Abstract
The present study region comprises granite and granite gneisses aquifer system constituted by Precambrian rocks. Groundwater is the primary source for drinking and other domestic purposes. Many developing regions in the world suffer from lack of safe drinking water. A rural part of Wanaparthy District in Telangana State, India, is one of them. For this reason, the groundwater samples collected from the study region were analyzed for pH, TDS, Ca2+, Mg2+, Na+, K+, HCO3−, Cl−, SO42−, NO3− and F− and evaluated groundwater quality criteria, using ionic spatial distribution (ISD), entropy water quality index (EWQI) and principal component analysis (PCA). The ISD maps show that some locations are not suitable for drinking purpose due to exceeding concentrations of TDS, Mg2+, Na+, K+, HCO3−, Cl−, NO3−and F−, compared to those with national drinking water quality standards. According to the EWQI, about 3%, 47%, 43% and 7% of the total area come under the excellent, good, medium and extremely poor water quality types for drinking purpose, respectively. Chadha’s diagram classified the area as carbonate hardness (63%), non-carbonate alkali (17%), carbonates alkali (13%) and non-carbonate hardness (7%) zones. The binary diagrams (Na+ + K+ vs TC, Na+ vs Ca2+ and HCO3− vs TC) indicate that the quality of groundwater is controlled by influences of water–rock interactions, mineral weathering and dissolution, ion exchange and evaporation as well as the impact of anthropogenic sources. The PCA transferred the chemical variables into three principal components accounts for about 81% of the total variance. The high positive loadings of PC1 (Cl−, TDS, SO42−, Na+, NO−3, Mg2+ and HCO3−) stand for processes of silicate weathering and dissolution, ion exchange and evaporation, and the influence of domestic waste waters, irrigation return flows and chemical fertilizers on the groundwater system, the PC2 (F− and pH) signifies the alkaline nature of groundwater, which causes fluorosis, and the PC3 (K+) is a result of potassium fertilizers. The study helps to take remediate measures at a specific site and hence suggests the treatment of water before its drinking and also the recharge of the aquifer artificially to improve the groundwater quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Especially in hard rock terrain aquifers, ions from the soil and weathered rocks are dispersed into the groundwater during soil–water–rock interactions (Subba Rao et al. 2017a). Hence, it is an imperative to study the processes controlling the chemistry of groundwater (Perrin et al. 2011; Ehya and Saeedi 2018; Subba Rao et al. 2018; Barakat et al. 2019). On the other hand, the availability of groundwater for drinking in many parts of the world is a problem due to not only over-exploitation of groundwater, but also contamination of groundwater (Subba Rao 2012; Bouderbala et al. 2016; Al-Abadi 2017; Adimalla et al. 2018a; Adimalla 2019a, b; Subba Rao and Chaudhary 2019; Subba Rao et al. 2019).
About 90% of the rural India’s drinking water comes from the groundwater resources (Adimalla et al. 2018a; Verma et al. 2018). Human interferences can contaminate the groundwater body (Subba Rao et al. 2017a, 2019). Consuming safe drinking water is a basic human right. However, the continuous usage of poor chemical quality of water for drinking leads to various health hazards (Mgbenu and Egbueri 2019; Wang et al. 2019). That’s why, the public awareness on quality of water is being increased everywhere due to its direct affect the human health. Geographic Information System (GIS) and Surfer software’s are being used to generate the spatial distribution maps for understanding and identifying the groundwater vulnerable zones (Ramachandran et al. 2012; Duraisamy et al. 2019; Verma et al. 2018). Entropy water quality index (EWQI) method was applied in various studies to assess the groundwater quality for various purposes (Adimalla et al. 2018a; Hamlat and Guidoum 2018).
The researchers in recent years are mainly concentrated to identify the groundwater suitability and its contamination sources all over the world. For instance, Singh et al. (2017) studied the groundwater quality investigations in New Delhi, India, and observed that the hydrogeochemical process (water–rock interactions, weathering, ion exchange and evaporation) and anthropogenic activities (domestic wastes) deteriorate the groundwater quality. A survey on quality of groundwater was carried out by Subba Rao et al. (2017a) for assessing the geochemical characteristics and controlling factors of chemical composition of groundwater in a part of Guntur district, Andhra Pradesh, India, using graphical and binary diagrams, principal component analysis and geochemical modeling, and stated that the composition of groundwater is mainly controlled by geogenic processes (rock weathering, mineral dissolution, ion exchange and evaporation) and anthropogenic sources (irrigation return flows, wastewaters, agrochemicals and constructional activities). Abboud (2018) assessed the geochemistry and quality of groundwater of the Yarmouk basin aquifer, north Jordan, and found that the mineral dissolution mechanism under the alkaline conditions releases ions into the groundwater for enrichment and anthropogenic sources are also the main reasons for contamination of groundwater. Further, the study shows that the some groundwater samples are unsuitable for drinking and irrigation purposes. Aminiyan (2018) evaluated the multiple water quality indices for drinking and irrigation purposes for the Karoon River, Iran, and observed that the most dominant water type belongs to sodium and chloride type and the water quality indices demonstrate a decline in water quality. An and Lu (2018) examined the hydrogeochemical processes and the causes of groundwater pollution in the northern Ordos Cretaceous Basin, China, and the results show that the leaching, evaporation and condensation, mixing and anthropogenic activities are the dominant factors to control the groundwater chemistry and the landfill leachate, domestic sewage and other organic pollutants, excessive use of pesticides and fertilizers are the secondary sources of groundwater pollution. Duraisamy et al. (2019) evaluated the hydrogeochemical characteristics of groundwater and its suitability for drinking water supply in Kangayam Taluk, Tirupur District, Tamil Nadu, India, and demarcated the groundwater quality