Abstract
Mercury (Hg) contamination of aquatic ecological units and subsequent bioaccumulation are major environmental problems of international scope. Moreover, the biogeochemistry of Hg in the remote alpine lakes aquatic ecosystem in the Himalayas remains largely unexplored. The current study investigated Hg concentrations in different environmental compartments such as water, fish, and sediments in the remote alpine lakes (RALs) including Glacial-fed Lake, Ice melting-fed Lake, and Rain-fed Lake in northern areas of Pakistan. The mean concentration of Hg in Rain-fed Lake water was (1.07 µg L−1), Ice melting-fed Lake (1.16 µg L−1), and Glacial-fed Lake (1.95 µg L−1). For fish muscle tissues, mean concentration of Hg was 1.02 mg kg−1 in the Rain-fed Lake, and 1.2 mg kg−1 for the Ice melting-fed Lake, and 1.51 mg kg−1 in the Glacial-fed Lake. Meanwhile, 0.27 mg kg−1 was observed for sediments in the Rain-fed Lake, 0.33 mg kg−1 for the Ice melting-fed Lake, and 0.38 mg kg−1 for the Glacial-fed Lake, respectively. Chronic daily intake (CDI) and potential health quotient (PHQ) for water showed high health risk in Glacial-fed Lake and low in Rain-fed Lake (PHQ < 1). The target hazard quotient (THQ) values for both the Brown and Rainbow trout in all the studied lakes water were less than 1, indicating no health risk. Furthermore, the Hg level showed high level of contamination in the sediments of all the studied lakes (190 ≤ RI < 380). Overall, Glacial-fed Lake water was more polluted with Hg, as compared to Rain-fed Lake and Ice melting-fed Lake. In the light of the abovementioned results, further research work is urgently needed to shed light on the biological and geochemical monitoring of Hg in arid high-altitude ecosystems along with source identification, mercury speciation, and other potential pollutants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is considered to be a major environmental contaminant worldwide, because of its complex biogeochemistry in nature, high atmospheric deposition, strong tendency of bio-magnification, and high potential toxicity (Santos et al. 2021; Melnyk et al. 2021; Xu et al. 2019; Zupo et al. 2019; Gruszecka-Kosowska et al. 2018; Alves et al. 2017). The heavy metal Hg exists in three chemical forms such as inorganic form (IHg), elemental form (Hgo), and organic form (Me-Hg and Et-Hg). The metallic Hg mostly occurs in white silver liquid form on the earth crust, and odorless at room temperature (Li et al. 2019; Beckers and Rinklebe 2017; Ullrich et al. 2001). These forms are capable of inter-convertible and create potential toxicity in the ecosystem (Wang et al. 2019). Hg is usually originating from natural sources such as weathering of rocks, volcanic eruptions, lixiviation, as well as erosion of soil particles, while anthropogenic sources include the use of fossil fuel, incineration, fungicides, catalyst processes, and industrial activities such as electric stuffs and asbestos fibers (Ponton et al. 2022; Tayemeh et al. 2020; UNEP 2018; Gerson et al. 2018; Jagtap and Maher 2015; Chiarelli and Roccheri 2014). Hg also originates from the mining activities on regional basis (Torres et al. 2018), and also release to the atmosphere by coal combustion process in the form of fly ash to the environment, and can adversely contaminate both the terrestrial and aquatic environments (Streets et al. 2018; Horowitz et al. 2017). As a result, it evaporates to the atmosphere and releases through precipitation to the earth surface, resulting from poor solubility of Hg compounds (Chiarelli and Roccheri 2014). Maximum proportion of water soluble (Hg+2) releases to terrestrial environment and returns back to the atmosphere in elemental Hg form (Amos et al. 2015), and could be remained for 6 months (Horowitz et al. 2017). Hg can be deposited in the aquatic and terrestrial ecosystem by precipitation process in the atmosphere (UNEP 2008), and it can be transferred from one region to another region, depending on its chemical behavior and wind flow in the atmosphere (Pacyna et al. 2016; Cohen et al. 2004). Moreover, Hg can be transfer to remote areas due to less degradation and its volatile ability in nature (Lindberg et al. 2007). In Asia, Hg has been highly contributed about (67%) emissions through anthropogenic activities, followed by North America and Europe (EFSA 2012). In 1990s, the Asia including India and China were dominantly responsible for Hg emissions (Pacyna et al. 2010), and the coal-fired plants were primary source of Hg emissions in Asia (Driscoll et al. 2013).
Fish consumption was estimated about 154 million t in 2011 worldwide, produced (50%) by aqua-farming, which has significant impacts on countries economy (FAO 2012). Fish is enriched of essential nutrients such as proteins, vitamins, omega-3, fatty acids, and other minerals (Storelli 2008). Since, the exposure of Hg to humans and animals has been increased, due to consumption of fish through bio-magnification process (Melnyk et al. 2021; Ki-Hyun et al. 2016). Hg has been listed as a high priority contaminant worldwide, recommended by the US EPA (2010). Due to the fact that Hg can be accumulated by fish in aquatic ecosystems through bio-magnification and thereby affects the human health via the food chain (Gruszecka-Kosowska et al., 2018). Previously, elevated Hg levels have been reported in fish species, and have posed adverse health issues in riparian people, due to the main source of fish diet (Lino et al. 2018; Salazar-Camacho et al. 2017). For instance, the first infected case was reported in 1965, caused by Me-Hg form in Minamata city, Japan (Ekino et al. 2007). Ingestion of Hg poses adverse effects to human health risk, resulting to impair and suffer the spoken and hearing ability, contributes to cause cancer, ataxia, and tremors in the body, disorders the sensory and vision features, and was briefly highlighted by Minamata Convention Treaty in 2013, and the main objectives were to protect and mitigate the Hg contamination in the environment (Bhattacharya 2020; Yang et al. 2020; Selin 2014). The majority of the studies have been conducted regarding Hg concentrations in field reservoirs and lakes water, sediments, and fish species. In recent studies, Ni et al. (2021) investigated elevated Hg concentrations in lakes water, sediments, and fish in microcosm experiments under the controlled conditions of water level fluctuations. Despite the significant progress in understanding of Hg contamination in different ecosystems, there are still considerable scientific research gaps of Hg contamination in alpine lakes of Himalayas region. Therefore, it is important to comprehensively investigate the abundance and composition of Hg in alpine lakes water, sediments, and in fish species in the Himalayas region.
Data on Hg enrichment in natural aquatic lakes ecosystems are available, but it will be the first study to investigate Hg composition and its bioaccumulation in fish species in alpine lakes of Pakistan. The objectives of this study were to investigate Hg concentrations in water, sediments, and fish species in remote alpine lakes including Glacial-fed Lake, Ice melting-fed Lake, and Rain-fed Lake in northern areas of Pakistan. This study further evaluated the health risk associated with consumption of Hg through water and fish in lakes while the concerned study is the baseline for future studies in remote alpine lakes of Pakistan.
Materials and methods
Study area description
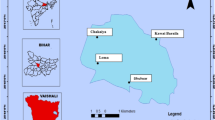
The study area of remote alpine lakes is located in Northern region of Himalayas in Pakistan, as shown in Fig. 1. Three natural lakes were selected for water, sediments, and fish sampling in remote Alpine region including Mahodand (Glacial-fed Lake), Satpara (Ice melting-fed Lake), and Kachura (Rain-fed Lake). The Mahodand Lake is located at 35.7139° N latitude and 72.6510° E longitude in Matiltan valley of upper Usho on a distance of 40 km away from Kalam district Swat, Khyber Pakhtunkhwa, Pakistan. The lake receives water from melting of glaciers and mainsprings of Hindu Kush mountain ranges, and exists with elevation of total 2865 m above (2 km in length). The Satpara Lake is situated at 35° 26′ 40′′ N latitude and 75° 38′ 15′′ E longitude in Northern area of Skardu, with total distance of 9 km in Gilgit Baltistan region. The Satpara Lake mainly receives water from the ice melting in the Deosai plains and water runoff flowing into the Skardu town from the lake. The lake receives 5.4-, and 7.4-in. annual precipitation on average in winter and summer seasons, respectively. The Kachura Lake is located at 35° 25′ 36′′ N latitude and 75° 27′ 18′′ E longitude with elevation of 2499 m at Kachura region. The source of this lake is mostly the rainfall, and lake receives monthly precipitation of about 4.4 in. with temperature ranges from − 6 to 20 °C. The temperature reaches to 15 °C in Kachura Lake during the summer season and gets frozen in the cold winter season. The lake environments are severely cold in winter season due to high elevation above the sea level with annual average of temperature of 0 °C. The alpine lakes are mostly covered with ice during December to April months, and the lakes contain high abundance of trout fishes in fishing grounds for tourist worldwide.
Samples collection and preparation
The water, sediments and fish samples were collected in April 2019, in aforementioned Northern remote alpine areas of Pakistan (Fig. 1). Total of 100 samples were randomly collected including water samples (n = 40), sediment samples (n = 40), and fish samples (n = 20) from selected lakes, respectively. For Hg sampling, one offshore surface water sample (10–15 cm) was collected at each lake site. Water samples were collected in 50-mL new polypropylene falcon tubes, and were acidified to 0.4% with ultrapure nitric acid (Sigma Aldrich) on site. Field blank samples were obtained by filling ultrapure water in situ for each sampling lake and were handled as samples. All samples were tightly sealed, double-packed in Ziploc bags and then stored in a cooler before transported back to the laboratory.
