Abstract
In the current study, a protected subtropical wetland in Bihar (India), Baraila Lake, was investigated for heavy metal (Pb and Hg) status. These metals tend to bioaccumulate in fish, posing a concern to human health. This study reported the concentration of lead and mercury in water, sediment, and fish muscles of Baraila Lake in the year 2022. The samples were collected from pre-monsoon and post-monsoon seasons at four sampling locations, i.e., Loma, Dhulwar, Chakaiya, and Kawai Baraila, and were analyzed in triplicates. Lead concentration in water samples of all four sites of Baraila Lake observed during pre-monsoon and post-monsoon season exceeded the permissible limit for drinking water, while the mercury concentration of all sites was under the permissible limit in both seasons as prescribed by WHO. The extent of elemental pollution was evaluated using the Geo-accumulation index (Igeo), contamination factor (CF), contamination degree (Cd), ecological risk factor (Er), and the potential ecological risk index (Ri). Lead concentration in fish muscles of both seasons exceeded the permissible limit, while the concentration of mercury exceeded in Xenentodon cancila (0.55 ± 0.07 µg/g) during the pre-monsoon season. Also, estimated daily intake (EDI), target hazard quotient (THQ), and hazard index (HI) were calculated in different fish muscles to assess potential human health risks. A higher THQ value of 1.303 was observed in carnivore fish during the pre-monsoon season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands play a critical role in maintaining ecological harmony and fostering diverse aquatic life. However, wetlands across the globe are experiencing continuous degradation primarily due to the escalating intensity of agricultural activities, which involves widespread use of pesticides and fertilizers, as well as improper disposal of untreated sewage which significantly contributes to hazardous heavy metals in aquatic environments [1,2,3]. Besides this, industrial activities [4], mining and smelting [5], combustion of fossil fuel refining [6], and discharge and disposal of domestic and municipal wastes [7] also lead to heavy metal pollution. Hence, it is crucial to evaluate the dispersion of heavy metals and determine their degree of contamination to understand how these substances accumulate and are transported into the aquatic environment.
The entrance of heavy metals into wetland ecosystems, impacting both water quality and the trophic structure as well as the function of communities, occurs through various pathways [8,9,10]. Moreover, within the aquatic environment, heavy metals undergo substantial deposition into sediments, facilitated by processes such as adsorption, precipitation, diffusion, chemical reactions, and biological activity [11]. Sediments also serve as repositories for heavy metals, accumulating them through processes such as the chemical and physical breakdown of rocks, soil percolation, and the physiological activities of plants [12].
The presence of heavy metal contamination in wetlands not only degrades water and sediment quality but also the infiltration of heavy metals into the food chain poses a significant threat to aquatic ecosystems, as highlighted by Pandiyan et al. [13]. Organisms inhabiting wetland environments face the risk of accumulating pollutants over time, exposing them to both lethal and sublethal effects, particularly from heavy metals [14]. Accumulation of heavy metals in fish and other aquatic organisms occurs through both direct and indirect means. Direct exposure involves the consumption of contaminated water and food through the digestive system, while indirect exposure occurs through permeable membranes such as the skin and gills. The detrimental effects typically manifest when the rate of metal uptake surpasses the capacity of the organism’s metabolism, storage, and detoxification mechanisms [15, 16]. Due to their position at the top of the food chains within wetland ecosystems, fish play a crucial role as reliable indicators of metal pollution levels in aquatic settings. Consequently, fish are extensively utilized for evaluating the overall health of aquatic ecosystems, given that pollutants tend to accumulate in the food chain, resulting in detrimental effects and fatalities within aquatic systems [17, 18]. The accumulation of heavy metals in aquatic organisms, marked by their elevated levels of toxicity, persistence, and the potential for accumulation within the human body following the consumption of contaminated fish, poses significant health risks to humans [19,20,21,22,23,24,25,26,27]. Consequently, it remains crucial and necessary to assess the accumulation of heavy metal content in economically important fish species widely consumed by humans.
Baraila Lake, a wetland of immense importance plays a pivotal role as a vital freshwater ecosystem, serving as a primary water source for domestic and agricultural needs and providing habitat for various aquatic species, including fish. It holds significant importance as the sole surface water resource for approximately 100 villages, catering to the escalating water demands for irrigation, livestock maintenance, and long-term use amid a rapidly growing population [28]. Additionally, the lake recharges groundwater and sustains the livelihoods of 24 villages spanning 20,000 hectares. Eco-sensitive villages, numbering 10, with a population of 49,819 as per the 2011 census in Vaishali District, heavily rely on the services of this wetland [29]. However, concerns have arisen due to the rapid urbanization, agricultural activities, vehicles, pollutants coming from Noon River, and establishments such as poultry farms, prompting an evaluation of potential heavy metal contamination in this environmentally fragile ecosystem. The understanding of the impact of heavy metals in Baraila wetlands is limited, with no comprehensive studies on heavy metal concentrations in water, sediment, and fish muscles. Thus, this study aims to address this gap by (i) assessing levels of lead and mercury in Baraila Lake’s water, sediment, and fish muscles; (ii) gauging the extent of elemental pollution through indices such as Geo-accumulation index (Igeo), contamination factor (CF), contamination degree (Cd), ecological risk factor (Er), and potential ecological risk index (Ri); and (iii) identifying potential risks associated with heavy metal exposure to aquatic life and local communities, using the evaluation of target hazard quotient (THQ) and hazard index (HI) for persistent pollutants like lead and mercury.