zones for drinking purpose. Khound and Bhattacharyya (2019) studied the groundwater quality, using multivariate statistical techniques, for identification of pollution sources and also for assessment of spatial variations in water quality in the north Brahmaputra plain, India. Rezaei and Hassani (2018) studied the chemistry and quality of groundwater in the north of Isfahan, Iran, and assessed that the chemical weathering of rock-forming minerals is the main factor controlling the water chemistry, using correlation matrix and principal component analysis, and the quality of water is suitable for drinking and irrigation purposes. Adimalla (2019b) studied the controlling factors and mechanism of groundwater quality variation in semiarid region of South India and found that the weathering of rocks and dissolution of fluoride-bearing minerals and anthropogenic sources are the major reasons for deterioration of groundwater quality and also observed the hazard risk. Chitsazan et al. (2019) assessed the hydrochemical processes, quality change and groundwater pollution in the suburban area of Urmia City, Iran, and found that the mineral weathering, ion exchange and anthropogenic activity (domestic wastes and chemical fertilizers) lead to groundwater contamination. Khanoranga and Khalid (2019) studied the groundwater quality in an arid region of Balochistan Province, Pakistan, using multivariate statistical approaches, and the results show the contribution of both natural and anthropogenic activities in altering the hydrochemistry of the groundwater. In a study, Sajil Kumar and James (2019) applied the geostatistical and geochemical models to distinguish the groundwater quality flow pattern and also to identify the vulnerable spatial variations in the Coimbatore District, South India. Subba Rao and Chaudhary (2019) studied the groundwater quality from an area of Visakhapatnam, Andhra Pradesh, India, and evaluated the controlling processes of groundwater chemistry spatially, depending upon the geogenic processes and the anthropogenic activities, which lead to groundwater contamination.
From the literature, it is concluded that the evaluation of groundwater quality is essential, as it is directly related to human health and also provides baseline information for establishment of long-term groundwater monitoring programs for sustainable development of any area. Since the quality of groundwater in rural parts of Wanaparthy District, Telangana State, India (Fig. 1) is not potable in general, a part of the district is chosen for this purpose. Therefore, the objectivities of the present study are (1) to delineate the spatial distribution of various chemical variables on the basis of national drinking water quality standards, (2) to classify the area into groundwater quality types on the basis of entropy water quality index (EWQI) for drinking purpose and (3) to assess the hydrogeochemical processes controlling the groundwater quality, using Chadha’s and binary diagrams, and principal component analysis. The outcome of the present study helps the local residents and also the decision makers for taking the protection and management of groundwater resources at a specific site.
Study region
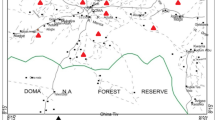
The study region located in Wanaparthy District, Telangana State, India (Fig. 1) experiences a semiarid climate. The average annual temperature varies from 16.9 °C in winter to 41.5 °C in summer. The average annual rainfall is 605 mm. Southwest monsoon contributes 61% of the total rainfall. The River Sarala vagu draining through the region shows sub-dendritic type (Fig. 2).
Sandy soils, black cotton soils and sandy loamy soils are the important soil types. Calcium carbonate concretions occur in the soil zone. The study area is underlain by Precambrian rocks (Fig. 3). The rocks include granite and granite gneiss with dykes, pegmatites and quartzite veins, which are medium to coarse grained and equigranular in texture. They are composed of quartz, plagioclase and potash feldspars, apatite, biotite and hornblende. The rocks show N–S or E–W direction, with a deviation toward the E–W, NW–SE and NE–SW with a dip of 55°–70° southeast. The soils and alluvium occur over the basement rocks.
Geology of the study region (after CGWB 2013)
Groundwater is extracted through dug wells and bore wells. Groundwater occurs under unconfined conditions in the weathered rocks and under semi-confined conditions in the fractured rock portions. The depth to groundwater level varies from 5.1 to 19.5 m bgl (Table 1). Comparatively, the shallow depth to groundwater level is observed at low-lying areas and the deep depth to groundwater level at topographic highs. The yields of the wells range between 250 and 350 cubic meter per day (m3/day; CGWB 2013).
About 49% of the study region is occupied by agricultural land, 44% by waste land, 4% by built-up land and 3% by water bodies. Agriculture is the main occupation of the people. Irrigation practice is intensive and time-consuming. Agrochemicals are used for improvement in soil permeability and also for higher crop yields. The important crops grown are cotton, chilly, paddy, pulses and millets. Dumping of wastes on empty places, leakage of drainage channels and septic tanks are very common.
Methodology
Groundwater samples were collected from 30 locations during summer (May) 2015 (Fig. 1), following the procedures of American Public Health Association (APHA 2012). The groundwater samples were analyzed for hydrogen ion concentration (pH), electrical conductivity (EC), total dissolved solids (TDS), total hardness ((as CaCO3), calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), bicarbonate (HCO3−), chloride (Cl−), sulfate (SO42−), nitrate (NO3−) and fluoride (F−), following the standard methods of APHA (2012). The pH and EC were measured, using their portable meters. The TDS was computed, using EC × 0.65 (Hem 1991). The TH and Ca2+ were estimated by EDTA titration method. The Mg2+ was computed, taking the difference between TH and Ca2+. A flame photometer was used for estimation of Na+ and K+ ions. The HCO3− was measured by HCl volumetric method. The Cl− was analyzed by AgNO3 titration method. The SO42− was determined, using turbidimetric procedure, the NO3−, using colorimetric method, and the F−, using specific ion analyzer. The units of chemical variables (except pH) are expressed in milligrams per liter (mg/L).
