and their risks to human health: a case study of the Shahu Lake tourist area, Northwest China. Expo Health 9:213–225. https://doi.org/10.1007/s12403-016-0237-3
Liu CW, Lin KH, Kuo YM (2003) Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci Total Environ 313:77–89. https://doi.org/10.1016/S0048-9697(02)006836
Liu R, Zheng X, Li M, Han L, Liu X, Zhang F, Hou X (2019) A three chamber bioelectrochemical system appropriate for in-situ remediation of nitrate-contaminated groundwater and its reaction mechanisms. Water Res 158:401–410
Liu L, Wu J, He S, Wang L (2021) Occurrence and distribution of groundwater fluoride and manganese in the Weining Plain (China) and their probabilistic health risk quantification. Expo Health. https://doi.org/10.1007/s12403-021-00434-4
Long J, Luo K (2020) Elements in surface and well water from the central North China Plain: enrichment patterns, origins, and health risk assessment. Environ Pollut 258:113725. https://doi.org/10.1016/j.envpol.2019.113725
Magesh NS, Chandrasekar N, Elango L (2018) Trace element concentrations in the groundwater of the Tamiraparani river basin, South India: insights from human health risk and multivariate statistical techniques. Chemosphere 185:468–479. https://doi.org/10.1016/jchemosphere.2017.07.044
Mahammad S, Islam A (2021) Evaluating the groundwater quality of Damodar Fan Delta (India) using fuzzy-AHP MCDM technique. Appl Water Sci 11(7):1–17. https://doi.org/10.1007/s13201-021-01408-2
Mahammad S, Islam A, Shit PK (2022) Geospatial assessment of groundwater quality using entropy-based irrigation water quality index and heavy metal pollution indices. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-20665-5
Maurya J, Pradhan SN, Seema GAK (2020) Evaluation of ground water quality and health risk assessment due to nitrate and fluoride in the Middle Indo-Gangetic plains of India. Hum Ecol Risk Assess Int J 11:1–7. https://doi.org/10.1080/10807039.2020.1844559
Mbissik A, Elghali A, Ouabid M, Raji O, Bodinier J-L, El Messbahi H (2021) Alkali-hydrothermal treatment of K-rich igneous rocks for their direct use as potassic fertilizers. Minerals 11(2):140. https://doi.org/10.3390/min11020140
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287(5):401–428
Meyers D (1975) Mortality and water hardness. Lancet 1:398–399. https://doi.org/10.1016/S0140-6736(75)91318-5
Mthembu PP, Elumalai V, Brindha K, Li P (2020) Hydrogeochemical processes and trace metal contamination in groundwater: impact on human health in the Maputaland Coastal Aquifer, South Africa. Expo Health. https://doi.org/10.1007/s12403-020-00369-2
Noori R, Berndtsson R, Hosseinzadeh M, Adamowski JN (2019) Abyaneh, M R (2019)A critical review on the application of the National Sanitation Foundation Water Quality Index. Environ Pollut 244:575–587. https://doi.org/10.1016/j.envpol.2018.10.076
Paliwal KV (1967) Effect of gypsum application on the quality of irrigation waters. Madras Agric J 59:646–647
Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, Sultana R, Sultana R, Islam T, Levy D, Mey JL, van Geen A, Khan KM, Kline J, Ahsan H, Graziano JH (2011) Arsenic exposure and motor function among children in Bangladesh. Environ Health Perspect 119:1665–1670. https://doi.org/10.1289/ehp.1103548
Patel MP, Bharat G, Akash P, Pankaj P, Beena P (2020) Climatic and anthropogenic impact on groundwater quality of agriculture dominated areas of southern and central Gujarat, India. Groundw Sustain Dev 10(3):100306. https://doi.org/10.1016/j.gsd.2019.100306
Piper AM (1944) A graphical interpretation of water analysis. EOS Trans Am Geophys Union 25:914–928. https://doi.org/10.1029/TR025i006p00914
Prasad G, Reshma AS, Ramesh MV (2021) Assessment of drinking water quality on public health at Alappuzha district, southern Kerala, India. Mat Today: Proc 46:3030–3036. https://doi.org/10.1016/j.matpr.2021.01.302