The surface sediment samples were collected by gravity corer at the bottom of the lakes, and sediments were pulled out by corer under vacuum pressure and were kept in polythene bags. All the water and sediment samples were transferred to laboratory for further analysis. The sediment samples were air dried on ground and sieved through a 74-μm stainless steel wire sieve, according to procedure adopted by Lino et al. (2019). Total of 20 fish samples were collected from selected lakes with the help of local fisherman. Fish species samples include Brown trout (Salmo trutta), belongs to Salmonidae family, collected from Glacial-fed Lake. While, Rainbow trout (Oncorhynchus mykiss) also belongs to Salmonidae family, collected from Ice melting-fed Lake and Rain-fed Lake. The fish samples were kept in plastic bags and stored at − 18 °C on site before transfer to laboratory. The fish species’ heads, skin, their internal organs, and tails were removed because these parts cannot be generally consumed, and only the edible portions of muscles were considered for Hg analysis in the present study. The edible parts of all selected fish were cut into small pieces by stainless steel knife, and were three times washed with deionized water for further analysis. All the reagents and chemicals were used for analytical grade.
Sample digestion and Hg analysis
For the analysis of Hg concentrations, 0.5 g of sediments was digested with 15 ml of sulfuric acid (H2SO4) and nitric acid (HNO3) on ratio of (2:1 v:v) in water bath at 60 °C temperature. The clear suspension was made in the extraction process, and 6% potassium permanganate (KMnO4) was added until the color changed to purple, and all the extracted samples were then kept in the ice bath. Hg concentrations were determined in all water samples by adding bromine monochloride (100 μl) to oxidized Hg forms, followed by reduction with stannous chloride (20% w/v) and hydroxylamine hydrochloride (HONH2·HCl) (30% w/v) (USEPA 2002). The pH of all the samples was recorded on-site using digital pH meter (W2015, Sinowell Company, Shanghai, China). Electrical conductivity (EC) and total dissolved solid (TDS) were measured using conductivity meter (Model HI 98,303) and TDS meter (Model S 518,877), respectively. Turbidity was determined on the field by electrochemical analyzer instrument (CONSORT C-931) which was calibrated before use. Fish muscle samples were extracted with 8 ml of HNO3 and H2SO4 on the ratio of (2:1 v:v) for 3 h at 25 °C, and further digested for 5 h at 60 °C. In addition, (30%) of hydrogen peroxide (H2O2) was added to increase the sample to (0.5 mL) with time in extraction, following the protocol described by Azevedo et al. (2020). Temperature was further increased to 65 °C until the samples became colorless. After digestion, the digested mixture was filtered using a membrane filter paper (0.45 μm). The samples were then analyzed for Hg concentrations in water, sediments, and fish muscle samples through cold vapor atomic fluorescence spectrometry, by following USEPA Method 1631 (USEPA 2002).
Exposure risk assessments
In this study, the general exposure equations were followed to assess the potential risk assessments of water, sediments, and fish. These exposure equations were based on recommendations presented by various Canadian and American publications (Health Canada 2010; USEPA 1989, 1992, 2003).
Water exposure risk assessments
Hg was detected in each lake for the identification of risk assessments, while daily intake of Hg and health quotient were calculated for health risks assessment in water by the following Eqs. (1) and (2), respectively.
Chronic daily intake (CDI)
Health quotient (HQ)
where “C” represents Hg concentrations (µg L−1), DI shows the daily intake of consumption of water whereas 2 L for adults and 1 L for children per day, respectively (USEPA 2011). The BW represents average body weight for children (32.7 kg) and adults (70 kg), respectively. The RfD shows the reference dose value for Hg (0.03 µg−1 kg−1 day−1) described by (USEPA 1995). The observed values of HQ (< 1) are considered as safe permissible level (Gul et al. 2016).
Exposure risk assessments of fish
Estimated daily intake (EDI)
EDI of Hg (mg kg−1) via consumption of fish can be calculated by the following Eq. (3):
where DC represents daily fish consumption rate in gm day−1 person−1 (10 gm day−1 person−1 for local people and 100 gm day−1 person−1 for fishermen), C is the concentrations of Hg in fish samples, and ABW indicated average body weight for adult human 60 kg (Nawab et al. 2018). If the EDI values were observed in the range of 1 × 10−6 to 1 × 10−4 considered tolerable level (Chen et al. 2015).
Target hazard quotient (THQ)
THQ helps to evaluate human health risks through consumption of fish caused by Hg concentrations USEPA (2000). Thus, THQ was followed widely in some recent studies (Ahmad et al. 2015), expressed as follows:
where EF represents exposure frequency of 365 days year−1, ED is the exposure duration of 70 years for Hg concentrations of fish in mg kg−1, \(\mathrm{RfD}\) is the oral reference dose for Hg (0.004 µg gm−1 day−1 (USEPA 2010, 2000, 1997), and FIR shows the food ingestion rate 10 gm day−1 person−1 for local people and 100 gm day−1 person−1 for fishermen. WAB is the adult body weight approximately 60 kg and AT showed average time per year (Nawab et al. 2018). Although, some international agencies set limits for Hg concentration to protect against Hg toxicity to human health through consumption of fish and fish-related products. Moreover, the maximum allowable limit for Hg for fish consumption was 1 µg gm−1 wet wt suggested by US food and Drug Administration (FDA). Similarly, fish containing Hg (0.4 µg gm−1 wet wt) concentration has been considered toxicant for human health in Japan. Furthermore, MAL for Hg concertation was 0.5 µg gm−1 set by WHO/FAO (2003) and in Europe as well. Moreover, the concentrations of Hg were greater than MAL in some tropical countries (Burger et al. 2013; Nakagawa et al. 1997).
Ecological risk assessments of sediments
The pollution indices are useful tools to evaluate sediments contaminations. Therefore, several equations have been used in the study to assess pollution degree and ecological risks in the study area (Bourliva et al. 2018; Jafarabadi et al. 2017; Christophoridis et al. 2009).
Contamination factor (CF)
CF is used to calculate the Hg contamination level in the water of each lake, by the given Eq. (5):
Here CMc shows the Hg concentrations in contaminated sites, and CMb is Hg (0.25 mg kg−1) concentrations in the standard pre-industrial reference level (Hakanson 1980). However, CF categorized the values based on contamination levels as (i) CF < 1 is considered as low contamination, (ii) 1 ≤ CF ≤ 3 represents the moderate contamination, and (iii) 3 ≤ CF ≤ 6 shows the high contamination, while (iv) CF > 6 exhibits the significant contamination level (Mmolawa et al. 2010; Chen et al. 2005).
Geo-accumulation index (Igeo)
Igeo can be used to identify the extent of heavy metal concentrations in water sediments, developed by Muller and Geyer (1969), and Igeo classifies the quality of sediments (Nowrouzi and Pourhabbaz 2014).
Here CMc represents the Hg concentration in contaminated lakes site, and CMb shows Hg concentrations in reference sediments level, and 1.5 used as constant value due to variation in metal concentration of background sediments (Nowrouzi and Pourhabbaz 2014). Although, Igeo categorized the contamination levels in sediments as (i) Igeo < 0 shows the low contamination, (ii) 0 ≤ Igeo < 1 exhibits moderate contamination, (iii) 2 ≤ Igeo < 3 presents moderate-high contamination, (iv) 3 ≤ Igeo < 4 shows high contamination, and (v) 4 ≤ Igeo < 5 indicates high-significant contamination, while (vi) Igeo > 5 represents the significant contamination level (Hu et al. 2019).
Enrichment factor (EF)
EF is used to evaluate the variation in metals concentration in contaminated sediments and in the background sites, described by Simex and Helz (1981). The EF can be estimated by the following Eq. (7):
where CMc represents the Hg concentrations in each contaminated lake site, and “CMb” is the Hg concentrations in the background site. EF classified the observed values as (i) EF < 2 shows low enrichment level, (ii) 2 ≤ EF < 5 is normal enrichments level, and (iii) 5 ≤ EF < 20 is high enrichments level, whereas (iv) 20 ≤ EF < 40 is significant enrichments level (Sutherland 2000). Although, iron (Fe) oxidizes to iron hydroxide [Fe (OH)3] in aquatic environment, thereby tends to major sink for heavy metals. Even low level of [Fe (OH)3] has effects on the distribution of metal in aquatic ecosystem (Forstner and Wittmann 1983). The EF values range (from 0.5 to 1.5) might be considered in the natural geogenic sources whereas above from (EF < 1.5) considered anthropogenic sources (Elias and Gbadegesin 2011). Some studies evaluated the HMs pollution, to their average shale for quantification of the concentrations and degree of Hg contaminations (Muller and Geyer 1969; Forester and Muller 1973).
