Abstract
The present study describes the source of chemical ions and quality of groundwater in parts of Srinagar district, Kashmir valley, India. About 140 water samples from bore wells, dug wells, and springs were collected during December, 2004, and June–July, 2005. The physicochemical parameters analyzed include pH, EC, TDS, total hardness, Ca2+, Mg2+, Na+, K+, Cl−, SO42−, HCO3−, and NO3−. Statistical analyses, i.e., descriptive statistics, correlation matrix, and factor analysis (FA), were carried out to understand the specific processes and sources that could control the chemistry of groundwater of the area. Overall, a high positive correlation among the chemical variables indicated a dominant influence of carbonate lithology as compared to the least role of silicate lithology on the chemical nature of the water. However, positive correlations of K+ (0.57, 0.60), Na+ (0.49, 0.42), SO42− (0.41, 0.55), and Cl− (0.41, 0.51) with NO3− manifest the possible anthropogenic inputs that have a modifying influence to the chemistry of groundwater. The FA separates the variables into three factors, which together account for 75.68% and 82.75% of the total variance in pre- and post-melting seasons, respectively. The F1 is considered as lithologically controlled factor, F2 as both lithologically and non-lithologically controlled factor, whereas F3 as non-lithologically controlled factor that is anthropogenic factor. The factor 3 includes K+, NO3−, and SO42− indicating possible input from anthropogenic sources via agricultural farmlands, domestic wastes, leakage from sewer drains, and polluted surface water bodies and vegetable gardens. The concentrations of major ions of groundwater are well within the maximum permissible limits of WHO (2006) and ISI (2012) for drinking purpose. Nevertheless, the increasing tendency and enrichment of water with respect to K+, NO3−, and Cl− at some locations is an alarming indication of contamination of shallow aquifers. The low sodium and low-slightly high salinity hazard along with safe-moderate category of water in terms of residual sodium carbonate (RSC) document mostly the suitability of groundwater for agricultural purposes. Relatively an increase in salinity at some locations is a matter of concern.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Groundwater is considered the largest resource of freshwater after the glaciers and ice sheets of the polar regions of the world. It plays a significant role in shaping the socioeconomic conditions of the human life. In Kashmir valley, the ground has a huge potential to provide major resource of freshwater supply that can be used for all domestic, horticultural, agricultural, and hydropower generation purposes significantly. However, the value of groundwater is not determined by its widespread occurrence and availability, but its value depends on its consistent and good quality (UNESCO 2000; Jeelani et al. 2014; Mir and Jeelani 2015). The suitability of groundwater for different uses depends upon the inputs of chemical species from the atmosphere, from soil and rock weathering, as well as from anthropogenic activities (Jeelani et al. 2014; Mir and Jeelani 2015). Nearly all groundwater originates as rain, snow melt, or surface water that infiltrates through the soil or geological material and reaches the phreatic zone. Rain and snow are naturally slightly acidic due to chemical reactions with CO2 in the atmosphere. However, it is in the soil zone that the water interacts with the inorganic and organic solids while infiltrating through it. The inorganic material may be fairly fresh rock material as found on the steeper slopes of a mountain, or more likely, it is weathered material composed of fairly reactive secondary minerals that may equilibrate with the infiltrating water, thus imparting its chemistry (Deutsch 1997; Jeelani et al. 2014). The groundwater’s chemical composition is determined by its sources and sinks along its flow path (Deutsch 1997). Moreover, the relative concentration of dissolved constituents is also a function of its availability from the solid phases as well as its solubility of secondary minerals produced due to weathering processes. In general, the groundwater during its flow tends to acquire a similar chemical composition as that of the seawater. This tendency is generally associated with a significant change from a dominant bicarbonate (HCO3−) type or anion species to a chloride (Cl−) type (Chebotarev 1955). During recent decades, the anthropogenic activities have significantly affected the groundwater resources and are therefore considered a major hazard in the evolution of chemical quality of groundwater. The increased and rapid urbanization and conversion of agricultural lands into the urbanized settlements have resulted in the dreadful conditions of water quality (Mir et al. 2016). Rapid and increased use of fertilizers, pesticides, huge production of house hold effluents or effluents discharged from industries, and other municipal sewage are the main contaminants of groundwater (Mir and Gani 2019).
Nonetheless, any imbalance in the chemical quality of groundwater is responsible for many diseases in humans. For example, the lower values of pH can cause gastrointestinal disorders, hence limiting its domestic uses. Similarly, the groundwater with higher TDS values cannot be used for irrigation and drinking purposes (Fetters 1990; Freeze and Cherry 1979; Jehangir et al. 2011). The presence of F higher than a threshold value of 1.5 ppm results in fluorosis (Subba and John 2003), and the presence of nitrate, nitrite, and phosphate above certain permissible limits makes water unsuitable for drinking purpose (Lee et al. 2003; Rajmohan and Elango 2005). Furthermore, the study of major ions explains the source of ions in groundwater and gives an insight into the level of contamination that is either from natural or anthropogenic sources (Jalali 2005; Subba 2006; Jeelani et al. 2014). For this purpose, several indices, ratios, and diagrams are used. For example, the Wilcox diagram and US Salinity Laboratory diagrams are used to find out the nature of water and to check out whether the water is useful for irrigation (Subramani et al. 2005; Singh et al. 2005; Jeelani et al. 2014; Mir and Jeelani 2015). Thus, using the chemical budget of major ions, the quality of water can be easily assessed (Mir and Jeelani 2015; Mir et al. 2016).
Thus keeping in view the above discussion, the present study has been carried out in parts of Srinagar district, Kashmir valley, northwest Himalaya, India. The main objectives of the study include to determine sources of the major ions of the groundwater of the area using statistical analysis and to determine the suitability of the groundwater for domestic as well as agricultural purposes using the standard indices and other international guidelines.