Raghunath HM (1987) Ground water, 2nd edn. Wiley Eastern Ltd., New Delhi, pp 344–369
Rahman SM, Kippler M, Tofail F, Bölte S, Derakhshani Hamadani J, Vahter M (2017) Manganese in drinking water and cognitive abilities and behavior at 10 years of age: a prospective cohort study. Environ Health Perspect 125(5):057003. https://doi.org/10.1289/EHP631
Rahmanian N, Ali SHB, Homayoonfard N, Ali NJ, Rehan M, Sadef Y, Nizami AS (2015) Analysis of physiochemical parameters to evaluate the drinking water quality in the state of Perak, Malaysia. J Chem 2015: Article ID 716125. https://doi.org/10.1155/2015/716125
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ Geol 46:47–61. https://doi.org/10.1007/s00254-004-1012-5
Rao NS, Rao PS, Reddy GV, Nagamani M, Vidyasagar G, Satyanarayana NLVV (2012) Chemical characteristics of groundwater and assessment of groundwater quality in Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environ Monit Assess 184(8):5189–5214. https://doi.org/10.1007/s10661-011-2333-y
Ravindra K, Thind PS, Mor S, Singh T, Mor S (2019) Evaluation of groundwater contamination in Chandigarh: source identification and health risk assessment. Environ Pollut 255:113062. https://doi.org/10.1016/j.envpol.2019.113062
Richards LA (1954) Diagnosis and improvement of saline alkali soils, agriculture handbook 60. US Department of Agriculture, Washington DC, p 160
Rina K, Datta PS, Singh CK (2012) Mukherjee S (2012) Characterization and evaluation of processes governing the groundwater quality in parts of the Sabarmati basin, Gujarat using hydrochemistry integrated with GIS. Hydrol Process 26:1538–1551. https://doi.org/10.1002/hyp.8284
Sandhu C, Grischek T, Schoenheinz D, Prasad T, Thakur AK (2011) Evaluation of bank filtration for drinking water supply in Patna by the Ganga River, India. In: Shamrukh M (ed) Riverbank filtration for water security in desert countries. NATO Science for Peace and Security Series C: Environmental Security. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0026-0_12
Sarafraz M, Ali S, Sadani M, Heidarinejad Z, Bay A, Fakhri Y, Mousavi KA (2020) A global systematic, review-meta analysis and ecological risk assessment of ciprofloxacin in river water. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1791330
Sasikaran S, Sritharan K, Balakumar S, Arasaratnam V (2012) Physical, chemical and microbial analysis of bottled drinking water. Ceylon Med J 57(3):111–116. https://doi.org/10.4038/cmj.v57i3.4149
Satyanarayana E, Dhakate R, Laxman Kumar D, Ravindar P, Muralidhar M (2017) Hydrochemical characteristics of groundwater quality with special reference to fluoride concentration in parts of Mulugu-Venkatapur Mandals, Warangal district, Telangana. J Geol Soc India 89:247–258. https://doi.org/10.1007/s12594-017-0597-8
Selvam S, Ravindran AA, Venkatramanan S, Singaraja C (2017) Assessment of heavy metal and bacterial pollution in coastal aquifers from SIPCOT industrial zones, Gulf of Mannar, South Coast of Tamil Nadu, India. Appl Water Sci 7(2):897–913. https://doi.org/10.1007/s13201-015-0301-3
Shakeri A, Hosseini H, Mehr MR, Barmaki MD (2022) Groundwater quality evaluation using water quality index (WQI) and human health risk (HHR) assessment in Herat aquifer, west Afghanistan. Hum Ecol Risk Assess Int J 28(7):711–733. https://doi.org/10.1080/10807039.2022.2075316
Singh UK, Kumar B (2017) Pathways of heavy metals contamination and associated human health risk in Ajay River Basin, India. Chemosphere 174:183–199. https://doi.org/10.1016/j.chemosphere.2017.01.103
Singh CK, Kumar A, Shashtri S, Kumar A, Kumar P, Mallick J (2017) Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. J Geochem Explor 175:59–71