Nemerow’s pollution index (NPI)
NPI was used to determine the overall degree of metals contamination in contaminated sites, used by (Nemerow 1991). NPI can be identified by the given Eq. (8) as follows:
CFAve represents the average contamination factor and CFMax shows the maximum contamination factor. The NPI classified degree of contamination as (i) NPI < 1 is low contamination, (ii) 1 ≤ NPI < 2.5 is low contamination, (iii) 2.5 ≤ NPI < 7 is normal contamination, and (iv) NPI ≥ 7 is high contamination level (Yan et al. 2016).
Ecological risk index assessment
Ecological risk index (ERI) used to calculate the ecological risk degree related with metal concentrations (Hakanson 1980). ERI can be calculated by Eq. (9) below:
where n shows the number of analyzed Hg in the ecological risk factor, which can be calculated by Eq. (10):
Tri represents the toxic response factor of T-Hg is 40, while CF is the contamination factor for analyzed Hg concentrations in sediments of each lake. Whereas, the ERI categorized ecological risk degrees as (i) ERI < 95 shows low ecological risk, (ii) 95 ≤ RI < 190 is moderate ecological risk, (iii) 190 ≤ RI < 380 is high ecological risk, and (iv) RI > 380 is significant ecological risk; and for Eri, (i) Eri < 40 is low ecological risk, (ii) 40 ≤ Eri < 80 is moderate ecological risk, (iii) 160 < Eri ≤ 320 is high ecological risk, and (iv) Eri > 320 is significant ecological risk (Hu et al. 2019; Hakanson 1980).
Quality control and assurance
Quality control and assurance of the analysis were made through a combined measurement and assessment of replicate, field blanks, method blanks, and ongoing precision and certified reference materials. In this study, the method detection limit (MDL), defined as three times the standard deviation of ten replicate measurements of a blank solution, was less than 0.25 ng L−1. During Hg determination, a method blank and standard of 5 ng L−1 for water and 100 ng Hg kg−1 for (fish muscles and sediments) were loaded with a batch of 10–15 samples to check instrument operation. Results showed that method and field blanks were below the MDL with only few of field blank samples show lower than 0.2 ng L−1 and 5 ng Hg kg−1, representing insignificant pollution for the period of sampling, its transport, and analysis. The percentage recovery of OPR was 90–107% of the certified value for water, and 85–105% for fish muscles and sediments. The certified reference materials such as (SRM 1641e) for water, (ERM-CE464) for fish, and (CRM 580) for sediments were used for the recovery rate of Hg and verify the analytical procedure. The percentage recovery of reference materials was 90–107% of the certified value for water, and 85–105% for fish muscles, and 84–92% for lake sediments.
Data analysis
All the data were prepared in Microsoft Excel, and statistical software (SPSS 21) version was used for calculation of the data. The graphs and figures were prepared in Origin 9 (Origin Lab Corporation, USA). Arc-GIS was used for geographical map for the study area.
Results and discussion
Characteristics of physiochemical parameters
The description of pH, electric conductivity (EC µs/cm), total dissolved solid (TDS mg L−1), and turbidity of water in each selected lake is summarized in Table 1. In the present study, the pH ranged from 7.4 to 7.6, 7.5 to 7.6, and 7.2 to 7.4, with mean values of 7.54, 7.56, and 7.32 observed for Rain-fed Lake, Ice melting-fed Lake, and Glacial-fed Lake, respectively. Similar pH values were recorded for the previous study of surface water of alpine streams in Khunjerab National Park of Gilgit, Pakistan (Ali et al. 2017). The pH values in lakes were slightly neutral, while the maximum pH value was detected for Ice melting-fed Lake, which is associated with the increasing rate of algal productivity in lakes ecosystem, as demonstrated by high accumulation of algal bloom in lake sediments (Michelutti et al. 2005). Although, the surface runoff is attributed to slightly increase level in pH because runoff brings organic matter which can be decomposed in water, releasing carbon dioxide with the addition of carbonate and bicarbonate, which may lead to increase of alkalinity (Castrillon-Munoz et al. 2022). The turbidity for each Lake was (> 5), i.e., the turbidity of Glacial-fed Lake, Ice melting-fed Lake, and Rain-fed Lake was within maximum allowable limit (MAL) levels. The electric conductivity (EC) ranged from 0.13 to 0.17, 0.01 to 0.11, and 0.1 to 0.3 µs/cm, while mean values were 0.14, 0.08, and 0.02 µs/cm for Rain-fed Lake, Ice melting-fed Lake, and Glacial-fed Lake, respectively. The concentrations of Hg in lakes water were found in the decreasing order of Glacial-fed Lake < Ice melting-fed Lake < Rain-fed Lake. Moreover, the high EC in Rain-fed Lake is attributed to the precipitation and surface runoff from the mountains and high level of organic matters lead to increase in EC. The low TDS concentrations were detected in surface water ranged from 0.06 to 0.8, 0.4 to 0.5, and 0.01 to 0.02 mg L−1, with mean values of 0.07, 0.04, and 0.01 mg L−1 for Rain-fed Lake, Ice melting-fed Lake, and Glacial-fed Lake, respectively. These TDS values were found in very low concentration in comparison with the previous study of drinking water sources and surface water in Malakand Agency, Pakistan (Nawab et al. 2016). However, TDS concentrations rely on ions in surface water (Baig et al. 2009), and the presence of TDS in surface water reflects the ions dissolution and the gradual deposition of minerals (Chabukdhara et al. 2017). Results showed that the physiochemical parameters of Rain-fed Lake, Ice melting-fed Lake, and Glacial-fed Lake were found within MAL of WHO. High turbidity indicated that lakes prone to surface runoff from the surrounding environment were due to population and other human activities which increased suspended solid leading to increase in turbidity (Sharma and Singh 2019).
Hg concentration level in surface water and sediments
The substantial variation has been observed in Hg concentrations detected in lakes surface water ranged from 0.5 to 1.8, 0.3 to 2.6, and 1.1 to 3.1 µg L−1, with mean values of 1.07, 1.16, and 1.95 µg L−1 for the Rain-fed Lake, Ice melting-fed Lake, and the Glacial-fed Lake, respectively. The total Hg concentrations were found in the decreasing order of Glacial-fed Lake > Ice melting-fed Lake > Rain-fed Lake surface water, as shown in Table 2. However, the above results exceeded the MAL of (≤ 1 µg L−1) set by Pak EPA (2008), WHO guideline (1 µg L−1), and pre-industrial acceptable limit of EU and American Lakes (0.25 ppm) (Hakanson 1980). All the Hg values were exceeded than the previous study of 30 ng/L, conducted by (Babiarz et al. 2003), and apparently lower than the surface water study of Tapajós River basin in the Brazilian Amazon (Lino et al. 2019). However, surface water contaminated by Hg is attributed to the direct atmospheric depositions or originated from the sediments (Biber et al. 2015). Hence, the exposure of these Hg concentrations results in affecting the surface water quality and aquatic ecosystem and may pose harmful effects to human health (Zhong et al. 2018) and could ultimately cause disorders and death after exposure to elevated level of Hg. For instance, experimental study of Soni et al. (2012) demonstrated that high Hg concentration (27 mg m−3) in gaseous state, led to the instant death of rats and rabbits after exposure to Hg for 2 h and 20 h, respectively. Other influencing factors of high Hg in lakes water might be related to geogenic sources of Hg, vegetation cover, local climate, and alteration in topography (Nasr et al. 2011). In addition, flooding of snowmelt in elevated alpine lake areas could be another reason of Hg important source, releasing to the lakes water. For instance, Chetelat et al. (2015) stated that snowmelt was an important source of Hg to lakes water in the Canadian Arctic Archipelago region. It has also been reported that concentrations of Hg and Me-Hg were highest in lakes water during spring season due to snowmelt inputs in Cornwallis Island, Canada (Loseto et al. 2004).
Less variation has been observed in total Hg concentrations in selected lakes of sediments, ranged from 0.11 to 0.45, 0.13 to 0.5, and 0.25 to 0.53 mg kg−1, with mean concentrations of 0.27, 0.33, and 0.38 mg kg−1 in Rain-fed Lake, Ice melting-fed Lake, and Glacial-fed Lake, respectively. The detected mean concentrations in lake sediments were found in the order of Glacial-fed Lake > Ice melting-fed Lake > Rain-fed Lake presented in Table 2. Although, the maximum concentrations in Glacial-fed Lake may be due to anthropogenic activities involved in residential area around it and runoff during high precipitations in Rain-fed Lake region. Hence, Hg deposits in the bottom of sediments, thereby act as a reservoir in waterbodies, and additionally the carbon contents also control and distribute Hg level in sediments (Soto-Jimenez and Paez-Osuna 2001). In addition, moderate pH levels have been observed in the present study. Therefore, Hg can also release from the sediments when pH becomes low (Duarte et al. 1991; Schindler et al. 1980). Elevated level of Hg in sediments accumulates to aquatic ecosystem, resulting to prone ecological toxicity by its spontaneous source of high contamination (Shen et al. 2017). Whereas, low level of Hg contamination in Ice melting-fed Lake sediments is attributed to its high elevations (2636 m) that is situated between the sidewise of high mountains. Moreover, low Hg in sediments can also be associated with resuspension process that transfers Hg to overlying surface water column from the sediments (Gosnell et al. 2016). Fujji (1976) reported that the worldwide background level of Hg was recorded to be 50, 100–300, and 50–80 µg/kg in river, lake, and sea sediments, which has been higher than the present study. The mean concentrations of Hg in Glacial-fed Lake, Rain-fed Lake, and Ice melting-fed Lake were lower than the previous studies conducted by Zhang et al. (2018), Engels et al. (2018), and Tong et al. (2013), but were exceeded than the studies of El-Kady et al. (2019) and Salem et al. (2014).