Material and Methods
Study Area
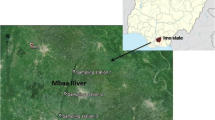
The Baraila Wetland, located in the northeastern region of India, holds significant importance for biodiversity and urgently requires attention. Not only does it serve as a habitat for both native and migratory bird species, but it also stands out as a host for the largest international bird communities in India, originating from regions such as Siberia, Mongolia, Africa, Eurasia, and Japan [30]. Encompassing an area of 12.7 square kilometers, the Baraila wetland experiences seasonal flooding and receives monsoon water from three blocks of the Vaishali District through the Noon River, affecting a population of around 1.18 million people [31]. Additional water sources include the Baya River and Gandak River [32]. The study area includes four sites, namely Loma, Dhulwar, Chakaiya, and Kawai Baraila, each recognized for their significant ecological and environmental value (Map 1). These sites were selected because their accessibility facilitated comprehensive fieldwork and data collection. Secondly, despite the prevalence of weed infestation affecting more than three-fourths of the Baraila wetland area, these selected sites were comparatively less impacted by this issue. To address the need for conservation, the Government of Bihar designated the wetland as a sanctuary in 1997, later officially naming it the Baraila Lake Salim Ali Jubba Sahni Bird Sanctuary in 2016 [33]. In collaboration with the World Bank and the United Nations Development Programme (UNDP), the Government of India has outlined a plan for Baraila wetland conservation (State Action Plan on Climate Change) [34, 35].
Collection and Preservation of Water, Sediment, and Fish Samples
Samples were collected from four distinct sites in Baraila Lake, namely Site 1 (Loma), Site 2 (Dhulwar), Site 3 (Chakaiya), and Site 4 (Kawai Baraila), using the methodology recommended by APHA [36] and de Zwart and Trivedi [37]. The collection of water samples was carried out in triplicates. For lead analysis, thoroughly cleaned PTFE bottles were soaked in 1 + 1 conc. HNO3 for 24 h then was washed with distilled water. Then, water samples were preserved by the addition of 1 ml HNO3. Similarly for the analysis of mercury, thoroughly cleaned Scott Durham bottles were first soaked in 1 + 1 conc. HNO3 for 24 h then was washed with distilled water and was then preserved with 5 ml K2Cr2O7 and 2 ml HNO3.
Sediment samples of each site, intended for the analysis of heavy metals (Pb and Hg), were obtained using a core or grab sampler and were transported to the laboratory in airtight plastic bags at 4 °C. Subsequently, the sediment samples were subjected to air drying; then, it was ground to powder in a mortal pestle, followed by its sieving through a 2-mm sieve.
Fish samples designated for heavy metal analysis (Pb and Hg) were procured from fishermen of Baraila Lake, and then their length and weight were estimated; after, that the fish samples were transported to the laboratory in an ice box. A total of eighty-seven (87) fish samples were collected during the pre-monsoon season, encompassing seven distinct species: Labeo bata, Channa punctatus, Xenentodon cancila, and Cabdio morar, each obtained in triplicates, while Pethia phutunio, Esomus danrica, and Trichogaster fasciata were collected in quantities of 25. Eleven (11) fish samples representing 5 species were collected during the post-monsoon season, revealing the presence of Channa punctatus, Heteropneustes fossilis, and Puntius sophore in triplicates and Labeo catla and Cirrhinus mrigala in singular quantities. Upon arrival at the laboratory, the samples underwent a thorough wash with fresh water to eliminate mud or other impurities. The muscle tissue of each fish sample was then extracted and cut into pieces using a sterilized stainless knife. The muscles were subsequently subjected to oven drying. While sampling, samples were carefully handled to avoid any contamination. To confirm that no particles, such as sediment or other external particles, were included, fish samples were thoroughly washed with clean water as soon as possible after sampling. Fish of almost the same size and weight were considered for sampling. The collected samples were washed several times with distilled water. The muscle tissue of each fish sample was then extracted and cut into pieces using a sterilized stainless knife. The muscles were subsequently subjected to oven drying. Later, the dried samples were ground using a mortar and pestle and stored in a polybag pack in plastic bottles at—20 °C until further analysis. The working procedure started within 24 h of the samples being stored [38].
Digestion of Water, Sediment, and Fish Samples
Water, sediment, and fish samples for heavy metal analysis (Pb and Hg) were analyzed as per the methods given by de Zwart and Trivedi [37].
For the digestion of the water sample for lead analysis, 50 ml of the sample underwent evaporation with 5 ml concentrated HNO3, supplemented by an additional 5 ml HNO3 and subsequent dilution to 100 ml with distilled water. For sediment samples, 5 gm were treated with a mixture of concentrated HNO3 and perchloric acid, followed by drying, addition of more HNO3, and dilution to 100 ml. Fish samples, comprising 0.5 gm, underwent digestion with concentrated HNO3, HCl, and distilled water, followed by filtration and adjustment to 100 ml. In each case, the prepared solutions were analyzed in triplicates using atomic absorption spectrophotometry (AAS) (Perkin Elmer-Analyst 200). The AAnalyst 200 Atomic Absorption Spectrophotometer is a double-beam atomic absorption spectrophotometer for analysis.
For the analysis of inorganic mercury, 100 ml of water samples were heated in a water bath with 5 ml of 50% H2SO4 and 5% KMnO4 for 7–8 h. Then, after standing the samples at room temperature for 24 h, 10% hydroxylamine was added until the solution turned colorless. For sediment samples, 0.1–0.5 gm of powdered dried sediment was treated with 10 ml of conc. H2SO4 and then after 1 h, samples were heated in a water bath for 20 h with occasional shaking. Then, 2 ml of 50% KMnO4 was added to the samples, and then 1 ml of HNO3 followed by 10% hydroxylamine solution was added to the sample. And 0.5 gm of fish samples underwent digestion in a water bath with 4 ml H2SO4. Then, samples were added with 15 ml of 6% KMnO4 [39]. Afterward, the solution was cooled to room temperature and added with 5 mL of Tin (II) chloride (SnCl2). The samples were then analyzed in cold vapor atomic absorption spectrophotometry (CVAAS): Motras Scientific mercury analyzer (MS HG100).