The ionic balance error (IBE) was computed between the cations (Ca2+, Mg2+, Na+ and K+) and anions (HCO3−, Cl−, SO42−, NO3− and F−) to verify the accuracy of a complete chemical analysis of each groundwater sample. The IBE was observed to be within the standard limit of ± 5% (Domenico and Schwartz 1990).
Entropy water quality index (EWQI)
Entropy water quality index (EWQI) is a comprehensive tool, which is widely used for quantification of water quality for drinking purpose (Zhou et al. 2016; Wu et al. 2017; Alizadeh et al. 2018; Su et al. 2018). The steps of entropy, entropy weight and EWQI are as follows:
The eigenvalue matrix, X, (Eq. 1) associated with groundwater quality data for “m” samples and “n” parameter is as shown as follows (Su et al. 2018):
Then, using Eq. (2), the eigenvalue matrix X is converted into a standard-grade matrix Y in Eq. (3):
The information entropy (ej) is calculated by the following equations (Su et al. 2018):
The entropy weight (wj) and quality rating scale (qi) are computed, using the following equations, respectively:
where Cj is the concentration of chemical parameters j (mg/L) and Sj is the permissible limits of World Health Organizations (WHO) and Bureau of Indian standards (BIS) of parameter j (mg/L). However, the final EWQI is computed using Eq. (8):
Principal component analysis
Principal component analysis (PCA) is a powerful tool to explain a variance of interrelated variables for reducing the dimensionality of the data set (Subba Rao et al. 2007; Subba Rao 2014; Wu et al. 2014). The chemical variables, such as pH, TDS, Ca2+, Mg2+, Na+, K+, HCO3−, Cl−, SO42−, NO3− and F−, measured from the present study area were used for PCA, using STATISTICA (version 6). The varimax rotation procedure was applied to principal components (PCs) with Kaiser’s criterion, which was used with eigenvalue greater than one (Kaiser 1958). PC1 gives the largest eigenvalue and explains the greatest amount of variance in the data set. PC2 (orthogonal and uncorrelated with the PC1) represents the low eigenvalue and explains the greatest of the remaining variance and so forth. The PC loadings, which are the uncorrelated variables obtained by multiplying the original correlated variables, are used to explain the relative contribution of chemical species over the quality of groundwater.
Results and discussion
General chemistry and spatial distribution of groundwater
Detailed analyzed chemical variables of 30 groundwater samples of the present study region and their drinking water quality standards are listed in Tables 1 and 2, respectively. The values of pH vary from 7.1 to 8.6 with an average of 7.56, indicating an alkaline water quality, which is within its safe limit of 6.5–8.5 prescribed for drinking water (BIS 2012). The eastern and western parts of the study region show alkaline groundwater with pH more than 7.5 (Fig. 4).
The TDS ranges from 200 to 2106 mg/L, and its average is 583.60 mg/L (Table 1), which shows the differences in the degree of water quality due to gradual increase in foreign matter into the aquifer system (Subba Rao 2018). The TDS (701–2106 mg/L) is 0.40–4.21 times higher than its recommended limit of 500 mg/L in about 33% of the total groundwater samples (Table 2). The higher TDS decreases palatability and causes gastrointestinal irritation on human beings (BIS 2012). Generally, the lower TDS indicates a rock–water interactions, while the higher TDS is a result of the influence of domestic wastewaters, irrigation return flows and chemical fertilizers (Subba Rao et al. 2017a, 2019; Duraisamy et al. 2019; Subba Rao 2018; Adimalla 2019b). Further, the spatial distribution map of TDS shows that more than 50% of the study region has TDS less than 500 mg/L (Fig. 5), indicating that the groundwater source from this part is suitable for drinking purpose. The safe drinking water range of TDS (500–1000 mg/L) is observed from the western and eastern parts. The TDS more than 1000 mg/L is called brackish water type, which is observed from the eastern part of the study region. The gradual increasing of TDS from freshwater to brackish water could be due to influence of anthropogenic source on the groundwater system.
Ferromagnesium minerals, ion exchange (between Na+ and Ca2+) and precipitation of CaCO3 are the main causes for the concentration of Mg2+ (19 and 119 mg/L with an average of 39.30 mg/L) than that of Ca2+ (18–67 mg/L with an average of 41.63 mg/L) in the groundwater (Table 1). The Mg2+ (31–60 gm/L) is 1.07–3.96 times higher than its standard safe limit of 30 mg/L allowed for drinking water in 60% of the total groundwater samples (Table 2). The concentration of Ca2+ in all groundwater samples is below its desirable limit of 75 mg/L (BIS 2012). Both Ca2+ and Mg2+ ions develop scale formation on well structures. Spatial distribution map of Ca2+ (Fig. 6) shows that the entire study region comes under the potable zone (acceptable limit) for drinking purpose. About 40% of the study region (western and eastern parts) shows Mg2+ below its desirable limits for drinking use (Fig. 7). About forty percent of the region with Mg2+ between 30 and 60 mg/L is observed from the western and eastern parts, while the rest of the area (20%) shows Mg2+ more than 60 mg/L, which is spread in the eastern part of the study region.