Hg concentration level in fish species
In the present study, the Hg concentrations in fish species ranged from 0.79 to 1.49, 0.79 to 1.69, and 1.36 to 1.72 mg kg−1, with average concentrations of 1.02, 1.2, and 1.51 mg kg−1 in Rain-fed Lake, Ice melting-fed Lake, and Glacial-fed Lake, respectively. The total concentrations of Hg in fish were observed in the order of Glacial-fed Lake > Ice melting-fed Lake > Rain-fed Lake as shown in Table 2. The maximum concentrations of Hg were observed in S. trutta, while minimum concentrations were detected in O. mykiss species. The maximum level of Hg in S. trutta fish could be associated with the high exposure level or having the efficiency of strong physiological system to accumulate high Hg in its body organs. Besides, all the values were exceeded from MAL (0.5 µg/g) set by WHO/FAO (2009), and EU (2001), respectively. Additionally, the present results of Hg concentrations in fish species were similar with the previous study of southeast Peru in Amazon rainforest (Martinez et al. 2018) and were also compared with the other studies conducted in Lakes (Table 3), respectively. In the current study, the Hg levels in fish may pose potential disorders in humans via consumption, such as the previous study of Barregard et al. (1994) who reported that high level of Hg concentration in blood resulted to disrupt the male reproductive system and hampered infertility status. High Hg exposure to animals may defect and stimulate sperm abnormalities, as previously reported by Choy et al. (2002) and Homma-Takeda et al. (2001). It has also been identified in experimental studies that Hg concentrations were adversely affected the female reproductive system (Bloom et al. 2010).
However, similar results were found in the previous study of same fish species in freshwater lake ecosystems, and their bioaccumulation factor was associated with atmospheric Hg deposition in Canadian Arctic (Chetelat et al. 2015). Hence, the Hg was moderately enriched in the present study of S. trutta species at northern area of Glacial-fed Lake (Mahodand Lake) as compared to other fish species. This demonstrates that Hg ultimately releases to the lakes ecosystem by atmospheric deposition (Meili et al. 2003), and transporting factor of Hg in lakes water is highly complex before being bio-accumulated to fish, indicating the weak relationships between the degree of Hg concentrations in lakes water and fish (Sorensen et al. 1990).
Water contamination assessment
The CDI values of Hg via surface water consumption for adults were 0.030, 0.033, and 0.055, while CDI values for children were 0.032, 0.035, and 0.059 in Rain-fed Lake, Ice melting-fed Lake, and Glacial-fed Lake water samples, respectively as shown in (Fig. 2). Whereas, PHQ values were calculated for adults as (1.0, 0.09, and 1.8) and (1.07, 1.16, and 1.96) for children via surface water consumption in Rain-fed Lake, Ice melting-fed Lake, and Glacial-fed Lake respectively (Fig. 3). The maximum PHQ values for both the adults and children were observed in Glacial-fed Lake, while low PHQ value was indicated for Rain-fed Lake. The PHQ values were recorded higher than the recommended acceptable value of (HQ < 1), indicating the water nearly contaminated with Hg as presented in (Fig. 3). While in comparison, the results of CDI and PHQ values were consistent in the previous studies (Riaz et al. 2019; Shakir et al. 2016; Liu et al. 2012). Since high Hg concentrations have been reported in the present study, as a result, CDI and PHQ values were also high for the adults and children. Whereas, high level of Hg interferes in the body and disturbs the nervous system, hinders the thyroid and testosterone production, and disrupts the neurotransmitter production (Fatoki and Awofolu 2003), and also causes chronic effect, irritates respiratory tract, and lung inflammation (Gary 2012). Furthermore, high level of Hg exposure may hinder children growth and cause health problems such as cognitive impairments, kidney diseases, cough and sputum, neurasthenia, tremor disease, headache and tiredness, skin allergies, chest pain, hyporeflexia, poor vision of nyctalopia, and other sensory problems (Khan et al. 2012). For instance, night blindness of nyctalopia has been reported in biological samples due to exposure to elevated level of Hg in Pakistan (Afridi et al. 2015).
Sediments contamination assessment
Several international guidelines have been developed for the sediments quality assessment. Hg concentrations of sediments in selected lakes were compared with the background values of USEPA (1997). However, Hg concentrations in sediments of Rain-fed Lake, Ice melting-fed Lake, and Glacial-fed Lake have been compared with the sediments quality guidelines in Table 5.
Numerous indices were used comprehensively to understand the Hg contamination in the alpine lake sediments. The indices such as CF, Igeo, EF, NPI, Eri, and RI were applied to assess the sediments quality in Rain-fed, Ice melting-fed, and Glacial-fed Lakes. The CF mean values were 1.39, 1.65, and 1.95 in Rain-fed, Ice melting-fed, and Glacial-fed Lakes as shown in Table 4. The results revealed moderate level of Hg contamination according to CF classifications (Hakanson 1980). The observed CF values were higher than the other previous studies (El-Kady et al. 2019; Mills et al. 2009; Wiener et al. 2006). Likewise, the Igeo values were 0.73, 0.99, and 1.23 for the same lakes. The Igeo values were found below the acceptable limit of (Igeo < 1) indicating, low level of Hg contamination in the studied lakes except Glacial-fed Lake. The Glacial-fed Lake indicating nearly high contamination. Comparatively, other studies also reported that Igeo values of Hg were below zero, i.e., Igeo < 0 and showed low level of contamination (El-Kady et al. 2019; Tripathee et al. 2018, 2016; Sheng et al. 2012).
On the other hand, EF values of Hg were as 0.57, 1.03, and 1.66 for Rain-fed, Ice melting-fed, and Glacial-fed Lakes, respectively. Although, Rain-fed and Ice melting-fed Lakes values were between 0.5 and 1.5 indicating natural origin while Glacial-fed Lake showed above from 1.5 suggested that Hg source has more likely as anthropogenic origin. Low enrichments of sediments for Hg were identified and showed low enrichments than guideline value of (EF < 2), according to classifications of Sutherland (2000). Moreover, the NPI values of Hg were 1.05, 1.19, and 1.31 for Rain-fed, Ice melting-fed, and Glacial-fed Lakes and were found consistent with CF by with the order of Glacial-fed Lake > Ice melting-fed Lake > Rain-fed Lake, respectively. The NPI values for Hg were found (1 ≤ NPI < 2.5), indicating that overall degree of Hg contamination was showed low level contaminations. At the same time, the EF and PI values were less than for Himalayas, demonstrating that low Hg concentrations are related to atmospheric deposition (Tripathee et al. 2018) and naturally originated from regional parent bed rocks materials (Raut et al. 2017). The ecological risk factors (Eri) for Hg were 44.3, 52.8, and 62.0 at Rain-fed, Ice melting-fed, and Glacial-fed Lakes (Table 4). The Eri values were between (40 ≤ Eri < 80), showing moderate ecological risk factor. Furthermore, the potential ecological risk index (RI) was 215 for the studied lakes (190 ≤ RI < 380) which is high ecological risk, hence, poses high potential risk to the lakes aquatic ecosystem in the study area. The potential ERI values for Rain-fed, Ice melting-fed, and Glacial-fed Lake values were greater than the past study conducted by Tripathee et al. (2018). The natural low degree of Hg contamination could be associated with the transportation of aerosol particles to the atmosphere by domestic emissions, which mostly contain black carbon contents release over the Himalayas hills regions during spring season (Lau et al. 2010).
In addition, burning of high African biomass and combustion of fossil fuel on Eastern sites may possibly transfer the atmospheric aerosols to the high Himalayas regions (Kopacz et al. 2011) and surrounding regions of Indian subcontinent also contributed to release aerosols in the atmosphere, as demonstrated by previous observations of Lau and Kim (2006). Consequently, the present study suggested that natural processes such as atmospheric depositions, long-range atmospheric transport, particulate matters, and other less human intrusions contributed to release Hg in the study area of alpine lakes in the Himalayas region of Pakistan.
Fish species contamination assessment
EDI and THQ for Hg were calculated via consumption of fish for adults and children. EDI results were also compared with the tolerable daily intake (TDI) in the studied Lakes, as shown in (Fig. 4). The EDI’s of Hg for local people were 0.17, 0.20, and 0.25 µg/kg day−1, and 1.7, 2.0, and 2.5 µg/kg day−1 for fishermen in Rain-fed, Ice melting-fed, and Glacial-fed Lakes, respectively. High EDI values were found for fish in Glacial-fed Lake, while lowest EDI values were observed for Rain-fed Lake fish. EDI’s were found lower than the tolerable daily intake (0.71 µg/kg day−1 and 40 µg/kg day−1), set by WHO (1990) and Ostapczuk et al. (1987), while also lower than the previous study of Cheng et al. (2009).