Snousy MG, Wu J, Su F, Abdelhalim A, Ismail E (2021) Groundwater quality and its regulating geochemical processes in Assiut Province, Egypt. Expo Health 14:305–323. https://doi.org/10.1007/s12403-021-00445-1
Srinivasamoorthy K, Gopinath M, Chidambaram S, Vasanthavigar M, Sarma VS (2014) Hydrochemical characterization and quality appraisal of groundwater from Pungar sub basin, Tamilnadu, India. J King Saud Univ-Sci 26(1):37–52. https://doi.org/10.1016/j.jksus.2013.08.001
Srivastava SK, Ramanathan AL (2008) Geochemical assessment of groundwater quality in vicinity of Bhalswa landfill, Delhi, India, using graphical and multivariate statistical methods. Environ Geol 53:1509–1528. https://doi.org/10.1007/s00254-007-0762-2
Su H, Wang J, Liu J (2019) Geochemical factors controlling the occurrence of high-fluoride groundwater in the western region of the Ordos basin, north-western China. Environ Pollut 252:1154–1162. https://doi.org/10.1016/j.envpol.2019.06.046
Subba Rao N (2018) Groundwater quality from a part of Prakasam district, Andhra Pradesh, India. Appl Water Sci 8:1–18
Subba Rao N, Deepali M, Dinakar A, Chandana I, Sunitha B, Ravindra B, Balaji T (2017) Geochemical characteristics and controlling factors of chemical composition of groundwater in a part of Guntur district, Andhra Pradesh, India. Environ Earth Sci 76:747. https://doi.org/10.1007/s12665-017-7093-8
Subba Rao N, Sunitha B, Adimalla N, Chaudhary M (2020) Quality criteria for groundwater use from a rural part of Wanaparthy District, Telangana State, India, through ionic spatial distribution (ISD), entropy water quality index (EWQI) and principal component analysis (PCA). Environ Geochem Health 42:579–599. https://doi.org/10.1007/s10653-019-00393-5
Subba Rao N, Chaudhary Maya (2019) Hydrogeochemical processes regulating the spatial distribution of groundwater contamination, using pollution index of groundwater (PIG and hierarchical cluster analysis (HCA)): a case study. GSD 9. https://doi.org/10.1016/j.gsd.2019.100238
SubbaRao N, Sunitha B, Rambabu R, Rao PVN, Rao PS, Spandana BD, Sravanthi M, Marghade D (2018) Quality and degree of pollution of groundwater, using PIG from a rural part of Telangana State, India. Appl Water Sci 8:227. https://doi.org/10.1007/s13201.018.0864.x
Sukumaran D, Saha R, Saxena RC (2015) Ground water quality index of Patna, the capital city of Bihar, India. Am J Water Resour 3(1):17–21. http://pubs.sciepub.com/ajwr/3/1/3
Sulin VA (1946) Oil water in the system of natural groundwater. Gostopichezdat, Moscow USSR 30:37–45
Thomas KS, Sach TH (2000) A multicentre randomized controlled trial of ion-exchange water softeners for the treatment of eczema in children: protocol for the Softened Water Eczema Trial (SWET) (ISRCTN: 71423189). Br J Dermat 159(3):561–566. https://doi.org/10.1111/j.1365-2133.2008.08704.x
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, New York, p 315
Uddin MG, Nash S, Olbert AI (2021) A review of water quality index models and their use for assessing surface water quality. Ecol Indic 122:107218. https://doi.org/10.1016/j.ecolind.2020.107218
USGS (2022) Maps showing geology, oil and gas fields and geologic provinces of South Asia. https://pubs.usgs.gov/of/1997/ofr-97-470/OF97-470C/ofr97470C.pdf
USSL (1954) Diagnosis and improvement of saline and alkali soils. USDA Handbook 60:147
Ustaoğlu F, Tepe Y (2019) Water quality and sediment contamination assessment of Pazarsuyu stream, Turkey using multivariate statistical methods and pollution indicators. Int Soil Water Cons Res 7:47–56. https://doi.org/10.1016/j.iswcr2018.09.001
Van Geen A, Farooqi A, Kumar A, Khattak JA, Mushtaq N, Hussain I, Ellis T, Singh CK (2019) Field testing of over 30,000 wells for arsenic across 400 villages of the Punjab plains of Pakistan and India: implications for prioritizing mitigation. Sci Total Environ 654:1358–1363. https://doi.org/10.1016/j.scitotenv.2018.11.201