The concentration of Na+ is from 33 to 434 mg/L, being an average of 117.87 mg/L (Table 1). Moreover, Na+ is the dominant ion among the other cations (Ca2+, Mg2+ and K+), which indicates the silicate rock weathering and/or dissolution of soil salts caused by evaporation and anthropogenic activities on the aquifer system (Stallard and Edmond 1983; Subba Rao et al. 2012, 2017a, 2019). The higher Na+ (average: 117.87 mg/L) than the Ca2+ (average: 41.63 mg/L) is expected due to a result of ion exchange and precipitation of CaCO3 (Saxena and Ahmed 2003; Chitsazan et al. 2019; Sajil Kumar and James 2019). The Na+ (238–434 mg/L) is 1.19–2.17 times higher than its threshold limit of 200 mg/L in 20% of the total groundwater samples (Table 2). It causes hypertension (Qasemi et al. 2018). Spatial distribution of Na+ is shown in Fig. 8. About eighty percent of the study region shows Na+ content is below the safe limit (< 200 mg/L) and the rest of the study region (20%) is above its safe limit (> 200 mg/L) mainly in the eastern part. The lower concentration of K+ (1–18 mg/L with an average of 4.10 mg/L; Table 1) compared to other cations is a result of potassium feldspars, which are more resistant to chemical weathering and its fixation on clay products (Chitsazan et al. 2019). The K+ (14–18 mg/L) is 1.16–1.50 times higher than its normal limit of 12 mg/L in the groundwater in about 7% of the total groundwater samples (Table 2), spreading in the southern part as isolated zones (Fig. 9).
Among the anions (HCO3−, Cl−, SO42−, NO3− and F−), the HCO3− varies from 13 to 453 mg/L and its average of 137.87 mg/L (Table 1). The soil CO2 is the main source of HCO3− in the groundwater system (Subba Rao et al. 2012). The HCO3− should not exceed 300 mg/L in potable water (BIS 2012). In 6.67% of the total groundwater samples, the concentration of HCO3− (400–453 mg/L) is 1.33–1.51 times higher than its safe limit (Table 2). The spatial distribution of HCO3− shows (Fig. 10) that about 93% of the study region has HCO3− below the drinking water quality standard limit of 300 mg/L. The rest of the study region (7%) shows HCO3− above its safe limit in the southern part.
The clay products, irrigation return flows and domestic waste waters are the prime source of Cl− content (20–680 mg/L with an average of 119.27 mg/L) in the groundwater (Table 1). The desirable limit of Cl− is 250 mg/L for drinking purpose (BIS 2012). The Cl− content (278–680 mg/L) is 1.13–2.72 times greater than its recommended limit in 20% of the total groundwater samples (Table 2). Figure 11 illustrates the spatial distribution of Cl− content. Like Na+, about 80% of the study region comes under the safe limit zone of 250 mg/L with respect to Cl− content. The rest of the region (20%) shows a higher concentration of Cl− (> 250 mg/L) mainly in the north to south parts due to influences of geogenic (clays) and anthropogenic sources (agricultural activities and waste waters) on the aquifer system.
The application of gypsum and nitrogen fertilizers appears as the main source of SO42− (14–200 mg/L with an average of 51.53 mg/L) and NO3− (0.1–59 mg/L with an average of 7.50 mg/L) in the groundwater (Table 1). The desirable limits of SO42− and NO3− are 200 and 45 mg/L, respectively, for drinking purpose (BIS 2012). The concentration of SO42− is below its safe limit in all groundwater samples, while that of NO3− (48.24–59 mg/L) is 1.07–1.31 times higher than its standard limit in 6.67% of the total groundwater samples (Table 2), which causes blue baby syndrome (Stuart et al. 2011; Menció et al. 2016; Adimalla 2019b). As shown in Figs. 12 and 13, the entire study region comes under the permissible limit of SO42− (200 mg/L), while one isolated zone in the eastern part shows the concentration of NO3− more than its desirable limit of 45 mg/L prescribed for drinking purposes.
The geogenic (apatite, biotite, hornblende and clays) and anthropogenic (chemical fertilizers) origins, in addition to the higher rate of evaporation and longer contact of water with the aquifer system, are the prime sources for the concentration of F− content (0.26–2.30 mg/L with an average of 0.99 mg/L; Table 1) under alkaline environment in the groundwater (Subba Rao and John Devadas 2003; Narsimha and Sudarshan 2017a, b; Subba Rao 2017; Adimalla 2019b). The F− content (1.84–2.30 mg/L) is 1.22–1.53 times higher than the allowable limit of 1.5 mg/L (BIS 212) in 20% of the total groundwater samples (Table 2), which causes fluorosis. The lower fluoride content (< 0.6 mg/L) is observed from the eastern, western and central parts as isolated zones (Fig. 14), causing dental caries. The safe range of F− (0.6–1.5 mg/L) for drinking is spread in 80% of the study region. A few isolated zones show the F− content more than 1.5 mg/L in the entire study region, indicating that the groundwater is not suitable for drinking from these parts.
EWQI and its distribution
The computed data of EWQI and its classification are presented in Tables 3 and 4. The values of EWQI range from 22.70 to 170.69 with an average of 59.33. As per the classification of EWQI, the groundwater quality can be categorized into five types, which are excellent (rank 1), good (rank 2), medium (rank 3), poor (rank 4) and extremely poor (rank 5) water quality types. When the values of EWQI are more than 100, the groundwater is not suitable for drinking purpose (Zhou et al. 2016; Wu et al. 2017; Alizadeh et al. 2018; Su et al. 2018).
According to the EWQI, 3.33%, 46.67%, 43.33% and 6.67% of the total groundwater samples come under the excellent, good, medium and extremely poor water quality types for drinking purpose, respectively. Excellent and good water quality types are fit for drinking purpose. Medium water quality type is fit only for the domestic purpose. The poor and extremely poor water quality types are totally unsuitable for drinking purpose.
Spatial distribution of EWQI is shown in Fig. 15. Excellent water quality type is observed from the extreme northwestern and western parts, good water quality type from the center to eastern, western and northern parts, medium water quality type from eastern, central, western, northern and southern parts, and extremely poor water quality type from the eastern part as an isolated zone. The increase in water quality from excellent to extremely poor types could be due to influences of anthropogenic sources, which increase the concentrations of TDS, Mg2+, Na+ and Cl− compared to other chemical parameters (Subba Rao et al. 2017a; Subba Rao and Chaudhary 2019).