THQ of Hg were found varied for studied fish species in Rain-fed, Ice melting-fed, and Glacial-fed Lakes (Fig. 5). The THQ values for local people were 0.07, 0.34, and 0.64, and fishermen were 0.06, 0.30, and 0.57 in Rain-fed, Ice melting-fed, and Glacial-fed Lakes, respectively. Comparatively, THQ values for ingested fish species in the present study were found lower than the previous study of Dongting Lake, China (Torres et al. 2018). Maximum THQ values were recorded for BT in Glacial-fed Lake, whereas the lowest THQ values were observed for RT in Rain-fed Lake. The THQ values were found less than the acceptable range of (THQ < 1). If the EDI values were in the range of 1 × 10−6 to 1 × 10−4 considered tolerable level (Chen et al. 2015). The low concentration of Hg in BT and RT in the studied lakes may be due to the low atmospheric transport to the elevated height of approximate 2500 to 3000 m. The other possible reason may be the Indian monsoon, which cannot reach to the northern parts of Pakistan due to height of Himalayas which changes the direction of Indian monsoon. So the Hg concentration in these remote alpine lakes may be due to the local sources as well as transportation of Hg through valleys to the colder regions.
Conclusion
The extent of Hg contamination in water, sediments, and fish species (S. trutta and O. mykiss) was investigated in remote alpine lakes of Glacial-fed Lake, Rain-fed Lake, and Ice melting-fed Lake along with the potential risk assessment. This report is the first one in the northern areas of Pakistan related to Hg concentration in water, sediments, and their bioaccumulation in S. trutta and O mykiss species. Moderate levels of Hg were above the MAL of Pak-EPA, WHO, and EU acceptable limits in water and fish species in the present study. The HQ results indicated that Glacial-fed Lake showed higher level of contamination as compared to the other studied Lakes. Several indices results showed the moderate level of pollution in all the lake sediments as compared with other previous studies. Moreover, all the indices observed values were also compared with the sediments quality guidelines (SQGs), and consequently exceeded the SQGs acceptable limits (Table 5). In addition, the present study also represents a useful dataset for Hg compositions in water, sediments, and fish species and their risk assessment as well as background level for future studies in northern areas of Pakistan. Nevertheless, future studies are necessary for shedding further light on the influence of Hg transport to higher altitude areas such as forest soil, trees barks, melting glaciers, and pastures and also the tropic transfer such as bio-magnification mechanisms.
Data availability
The analyzed datasets used are available on reasonable request from the corresponding author during the current study.
References
Afridi HI, Kazi TG, Talpur FN, Kazi A, Arain SS, Arain SA, Brahman KD, Panhwar AH, Khan N, Arain MS, Ali J (2015) Assessment of selenium and mercury in biological samples of normal and night blindness children of age groups (3–7) and (8–12) years. Environ Monit Assess 187(3):1–1. https://doi.org/10.1007/s10661-014-4201-z
Ahmed MK, Shaheen N, Islam MS, Habibullah-al-Mamun M, Islam S, Mohiduzzaman M et al (2015) Dietary intake of trace elements from highly consumed cultured fish (Labeo rohita, Pangasius pangasius and Oreochromis mossambicus) and human health risk implications in Bangladesh. Chemosphere 128:284–292. https://doi.org/10.1016/j.chemosphere.2015.02.016
Ali S, Gao J, Begum F, Rasool A, Ismail M, Cai Y, Ali S, Ali S (2017) Health assessment using aqua-quality indicators of alpine streams (Khunjerab National Park), Gilgit, Pakistan. Environ Sci Pollut Res 24(5):4685–4698. https://doi.org/10.1007/s11356-016-8186-8
Alves AC, Monteiro MS, Machado AL, Oliveira M, Boia A, Correia A, Oliveira N, Soares AMVM, Loureiro S (2017) Mercury levels in parturient and newborns from Aveiro region, Portugal. J Toxicol Environ Health A 80(13–15):697–709. https://doi.org/10.1080/15287394.2017.1286926
Amos HM, Sonke JE, Obrist D, Robins N, Hagan N, Horowitz HM, Mason RP, Witt M, Hedgecock IM, Corbitt ES, Sunderland EM (2015) Observational and modeling constraints on global anthropogenic enrichment of mercury. Environ Sci Technol 49(7):4036–47. https://doi.org/10.1021/es5058665
ANZECC (1997) Australian and New Zealand environment and conservation council, MCFFA National Forest Policy Statement Implementation sub-committee. Nationally agreed criteria for the establishment of a comprehensive, adequate and representative reserve system for forests in Australia. In: Canberra, Australian and New Zealand Environment and Conservation Council and Ministerial Council on Forestry. Fisheries and Aquaculture
Azevedo LS, Pestana IA, da Costa Nery AF, Bastos WR, Souza CMM (2020) Mercury concentration in six fish guilds from a floodplain lake in western Amazonia: interaction between seasonality and feeding habits. Ecol Indic 111:106056. https://doi.org/10.1016/j.ecolind.2019.106056
Babiarz CL, Hurley JP, Krabbenhoft DP, Gilmour C, Branfireun BA (2003) Application of ultrafiltration and stable isotopic amendments to field studies of mercury partitioning to filterable carbon in lake water and overland runoff. Sci Total Environ 304(1–3):295–303. https://doi.org/10.1016/S0048-9697(02)00576-4
Baig JA, Kazi TG, Arain MB, Afridi HI, Kandhro GA, Sarfraz RA, Jamal MK, Shah AQ (2009) Evaluation of arsenic and other physico-chemical parameters of surface and ground water of Jamshoro, Pakistan. J Hazard Mater 166(2–3):662–669. https://doi.org/10.1016/j.jhazmat.2008.11.069
Barregård L, Lindstedt G, Schütz A, Sällsten G (1994) Endocrine function in mercury exposed chloralkali workers. Occup Environ Med 51(8):536–540. https://doi.org/10.1136/oem.51.8.536
Beckers F, Rinklebe J (2017) Cycling of mercury in the environment: sources, fate, and human health implications: a review. Crit Rev Environ Sci Technol 47:693–794. https://doi.org/10.1080/10643389.2017.1326277
Bhattacharya S (2020) The role of probiotics in the amelioration of cadmium toxicity. Biol Trace Elem Res 197(2):440–444. https://doi.org/10.1007/s12011-020-02025-x
Biber K, Khan SD, Shah MT (2015) The source and fate of sediment and mercury in Hunza River basin, northern areas, Pakistan. Hydrol Process 29(4):579–587. https://doi.org/10.1002/hyp.10175
Bloom MS, Parsons PJ, Steuerwald AJ, Schisterman EF, Browne RW, Kim K, Coccaro GA, Conti GC, Narayan N, Fujimoto VY (2010) Toxic trace metals and human oocytes during in vitro fertilization (IVF). Reprod Toxicol 29(3):298–305. https://doi.org/10.1016/j.reprotox.2010.01.003
Bourliva A, Kantiranis N, Papadopoulou L, Aidona E, Christophoridis C, Kollias P, Evgenakis M, Fytianos K (2018) Seasonal and spatial variations of magnetic susceptibility and potentially toxic elements (PTEs) in road dusts of Thessaloniki city, Greece: a one-year monitoring period. Sci Total Environ 639:417–427. https://doi.org/10.1016/j.scitotenv.2018.05.170
Braaten HF, Åkerblom S, de Wit H, Skotte G, Rask M, Vuorenmaa J, Kahilainen KK, Malinen T, Rognerud S, Lydersen E, Amundsen PA (2017) Spatial and temporal trends of mercury in freshwater fish in Fennoscandia (1965–2015). http://hdl.handle.net/11250/2467116
Burger J, Jeitner C, Donio M, Pittfield T, Gochfeld M (2013) Mercury and selenium levels, and selenium: mercury molar ratios of brain, muscle and other tissues in bluefish (Pomatomus saltatrix) from New Jersey, USA. Sci Total Environ 443:278–286. https://doi.org/10.1016/j.scitotenv.2012.10.040
Castrillon-Munoz FJ, Gibson JJ, Birks SJ (2022) Carbon dissolution effects on pH changes of RAMP lakes in northeastern Alberta. Canada Journal of Hydrology: Regional Studies 40:101045. https://doi.org/10.1016/j.ejrh.2022.101045
Chabukdhara M, Gupta SK, Kotecha Y, Nema AK (2017) Groundwater quality in Ghaziabad district, Uttar Pradesh, India: multivariate and health risk assessment. Chemosphere 179:167–178. https://doi.org/10.1016/j.chemosphere.2017.03.086
Chapman PM, Allard PJ, Vigers GA (1999) Development of sediment quality values for Hong Kong special administrative region: a possible model for other jurisdictions. Mar Pollut Bull 38(3):161–169. https://doi.org/10.1016/S0025-326X(98)00162-3