3.2 Groundwater Pollution in Kashmir
There are a number of studies which reported a general degradation and deteriorating conditions of water resources of the Kashmir valley (Mushtaq and Pandey 2014; Showqi et al. 2014, Rashid et al. 2017; Rashid and Romshoo 2013). There are several studies on the chemical quality of the River Jhelum and its tributaries also which suggest that the water of the river is chemically good but the nature is trending toward degrading quality (Mir et al. 2016; Mir and Jeelani 2015; Mir and Gani 2019; Rather et al. 2016). However, there are a least number of studies on the groundwater resources of the Kashmir valley and its quality status. Some sporadic studies have been carried out previously on the groundwater resources of the Kashmir valley. For instance, Jehangir et al. (2011) studied groundwater quality for irrigation in southern parts of the Kashmir valley. They suggested that majority of groundwater in the aquifers of this area is of marginal to harmful quality for irrigation purposes. Gulzar et al. (2017) studied the groundwater quality of Anantnag district, Kashmir valley, and suggested that although the water is suitable for domestic and irrigational purposes, there are certain locations which indicated its unsuitable nature. Bhat et al. (2013) studied the water quality of the Srinagar district, Jammu and Kashmir, and concluded on the basis of physicochemical properties that the groundwater of the area is suitable for domestic as well as agricultural purposes with few exceptions. Sarah et al. (2011) studied the water quality of a groundwater-fed Manasbal Lake of Kashmir Himalayas and concluded that the water is safe for domestic uses based on chemical quality. Jeelani et al. (2014) carried out a detailed hydrochemical assessment of groundwater throughout the Kashmir valley and suggested that the groundwater of shallow as well as deep aquifers is good for drinking, livestock, and irrigation purposes with the exception of one site located in Sopore areas of the valley.
Central Groundwater Board has reported a groundwater potential of the valley of 2400 million m3/year which is being currently exploited at 2.4 million m3/year (Gulzar et al. 2017). This groundwater resource poses a huge potential to supply water for both urban and rural areas in the valley. However, keeping in view the higher demand for water in response to rapid population growth and industrialization, high tourism pressures and continuous landform degradation (Mushtaq and Pandey 2014; Zaz and Romshoo 2012), uncontrolled use of fertilizers and pesticides (Mir et al. 2016), spontaneous urbanization (Sajjad and Iqbal 2012), frequent expansion of the settlements into the agricultural lands vis-à-vis shifting the land use land cover pattern, and overall ever-rising anthropogenic activities (Mir et al. 2016, Mir and Jeelani 2015) in the valley, the health of the groundwaters is highly jeopardized, and its biological as well as physicochemical properties may also be seriously affected.
3.3 Location of the Study Area
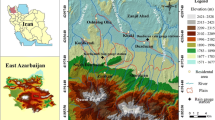
The study area covers a part of the Srinagar district of the Kashmir valley, northwest Himalaya, India. The area comprising both urban and rural areas lies between 34° 3′–34° 20′ N latitudes and 74° 40′–75o15′ E longitudes and covers an area of 2228 km2 (Fig. 3.1). The study area is connected to the other parts of the valley by all-weather motorable roads and to the rest of India by National Highway No.1A (NH 1A) and the air route. The study area is bounded in the north by Sonawari (Baramulla district), in the northeast and east by district Kargil, in the south by district Pulwama, and in the southwest and west by Budgam and Baramulla districts, respectively. The total population of the district is ~12,02,447 which comprises a rural population of ~17,313,281 and urban population of ~121,9516 (Census 2001). In the study area, hand pumps are being widely used to pump out groundwater for domestic and other related purposes. Most of the wells are shallow, hence liable to contamination from the sources like sewage drains, septic tanks, polluted surface water bodies, and agricultural farmlands and/or floating gardens of the Dal Lake where huge pesticides are being used.
3.4 Climate of the Area
The study area has a distinctive character with its own climatic peculiarities. The area witnesses a continental climate system, characterized with marked seasonality linked with the mechanism of weather in the Indian sub-continent (Hussain 2005). In general, it has a long period of winter and spring seasons. Based on general characteristics of weather, i.e., precipitation and temperature, four seasons have been described for the area as (a) winter season (November–February), (b) spring season (March–mid-May), (c) summer season (mid-May–mid-September), and (d) autumn season (mid-September–mid-November). January is the coldest month whereas the month of July is generally the warmest. Normally, the temperature varies between −5 and >30 °C in the area. The precipitation is highly variable round the year with more rains occurring during summer season and snowfall during winter season. Overall, the precipitation is higher during winter season with a total annual precipitation of ~1100 mm. March receives maximum rainfall and October the least and September–November is usually a dry season (Mir and Jeelani 2015).
3.5 Geology of Study Area
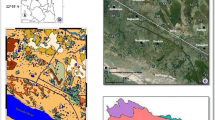
The main lithological units exposed in the area include the Agglomeratic Slate, Panjal volcanic, Gangmopteris beds, Zewan Formation, Triassic Formation, Karewa Formation, and Alluvial deposits (Fig. 3.2). The Agglomeratic Slate is comprised of pyroclastic slates and conglomerates and underlies the lower part of the Panjal volcanics that are generally basaltic in composition with grayish to dark green in color. The Panjal volcanics are called the Panjal Traps generally. Thin Gangmopteris beds of light-colored shale conformably overlie the Panjal volcanics. The Panjal volcanics are overlain by Gangmopteris beds which are followed by the Zewan Formation. The Zewan Formation is followed by the homogeneous compact, light gray-colored limestone of Triassic Formation with shale in the lower parts, shale and sandstone intercalated with limestone in the middle parts, and massive limestone in the upper part. The Plio-Pleistocene deposits called as Karewa sediments overlie the Triassic and pre-Triassic rocks, flanking the surrounding mountain precipices of the study area. The Lower Karewas comprise blue, gray, and buff silts and sands, conglomerates, alternating laminated yellow marls, and silts and sands. In the low-lying areas, adjoining the Jhelum River and its local streams, the recent alluvium comprised of the finely compacted detrital sediments of loam, clay, silt, and sand is present. The geological map is shown in Fig. 3.2.