Spatial distribution of EWQI gives baseline information on the occurrence of vulnerable zones at a specific site. Accordingly, it can be possible to take the protection and management measures at specific site for sustainable development of groundwater resources.
Hydrogeochemical types
Basically, Piper (1944) introduced the concept of hydrogeochemical types, which provide significant information on compositional classes that are presented dominant ions in the groundwater system. Later on, Chadha (1999) simplified the concept of hydrogeochemical types in a different perspective (Fig. 16).
Chadha’s diagram showing the hydrochemical facies of groundwater in the study region. (1) Alkaline earths (Ca2+ and Mg2+) exceed alkalies (Na+ and K+), (2) alkalies exceed alkaline earths, (3) weak acids (CO32− and HCO3−) exceed strong acids (Cl− and SO42−), (4) strong acids exceed weak acids, (5) HCO3−–Ca2+–Mg2+, HCO3−–Ca2+ or HCO3−–Mg2+ types, (6) Cl—SO42−–Ca2+–Mg2+, Cl−–Ca2+–Mg2+ or SO42−–Ca2+–Mg2+ types (7) SO42−–Cl−–Na+ or SO42−–Na+ types and (8) HCO3−–Na+ type
As shown in Fig. 16, alkaline earths (Ca2+ and Mg2+) and weak acids (HCO3− and CO3) exceed the alkalies (Na+ and K+) and strong acids (Cl− and SO42−) in 63.33% of the total groundwater samples collected from the present investigated region, which fall in zone 5, indicating the carbonate or temporary hardness or freshwater type (Ca2+–Mg2+–HCO−3 type). The temporary hardness can be easily removed by boiling of water. Further, 16.67% of the groundwater samples are observed from zone 7 (non-carbonate alkali or saline water type; Na+–Cl− type), which shows more alkalies (Na+ and K+) and strong acids (Cl− and SO42−) than alkaline earths and weak acids. This type develops salinity problems in drinking water. Some groundwater samples (13.33%) fall in zone 8, which is characterized by more alkalies and weak acids than alkaline earths and strong acids. This type is called carbonate alkali water type (Na+–HCO3− type), which causes foaming problems in domestic water. A few groundwater samples (6.67%) are observed from zone 6, which shows non-carbonate or permanent hardness water type (Ca2+–Mg2+–Cl− and SO42− type) that is characterized by more alkaline earths and strong acids than alkalies and weak acids. The permanent hardness cannot be easily removed by boiling of water.
Hydrogeochemical proceseses controlling the groundwater quality
Stallard and Edmond (1983), Subba Rao (2002) and Subba Rao et al. (2017a) suggested a diagram, Na+ + K+ versus TC, to explain the contribution of silicate weathering and/or soil salts stored by evaporation as a supply of cations to the groundwater system. All groundwater sampling points fall below the theoretical line of Na+ + K+ versus TC (Fig. 17a). It indicates that the rock weathering and/or soil salts contribute to the groundwater system.
Since the clay occurs in the present study region, it causes ion exchange between Na+ and Ca2+, leading to higher concentration of Na+ in the groundwater system. This phenomenon is verified by plotting of Na+ against Ca2+ (Fig. 17b). The concentration of Ca2+ varies from 1 to 4 meq/L, while that of Na+ is from 2 to 20 meq/L. This supports the ion exchange process taking place in the present study region.
The HCO3− is plotted against TC (Fig. 17c) to further explain not only the role of water–rock interactions, but also the influence of anthropogenic sources on the groundwater system (Subba Rao et al. 2017a). According to Kim (2003), the sampling points should be close to the equiline of HCO3−: TC, if there is dissolution of minerals as a dominant source over the controlling process of chemical quality of groundwater. In the present study region, all groundwater sampling points fall below the uniline of HCO3− versus TC, indicating the deviation from the groundwater quality formed by geogenic origin. Thus, it could be due to a result of influence of anthropogenic sources (domestic wastewaters, irrigation return flows and chemical fertilizers) on the aquifer system.
From these observations, it can be said that the water–rock interactions, mineral weathering and dissolution, ion exchange and evaporation are the primary processes and the anthropogenic activities are the secondary sources controlling the groundwater quality.
Principal component loadings
The computed data of principal components analysis are presented in Table 5. The chemical parameters (pH, TDS, Ca2+, Mg2+, Na+, K+, HCO3−, Cl−, SO42−, NO3− and F−) have yielded three principal components (PCs), depending upon the eigenvalues, which are more than 1 (Fig. 18).
The PC1 accounts for 52.252% of the total variance, which exhibits significant positive loadings of Cl− (0.956), TDS (0.952), SO42− (0.937), Na+ (0.934), NO−3 (0.853), Mg2+ (0.835) and HCO3− (0.613). The Na+, Mg2+ and HCO3− stand for processes of rock–water interactions, silicate weathering and dissolution, ion exchange and evaporation, while those of Cl−, SO42−, Na+ and NO−-3represent the influence of domestic wastewaters, irrigation return flows and chemical fertilizers on the groundwater system. The results of this study are in accordance with Subba Rao et al. (2007, 2019), Adimalla and Venkatayogi (2018) and Subba Rao and Chaudhary (2019). The second component PC2 is much concerned with F−(0.860) and pH (0.859), and its total variance is 19.273%, which signifies the alkaline nature of groundwater. The negative loading of Ca2+ (− 0.519) in PC2 is characteristically triggering the concentration of F− in the groundwater (Adimalla and Venkatayogi 2017: Subba Rao 2017; Subba Rao et al. 2017a, b; Adimalla 2018; 2019b). The rocks of granites containing the fluoride-rich minerals (apatite, biotite and hornblende) are the main sources of fluoride content in the groundwater (Subba Rao et al. 2017a, b; Adimalla and Li 2018; Adimalla et al. 2018b). The PC3 accounts for 9.270% with a high positive loading of K+ (0.766), which is mainly caused by the impact of potassium fertilizers on the groundwater system (Subba Rao et al. 2017a).