Chen TB, Zheng YM, Lei M, Blais JM, Depew D, Emmerton CA, Evans M, Gamberg M, Gantner N, Girard C, Graydon J (2005) Assessment of heavy metal pollution in surface soils of urban parks in Beijing. China Chemosphere 60(4):542–551. https://doi.org/10.1016/j.chemosphere.2004.12.072
Chen H, Teng Y, Lu S, Wang Y, Wang J (2015) Contamination features and health risk of soil heavy metals in China. Sci Total Environ 512:143–153
Cheng J, Gao L, Zhao W, Liu X, Sakamoto M, Wang W (2009) Mercury levels in fisherman and their household members in Zhoushan, China: impact of public health. Sci Total Environ 407(8):2625–2630. https://doi.org/10.1016/j.scitotenv.2009.01.032
Chételat J, Amyot M, Arp P et al (2015) Mercury in freshwater ecosystems of the Canadian Arctic: recent advances on its cycling and fate. Sci Total Environ 509:41–66. https://doi.org/10.1016/j.scitotenv.2014.05.151
Chiarelli R, Roccheri MC (2014) Marine invertebrates as bio indicators of heavy metal pollution. . https://doi.org/10.4236/ojmetal.2014.44011
Choy CM, Lam CW, Cheung LT, Briton-Jones CM, Cheung LP, Haines CJ (2002) Infertility, blood mercury concentrations and dietary seafood consumption: a case–control study. BJOG An Int J Gynaecol Obstet 109(10):1121–5. https://doi.org/10.1016/S1470-0328(02)02984-1
Christophoridis C, Dedepsidis D, Fytianos K (2009) Occurrence and distribution of selected heavy metals in the surface sediments of Thermaikos Gulf, N. Greece. Assessment using pollution indicators. J Hazard Mater 168(2–3):1082–1091. https://doi.org/10.1016/j.jhazmat.2009.02.154
Cohen M, Artz R, Draxler R, Blanchard P, Gustin MS, Han YJ, Holsen TM, Jaffe DA, Kelley P, Lei H, Loughner CP (2004) Modeling the atmospheric transport and deposition of mercury to the Great Lakes. Environ Res 95(3):247–265. https://doi.org/10.1016/j.envres.2003.11.007
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47(10):4967–4983. https://doi.org/10.1021/es305071v
Duarte AC, Pereira ME, Oliveira JP, Hall A (1991) Mercury desorption from contaminated sediments. Water Air Soil Pollut 56(1):77–82
EFSA Panel on Contaminants in the Food Chain (CONTAM) (2012) Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J 10(12):2985
EU (2001) European Union, Maximum concentrations of certain industrial Mercury discharges
FAO (2012) The state of world fisheries and aquaculture, Rome
FDA (2001) Recommendations of the United States public health service food and drug administration food code:2001
Fjeld E, Rognerud S (2009) Contaminants in freshwater fish, 2008. NIVA Report, OR-5851, 573(66):574
Ekino S, Susa M, Ninomiya T, Imamura K, Kitamura T (2007) Minamata disease revisited: an update on the acute and chronic manifestations of methyl mercury poisoning. J Neurol Sci 262(1–2):131–144. https://doi.org/10.1016/j.jns.2007.06.036
Elias P, Gbadegesin A (2011) Spatial relationships of urban land use, soils and heavy metal concentrations in Lagos Mainland Area. J Appl Sci Environ Manag 15(2). https://doi.org/10.4314/jasem.v15i2.68533
El-Kady AA, Wade TL, Sweet ST, Klein AG (2019) Spatial distribution and ecological risk assessment of trace metals in surface sediments of Lake Qaroun, Egypt. Environ Monit Assess 191(7):1–3. https://doi.org/10.1007/s10661-019-7548-3
Engels S, Fong LS, Chen Q, Leng MJ, McGowan S, Idris M, Rose NL, Ruslan MS, Taylor D, Yang H (2018) Historical atmospheric pollution trends in Southeast Asia inferred from lake sediment records. Environ Pollut 235:907–917. https://doi.org/10.1016/j.envpol.2018.01.007
Fatoki OS, Awofolu RJWS (2003) Levels of Cd, Hg and Zn in some surface waters from the eastern Cape Province, South Africa. Water SA 29(4):375–380
Förstner U, Müller G (1973) Heavy metal accumulation in river sediments: a response to environmental pollution. Geoforum 4(2):53–61. https://doi.org/10.1016/0016-7185(73)90006-7
Forstner U, Wittmann GT (1983) Metal pollution in the aquatic environment. Springer Vereleg, Berlin, pp 110–112
Fujji M (1976) Mercury distribution in lithosphere and atmosphere. Mercury. Kodansha Scientific, Tokyo
Gary F (2012) Common heavy metals: sources and specific effects
Gerson JR, Driscoll CT, Hsu-Kim H, Bernhardt ES, Chadwick O (2018) Senegalese artisanal gold mining leads to elevated total mercury and methylmercury concentrations in soils, sediments, and rivers. Elementa Sci Anthropocene 1:6. https://doi.org/10.1525/elementa.274
Gosnell K, Balcom P, Ortiz V, DiMento B, Schartup A, Greene R, Mason R (2016) Seasonal cycling and transport of mercury and methylmercury in the turbidity maximum of the Delaware estuary. Aquat Geochem 22(4):313–336
Gruszecka-Kosowska A, Baran A, Czesława J (2018) Content and health risk assessment of selected elements in commercially available fish and fish products. Hum Ecol Risk Assess Int J 24(6):1623–1641. https://doi.org/10.1080/10807039.2017.1419817
Gul N, Khan S, Khan A, Nawab J, Shamshad I, Yu X (2016) Quantification of hg excretion and distribution in biological samples of mercury-dental-amalgam users and its correlation with biological variables. Environ Sci Pollut Res 23(20):20580–20590. https://doi.org/10.1007/s11356-016-7266-0
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14(8):975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Health Canada (2010) Federal contaminated site risk assessment in Canada, Part V: guidance on human health detailed quantitative risk assessment for chemicals (DQRAChem.)
Homma-Takeda S, Kugenuma Y, Iwamuro T (2001) Impairment of spermatogenesis in rats by methylmercury: involvement of stage-and cell-specific germ cell apoptosis. Toxicology 169(1):25–35. https://doi.org/10.1016/S0300-483X(01)00487-5
Horowitz HM, Jacob DJ, Zhang Y (2017) A new mechanism for atmospheric mercury redox chemistry: implications for the global mercury budget. Atmos Chem Phys 17(10):6353–6371. https://doi.org/10.5194/acp-17-6353-2017
Hu J, Lin B, Yuan M (2019) Trace metal pollution and ecological risk assessment in agricultural soil in Dexing Pb/Zn mining area, China. Environ Geochem Health 41(2):967–980. https://doi.org/10.1007/s10653-018-0193-x
Jafarabadi AR, Bakhtiyari AR, Toosi AS, Jadot C (2017) Spatial distribution, ecological and health risk assessment of heavy metals in marine surface sediments and coastal seawaters of fringing coral reefs of the Persian Gulf, Iran. Chemosphere 185:1090–1111. https://doi.org/10.1016/j.chemosphere.2017.07.110
Jagtap R, Maher W (2015) Measurement of mercury species in sediments and soils by HPLC–ICPMS. Microchem J 121:65–98. https://doi.org/10.1016/j.microc.2015.01.010
Khan S, Shah MT, Din IU, Rehman S (2012) Mercury exposure of workers and health problems related with small-scale gold panning and extraction. J Chem Soc Pak 34(4)
Ki-Hyun K, Ehsanul K, Jahan Ara S (2016) A review on the distribution of Hg in the environment and its human health impacts. J Hazard Mater 306:376–385. https://doi.org/10.1016/j.jhazmat.2015.11.031
Kopacz M, Mauzerall DL, Wang J (2011) Origin and radiative forcing of black carbon transported to the Himalayas and Tibetan Plateau. Atmos Chem Phys 11(6):2837–2852. https://doi.org/10.5194/acp-11-2837-2011
Lau KM, Kim KM (2006) Observational relationships between aerosol and Asian monsoon rainfall, and circulation. Geophys Res Lett 33(21). https://doi.org/10.1029/2006GL027546
Lau WK, Kim MK, Kim KM (2010) Enhanced surface warming and accelerated snow melt in the Himalayas and Tibetan Plateau induced by absorbing aerosols. Environ Res Lett 5(2):025204
Laybauer L (2002) Estudo do risco ambiental e da dinâmica sedimentológica e geoquímica da contaminação por metais pesados nos sedimentos do Lago Guaíba, Rio Grande do Sul, Brasil. Universidade Federal do Rio Grande do Sul
Li ZW, Huang M, Luo NL, Wen JJ, Deng CX, Yang R (2019) Spectroscopic study of the effects of dissolved organic matter compositional changes on availability of cadmium in paddy soil under different water management practices. Chemosphere 225:414–423. https://doi.org/10.1016/j.chemosphere.2019.03.059
Lindberg S, Bullock R, Ebinghaus R, Engstrom D, Feng X, Fitzgerald W, Pirrone N, Prestbo E, Seigneur C (2007) A synthesis of progress and uncertainties in attributing the sources of mercury in deposition. Ambio 1:19–32. https://doi.org/10.1579/0044-7447(2007)36[19:asopau]2.0.co;2