Analytical Quality Control
All glassware underwent washing with 1 + 1 concentrated nitric acid (HNO3) followed by soaking in 10% nitric acid for 48 h and thorough rinsing with deionized water. Throughout the analysis of samples, high-quality analytical grade reagents (Merck: Germany) and deionized distilled water were utilized. The calibration curve was established with one blank and three standards, adhering to the optimal detection limit recommended by APHA [36]. Analysis was meticulously conducted to mitigate any potential contamination influence, achieving a correlation coefficient above 0.995 (observed r2 = 0.998) and maintaining the percent relative standard deviation (%RSD) below 10% throughout the testing process. Additionally, quality control measures were upheld by running a blank after every three samples and repeated comparison with a standard.
The 1000 ppm stock solutions were prepared using 0.1719 g of PbCl2 (Fisher Scientific, CAS No: 35658–65-2) and 0.1354 g of HgCl2 (Sisco Research Laboratory, CAT No: 7487–94-7) in 100 ml of deionized water [36]. The standard solution within the limit of detection of 1 to 20 mg/l of 20 to 100 ng was prepared for Pb and Hg respectively [36]. The same has been used as a control reference material (CRM) during the analysis. Further, quality control (QC) was maintained by running a blank after every three samples and repeated comparison with a standard.
Sediment Quality Assessment
It plays a crucial role in the thorough evaluation of the level of pollution in sediment, as suggested by Mazurek et al. [40].
Geo-accumulation Index (I-geo)
The geo-accumulation index (Igeo) is defined by the following equation:
Here, Cn represents the concentration of metals analyzed in sediment samples, while Bn denotes the geochemical background concentration of the respective metal (n). The factor 1.5 accounts for the correction of the background matrix, considering lithospheric effects [41].
Contamination Factor (CF)
This is derived by comparing the concentration of heavy metals measured in the sediment of the water body to the pre-industrial reference value for the corresponding metal.
In this context, Ci represents the observed concentration of heavy metals in sediment, while Cin stands for the established pre-industrial reference level (measured in mg/kg), set at 70 for lead and 0.25 for mercury, as documented by [42].
Contamination Degree (Cd)
Hakanson [42] proposed a methodology employing a diagnostic indicator known as the contamination degree (Cd). Cd is determined by summing the individual contamination factors (Cif) for each sample, as per the following equation:
Hakanson [42] introduced a classification system for the contamination degree, where Cd values are categorized as follows: Cd < 6 signifies a low level of contamination; 6 < Cd < 12 indicates a moderate level of contamination; 12 < Cd < 24 suggests a substantial level of contamination; and Cd > 24 points to a high level of contamination, signifying severe anthropogenic pollution.
Ecological Risk Factor (E.ir) and Potential Ecological Risk Index (Ri)
In this study, the method developed by Hakanson [42] for potential ecological risk index was utilized. According to this approach, the potential ecological risk coefficient (Eir) for an individual element and the potential ecological risk index (Ri) for multiple elements can be calculated using the following equations:
Here, Cif represents the cumulative coefficient of element i, and Tif stands for the toxic-response factor of element i, which reflects its toxicity levels and the sensitivity of bio-organisms to it. The toxic-response factors for the prevalent heavy metals, such as Pb and Hg, were 5 and 40, respectively, as documented by [42, 43].
Health Risk Assessment
Estimated Daily Intakes (EDI)
Song et al. [44] were followed to calculate the estimated daily intake (EDI) of heavy metals.
In this context, C represents the average heavy metal concentration in fish muscle (µg/g) based on dry weight. FIR (food ingestion rate) denotes the daily consumption of freshwater fish per capita, set at 19.5 × 10–3 kg/day as per previous studies [45]. For this study, the ingestion rate is considered as 27 × 10–3 kg/person/day. BW stands for the average body weight, assumed to be 70 kg for adults, following the guidelines from USEPA [46].
Non-carcinogenic Risk
Non-carcinogenic risk assessments are commonly carried out to evaluate the potential health risks posed by pollutants, employing the target hazard quotient (THQ). The THQ values, determined through the consumption of fish species by the local population, can thus be evaluated for each heavy metal using the following equation, as indicated by USEPA [46] and Islam et al. [47].
In this equation, RfD stands for the oral reference dose (mg kg−1 d−1), which evaluates the health risks associated with consuming fish, as outlined by USEPA [46, 48]. The guideline reference doses for lead and mercury are 0.035 and 0.0003, respectively, following the guidelines set by USEPA [46, 48]. When the THQ value is less than 1, it indicates that the exposed population is unlikely to face any adverse health hazards. Conversely, if the THQ is equal to or higher than 1, there is a potential health risk, as noted by Wang et al. [49]. It has been observed that exposure to two or more pollutants may lead to additive and/or interactive effects [50]. Therefore, in this study, the cumulative health risk was assessed by summing the THQ values for individual metals and expressing it as a hazard index (HI), following USEPA guidelines [46].
Bio-Accumulation Factor (BAF)
The bio-accumulation factor was calculated following Fairbrother et al. [51], which is as follows:
In this context, X represents the concentration of metal in biota or sediment, while Y stands for the concentration of the same metal in water.
Data Analyses
Mean values of metal concentration in the samples of Baraila Lake were examined for statistical significance using Student’s t-test in which p < 0.05 was employed for comparing seasonal differences in metal concentration in the samples. For site-wise and species-wise variation, one-way ANOVA test was applied (p < 0.05). All statistical and graphical analysis was computed using IBM SPSS version 22 and MS Excel 2021.