Conclusions
The following conclusions were from the present study carried out from a rural part of Wanaparthy District, Telangana State, India:
Groundwater quality is of the alkaline condition, with a dominance of carbonate hardness water type (Ca2+–Mg2+–HCO3− type) in about 63% of the total study region. The spatial distributions of various chemical parameters show that some locations are not suitable for drinking purpose, where the TDS, Mg2+, Na+, HCO3−, Cl−, NO3− and F− exceed their safe limits for drinking purpose. As per the classification of EWQI, about 3%, 47%, 43% and 7% of the total area come under the excellent, good, medium and extremely poor water quality types for drinking purpose, respectively.
The binary diagrams (Na+ + K+ vs TC, Na+ vs Ca2+ and HCO3− vs TC) indicate the influences of water–rock interactions, mineral weathering and dissolution, ion exchange and evaporation as the primary source and the impact of anthropogenic activity as the secondary source controlling the groundwater quality.
The PCA was transferred the chemical variables (pH, TDS, Ca2+, Mg2+, Na+, K+, HCO3−, Cl−, SO42−, NO3− and F−) into three principal components (PCs), which show eigenvalues more than 1. They account for about 81% of the total variance. The PC1 shows the high positive loadings of Cl−, TDS, SO42−, Na+, NO3−, Mg2+, and HCO3− representing the influences of geogenic origin (rock–water interactions, silicate weathering and dissolution, ion exchange and evaporation) and anthropogenic sources (wastewaters, irrigation return flows and chemical fertilizers) on the groundwater system. The high positive loadings of F− and pH in PC2 stand for alkaline condition of groundwater, which causes a higher concentration of F− content in the groundwater. The high positive loading of PC3 associated with K+ is caused by the influence of potassium fertilizers.
Since the groundwater is the prime source for drinking purpose in the present study region, the continuous usage of inferior water quality will lead to various health disorders. Therefore, the present study helps the civic authorities for taking the protection and management of groundwater resources at a specific site. In view of this, artificial recharging of aquifers is imperative for sustainable development of the rural area, which improves the groundwater conditions, including groundwater quality. Meanwhile, it is also essential to treat the poor groundwater quality before its drinking.
References
Abboud, I. A. (2018). Geochemistry and quality of groundwater of the Yarmouk basin aquifer, North Jordan. Environmental Geochemistry and Health,40, 1405–1435. https://doi.org/10.1007/s10653-017-0064-x.
Adimalla, N. (2018). Groundwater quality for drinking and irrigation purposes and potential health risks assessment: A case study from semi-arid region of South India. Exposure and Health. https://doi.org/10.1007/s12403-018-0288-8.
Adimalla, N. (2019a). Spatial distribution, exposure, and potential health risk assessment from nitrate in drinking water from semi-arid region of South India. Human and Ecological Risk Assessment: An International Journal,00, 1–25. https://doi.org/10.1080/10807039.2018.1508329.
Adimalla, N. (2019b). Controlling factors and mechanism of groundwater quality variation in semiarid region of South India: An approach of water quality index (WQI) and health risk assessment (HRA). Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-019-00374-8.
Adimalla, N., & Li, P. (2018). Occurrence, health risks, and geochemical mechanisms of fluoride and nitrate in groundwater of the rock-dominant semi-arid region, Telangana State, India. Human and Ecological Risk Assessment: An International Journal. https://doi.org/10.1080/10807039.2018.1480353.
Adimalla, N., Li, P., & Venkatayogi, S. (2018a). Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environmental Processes,5(2), 363–383.
Adimalla, N., Vasa, S. K., & Li, P. (2018b). Evaluation of groundwater quality, Peddavagu in Central Telangana (PCT), South India: An insight of controlling factors of fluoride enrichment. Modeling Earth Systems and Environment,4(2), 841–852.
Adimalla, N., & Venkatayogi, S. (2017). Mechanism of fluoride enrichment in groundwater of hard rock aquifers in Medak, Telangana State, South India. Environmental Earth Sciences,76(1), 45. https://doi.org/10.1007/s12665-016-6362-2.
Adimalla, N., & Venkatayogi, S. (2018). Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangana State, South India. Applied Water Sciences, 8, 44. https://doi.org/10.1007/s13201-018-0682-1.
Al-Abadi, A. M. (2017). Modeling of groundwater productivity in northeastern Wasit Governorate, Iraq using frequency ratio and Shannon’s entropy models. Applied Water Science,7(2), 699–716.
Alizadeh, Z., Yazdi, J., & Moridi, A. (2018). Development of an entropy method for groundwater quality monitoring network design. Environmental Processes,5(4), 769–788.
Aminiyan, M. M. (2018). Evaluation of multiple water quality indices for drinking and irrigation purposes for the Karoon river, Iran. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-018-0135-7.
An, Y., & Lu, N. (2018). Hydrogeochemical processes identification and groundwater pollution causes analysis in the northern Ordos Cretaceous Basin, China. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-017-0037-0.
APHA. (2012). Standard methods for the examination of water and wastewater (22nd ed.). Washington, DC: American Public Health Association.
Barakat, A., Hilali, A., Baghdadi, M. E., & Touhami, F. (2019). Assessment of shallow groundwater quality and its suitability for drinking purpose near the Béni-Mellal wastewater treatment lagoon (Morocco). Human and Ecological Risk Assessment: An International Journal. https://doi.org/10.1080/10807039.2019.1584029.