3.6 Materials and Methods
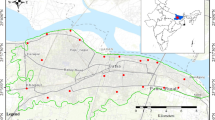
In this study, 140 groundwater samples were collected seasonally, 70 each in pre-melting (December, 2004) and post-melting (June/July, 2005) seasons between 10.00 h and 15.00 h from different sampling stations in one liter polyethylene bottles (Fig. 3.3). Samples were collected from bore wells, springs, and dug wells that represent the main sources of groundwater in the area. In order to avoid the stagnant water and to collect the rust-free and running water samples from bore wells, the pumping was carried out for 5 to 10 min, while in the case of springs and dug wells to avoid floating debris, the samples were collected at depths greater than 30 cm below the water surface. Prior to sample collection, the containers were also washed with conc. HNO3 followed by complete removal with distilled water. The sample bottles were also rinsed by the sample water that was to be sampled. Water temperature, pH, and electrical conductivity (EC) measurements were carried out in situ at the sampling sites, whereas further chemical analysis was carried out at the Center of Research for Development (CORD) and Department of Geology and Geophysics, University of Kashmir, Srinagar.
The chemical analysis was carried out following the standard methods of Trivedy and Goel (1984) and APHA (2001). Water temperature, pH, and EC were determined by the potable laboratory thermometer, digital pH meter, and conductivity meter in situ. The Ca2+ and Mg2+ ions were determined by EDTA titration wherein the Eriochrome Black T and murexide were used as indicators. The Cl− ion was determined by titration against AgNO3 (0.02 N) using potassium chromate (5%) as indicator, whereas the HCO3− ion was determined by again titration method against HCl (0.01 N) using methyl orange as an indicator. Na+ and K+ ions were estimated by Flame Emission Photometry. The Spectrophotometric method was used for the determination of SO42− and NO3− ions. It is important to mention that the results were obtained in mg/L that was converted into milliequivalents during the data processing and interpretations. Furthermore, a standard computer program Minitab was used for statistical data analysis such as for the determination of descriptive statistics, correlation matrix, and principal component or factor analysis (FA). Prior to conducting FA, the raw data was normalized as the first step in factor coefficient matrix. By examining the factor loadings and their Eigen values, the variables belonging to a specific process were identified and explained.
3.7 Findings
3.7.1 Geochemistry of Groundwater
The physicochemical parameters analyzed in this study include pH, EC, total dissolved solids (TDS), total hardness, Ca2+, Mg2+, Na+, K+, Cl−, SO42−, HCO3−, and NO3−. The statistical overview of the results is presented in Table 3.1.
The temperature of groundwater ranged from 11° C to 15° C with a mean of 13.06 °C. The TDS of groundwater samples ranged from 107 to 710 mg/L with a mean value of 382.21 mg/L, being low in springs (107 mg/L–429 mg/L) and moderate in dug wells (141–704 mg/L) and high in tube wells (136–710 mg/L). The EC of groundwater varied between 168 μS/cm and 1110 μS/cm with a mean of 587.65 μS/cm, being lower in springs (168–670 μS/cm) and higher in dug wells (220–1100 μS/cm) and tube wells (212– 1110 μS/cm). The pH of the groundwater samples was moderately alkaline and ranged between 7.10 and 8.30 with an average of 7.62. pH of the springs ranged between 7.1 and 8.07; that of dug wells ranged between 7.16 and 8.03, while that of the tube wells ranged between 7.10 and 8.30. The groundwater hardness ranged between 86 and 410 mg/L with an average of 218 mg/L. The lower values have been found in spring water with the exception of location no. 29 (340 mg/L) and high in tube wells with the exception of well no. 4 (94 mg/L).
The Ca2+ concentration in groundwater varied from 20 mg/L to 127 mg/L with an average of 73.77 mg/L, being high in tube wells (20–127 mg/L) with the exception of location no. 4 (20 mg/L); moderate in springs (36–105 mg/L) with the exception of location no. 29 (105 mg/L); and relatively low in dug wells (27–100 mg/L) with the exception of well no. 50 and 58 (100 mg/L). The Mg2+ concentration of groundwater ranged from 5 mg/L to 35 mg/L with an average of 17.34 mg/L. Mg2+ did not fluctuate much, being low in springs (5 mg/l–25 mg/L) with the exception of location no. 53 (25 mg/L), intermediate in dug wells (8 mg/L–28 mg/L), and relatively high in tube wells (6–35 mg/L) with the exception of well no. 5 (7 mg/L) and 6 and 7 (6 mg/L). Na+ concentration in groundwater varied from 3 mg/L to 80 mg/L with an average value of 28.28 mg/L, being high in tube wells (6–80 mg/L) with the exception of location no. 14 (9 mg/L), 15 (10 mg/L), 19 and 20 (10 mg/L), 27 (9 mg/L), 30 (6 mg/L), 31 (8 mg/L), 32 (10 mg/L), and 34 (8 mg/L), moderate in dug wells (7–70 mg/L) with the exception of location no. 50 (70 mg/L), and low in springs (3–42 mg/L) with the exception of location no. 52 (42 mg/L). The K+ concentration found in groundwater varied from 0 to 10 mg/L with the mean of 3.67 mg/L. K+ exhibited rather uniformity in variation, ranging from 0.5 mg/L to 9 mg/L in springs being relatively higher at location no. 39 (9 mg/L), 2.2 mg/L to 8.0 mg/L in dug wells being higher at location no. 10 and 50 (8 mg/L), and 0 to 10 mg/L in tube wells being higher at location no. 12 (10 mg/L). However, the mean concentration is relatively high in dug wells (5.94 mg/L); moderate in tube wells (3.58 mg/L); and low in springs (3.07 mg/L).
Among the anions, HCO3− was the dominant anion and represented the total alkalinity of the water in the study area. The phenolphthalein alkalinity was found to be zero. HCO3− in groundwater varied between 95 and 455 mg/L with an average value of 2.99.87 mg/L, being high in dug wells (185 to 455 mg/L) with the exception of location no. 10 (185 mg/L); moderate in tube wells (95–450 mg/L) with the exception of location no. 6 and 7 (95–105 mg/L), respectively; and low in springs (120–350 mg/L) with the exception of location no. 29 (350 mg/L) and 37 (345 mg/L). The Cl− content in groundwater varied from 7 to 120 mg/L with a mean of 39.7 mg/L. The higher values have been found in tube wells with the exception of well no. 14, 15, and 17 (7 mg/L) and 13 (9 mg/L), moderate in dug wells with anomalous values at location no. 50 (93 mg/l) and 58 (60 mg/L), and low in springs with the exception of location no. 51 (60 mg/L), 52 (50 mg/L), and 53(55 mg/L). SO42− in groundwater has been found to be at lower levels ranging from 1.5 mg/L to 6.6 mg/L with a mean value of 3.15 mg/L. No exceptional concentration has been found in the study area. The NO3− levels found in groundwater varied from 0.4 mg/L to 8.5 mg/L with a mean concentration of 2.70 mg/L. The lower values have been found in spring waters (0.9–8.5 mg/L) with a relatively high values at location no. 29 (4 mg/L) and 39 (3.9 mg/L); high concentration in waters of dug wells (0.9 to 8.5 mg/L) with the exception of well 18(0.9 mg/L); and moderate values of waters in tube wells (0.4 mg/L–6.8 mg/L) with relatively high values at location no. 12 (6.00 mg/L), 47 (6.80 mg/L), 56 and 57 (4.0 mg/L), 64 (4.10 mg/L), and 69 (5.0 mg/L).