Results
Trace Metal Concentrations in Water and Sediment Samples of Baraila Lake
Lead concentration in water samples of all four sites of Baraila Lake observed during pre-monsoon and post-monsoon periods exceeded the permissible limit of 0.01 µg/l [52, 53] as given in Table 1. Mercury concentration in water samples of all four sites of Baraila Lake observed during the pre-monsoon and post-monsoon periods was under the permissible limit of 0.001 µg/l and 0.006 µg/l as prescribed by [52, 53]. In water samples lead concentration ranged from 0.0175 µg/l (Site 2) to 0.0465 µg/l (Site 3) during pre-monsoon season and 0.020 (Site 4) to 0.045 (Site 1) during post-monsoon season, while mercury concentration ranged from 0.00014 µg/l (Site 3 and 4) to 0.00022 µg/l (Site 2) during pre-monsoon season and 0.00007 µg/l (Site 3 and 4) to 0.00024 µg/l (Site 1) during post-monsoon season. The Student’s t-test revealed that the mean concentrations of lead (µg/l) and mercury (µg/l) in water samples did not differ significantly during both seasons in Baraila Lake. The concentration of lead in sediment samples (Table 1) was under the threshold effect concentrations (TECs) of 35.8 µg/g [54] in all four sites of Baraila Lake during both seasons. However, the threshold effect concentrations (TECs) of mercury, i.e., 0.18 µg/g [54] in sediment samples exceeded at all sites during both pre-monsoon and post-monsoon periods except for Site 3 (0.0875 µg/g) during pre-monsoon season and Site 2 (0.08336 µg/g) during post-monsoon season. In sediment samples lead concentration ranged from 1.028 µg/g (Site 2) to 2.837 µg/g (Site 4) during the pre-monsoon season and 2.171 µg/g (Site 1) to 3.345 µg/g (Site 4) during post-monsoon season, while mercury concentration ranged from 0.0875 µg/g (Site 3) to 0.8632 µg/g (Site 1) during pre-monsoon season and 0.08336 µg/g (Site 2) to 0.58061 µg/g (Site 3) during post-monsoon season. The Student’s t-test revealed that the mean concentrations of mercury (µg/g) in sediment did not differ significantly during both seasons in Baraila Lake. However, the mean concentration of lead (µg/g) in the sediment of Baraila Lake (p = 0.046) differed significantly during both seasons, i.e., pre-monsoon and post-monsoon.
The results obtained for the level of lead and mercury in water and sediment samples of Baraila Lake were analyzed using One-way ANOVA between the sampling sites. No such significant differences in lead and mercury concentrations were observed in the water samples collected from Baraila Lake (p > 0.05). However, a statistically significant difference in the concentration of lead was observed (p = 0.002), while no significant difference in mercury concentration was observed in the sediment samples of Baraila Lake.
Sediment Quality Indices
To evaluate pollution levels and potential ecological risks linked to these heavy metals, the study employed established indices such as the geo-accumulation index (Igeo), contamination factor (CF), contamination degree (Cd), and the potential ecological risk index (Ri). The extent of metal pollution (geo-accumulation index) was characterized according to Abraham and Parker’s [55] enrichment classes: Igeo value > 5 (extremely contaminated); 4–5 (strongly to extremely contaminated); 3–4 (strongly contaminated); 2–3 (moderately to strongly contaminated); 0–1 (uncontaminated to moderately contaminated); and 0 < (uncontaminated). The geo-accumulation index values (Table 2) for Pb ranged from 0.0096 at site 2 to 0.0282 at site 4 during the pre-monsoon period and from 0.0204 at site 1 to 0.0318 at site 4 during the post-monsoon period. The values of the geo-accumulation index of Pb in Baraila Lake, Bihar, were found in the range of 0–1 which signifies uncontaminated to moderately contaminated sediment. While geo-accumulation index values for Hg ranged from 0.873 (uncontaminated to moderately contaminated) at site 3 to 8.6319 (extremely contaminated) at site 1 during pre-monsoon season and from 0.831 (uncontaminated to moderately contaminated) at site 2 to 5.805 (extremely contaminated) at site 3 during post-monsoon season.
The result of the present study shows that the contamination factor (CF) values of Pb in the study areas are low (> 1) which indicates that the sediments of all four sites are not polluted by lead. However, the contamination factor of mercury ranged from 0.35 (low contamination) at site 3 to 3.452 (considerable contamination) at site 1 during pre-monsoon season. The contamination factor of mercury ranged from 0.333 (low contamination) at site 2 to 2.322 (moderate contamination) at site 3 during post-monsoon season.
Regarding the contamination degree (Cd), Hakanson [42] proposed the following classification: When the level of contamination is less than 6, it is considered low; when it is between 6 and 12, it is considered moderate; when it is between 12 and 24, it is considered significant; and when it is greater than 24, it is considered high, signifying significant human-caused pollution. In the present study, the values of contamination degree (Cd) for both Pb and Hg at all four sites of Baraila Lake were less than 6, indicating a low degree of contamination. The contamination degree ranged from 0.385 at Site 3 to 3.478 at Site 1 during pre-monsoon season, while the contamination degree during post-monsoon season ranged from 0.365 at Site 2 to 2.367 at Site 3.
The potential ecological risk index method was proposed by Hakanson [42, 56] to assess the characteristics and environmental behavior of heavy metal contaminants in sediment. The potential ecological risk index (Ri) was employed to evaluate the level of heavy metal pollution in sediments, as introduced by Hakanson [42]. According to this classification, Eri < 40 indicates a low potential ecological risk, 40 < Eri < 80 is a moderate ecological risk, 80 < Eri < 160 is a considerable ecological risk, 160 < Eri < 320 is a high ecological risk, and Eri > 320 is a very high ecological risk. Similarly, Ri < 95 indicates a low potential ecological risk, 95 < Ri < 190 is a moderate ecological risk, 190 < Ri < 380 is a considerable ecological risk, and Ri > 380 is a very high ecological risk. The present study on Baraila Lake shows the value of Eri < 40 for lead which indicates a low potential ecological risk at all sites during both seasons. The ecological risk factor for mercury ranged from 14 (low potential ecological risk) at Site 3 and 138.112 (considerable ecological risk) at Site 1 during the pre-monsoon season, while during post-monsoon season values of ecological risk factor ranged from 13.32 (low potential ecological risk) at Site 2 and 92.88 (considerable ecological risk) at Site 3. While potential ecological risk index for lead and mercury ranged from 14.175 (low potential ecological risk; Ri < 95) at Site 3 and 138.242 (moderate ecological risk; 95 < Ri < 190) at Site 1 during pre-monsoon season, while during post-monsoon season, values of potential ecological risk factor ranged from 13.48 at Site 2 and 93.105 at Site 3 (low potential ecological risk; Ri < 95).