Vijay Shankar PS, Kulkarni H, Krishnan S (2011) India’s groundwater challenge and the way forward. Econ Pol Wkly 56(2):37–45
Wagh VM, Panaskar DB, Mukate SV, Gaikwad SK, Muley AA, Varade AM (2018) Health risk assessment of heavy metal contamination in groundwater of Kadava River Basin, Nashik, India. Model Earth Syst Environ 4:969–980. https://doi.org/10.1007/s40808-018-0496-z
Wagh V, Mukate S, Muley A, Kadam A, Panaskar D, Varade A (2020) Study of groundwater contamination and drinking suitability in basaltic terrain of Maharashtra, India through PIG and multivariate statistical techniques. J Water Supply Res Technol AQUA 69(4):398–414. https://doi.org/10.2166/aqua.2020.108
WHO (2004) Guidelines for drinking-water quality volume 1: recommendations, 3rd edn. WHO, Geneva
Wilcox LV (1955) Classification and use of irrigation waters. US Department of Agriculture, Washington
World Health Organization (WHO) (2009) Guidelines for drinking water quality. World Health Organization, Geneva
World Health Organization (WHO) (2011) Guideline for drinking water quality (4th ed.). Geneva: World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/44584/9789241548151_eng.pdf?sequence=1
Yasmin G, Islam D, Islam MT, Shariot-Ullah M, Adham AKM (2019) Evaluation of groundwater quality for irrigation and drinking purposes in Barishal district of Bangladesh. Fundam Appl Agric 4(1):632–641
Zafar MM, Sulaiman MA, Prabhakar R, Kumari A (2022) Evaluation of the suitability of groundwater for irrigational purposes using irrigation water quality indices and geographical information systems (GIS) at Patna (Bihar), India. Int J Energy Water Resour:1–14. https://doi.org/10.1007/s42108-022-00193-1
Zaghlool E (2020) Geochemical modeling and statistical analysis for groundwater evolution assessment in Wadi Qasab, Sohag, Eastern Desert, Egypt. J Geosci Environ Protect 8:33–61. https://doi.org/10.4236/gep.2020.89003
Zhang Q, Xu P, Qian H (2019) Assessment of groundwater quality and human health risk (HHR) evaluation of nitrate in the Central Western Guanzhong Basin, China. Int J Environ Res Public Health 16(21):4246. https://doi.org/10.3390/ijerph16214246
Zhang Q, Xu P, Qian H (2020) Groundwater quality assessment using improved water quality index (WQI) and human health risk (HHR) evaluation in a semi-arid region of northwest China. Expo Health 12:487–500. https://doi.org/10.1007/s12403-020-00345-w
Acknowledgements
The authors would like to thank all the members who helped with water sampling and chemical analysis of groundwater samples. The laboratory facility extended by the Environmental Biology Laboratory, Department of Zoology, Patna University, is acknowledged. We acknowledge the support of the DST FIST-supported GIS Laboratory, Department of Environmental Science, Central University of Rajasthan, where the computational work and the graphics have been performed. The unanimous reviewer’s comments have helped improve the paper’s quality, and the authors express their gratitude for the constructive suggestions.
Author information
Authors and Affiliations
Contributions
Ramesh Kumar, Anupma Kumari, and Rajesh Kumar contributed to the concept and design of manuscripts. Mohammed Aasif Sulaiman, Mohammad Masroor Zafar, Ravi Prabhakar, Atar Singh, and Prity Singh Pippal were involved in water sampling and data analysis. Anupma Kumari and Rajesh Kumar supervised the research and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Xianliang Yi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, R., Kumari, A., Kumar, R. et al. Assessing the geochemical processes controlling groundwater quality and their possible effect on human health in Patna, Bihar. Environ Sci Pollut Res 30, 107138–107157 (2023). https://doi.org/10.1007/s11356-023-26203-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26203-1