3.8 Analyzing the Source of Ions Using Statistical Analysis
3.8.1 Correlation Matrix of Hydrogeochemical Data
The statistical correlation matrix developed for the geochemical data of groundwater for pre-melting and post-melting seasons is given in Table 3.2. The correlation shows high positive and significant relation between Ca2+ and HCO3− (0.86, 0.74), Ca2+ and hardness (0.94, 0.85), and HCO3− and hardness (0.92, 0.94) suggesting the predominant source of these ions be carbonate lithology mainly coming through dissolution of calcite, and a relatively lower positive correlation between Mg2+ and HCO3− (0.62, 0.73) and Mg2+ and hardness (0.62, 0.69) indicating some contribution coming through dissolution of dolomite as well. Besides, a positive correlation between Na+ and HCO3− (0.44, 0.73), Mg2+ and Na+ (0.31, 0.78), and Mg2+ and Cl− (0.43, 0.86) though relatively low in pre-melting season indicates the dissolution of silicates (primary and/or secondary silicates). The possible minerals furnishing these ions could be the pyroxenes, amphiboles (containing ferromagnesian minerals), and/or their altered products. Moreover, a positive and significant correlation between Na+ and Cl− (0.74, 0.87) cannot be related solely to the dissolution of soil salts; a significant contribution may be furnished from anthropogenic sources in the form of domestic wastes, polluted surface water bodies, and/or evaporation of waterlogged bodies, thereby increasing the salinity of groundwater. pH is moderately correlated with HCO3− (0.38, 0.51), Mg2+ (0.36, 0.37), Ca2+ (0.34, 0.36), Cl− (0.35, 0.38), and Na+ (0.15, 0.30), while K+ and SO42− show negative to very low correlation with pH. Ca2+ and K+ show negative correlation in both the seasons.
The positive correlations of K+ (0.57, 0.60), Na+ (0.49, 0.42), SO42− (0.41, 0.55), and Cl− (0.41, 0.51) with NO3− manifest the possible anthropogenic inputs from agricultural activities, domestic wastes, and seepage from polluted surface water bodies. The high positive correlation among the variables thus indicates the influence of lithology of the area particularly carbonate lithology and silicate lithology . However, a modifying influence to the chemistry of groundwater is also imparted through human influence.
3.8.2 Factor Analysis of Hydrogeochemical Data
The factor analysis of hydrogeochemical data explains the observed relations in simpler terms that are expressed as a new set of variants called factors. FA is used as a numerical method of discovering variables that are more important than others that represent parameter variation and identify the hydrochemical processes (Bakac 2000; Mir and Gani 2019). The results of the FA are given in Table 3.3. In order to have a better interpretation and understanding and to determine the mechanisms, processes, and sources that control the chemistry of the groundwater of the area, FA has been carried out in this study.
The FA performed on 12 different parameters indicated the 3 different trends in the present area. The FA of the groundwater samples explained that 75.68% of the total variability is accounted by first three factors with Eigen values greater than 1. Factor 1 explained 50.99% of total variance followed by 16.77% and 7.91% by the factors 2 and 3, respectively. Factor 1 includes EC, TDS, Ca2+, Mg2+, Cl−, HCO3−, and hardness with very high factor loadings of Ca2+, HCO3−, hardness, EC, and TDS and less pronounced values of Mg2+ and Cl− (also obvious from their moderate correlation with Ca2+). The possible source of Mg2+ and Cl− ions could be the carbonate minerals, pyroxenes, and/or amphiboles (present in the carbonate and silicate lithology in the catchment areas). Factor 1 therefore seems to be related to the dissolution of carbonate minerals/lithology dominantly calcite as indicated by the high factor loadings and strong positive correlation of Ca2+, HCO3−, and hardness. Factor 1, therefore, mainly measured the hardness, alkalinity, and dissolution of alkaline earths. Therefore, this factor seems to be lithologically controlled.
Factor 2 includes very high loading of Na+ and relatively less pronounced loading of Cl−. This factor indicated a possible dissolution of secondary silicates and soil salts, leading to the enrichment of Na+ and Cl− in finer detrital sediments comprising clay/silt/sandy-clay/sandy-silt. The fine-grade sediments provide a subsurface hydrogeological environment that extends residence time of groundwater due to low hydraulic conductivity and thereby promotes large residence time and contact surface, hence greater soil-water interaction. High positive correlation between Na+ and Cl− also indicated this phenomenon. Moreover, moderate loadings of Mg2+, K+, and NO3− on factor 2 (F2) and the moderate correlation between Na+ and NO3−, Cl− and NO3−, Na+ and K+, and Na+ and Mg2+ indicated some contribution from the possible anthropogenic sources (domestic wastes, waterlogged bodies, leaching from sewage drains, and/or agricultural inputs) to factor loading 2 (F2) as well. Factor 2, therefore, seems both lithologically and non-lithologically controlled.
Factor 3 includes SO42−, NO3−, and K+ with feeble loadings of Na+ and Cl−. This factor indicated influence of anthropogenic activities/sources on groundwater chemistry. The possible provenances of these nutrients include leaching from surface soils of the agricultural farmlands, domestic wastes, leakage from sewer drains, and/or influx from polluted surface water bodies.