Trace Metal Concentrations in Fish Muscles of Baraila Lake
Heavy metal concentration in the muscles of fish from Baraila Lake is presented in Table 3. The identification of fishes based on feeding habits is based on Hora and Pillay [57], Day [58], Jayaram [59], and Talwar et al. [60]. Among the studied fish, the highest concentration of lead was observed in carnivore fish in both seasons. However, the highest concentration of mercury was observed in carnivores followed by herbivores and omnivores during the pre-monsoon season, while during the post-monsoon period, higher concentration was observed in omnivores followed by carnivores and herbivores during the post-monsoon period. The concentration of heavy metals in the muscles of fish was as follows: herbivore fish (lead, 0.5 to 7.02 µg/g; mercury, 0.05511 to 0.07885 µg/g) during pre-monsoon season and (lead, 0.7 to 0.72 µg/g; mercury, 0.0255 to 0.032 µg/g) during post-monsoon season; in carnivore fish (lead, 0.5 to 22.7 µg/g; mercury, 0.0299 to 0.60416 µg/g) during pre-monsoon season and (lead, 0.92 to 2.212 µg/g; mercury, 0.02776 to 0.04592 µg/g) during post-monsoon period; and in omnivore fish (lead, 3.82 to 4.24 µg/g; mercury 0.01989 to 0.05562 µg/g) during the pre-monsoon period and (lead, 0.86 to 2.18 mg/kg; mercury, 0.0187 to 0.03458 µg/g) during post-monsoon period. The concentration of lead and mercury in the muscle tissue of different fish species of Baraila Lake did not vary significantly as the observed p value for the one-way ANOVA was above 0.05.
The mean heavy metal concentration in fish samples of Baraila Lake was found in the following order of Pb > Hg during both seasons, i.e., pre-monsoon and post-monsoon period (Table 3). The highest lead concentration was observed in Channa punctatus during the pre-monsoon period (22.37 µg/g) and the post-monsoon period (2.21 µg/g) which belong to the carnivore bottom-columnar species, while highest mercury concentration (0.5526 µg/g) was observed in Xenentodon cancila belonging to carnivore surface-feeder species during the pre-monsoon period (0.0425 µg/g) and in Channa punctatus belonging to the carnivore bottom-columnar species during the post-monsoon period. This shows a clear role of feeding behavior on the accumulation of lead and mercury in the muscle tissue of fish species of Baraila Lake. The levels of Pb in the muscles of fish samples exceeded the limit of 0.5 µg/g prescribed by FAO and WHO [61, 62] in all fish muscles during both seasons, while the concentration of mercury in fish muscles exceeded the limit of 0.5 µg/g as prescribed by Commission Regulation (EC) [63] in Xenentodon cancila during pre-monsoon season (Fig. 1).
Values of Estimated Daily Intake and Target Hazard Quotient (THQ)
The estimated daily intake values of lead (Pb) and mercury (Hg) for herbivore, carnivore, and omnivore fish were found to be below the guideline reference doses of 0.035 and 0.0003 as per the guidelines set by the USEPA [46, 48] except for EDI value of lead in carnivore fish (4.561 × 10–3) observed during pre-monsoon season (Table 4). In this case, the observed lead value exceeded the guideline reference dose of 0.035, signaling a potential concern for health risks associated with the consumption of carnivore fish during this specific season. The target hazard quotient (THQ) values suggest that the risk associated with lead (Pb) is higher than that for mercury (Hg) (Fig. 2). Specifically, in the present study, a higher THQ value of 1.303 was observed in carnivore fish during the pre-monsoon season, indicating a significant potential hazard for human populations that consume these fish.
The hazard index (HI) or total THQ (TTHQ) value of lead was recorded in the following sequence: carnivore > omnivore > herbivore during both seasons. The hazard index for mercury was in the following order: carnivore > herbivore > omnivore during both seasons. This implies that carnivore fish, regardless of the season, tend to have higher cumulative health risks compared with herbivores and omnivores.
Values of Bioaccumulation Factor (BAF) for Different Metals (Lead and Mercury)
The bioaccumulation factors (BAF) for lead (Pb) and mercury (Hg) were investigated in sediments and aquatic organisms during the pre-monsoon and post-monsoon periods (Fig. 3), revealing distinct patterns across trophic levels. In sediment, BAF values for Pb ranged from 0.53 at Site 3 to 0.708 at Site 1 during pre-monsoon season while 0.48 at Site 1 to 1.63 at Site 4 during post-monsoon season. However, BAF values for mercury in sediment ranged from 6.11 at Site 3 to 53.28 at Site 1 during pre-monsoon season, while 5.29 at Site 2 to 78.99 at Site 3 during post-monsoon season.
Values of BAF (Pb) for fish samples (Fig. 4) ranged from 0.94 (herbivore) to 3.65 (carnivore), and BAF values for mercury in fish samples ranged from 2.4 (omnivore) to 17.51 (carnivore) during pre-monsoon. However, during the post-monsoon period, BAF values for Pb ranged from 0.23 (herbivore) to 0.52 (carnivore) and BAF values for Hg ranged from 1.78 (omnivore) to 2.68 (carnivore). The bioaccumulation of Hg in sediments and fish muscles surpassed that of Pb during both seasons, emphasizing the differential behavior of these heavy metals.