BIS. (2012). Drinking water specifications (p. 10500). New Delhi: Bureau of Indian Standards, IS.
Bouderbala, A., Remini, B., Saaed Hamoudi, A., & Pulido-Bosch, A. (2016). Assessment of groundwater vulnerability and quality in coastal aquifers: A case study (Tipaza, North Algeria). Arabian Journal of Geosciences,9(3), 181. https://doi.org/10.1007/s12517-015-2151-6.
CGWB. (2013). Groundwater brochure, nalgonda district, central ground water board. New Delhi: Government of India.
Chadha, D. K. (1999). A proposed new diagram foe geochemical classification of natural waters and interpretation of chemical data. Hydrogeology Journal, 7, 431–439.
Chitsazan, M., Aghazadeh, N., Mirzaee, Y., & Golestan, Y. (2019). Hydrochemical characteristics and the impact of anthropogenic activity on groundwater quality in suburban area of Urmia city, Iran. Environment, Development and Sustainability,21(1), 331–351.
Domenico, P. A., & Schwartz, F. W. (1990). Physical and chemical hydrogeology (p. 824). New York: Wiley.
Duraisamy, S., Govindhaswamy, V., Duraisamy, K., Krishinaraj, S., Balasubramanian, A., & Thirumalaisamy, S. (2019). Hydrogeochemical characterization and evaluation of groundwater quality in Kangayam taluk, Tirupur district, Tamil Nadu, India, using GIS techniques. Environmental Geochemistry and Health, 41, 851–873.
Ehya, F., & Saeedi, F. (2018). Assessment of groundwater quality in the Garmez area (Southeastern Khuzestan province, SW Iran) for drinking and irrigation uses. Carbonates and Evaporites. https://doi.org/10.1007/s1314.
Hamlat, A., & Guidoum, A. (2018). Assessment of groundwater quality in a semiarid region of Northwestern Algeria, using water quality index (WQI). Applied Water Science,8(8), 220. https://doi.org/10.1007/s13201-018-0863-y.
Hem, J. D. (1991). Study and interpretation of the chemical characteristics of natural water: USGS Professional Paper Book 2254. Jodhpur: Scientific Publishers.
Kaiser, H. F. (1958). The varimax criterion for analytic rotation in factor analysis. Psychometrika, 23, 187–200.
Khanoranga, & Khalid, S. (2019). An assessment of groundwater quality for irrigation and drinking purposes around brick kilns in three districts of Balochistan province, Pakistan, through water quality index and multivariate statistical approaches. Journal of Geochemical Exploration,197, 14–26.
Khound, N. J., & Bhattacharyya, K. G. (2019). Assessment of water quality in and around Jia-Bharali river basin, North Brahmaputra Plain, India, using multivariate statistical technique. Applied Water Science,8(8), 221. https://doi.org/10.1007/s13201-018-0870-z.
Kim, K. (2003). Long-term disturbance of groundwater chemistry following well installation. Ground Water, 41, 780–789.
Menció, A., Mas-Pla, J., Otero, N., Regàs, O., Boy-Roura, M., Puig, R., et al. (2016). Nitrate pollution of groundwater; all right…, but nothing else? Science of the Total Environment,539, 241–251.
Mgbenu, C. N., & Egbueri, J. C. (2019). The hydrogeochemical signatures, quality indices and health risk assessment of water r esources in Umunya district, southeast Nigeria. Applied Water Science,9(1), 22. https://doi.org/10.1007/s13201-019-0900-5.
Narsimha, A., & Sudarshan, V. (2017a). Assessment of fluoride contamination in groundwater from Basara, Adilabad District, Telangana State. India. Applied Water Science,7(6), 2717–2725.
Narsimha, A., & Sudarshan, V. (2017b). Contamination of fluoride in groundwater and its effect on human health: A case study in hard rock aquifers of Siddipet, Telangana State, India. Applied Water Science,7(5), 2501–2512.
Perrin, J., Ahmed, S., & Hunkeler, D. (2011). The effects of geological heterogeneities and piezometric fluctuations on groundwater flow and chemistry in a hard-rock aquifer, southern India. Hydrogeology Journal,19(6), 1189–1201.
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water analyses. American Geophysical Union Transactions, 25, 914–923.
Qasemi, M., Farhang, M., Biglari, H., Afsharnia, M., Ojrati, A., Khani, F., et al. (2018). Health risk assessments due to nitrate levels in drinking water in villages of Azadshahr, northeastern Iran. Environmental Earth Sciences,77(23), 782. https://doi.org/10.1007/s12665-018-7973-6.
Ramachandran, M., Sabarathinam, C., Ulaganthan, K., Paluchamy, A., Sivaji, M., & Hameed, S. (2012). Mapping of fluoride ions in groundwater of Dindigul district, Tamilnadu, India—using GIS technique. Arabian Journal of Geosciences,5(3), 433–439.
Rezaei, A., & Hassani, H. (2018). Hydrogeochemistry study and groundwater quality assessment in the north of Isfahan, Iran. Environmental Geochemistry and Health,40, 583–608. https://doi.org/10.1007/s10653-017-0003-x.
Sajil Kumar, P. J., & James, E. J. (2019). Geostatistical and geochemical model-assisted hydrogeochemical pattern recognition along the groundwater flow paths in Coimbatore district, South India. Environment, Development and Sustainability,21(1), 369–384.
Saxena, V., & Ahmed, S. (2003). Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environmental Geology,43(6), 731–736.
Singh, C. K., Kumar, A., Shashtri, S., Kumar, A., Kumar, P., & Mallick, J. (2017). Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. Journal of Geochemical Exploration,175, 59–71.
Stallard, R. F., & Edmond, J. M. (1983). Geochemistry of the Amazon river—the influence of the geology and weathering environment on the dissolved load. Journal of Geophysical Research, 88, 9671–9688.