FA was also performed on the water samples collected in the post-melting season. About 82.75% of the total variability is accounted by three factors (F1, F2, F3) having Eigen value greater than unity. Factor 1 explained 58.40% of total variance followed by 14.83% and 9.52% by factors 2 and 3, respectively. Unlike pre-melting season, here, factor 1 (F1) showed the high loading of Na+ followed by Mg2+ and Cl− and also a moderate to low loadings of HCO3−, hardness, and NO3− as well. These variables also exhibit a high to moderate correlation coefficient. This factor may be attributed to the influence of weathering and dissolution of secondary silicates, thereby imparting their chemical character to the groundwater chemistry. Surface alluvium and/or clay/slit strata/sandy slit/sandy-clay lenses seem to be the most influential sources that provided the highly reactive secondary minerals and/or soil salts to groundwater moving vertically or horizontally.
Factor 2 exhibited very high loading of Ca2+, hardness, and HCO3−, reflecting dissolution of carbonate minerals particularly calcite, thereby controlling the alkalinity and hardness of groundwater. High positive correlation between Ca2+, hardness, and HCO3− also confirms the dissolution of carbonate minerals particularly calcite as the dominant contributor to the factor 2 (i.e., controlling hardness and alkalinity of groundwater). There is also a moderate influence of Cl− and Mg2+ as indicated by their moderate F2 values and correlation coefficient.
Like pre-melting season, factor 3 included K+, NO3−, and SO42− indicating possible input from anthropogenic sources, viz., agricultural farmlands, domestic wastes, leakage from sewer drains, and polluted surface water bodies and vegetable gardens. The above variables also showed good-moderate correlation coefficient indicating the possibility of their source being mainly non-lithological, i.e., anthropogenic sources.
3.9 Analyzing the Suitability of Groundwater for Various Uses
3.9.1 Suitability Criteria for Domestic Purposes
The quality of water plays a prominent role in promoting the standard human health as well as agricultural production. Whether groundwater of a given quality is suitable for a particular purpose depends on the criteria or standards of acceptable quality for the intended use (USEPA 1976). The chemical quality of water is an important factor to be considered in evaluating its usefulness for irrigation as well. Furthermore, the large numbers of health problems in developing areas are reported to be because of ignorance of water quality conditions (Niranjan et al. 1997). Human activity such as urbanization, industrialization, and mining may produce effluents, which pollute the environment as well as the groundwater. The quality standards established (WHO 2006; ISI 2012) for domestic purposes are summarized in Table 3.4.
In the present study, the range of the various parameters as given in the table showed that the concentrations of various constituents are well within the guidelines established by the WHO (2006) and ISI (2012) for drinking and domestic purposes. The results indicated that in the study area, the range of pH (7.1–8.36 and 6.95–8.36) of groundwater indicated an alkaline nature of groundwater. The groundwater is also colorless, odorless, and falls under freshwater (TDS <1000 mg/L) category (Carrol 1962; Freeze and Cherry 1979). Groundwater is moderately hard to very hard, with 14% of samples falling in moderately hard water and 37% as very hard water (Sawyer and MaCarty 1967). However, in the post-melting season, 23% of samples fall in moderately hard class, 63% in hard water class, and 14% in very hard water class (Tables 3.5 and 3.6). The overall hardness (86–410 and 79–410 mg/L) of all the samples fall within the maximum permissible limits (500 mg/L) of the WHO (2006) and ISI (2012). The concentration of Ca2+ and Mg2+ (alkaline earths) exceeds alkalis (Na + K). On the one hand, low concentration of Ca2+ may cause dental problems like tooth cavities and skeletal problems like bone fracture. On the other hand, high concentration is disadvantageous because of the hardness it imparts to the water that interferes with almost every cleaning task in the household and leads to incrustation of water-using appliances and boilers in industrial use. However, in this perspective, Ca2+ and Mg2+ concentrations found in groundwater are neither too low nor high and falls within the prescribed limits of the WHO and ISI. The range of Na+ (3–80 and 2.1–106 mg/L) in groundwater remains at lower levels and is quite below the permissible limits of the WHO and ISI. K+ concentration (0–9 and 0–11.8 mg/L) is relatively low; however, some locations showed increasing trend of K+ in groundwater which is an alarming indication of the increasing influx of K+ from agricultural farmlands. The concentration level, however, is within the maximum permissible limits of the WHO (12 mg/L).
In the case of anions, HCO3− is the dominant anion and exceeds over strong acids (Cl−, SO42− NO3−), thereby imparting basic nature to groundwater. Bicarbonates are not generally regarded as undesirable for municipal supplies, but are highly objectionable for some of the industrial uses because of the problem of scale formation and if used for sprinkler irrigation, it may cause white deposits on fruits and leaves (Subrahmanyam and Yadaiah 2001). The range of HCO3− (30–455 and 85–505 mg/L) in groundwater indicated generally the relatively lower levels and cannot pose any such nuisance as mentioned above. The concentration of Cl− (7–120 and 3–105 mg/L) falls within the permissible limits of the WHO and ISI. However, anomalous values at certain localized areas (particularly in urban Srinagar) are of concern because the inputs from anthropogenic sources have increased the concentration levels of Cl− against its natural background concentrations and/or atmospheric inputs. The high concentration at some locations may increase the salinity of groundwater, but the levels are much below the maximum permissible limits of the WHO and ISI and, therefore, cannot cause laxative effects and/or corrosive effects to equipments or pipelines. SO42− concentration (1.5–6.61 and 0.2–6.5 mg/L) of groundwater is very low and is well below the prescribed limits of the WHO and ISI. NO3− concentration (0.5–8.5 and 0–8.8 mg/L) in groundwater is also at lower levels and is quite below the maximum permissible limits of the WHO and ISI and does not pose any threat of methemoglobinemia in infants.
Since the dissolved loading of various ionic parameters is generally within the maximum acceptable limits of the WHO and ISI guidelines, the groundwater tapped in Srinagar and its adjoining rural areas can be regarded as potable and suitable for domestic and drinking purposes with respect to major ions. Nevertheless, the increasing tendency and enrichment of water with respect to K+, NO3−, and Cl− at some locations is an alarming indication of contamination of shallow aquifers. However, the extent of contamination has not yet reached the level of pollution and is still within the limits of potable water, in terms of national and international drinking water standards.