Discussion
The study has assessed the current levels of lead and mercury in both the biotic and abiotic components of Baraila wetland. Mercury was present in all water samples of Baraila Lake which might be due to domestic waste discharging in wetland [64]. However, the concentration of mercury was within the permissible limit, and there was no significant variation during pre-monsoon and post-monsoon seasons. Lead levels surpassed permissible limits at all sites throughout both pre-monsoon and post-monsoon periods. Although lead concentrations in water samples showed no significant variation, the pronounced lead levels at Sites 3 and 4 during the pre-monsoon season suggest a potential influence of meteorological conditions. Specifically, the intensified vaporization and reduced rainfall characteristic of the pre-monsoon season may have contributed to the elevated lead concentrations, whereas contrasting trends during the wet season could be attributed to dilution effects from rainfall [65]. These findings align with previous studies conducted by Salem et al. [66], Rajeshkumar et al. [67], Farsani et al. [68], and Manikandan et al. [69].
Elevated concentrations of lead and mercury stem from both natural and human-induced sources. Geological processes, influencing silicate composition, contribute to natural lead levels, typically below 50 mg/kg in the Earth’s crust [70]. Lead is subsequently removed from the atmosphere through rainfall, binding strongly with soil particles before entering water bodies, thereby perpetuating a continuous cycle [71]. Likewise, atmospheric inputs, particularly associated with rainfall, constitute the primary source of mercury in aquatic ecosystems [72]. Atmospheric deposition introduces various mercury forms, predominantly inorganic mercury [73, 74], while soil-bound mercury can be washed into surface waters during precipitation events. Human activities, such as the discharge of municipal wastes, application of fertilizers and pesticides, and runoff into lakes, can further elevate their levels [75, 76]. Poultry farms are another potential source, as they often utilize feed additives containing toxic metals like lead and mercury [77]. Mercury emissions, including burning waste, fossil fuel usage, and the use of fungicides, are additional anthropogenic sources [78,79,80]. Pollutants entering Baraila Lake from the Noon and Baya Rivers may also contribute to heavy metal contamination.
Significantly higher concentrations of lead were observed in sediment samples during the post-monsoon season, with a statistically significant difference (p = 0.002) between sampling sites using one-way ANOVA, and also differing significantly between seasons (p = 0.046) using t-test. The elevated concentrations, particularly at Sites 3 and 4, are primarily attributed to agricultural activities. Numerous studies have noted increased levels of heavy metals during the post-monsoon season, often linked to sediment accumulation [47, 67, 68, 81, 82]. Conversely, the highest concentrations of mercury in sediment samples were found during the pre-monsoon season, consistent with findings by Kwokal et al. [83, 84] and Ramasamy et al. [85]. Kwokal et al. [83] suggested that the highest mercury concentration observed at the surface/subsurface layers during the pre-monsoon season is due to post-depositional diagenetic processes, which mobilize the metal from deeper sediments and cause upward migration in the sediment column [86]. Sulphides also play a role as potential binding constituents involved in the cycling of mercury in sediments [87]. Additionally, bioturbation activities of macro zoobenthos in the mudflat of wetlands can induce physicochemical changes in the substrate, contributing to the remobilization of mercury from the bottom to the superficial sediments and into the water column [88].
The sediment in Baraila Lake indicated low to moderately contaminated levels (geo-accumulation index) of lead during both pre-monsoon and post-monsoon seasons, falling within the 0–1 range. Pradit et al. [89] found similar results in the Khuan Khi Sian Wetland of Thailand, where the average Igeo values for lead were less than 1. In contrast, mercury showed varying contamination levels, ranging from uncontaminated to extremely contaminated, indicating a more diverse pollution profile for this metal. Zhang et al. [90] also observed pollution in fish ponds in the West Lake of Hengshui Lake, with Pb and Hg showing moderate contamination due to population density and increased human social activities.
Contamination factor (CF) values, according to Hakanson’s [42] classification, indicate low contamination for lead, suggesting that the sediments were not significantly polluted by lead. Esmaeilzadeh et al. [91] found similarly low CF values for lead in the Siahkesheem Area of the Anzali Wetland, Iran. However, mercury exhibited low to considerable contamination at various sites, signifying a higher susceptibility to mercury pollution in certain areas of the lake. The high CF and Igeo values of Hg in the sediment suggested potential anthropogenic input and accumulation, consistent with previous studies indicating that high Hg concentration in sediment may originate from anthropogenic activity [92,93,94,95,96]. While the contamination degree (Cd) values indicated a low degree of contamination for both lead and mercury across all sites in Baraila Lake.
In Baraila Lake, low potential ecological risk for lead at all sites was observed. However, the potential ecological risk associated with mercury varied, with some sites showing low to moderate ecological risk levels, particularly during the pre-monsoon season. Similar findings were reported by Ben et al. [97], El Zrelli et al. [98], and Vahidipour [99].
Non-essential metals, such as lead and mercury, are not known to play any metabolic function; however, their bioaccumulation in fish can be toxic for humans, even at very low concentrations [100]. In Baraila Lake, fish have been found to accumulate heavy metals from runoff water, with lead concentrations in the muscles of various fish samples exceeding the limits set by FAO and WHO [61, 62]. Rashed [101] observed elevated lead concentrations in fish from freshwater ecosystems affected by activities like agriculture and poultry farming. However, mercury concentrations in all fish muscles remained within permissible limits, except for Xenentodon cancila during the pre-monsoon period. The heightened concentration of mercury in Xenentodon cancila, a carnivorous fish, during the pre-monsoon season may be attributed to factors such as trophic level, age, and size. Xenentodon cancila was measured at 21.2 ± 0.30 cm in length and 9.5 ± 0.21 gm in weight during the pre-monsoon season. Typically, older and larger fish tend to accumulate higher levels of mercury over time [102,103,104]. Moreover, fish at the top of the food chain often exhibit elevated mercury concentrations due to biomagnification [105, 106].