Stuart, M. E., Gooddy, D. C., Bloomfield, J. P., & Williams, A. T. (2011). A review of the impact of climate change on future nitrate concentrations in groundwater of the UK. Science of the Total Environment,409(15), 2859–2873.
Su, H., Kang, W., Xu, Y., & Wang, J. (2018). Assessing groundwater quality and health risks of nitrogen pollution in the Shenfu mining area of Shaanxi province, Northwest China. Exposure and Health,10(2), 77–97.
Subba Rao, N. (2002). Geochemistry of groundwater in parts of Guntur District, Andhra Pradesh, India. Environmental Geology,41(5), 552–562.
Subba Rao, N. (2012). PIG: A numerical index for dissemination of groundwater contamination zones. Hydrological Processes,26, 3344–3350.
Subba Rao, N. (2014). Spatial control of groundwater contamination, using principal component analysis. Journal of Earth System Science., 123, 715–728.
Subba Rao, N. (2017). Controlling factors of fluoride in groundwater in a part of South India. Arabian Journal of Geosciences,10(23), 524. https://doi.org/10.1007/s12517-017-3291-7.
Subba Rao, N. (2018). Groundwater quality from a part of Prakasam District, Andhra Pradesh, India. Applied Water Science,8(1), 30. https://doi.org/10.1007/s13201-018-0665-2.
Subba Rao, N., & Chaudhary, M. (2019). Hydrogeochemical processes regulating the spatial distribution of groundwater contamination, using pollution index of groundwater (PIG) and hierarchical cluster analysis (HCA): A case study. Groundwater for Sustainable Development. https://doi.org/10.1016/j.gsd.2019.100238.
Subba Rao, N., & John Devadas, D. (2003). Fluoride incidence in groundwater in an area of Peninsular India. Environmental Geology,45(2), 243–251.
Subba Rao, N., Marghade, D., Dinakar, A., Chandana, I., Sunitha, B., Ravindra, B., & Balaji, T. (2017a). Geochemical characteristics and controlling factors of chemical composition of groundwater in a part of Guntur district, Andhra Pradesh, India. Environ Earth Sci,76(21), 747. https://doi.org/10.1007/s12665-017-7093-8.
Subba Rao, N., Prakasa Rao, J., & Subrahmanyam, A. (2007). Principal component analysis in groundwater quality in a developing area of Andhra Pradesh. Journal of the Geological Society of India, 69, 959–969.
Subba Rao, N., Srihari, C., Deepthi Spandana, B., Sravanthi, M., Kamalesh, T., & Abraham Jayadeep, V. (2019). Comprehensive understanding of groundwater quality and hydrogeochemistry for the sustainable development of suburban area of Visakhapatnam, Andhra Pradesh, India. Human and Ecological Risk Assessment: An International Journal,25, 52–80. https://doi.org/10.1080/10807039.2019.1571403.
Subba Rao, N., Subrahmanyam, A., Ravi Kumar, S., Srinivasulu, N., Babu Rao, G., Rao, P. S., et al. (2012). Geochemistry and quality of groundwater of Gummanampadu sub-basin, Guntur District, Andhra Pradesh, India. Environmental Earth Sciences,67(5), 1451–1471.
Subba Rao, N., Sunitha, B., Rambabu, R., Nageswara Rao, P. V., Surya Rao, P., Deepthi Spandanam, B., et al. (2018). Quality and degree of pollution of groundwater, using PIG from a rural part of Telangana State, India. Applied Water Science,8, 227. https://doi.org/10.1007/s13201-018-0864-x.
Subba Rao, N., Surya Rao, P., Dinakar, A., Nageswara Rao, P. V., & Marghade, Deepali. (2017b). Fluoride occurrence in the groundwater in a coastal region of Andhra Pradesh, India. Applied Water Science,7, 1467–1478.
Verma, D. K., Bhunia, G. S., Shit, P. K., & Tiwari, A. K. (2018). Assessment of groundwater quality of the Central Gangetic Plain Area of India using Geospatial and WQI Techniques. Journal of the Geological Society of India,92(6), 743–752.
Wang, M., Yang, J., He, W., Li, J., Zhu, Y., & Yang, X. (2019). Vertical distribution of fluorine in farmland soil profiles around phosphorous chemical industry factories. Environmental Science and Pollution Research,26(1), 855–866.
WHO. (2011). Guidelines for drinking water quality. Geneva: World Health Organization.
Wu, J., Li, P., Qian, H., Duan, Z., & Zhang, X. (2014). Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: Case study in Laoheba phosphorite mine in Sichuan, China. Arabian Journal of Geosciences, 7(10), 3973–3982. https://doi.org/10.1007/s12517-013-1057-4.
Wu, J., Xue, Ch., Tian, R., & Wang, S. (2017). Lake water quality assessment: A case study of Shahu Lake in the semiarid loess area of northwest China. Environmental Earth Sciences, 76, 232. https://doi.org/10.1007/s1266.
Zhou, Y., Wei, A., Li, J., Yan, L., & Li, J. (2016). Groundwater quality evaluation and health risk assessment in the Yinchuan Region, Northwest China. Exposure and Health,8(3), 443–456.
Acknowledgements
The authors are thankful to the anonymous reviewers and the editor in chief for their valuable suggestions and useful comments to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Subba Rao, N., Sunitha, B., Adimalla, N. et al. Quality criteria for groundwater use from a rural part of Wanaparthy District, Telangana State, India, through ionic spatial distribution (ISD), entropy water quality index (EWQI) and principal component analysis (PCA). Environ Geochem Health 42, 579–599 (2020). https://doi.org/10.1007/s10653-019-00393-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-019-00393-5