3.9.2 Suitability Criteria for Agricultural Purposes
For agricultural purposes, the total concentration of dissolved matter in water (salinity), the relative proportion of sodium to other principal cations (Na %), and sodium adsorption ratio (SAR), bicarbonate, etc. are used for the evaluation (Hem 1985). Wilcox (1955) also classified groundwater for irrigation purposes based on percent sodium and EC. Eaton (1950) recommended the concentration of residual sodium carbonate (RSC) to determine the suitability of water for irrigation purpose. The US Salinity Laboratory of the Department of Agriculture adopted salinity hazard diagram for agriculture quality check (US Salinity Laboratory 1954). In the study area, the groundwater samples have been checked for both the irrigation and agricultural purposes. The different indices considered are discussed as sodium percent (Na%), residual sodium carbonate (RSC), sodium adsorption ratio (SAR), salinity hazard diagram, etc.
-
(a)
Sodium Percent: Na % was calculated after Wilcox (1955) equation:
The Na % values are generally low to intermediate (4.08 to 49.71, pre-melting, and 3.96 to 40.51, post-melting) in the groundwater and falls in excellent to permissible water class. The total calcium and magnesium ion content of groundwater exceeds that of sodium. Such water is suitable for irrigation, even though the total mineral content may be quite high.
-
(b)
Residual Sodium Carbonate (RSC): RSC is calculated using the following relation (Eaton 1950) where all ionic concentrations are expressed in epm.
According to the US Department of Agriculture, water having more than 2.5 epm of RSC is not suitable for irrigation purposes. Groundwater of the study area was classified based on RSC, and the results are presented in Table 3.7 for both seasons. Based on RSC values, over 69 samples (98.57%) fall in the safe category, and 1 sample (1.43%) falls in the moderate category (pre-melting season), while over 94% of the samples (RSC < 1.25) fall in the safe category and 6% in moderate (RSC: 1.25–2.5) category in the post-melting season.
-
(c)
Sodium Adsorption Ratio (SAR): The US Salinity Laboratory (1954) defined the sodium adsorption ratio (SAR) of water where the concentrations are expressed in milliequivalent per liter.
The classification of groundwater samples from the study area with respect to SAR is represented in Table 3.8. During both the sampling seasons, the SAR values of the samples were found to be less than 10 and fall in the “excellent” field.
The suitability of water for irrigation was evaluated based on the SAR and specific conductance by plotting the data in on the US Salinity Laboratory (USSL) diagrams as shown in Fig. 3.4a, b and Table 3.8. The USSL diagram best explains the combined effect of sodium and salinity hazard. According to this diagram, all the samples of pre- and post-melting season fall in low sodium (alkali) hazard and low to high salinity hazard. The waters are found mostly confined in three classes of water type, i.e., C2S1, C3S1, and C1S1. In the pre-melting season, 9% of samples fall under C1S1 class (low sodium and salinity hazard); 50% in C2S1 class (low sodium and medium salinity hazard); and 41% in C3S1 class (low sodium and high salinity hazard), whereas in the post-melting season, 13% of samples lie in C1S1 class; 70% in C2S1 class; and 17% in C3S1 class. Thus, the groundwater in the study area falls in the category of the good to moderate class in terms of the quality of water for irrigation. The water class C3S1 (high salinity and low sodium hazard) is found at locations that lie either in the vicinity of polluted water bodies and/or agricultural farmlands, thereby reflecting the possible influence of anthropogenic activities that lead to the slight enrichment of solutes. However, the high salinity waters possess low sodium hazard (SAR), with little danger to the development of harmful level of the exchangeable sodium. Thus, the groundwater can be used for irrigation in almost all soils. Moreover, the total Ca2+ and Mg2+ ion content of groundwater equals and/or exceeds that of Na+ that could favor the maintenance of good tilth and permeability despite the relatively high salinity (an index of dissolved solids concentration). Though there is an increase in salinity at some locations (6, 7, 47, 48, 49, 50, 62, 69), the SAR, %Na, and RSC are well below the permissible limits for the irrigation purposes, and the water is mostly suitable for agricultural purposes (Table 3.9).
3.10 Discussion
In the present study area, the higher concentration of Ca2+ and HCO3− ions in the groundwater indicated the intense chemical weathering and dissolution of minerals mainly carbonate rocks in the area. In general, the interaction of carbonate rocks with carbonic acid that is produced due to the reaction between the CO2 with meteoric water liberates abundance of HCO3− ions into water. This phenomenon may also produce Ca2+ ions resulting in the high concentration of Ca2+ in the groundwater of the area. The statistical analysis also suggested that Ca2+ ion is mainly derived by the dissolution of carbonate minerals such as the calcite minerals in the area. In spite of similarities in the inorganic chemistry of Ca2+ and Mg2+, there is no pronounced correlation between these two ions in the area. A moderate to weak correlation exists between the Ca2+ and Mg2+ (0.34; 0.23). Therefore, the origin of Mg2+ may probably be attributed to the dissolution of alumino-silicates, pyroxenes, and amphiboles of the volcanic rocks and their weathered products. An appreciable amount of volcanics rocks and their weathered products are present in the watershed areas of study area. However, the study of Ca/Mg ratio suggested the dissolution of calcite and some contribution from dolomite as well (Fig. 3.5). Maya and Loucks (1995) have reported that the dissolution of dolomite should happen if the ratio Ca/Mg =1, whereas a higher ratio indicates the greater calcite contribution. Furthermore, a higher Ca/Mg molar ratio (>2) indicates the dissolution of silicate minerals which contribute both Ca2+ and Mg2+ ions to groundwater (Katz et al. 1998). For the present study area, the location of the points closer to the line (Ca/Mg = 1) indicated the dissolution of dolomite (Fig. 3.5). Most of the points having a ratio between 1 and 2 and/or closer to 2 indicate the dissolution of calcite. Those with values on the higher side indicated pronounced input from the dissolution of silicate minerals. The ionic ratio of Ca/Mg, therefore, explained the contribution of dissolution of carbonate rocks as well as the silicate minerals as the possible sources of Ca2+ and Mg2+ in the water. However, the results also indicated some modifying influence of possible cation-exchange reactions on the Ca2+ and Mg2+ concentration in the weathered material and/or alluvium present within the sedimentary unconsolidated formations of the area.