The concentrations of heavy metals in fish can vary significantly depending on the species and the specific aquatic environments they inhabit [107]. The increased metal accumulation in fish during pre-monsoon could be attributed to heightened biological activity, breathing rates, and metabolic rates due to elevated water temperatures. Similar seasonal patterns were reported in studies by Rajeshkumar and Li [16], Rajeshkumar et al. [67], Kumar et al. [82], and Kalita et al. [108].
The target hazard quotient (THQ) values reveal a heightened risk associated with lead (Pb) compared with mercury (Hg). Notably, during the pre-monsoon season, carnivore fish displays a significantly higher THQ value of 1.303, signaling a substantial hazard for human populations consuming these fish. This elevated THQ, particularly for lead in carnivore fish, underscores concern regarding potential health hazards. Prolonged exposure to elevated levels of lead can lead to various health issues [109, 110], including neurological and developmental problems. Despite its low absorption rates, chronic exposure to lead can accumulate in the human body, resulting in lead poisoning or toxicity. Lead’s neurotoxic effects, which include interference with neurotransmitter release, underscore its detrimental impact on cognitive functions and synaptic communication within the brain. Additionally, lead exposure is associated with adverse effects on the cardiovascular system, generating reactive oxygen species (ROS) and causing oxidative stress, leading to conditions such as cardiovascular disease and cancer [111]. Mercury (Hg), a neurotoxin that accumulates in the environment from both natural and human activities, raises significant concerns due to its broad spectrum of toxicological effects on various bodily systems, including cellular, cardiovascular, hematological, pulmonary, renal, immunological, neurological, endocrine, reproductive, and embryonic pathways [112,113,114,115,116,117,118]. It is well-documented as a neurotoxic agent capable of profoundly impacting the development and function of the human central nervous system (CNS), and it is widely distributed in the environment [116]. Renu et al. [115] indicated that mercury can induce apoptosis in the liver and, through epigenetic mechanisms, lead to DNA methylation and disruption of post-transcriptional modifications. Hence, addressing and managing the health risks associated with consuming carnivore fish, particularly during the pre-monsoon season when THQ values peak, is crucial.
The bioaccumulation factor (BAF) of heavy metal ions from water and sediment to fish tissues is depicted in Figs. 3 and 4. Notably, BAFs from water were found to be significantly higher than those from sediment, consistently exceeding 1 for mercury at all sites during both seasons. The study’s BAFs revealed that the concentration of measured metal ions in fish tissues followed the order of Hg > Pb. The diverse range of BAF values for both Hg and Pb across trophic levels indicates the intricate dynamics of metal accumulation in aquatic organisms, reflecting variations in feeding habits and habitat preferences. These findings suggest the potential for biomagnification, highlighting the ecological significance of understanding metal dynamics in aquatic ecosystems.
The presence of heavy metal pollution in Baraila Lake has implications not only for its immediate surroundings but also for similar water bodies globally. To effectively address this issue, a multifaceted approach is necessary, encompassing enhanced monitoring, enforcement of regulations, and the expansion of conservation efforts. Integration of phytoremediation techniques into environmental policies, alongside research into ecosystem-based solutions and technological innovations for pollution control, is crucial. Collaborative partnerships, capacity-building initiatives, and public awareness campaigns are essential for sustainable management practices and community engagement. Ultimately, these measures aim to preserve ecosystem integrity and support the well-being of local communities dependent on the lake, highlighting its broader significance in the context of environmental conservation.
Conclusion
The comprehensive assessment of water, sediment, and fish samples from Baraila Lake underscores significant findings regarding heavy metal contamination and potential ecological and health risks. The consistent exceedance of the WHO permissible limit for lead concentrations in water samples from Baraila Lake during both pre-monsoon and post-monsoon periods raises concerns about water quality. On the other hand, mercury concentrations in Baraila Lake water generally adhered to WHO limits. Sediment analysis demonstrated that lead concentrations consistently remained below the threshold effect concentrations (TECs), affirming the absence of lead pollution in the lake sediments. Conversely, mercury concentrations exceeded the TECs in sediment samples across all sites during both seasons, with specific exceptions. Geo-accumulation index values classified the sediments as uncontaminated to moderately contaminated for lead and exhibited a range from low to extremely contaminated for mercury. Contamination factor (CF) and contamination degree (Cd) values were employed to evaluate pollution levels, revealing consistently low contamination for lead across all sites. In contrast, mercury displayed varying degrees of contamination at different sites and seasons. The potential ecological risk index (Ri) method underscored that lead posed a low potential ecological risk, while mercury presented a considerable to moderate ecological risk, exhibiting diverse levels across sites and seasons. Analysis of heavy metal concentrations in fish muscles identified carnivore fish as consistently displaying the highest lead concentrations, while mercury concentrations varied across trophic levels and seasons. Channa punctata emerged as the species accumulating the highest lead concentration, whereas Xenentodon cancila exhibited the highest mercury concentration in fish muscles. Notably, lead concentrations in fish muscles surpassed the limits stipulated by FAO and WHO, while mercury concentrations exceeded regulatory thresholds in Xenentodon cancila during the pre-monsoon season. The bioaccumulation factors (BAF) revealed distinctive patterns across trophic levels, emphasizing the differential behavior of lead and mercury in sediments and aquatic organisms. Thus, the findings emphasize the necessity for ongoing monitoring efforts and proactive mitigation measures, including improved waste management practices, to address contamination sources and safeguard both the environment and human health.
Data Availability
No datasets were generated or analyzed during the current study..