Lino AS, Kasper D, Guida YS, Thomaz JR (2018) Mercury and selenium in fishes from the Tapajós River in the Brazilian Amazon: an evaluation of human exposure. J Trace Elem Med Biol 48:196–201. https://doi.org/10.1016/j.jtemb.2018.04.012
Lino AS, Kasper D, Guida YS, Thomaz JR, Malm O (2019) Total and methyl mercury distribution in water, sediment, plankton and fish along the Tapajós River basin in the Brazilian Amazon. Chemosphere 235:690–700. https://doi.org/10.1016/j.chemosphere.2019.06.212
Liu B, Yan H, Wang C, Li Q, Guédron S, Spangenberg JE, Feng X, Dominik J (2012) Insights into low fish mercury bioaccumulation in a mercury-contaminated reservoir, Guizhou, China. Environ Pollut 160:109–117. https://doi.org/10.1016/j.envpol.2011.09.023
Loseto LL, Lean DR, Siciliano SD (2004) Snowmelt sources of methylmercury to high arctic ecosystems. Environ Sci Technol 38(11):3004–3010. https://doi.org/10.1021/es035146n
Macdonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ 39(1):20–31. https://doi.org/10.1007/s002440010075
Martinez G, McCord SA, Driscoll CT, Todorova S, Wu S, Araújo JF, Vega CM, Fernandez LE (2018) Mercury contamination in riverine sediments and fish associated with artisanal and small-scale gold mining in Madre de Dios, Peru. Int J Environ 15(8):1584. https://doi.org/10.3390/ijerph15081584
Meili M, Bishop K, Bringmark L, Johansson K, Munthe J, Sverdrup H, de Vries W (2003) Critical levels of atmospheric pollution: criteria and concepts for operational modelling of mercury in forest and lake ecosystems. Sci Total Environ 304(1–3):83–106. https://doi.org/10.1016/S0048-9697(02)00559-4
Melnyk LJ, Lin J, Kusnierz DH, Pugh K, Durant JT, Suarez-Soto RJ, Venkatapathy R, Sundaravadivelu D, Morris A, Lazorchak JM, Perlman G (2021) Risks from mercury in anadromous fish collected from Penobscot River, Maine. Sci Total Environ 781:146691. https://doi.org/10.1016/j.scitotenv.2021.146691
Michelutti N, Wolfe AP, Vinebrooke RD, Rivard B, Briner JP (2005) Recent primary production increases in arctic lakes. Geophys Res Lett 32(19). https://doi.org/10.1029/2005GL023693
Mills RB, Paterson AM, Blais JM, Lean DR, Smol JP, Mierle G (2009) Factors influencing the achievement of steady state in mercury contamination among lakes and catchments of south-central Ontario. Can J Fish Aquat 66(2):187–200. https://doi.org/10.1139/F08-204
Mmolawa KB, Likuku AS, Gaboutloeloe GK (2010) Reconnaissance of heavy metal distribution and enrichment around Botswana. In Fifth International Conference of Environmental Science & Technology, 12–16
Muller A, Geyer G (1969) Submicroscopic heavy metal localization in the prosecreta of the Paneth cells of the mouse. Gegenbaurs Morphol Jahrb 113(1):70–77
Nakagawa H, Umino T, Tasaka Y (1997) Usefulness of Ascophyllum meal as a feed additive for red sea bream Pagrus majo. Aquaculture 151(1–4):275–281. https://doi.org/10.1016/S0044-8486(96)01488-3
Nasr M, Ogilvie J, Castonguay M, Rencz A, Arp PA (2011) Total Hg concentrations in stream and lake sediments: discerning geospatial patterns and controls across Canada. Appl Geochem 26(11):1818–1831. https://doi.org/10.1016/j.apgeochem.2011.06.006
Nawab J, Khan S, Ali S, Sher H, Rahman Z, Khan K, Tang J, Ahmad A (2016) Health risk assessment of heavy metals and bacterial contamination in drinking water sources: a case study of Malakand Agency, Pakistan. Environ Monit Assess 188(5):286. https://doi.org/10.1007/s10661-016-5296-1
Nawab J, Khan S, Xiaoping W (2018) Ecological and health risk assessment of potentially toxic elements in the major rivers of Pakistan: general population vs. Fishermen Chemosphere 202:154–164. https://doi.org/10.1016/j.chemosphere.2018.03.082
Nemerow NL (1991) Stream, lake, estuary, and ocean pollution. https://doi.org/10.1002/ep.670130106.
Ni FJ, Bhavsar SP, Poirier D, Branfireun B, Petro S, Arts MT, Chong-Kit R, Mitchell CP, Arhonditsis GB (2021) Impacts of water level fluctuations on mercury concentrations in hydropower reservoirs: a microcosm experiment. Ecotoxicol Environ Saf 220:112354. https://doi.org/10.1016/j.ecoenv.2021.112354
Nowrouzi M, Pourkhabbaz A (2014) Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Hara Biosphere Reserve, Iran. Chem Speciat Bioavailab 26(2):99–105. https://doi.org/10.3184/095422914X13951584546986
Ostapczuk P, Valenta R, Rutzel H, Nurnberg HW (1987) Determination of heavy metals in environmental samples. Sci Total Environ 69:1–16
Pacyna EG, Pacyna JM, Sundseth K, Munthe J, Kindbom K, Wilson S, Steenhuisen F, Maxson P (2010) Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmos Environ 44(20):2487–2499. https://doi.org/10.1016/j.atmosenv.2009.06.009
Pacyna JM, Travnikov O, De-Simone F, Hedgecock IM, Sundseth K, Pacyna EG, Steenhuisen F, Pirrone N, Munthe J, Kindbom K (2016) Current and future levels of mercury atmospheric pollution on a global scale. Atmos Chem Phys 16(19):12495–12511. https://doi.org/10.5194/acp-16-12495-2016
Pak EPA (2008) National standards for drinking water quality. Pakistan Environmental Protection Agency, (Ministry of Environment) government of Pakistan
Ponton DE, Ruelas-Inzunza J, Lavoie RA, Lescord GL, Johnston TA, Graydon JA, Reichert M, Donadt C, Poesch M, Gunn JM, Amyot M (2022) Mercury, selenium and arsenic concentrations in Canadian freshwater fish and a perspective on human consumption intake and risk. J Hazard Mater Adv 100060. https://doi.org/10.1016/j.chemosphere.2019.124576
Raut R, Bajracharya RM, Sharma SCM, Kang S, Zhang Q, Tripathee L, Chen P, Rupakheti D, Guo J, Dongol BS (2017) Potentially toxic trace metals in water and lake-bed sediment of panchpokhari, an alpine lake series in the central Himalayan region of Nepal. Water Air Soil Pollut 228(8):1–4. https://doi.org/10.1007/s11270-017-3467-5
Riaz A, Khan S, Muhammad S, Shah MT (2019) Mercury contamination in water and sediments and the associated health risk: a case study of artisanal gold-mining. Miner Water Environ 38(4):847–854. https://doi.org/10.1007/s10230-019-00613-5
Salazar-Camacho C, Salas-Moreno M, Marrugo-Madrid S, Marrugo-Negrete J, Díez S (2017) Dietary human exposure to mercury in two artisanal small-scale gold mining communities of northwestern Colombia. Environ Int 107:47–54. https://doi.org/10.1016/j.envint.2017.06.011
Salem DM, Khaled A, El Nemr A, El-Sikaily A (2014) Comprehensive risk assessment of heavy metals in surface sediments along the Egyptian Red Sea coast. Egypt J Aquat Res 40(4):349–362. https://doi.org/10.1016/j.ejar.2014.11.004
Santos LN, Neves RA, Koureiche AC, Lailson-Brito J (2021) Mercury concentration in the sentinel fish species Orthopristis ruber: effects of environmental and biological factors and human risk assessment. Mar Pollut Bull 169:112508. https://doi.org/10.1016/j.marpolbul.2021.112508
Schindler DW, Hesslein RH, Wagemann R, Broecker WS (1980) Effects of acidification on mobilization of heavy metals and radionuclides from the sediments of a freshwater lake. Can J Fish Aquat Sci 37(3):373–377. https://doi.org/10.1139/f80-051
Selin H (2014) Global environmental law and treaty-making on hazardous substances: the Minamata Convention and mercury abatement. Glob Environ Polit 14(1):1–19. https://doi.org/10.1162/GLEP_a_00208
Shakir SK, Azizullah A, Murad W, Daud MK, Nabeela F, Rahman H, Häder DP (2016) Toxic metal pollution in Pakistan and its possible risks to public health. Rev Environ Contam Toxicol 242:1–60. https://doi.org/10.1007/978-3-319-51243-3
Sharma HK, Singh RK (2019) Comparative water quality assessment of Central Himalayan Lake: Nainital Technology
Shen F, Liao R, Ali A, Mahar A, Guo D, Li R, Xining S, Awasthi MK, Wang Q, Zhang Z (2017) Spatial distribution and risk assessment of heavy metals in soil near a Pb/Zn smelter in Feng County, China. Ecotoxicol Environ Saf 139:254–262. https://doi.org/10.1016/j.ecoenv.2017.01.044
Sheng J, Wang X, Gong P, Tian L, Yao T (2012) Heavy metals of the Tibetan top soils. Environ Sci Pollut Res 19(8):3362–3370
Simex SA, Helz GR (1981) Regional geochemistry of trace elements in Chesapeake Bay. Environ Geo 3:315–323
Smith SL, MacDonald DD, Keenleyside KA (1996) A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. J Great Lakes Res 22(3):624–638. https://doi.org/10.1016/S0380-1330(96)70985-1