Plot of Ca/Mg molar ratio (Maya and Loucks 1995)
Similarly, the lower Na+ and K+ content also reflected the major influence on their origin by the catchment lithology. Na+ fluctuated irregularly, while K+ showed a definite regularity in its seasonal distributional pattern which may be attributed to its low geochemical mobility. However, increasing concentration of K+ against its background levels at some locations (2, 3, 10, 12, 21, 23, 37, 39, 50, 58, 62, 64, 66, 69, and 70) indicated an alarming anthropogenic influence, however, yet in the offing. Soil amendments applied on paddy fields and floating gardens of Dal Lake are the possible secondary sources of increased K+ concentration relative to normal background low levels at certain locations. The Na+ ions in groundwater originate from the interaction of meteoric water with primary silicates in the outcrops of catchment areas and/or secondary alumino-silicates (clay minerals) with some modifying influence because of cation-exchange reactions as well. However, some enrichment from waterlogged bodies and anthropogenic sources cannot be overlooked, particularly in the low-lying areas (locations 6, 7, 47, 48, 49, 50, 62, and 69). This is also evident from the positive correlation and high factor loadings (F2, pre-melting, and F1, post-melting) of these two ions.
Relatively low concentration of Cl− manifests low background levels of the lithological source in the area. No clearly defined linear relationship between concentration of Cl− and Na+ is apparent in the groundwater samples under consideration. This suggests that the concentrations of the two constituents are heavily influenced by factors other than the dissolution of halite. Nevertheless, the anomalous concentrations observed in the lower reaches in urban localities and near polluted surface water bodies and/or waterlogged bodies are indicative of the increasing anthropogenic influence in the form of domestic wastes, via improper sewage disposal, faulty drainage systems, etc. as there are no apparent Cl− sources in the aquifer-matrix of the area. Hence, anthropogenic activities locally affect Cl− concentrations in groundwater in both the sampling seasons. This is also evident from the positive and significant correlation and high factor loadings in both the sampling seasons.
In Srinagar, NO3− levels are generally low and fall in the concentration ranges that represent the possible low natural/background levels (Madison and Brunett 1984). However, high concentrations of NO3−, which may suggest human influences, appear to occur in isolated wells and/or localized areas (locations 12, 29, 39, 47, 50, 56, 57, 58, 64, and 69). Moreover, seasonal variation of NO3− and K+ exhibited more or less identical behavior suggesting that relatively high levels of K+ are generally present in the areas where agricultural activities are more intensive. The relatively high concentration of NO3− suggested the possible input from domestic wastes coupled with leaching of the nutrients from agricultural farmlands as NO3− fertilizers are often added to cultivated soils as an important plant nutrient. K+ and NO3− may originate mostly from fertilizers and domestic wastes (point sources and non-point sources). However, due to the very different chemistry of K+ and NO3−, there may be a considerable time lag between their rates of movement in the unsaturated zone of the groundwater aquifer. This could also explain the anomalous concentration levels of these ions. The higher concentrations of these constituents, though not alarming at this stage, may go to higher levels in the near future. SO42− content in percentage of total anions has no tendency to increase from recharge to discharge areas. Ideally, the SO42− content in groundwater should increase toward the discharge areas (Toth 1966). The temporo-spatial variation (indicates general dilution effect, in the summer season) besides, no exceptional variation and concentration have been found in the study area. SO42− high (relatively) may be attributed to the possible input from swamp areas/waterlogged bodies and/or topsoil layers. Apart from atmospheric input, some enrichment from anthropogenic sources (though not conspicuous) cannot be overlooked as is evident from high loading of SO42− in factor 3 (possible anthropogenic factor).
3.11 Conclusion
The chemical quality of groundwater is very important in determining its suitability for a sustainable use such as public water supply, irrigation, and industrial applications. Furthermore, the physical and chemical parameters also play a significant role in classifying and assessing water quality. The physicochemical parameters of groundwater of the present study area after comparing with the prescribed specifications or standards show that groundwater is suitable for drinking and domestic purposes as most of the constituents are within the permissible limits. The values of TDS and EC fall in the freshwater and low salinity category. The range of hardness of groundwater samples indicates moderately hard to very hard water types and falls within the maximum permissible limits. The groundwater shows basic nature with alkaline earths and weak acids exceeding alkalis and strong acids. Overall the results indicated the suitability of the water for domestic purposes. Nevertheless, the increasing tendency and enrichment of water with respect to K+, NO3−, and Cl− at some locations is an alarming indication of contamination of shallow aquifers. The low sodium hazard and low to slightly high salinity along with safe to moderate category of water with respect to RSC indicated the portability and suitability of groundwater for agricultural purposes. However, relatively an increase in salinity at some locations is a matter of concern and should be assessed and monitored in the future.
References
APHA (2001) Standard methods for the examination of water and waste. American Public Health Association, Washington, DC
Bakac M (2000) Factor analysis applied to a geochemical study of suspended sediments from the Gediz river, western Turkey. Environ Geochem Health 22:93–111
Bhat FA, Irshad M, Mir AR, Parveez B, Iqbal M, Sana H (2013) Hydrogeochemistry and groundwater quality for drinking and agricultural purposes: a case study of Srinagar district, Jammu and Kashmir, India. Int J Cur Res Rev 15:5(10):1
Bhat MY, Mir RA (2014) Medical Geology: a case study of Kashmir. J Cen Asian Stud 21(1):109–118
Carrol D (1962) Rainwater as a chemical agent of geologic processes- a review, US Geological Survey water-supply paper 1535-G, p 18
Census Report (2001) District census book. Srinagar, pp 26
Chebotarev II (1955) Metamorphism of natural water in the crust of weathering. Geochemica et Casmochemica Acta: 22–48, 137–170, 190–212
Deutsch WJ (1997) Groundwater geochemistry: fundamentals and application to contamination. CRC, Boca Raton
Eaton EM (1950) Significance of carbonate in irrigation water. Soil Sci 69:123–133
Fetter CW (1990) Applied hydrogeology. CBS Publishers & Distributors, New Delhi
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall, Englewood Cliffs
Gulzar A, Mohammad AM, Rouf AB, Sartaj AG (2017) Assessment of ground water quality in Anantnag district of Kashmir valley (J&K) Asian. J Sci Technol 08(9):5803–5811
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water (Vol. 2254). Department of the Interior, US Geological Survey
Hussain M (2005) Geography of Jammu and Kashmir, 4th edn. Rajesh Publications, New Delhi
Indian Standards Institute (2012) Indian Standard Specification for drinking water IS: 10500
Jalali M (2005) Major ion chemistry of groundwaters in the Bahar area, Hamadan, Western Iran. Environ Geol 47:763–772
Jeelani GH, Rouf AS, Aadil H (2014) Hydrogeochemical assessment of groundwater in Kashmir Valley, India. J Earth Syst Sci 123(5):1031–1043
Jehangir A, Aasimah T, Yousuf AR, Akbar M, Aabid HN (2011) Geochemistry and irrigation quality of groundwater along river Jhelum in South Kashmir. India Rec Res Sci Tech 3(6):57–63
Katz BG, Coplen TB, Bullen TD, Davis JH (1998) Use of chemical and isotopic tracers to characterize the interaction between groundwater and surface water in mantled Karst. Groundwater 35(6):1014–1028
Lee SM, Min KD, Woo NC, Kim YJ, Ahn CH (2003) Statistical models for the assessment of nitrate contamination in urban groundwater using GIS. Environ Geol 44:210–221
Madison RJ, Brunett JO (1984) Overview of the occurrence of nitrate in groundwater of the U.S. In: National Water Summary 1984: USGS, Water Supply Paper 2275
Maya AL, Loucks MD (1995) Solute and isotopic geochemistry and groundwater flow in the Central Wasatch Range, Utah. J Hydrol 172:31–59
Mir RA, Gani MK (2019) Evaluation of water quality of upper part of river Jhelum basin using multivariate statistical techniques. Arab J Geosci 12:445
Mir RA, Jeelani G (2015) Hydrogeochemical assessment of river Jhelum and its tributaries for domestic and irrigation purposes, Kashmir valley, India. C Sci 109(2):311–322
Mir RA, Jeelani G, Dar FA (2016) Spatio-temporal variations and factors controlling the hydrogeochemistry of river Jhelum located in Kashmir valley, western Himalayas. Environ Mon Assess 188:438
Mushtaq F, Pandey AC (2014) Assessment of land use/land cover dynamics vis-à-vis hydrometeorological variability in Wular Lake environs Kashmir Valley, India using multitemporal satellite data. Arab J Geosci 17(11):4707–4715
Niranjan BP, Subba Rao N, Prakash Rao P, Chandra Rao P (1997) Groundwater quality and its importance in the land development programme: Ind. J Geol 20:37–41
Rajmohan N, Elango L (2005) Nutrient chemistry of groundwater in an intensively irrigated region of Southern India. Environ Geol 47:820–830
Rashid I, Romshoo SA (2013) Impact of anthropogenic activities on water quality of Lidder River in Kashmir Himalayas. Environ Asses 185(6):4705–4719
Rashid I, Romshoo SA, Amin M, Khanday SA, Chauhan P (2017) Linking human-biophysical interactions with the trophic status of Dal Lake, Kashmir Himalaya, India. Limnologica-Ecol Manage Inland Waters 62:84–96
Rather MI, Rashid I, Shahi N, Murtaza KO, Hassan K, Yousuf AR, Shah IY (2016) Massive land system changes impact water quality of the Jhelum River in Kashmir Himalaya. Environ Mon Assess 188(3):185
Sajjad H, Iqbal M (2012) Impact of urbanization on land use/land cover of Dudhganga watershed of Kashmir Valley, India. Int J Urban Sci 16(3):321–339
Sarah S, Jeelani GH, Ahmed S (2011) Assessing variability of water quality in a groundwater-fed perennial lake of Kashmir Himalayas using linear geostatistics. J Earth Syst Sci 120(3):399–411
Sawyer GN, McCarthy DL (1967) Chemistry of Sanitary Engineers, 2nd ed, McGraw Hill, New York, pp. 518
Showqi I, Rashid I, Romshoo SA (2014) Land use land cover dynamics as a function of changing demography and hydrology. Geo J 79(3):297–307
Singh AK, Mondal GC, Singh PK, Singh S, Singh TB, Tewary BK (2005) Hydrochemistry of reservoirs of Damodar River basin, India: weathering processes and water quality assessment. Environ Geol 48:1014–1028
Subba RN (2006) Seasonal variation of groundwater quality in a part of Guntur District, Andhra Pradesh, India. Environ Geol 49:413–429
Subba RN, John DD (2003) Fluoride incidence in groundwater in an area of Peninsular India. Environ Geol 45:243–251
Subrahmanyam K, Yadaiah P (2001) Assessment of the industrial effluents of water quality in Patancherm and environs, Medak district, Andhra Pradesh, India. Hydrogeol J 9:297–312
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110
Toth J (1966) Groundwater geology, movement, chemistry and resources near Olds, Alberta. Research Council of Alberta Bulletin, 17
Trivedy RK, Goel PK (1984) Chemical and biological methods for water pollution studies. Environment Publications, Karad, p 215
UNESCO (2000) Groundwater pollution; International Hydrological Programme
US Environmental Protection Agency (1976) Quality criteria for water. Washington, DC, pp 501
US Salinity Laboratory (1954) Diagnosis and improvement of saline and alkali soils. U.S.G.P.O., Washington, DC, 160pp
WHO (2006) International standards for drinking water. World Health Organization, Geneva
Wilcox LV (1955) The quality of water for irrigation use, US Department of Agricultural Technical Bulletin 1962, Washington, DC
Zaz SN, Romshoo SA (2012) Assessing the geoindicators of land degradation in the Kashmir Himalayan region, India. Nat Hazards 64(2):1219–1245
Acknowledgments
The Khurshid Ahmad Lone would like to thank HoD, Department of Geology and Geophysics, and Director, CORD (Centre for Research and Development) Kashmir University Srinagar for their support during the work.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mir, R.A., Lone, K.A. (2020). A Recent Scenario of Groundwater Quality in Kashmir, Northwest Himalaya, India. In: Bhat, R.A., Hakeem, K.R. (eds) Bioremediation and Biotechnology, Vol 4. Springer, Cham. https://doi.org/10.1007/978-3-030-48690-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-48690-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48689-1
Online ISBN: 978-3-030-48690-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)