Soni R, Bhatnagar A, Vivek R, Singh R (2012) A systematic review on mercury toxicity from dental amalgam fillings and its management strategies. J Sci Res 56:81–92
Sorensen JA, Glass GE, Schmidt KW, Huber JK, Rapp GR (1990) Airborne mercury deposition and watershed characteristics in relation to mercury concentrations in water, sediments, plankton, and fish of eighty northern Minnesota lakes. Environ Sci Technol 24(11):1716–1727. https://doi.org/10.1021/es00081a015
Soto-Jimenez M, Paez-Osuna F (2001) Distribution and normalization of heavy metal concentrations in mangrove and lagoonal sediments from Mazatlan Harbor (SE Gulf of California). Estuar Coast Shelf Sci 53:259–274. https://doi.org/10.1006/ecss.2000.0814
Storelli MM (2008) Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem Toxicol 46(8):2782–2788. https://doi.org/10.1016/j.fct.2008.05.011
Streets DG, Lu Z, Levin L (2018) Historical releases of mercury to air, land, and water from coal combustion. Sci Total Environ 615:131–140. https://doi.org/10.1016/j.scitotenv.2017.09.207
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ Geol 39(6):611–627. https://doi.org/10.1007/S002540050473
Swartz RC (1999) Consensus sediment quality guidelines for polycyclic aromatic hydrocarbon mixtures. Environ Toxicol Chem 18(4):780–787. https://doi.org/10.1002/etc.5620180426
Tayemeh MB, Esmailbeigi M, Shirdel I, Joo HS, Johari SA, Banan A, Nourani H, Mashhadi H, Jami MJ, Tabarrok M (2020) Perturbation of fatty acid composition, pigments, and growth indices of Chlorella vulgaris in response to silver ions and nanoparticles: a new holistic understanding of hidden ecotoxicological aspect of pollutants. Chemosphere 238:124576. https://doi.org/10.1016/j.chemosphere.2019.124576
Tong Y, Zhang W, Hu D, Ou L, Hu X, Yang T, Wei W, Ju L, Wang X (2013) Behavior of mercury in an urban river and its accumulation in aquatic plants. Environ Earth Sci 68(4):1089–1097. https://doi.org/10.1007/s12665-012-1810-0
Torres YP, Gallardo KC, Verbel JV (2018) Mercury pollution by gold mining in a global biodiversity hotspot, the Choco biogeographic region, Colombia. Chemosphere 193:421–430
Tripathee L, Kang S, Rupakheti D, Paudyal R, Huang J, Sharma CM, Zhang Q, Rupakheti D, Chen P, Ghimire PS, Gyawali A (2016) Spatial distribution, sources and risk assessment of potentially toxic trace elements and rare earth elements in soils of the Langtang Himalaya, Nepal. Environ Earth Sci 75(19):1–2. https://doi.org/10.1007/s12665-016-6140-1
Tripathee L, Guo J, Kang S et al (2018) Concentration and risk assessments of mercury along the elevation gradient in soils of Langtang Himalayas, Nepal. Hum Ecol Risk Assess. https://doi.org/10.1080/10807039.2018.1459180
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31(3):241–293. https://doi.org/10.1080/20016491089226
USEPA (1989) Office of Emergency and Remedial Response. Risk assessment guidance for Superfund: interim final (Vol. 1). Office of Emergency and Remedial Response. United States Environmental Protection Agency
USEPA (1992) National Center for Environmental Assessment. United States Environmental Protection Agency
USEPA (1995) Guidance for Risk characterization. Science Policy Council, United States Environmental Protection Agency
USEPA (1997) Mercury Study Report to Congress, Volume III: Fate and Transport of Mercury Fate in the Environment; USEPA: Washington, DC, USA, United States Environmental Protection Agency
USEPA (2002) Method 1631, revision E: mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry
USEPA (2003) National Center for Environmental Assessment. United States Environmental Protection Agency.
USEPA (2010) Guidance for implementing the January 2001 Methylmercury Water Quality Criterion. EPA 823-R-10 001. United States Environmental Protection Agency., Of- fice of Water, Washington, DC.National Research Council (NRC), Wahshington, DC. Committee on the Toxicological Effects of Methyl Mercury. National Academies Press
USEPA (2011) Exposure factors handbook: edition (EPA/600/R-09/052F)
UNEP (2018) Global Mercury Assessment 2018: sources, emissions, releases and environmental transport. United Nations Environment Programme, Chemicals Branch, Geneva, Switzerland
UNEP (2008) Assessment Programme (AMAP), and United Nations Environment Programme (UNEP): technical background report to the global atmospheric mercury assessment. Switzerland, Geneva
USEPA (2000) Risk-based concentration table. United States Environmental Protection Agency, Washington DC, Philadelphia
Wang F, Outridge PM, Feng X, Meng B, Heimburger-Boavida LE, Mason RP (2019) How closely do mercury trends in fish and other aquatic wildlife track those in the atmosphere?–implications for evaluating the effectiveness of the Minamata Convention. Sci Total Environ 674:58–70. https://doi.org/10.1016/j.scitotenv.2019.04.101
WHO/FAO (1990) Environmental Health Criteria. Methylmercury. vol. 101, Geneva, Switzerland. Available at: http://www.inchem.org/documents/ehc/ehc/ehc101.htm
WHO/FAO (2003) FAO/WHO Global forum of food safety regulators, Marrakech, Morocco
WHO/FAO (2009) Children’s exposure to mercury compounds. (NLM classification: QV 293)
Wiener JG, Knights BC, Sandheinrich MB, Jeremiason JD, Brigham ME, Engstrom DR, Woodruff LG, Cannon WF, Balogh SJ (2006) Mercury in soils, lakes, and fish in Voyageurs National Park (Minnesota): importance of atmospheric deposition and ecosystem factors. Environ Sci Technol 40(20):6261–6268. https://doi.org/10.1021/es060822h
Xu X, Bryan AL, Mills GL, Korotasz AM (2019) Mercury speciation, bioavailability, and biomagnification in contaminated streams on the Savannah River Site (SC, USA). Sci Total Environ 668:261–270. https://doi.org/10.1016/j.scitotenv.2019.02.301
Yan N, Liu W, Xie H, Gao L, Han Y, Wang M, Li H (2016) Distribution and assessment of heavy metals in the surface sediment of Yellow River, China. J Environ Sci 39:45–51. https://doi.org/10.1016/j.jes.2015.10.017
Yang L, Zhang Y, Wang F, Luo Z, Guo S, Strähle U (2020) Toxicity of mercury: molecular evidence. Chemosphere 245, Article 125586. https://doi.org/10.1016/j.chemosphere.2019.125586
Zhang W, Cao F, Yang L, Dai J (2018) Distribution, fractionation and risk assessment of mercury in surficial sediments of Nansi Lake, China. Environ Geochem Health 40(1):115–25. https://doi.org/10.1007/s10653-017-9922-9
Zhong W, Zhang Y, Wu Z, Yang R, Chen X, Yang J, Zhu L (2018) Health risk assessment of heavy metals in freshwater fish in the central and eastern North China. Ecotoxicol Environ Saf 157:343–349. https://doi.org/10.1016/j.ecoenv.2018.03.048
Zupo V, Graber G, Kamel S, Plichta V, Granitzer S, Gundacker C, Wittmann KJ (2019) Mercury accumulation in freshwater and marine fish from the wild and from aquaculture ponds. Environ Pollut 255:112975. https://doi.org/10.1016/j.envpol.2019.112975
Acknowledgements
The authors are thankful to the Institute of Urban Environment Chinese Academy of Sciences for Hg analysis
Funding
The financial support was provided by Pakistan Science Foundation under National Sciences Linkages Program Project No. (PSF/NSLP/KP-AWKUM (827).
Author information
Authors and Affiliations
Contributions
Javed Nawab, Sardar Khan, Muhammad Idress, and Ali Asghar collected the samples from remote alpine lakes, and analyzed and interpreted the data for Hg. Javed Nawab, Junaid Ghani, and Muhammad Idress helped in Hg data analysis and reviewed the manuscript. Muhammad Idress, Junaid Ghani, and Ali Asghar performed the Hg analysis and calculated the values. Javed Nawab, Syed Aziz Ur Rehman, Muhammad Luqman, Sardar Khan, and Ziaur Rahman play important role in writing, revising, and polishing the manuscript. All the authors carefully read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors are aware of this publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Severine Le Faucheur
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nawab, J., Ghani, J., Rehman, S.A.U. et al. Biomonitoring of mercury in water, sediments, and fish (brown and rainbow trout) from remote alpine lakes located in the Himalayas, Pakistan. Environ Sci Pollut Res 29, 81021–81036 (2022). https://doi.org/10.1007/s11356-022-21340-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21340-5