Change history
18 June 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12011-024-04267-5
References
Atafar Z, Mesdaghrinia A, Nouri J, Homaee M, Yunesian M, Ahmadimoghaddan M, Mahvi AH (2010) Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess 160:83–89
Ogunlade MO, Agbeniyi SO (2011) Impact of pesticides use on heavy metals pollution in cocoa soils of Cross-River State, Nigeria African. J Agric Res 6(16):3725–3728
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6(9):e04691. https://doi.org/10.1016/j.heliyon.2020.e04691
Chaoua S, Boussaa A, El Gharmali A, Boumezzough A (2019) Impact of irrigation with wastewater on accumulation of heavy metals in soil and crops in the region of Marrakech in Morocco. J Saudi Soc Agri Sci 18(4):429–436
Chen H, Teng Y, Lu S, Wang Y, Wang J (2015) Contamination features and health risk of soil heavy metals in China. Sci Total Environ 15(512–513):143–153
Muradoglu F, Gundogdu M, Ercisli S, Encu T, Balta F, Jaafar HZ, Muhammad ZUH (2015) Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol Res 48(11):1–7
Khan I, Ghani A, Rehman AU, Awan SA, Noreen A, Khalid I (2016) Comparative analysis of the heavy metal profile of Brassica campestris (L.) and Raphanus sativus (L.) irrigated with municipal wastewater of Sargodha city. J Clin Toxicol 6:1–4. https://doi.org/10.4172/2161-0495.1000307
Peters K, Bundschuh M, Schäfer R (2013) Review on the effects of toxicants on freshwater ecosystem functions. Environ Pollut 180:324–329
Tang W, Shan B, Zhang W, Zhang H, Wang L, Ding Y (2014) Heavy metal pollution characteristics of surface sediments in different aquatic ecosystems in Eastern China: a comprehensive understanding. PLoS ONE 9:e108996
Song Q, Jabeen S, Shamsi IH, Zhu Z, Liu X, Brookes PC (2016) Spatio-temporal variability of heavy metal concentrations in soil-rice system and its socio-environmental analysis. Int J Agric Boil 18:403–411
Ramirez M, Serena M, Frache R, Correa J (2005) Metal speciation and environmental impact on sandy beaches due to El Salvador copper mine. Chile Mar Pollut Bull 50:62–72
Al-Saad HT, Mostafa YZ, Al-Imarah FJ (1997) Distribution of trace metals in tissues of fish from Shatt Al-Arab Estuary. Iraq Mar Meso 11:15–25
Pandiyan J, Mahboob S, Jagadheesan R, Elumalai K, Krishnappa K, Al-Misned F, Kaimkhani ZA, Govindarajan M (2020) A novel approach to assess the heavy metal content in the feathers of shorebirds: a perspective of environmental research. J of King Saud University, Sci 32(7):3065–3071
Zhang WW, Ma JZ (2011) Waterbirds as bioindicators of wetland heavy metal pollution. Procedia Environ Sci 10:2769–2774
Baki MA, Hossain MM, Akter J, Quraishi SB, Haque Shojib MF, Atique Ullah AKM, Khan MF (2018) Concentration of heavy metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in Saint Martin Island, Bangladesh. Ecotoxicol Environ Saf 15(159):153–163. https://doi.org/10.1016/j.ecoenv.2018.04.035
Rajeshkumar S, Li X (2018) Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol Rep 5:288–295. https://doi.org/10.1016/j.toxrep.2018.01.007
Squadrone S, Prearo M, Brizio P, Gavinelli S, Pellegrino M, Scanzio T, Guarise S, Benedetto A, Abete MC (2013) Heavy metals distribution in muscle, liver, kidney and gill of European catfish (Silurus glanis) from Italian Rivers. Chemosphere 90(2):358–365. https://doi.org/10.1016/j.chemosphere.2012.07.028
Salem DMSA, Khaled A, Nemr AE, El-Sikaily A (2014) Comprehensive risk assessment of heavy metals in surface sediments along the Egyptian Red Sea coast. Egypt J Aquat Res 40(4):349–362
Karim Z, Qureshi BA, Mumtaz M (2015) Geochemical baseline determination and pollution assessment of heavy metals in urban soils of Karachi, Pakistan. Ecol Ind 48:358–364
Hwang J, Song W, Hong D, Ko D, Yamaoka Y, Jang S, Lee Y (2016) Plant ABC transporters enable many unique aspects of a terrestrial plants’ lifestyle. Mol Plant 9(3):338–355. https://doi.org/10.1016/j.molp.2016.02.003
Gope M, Masto RE, George J, Hoque RR, Balachandran S (2017) Bioavailability and health risk of some potentially toxic elements (Cd, Cu, Pb, and Zn) in street dust of Asansol, India. Ecotoxicol Environ Saf 138:231–241
Ullah H, Noreen S, Fozia RA, Waseem A, Zubair S, Adnan M, Ahmad I (2017) Comparative study of heavy metals content in cosmetic products of different countries marketed in Khyber Pakhtunkhwa, Pakistan. Arab J Chem 10(1):10–18. https://doi.org/10.1016/j.arabjc.2013.09.021
Abarshi MM, Mada SB, Oyedeji DE (2017) Bioaccumulation of heavy metals in some tissues of croaker fish from oil spilled rivers of Nigeria Delta region, Nigeria. Asian Pac J Trop Biomed 7(6). https://doi.org/10.1016/j.apjtb.2017.05.008
Khan R, Strand MA (2018) Road dust and its effect on human health: a literature review. Epidemiol Health 40:e2018013. https://doi.org/10.4178/epih.e2018013
Liu XY, Bai ZK, Shi HD, Zhou W, Liu XC (2019) Heavy metal pollution of soils from coal mines in China. Nat Hazards 99(2):1163–1177. https://doi.org/10.1007/s11069-019-03771-5
Men C, Liu R, Wang Q, Guo L, Miao Y, Shen Z (2019) Uncertainty analysis in source apportionment of heavy metals in road dust based on positive matrix factorization model and geographic information system. Sci Total Environ 652:27–39
Tian SH, Tao L, Li KX (2019) Road dust contamination in a mining area presents a likely air pollution hotspot and threat to human health. Environ Int 128:201–209
Kumari P, Babu NJ, Chandrasekar S, Raj A, Barik SK (2023) Multivariate statistical analysis for water quality variation in Baraila Lake, Bihar. India. Austin Environ Sci 8(1):1090
Vaishali District (2022) Vaishali district population of rural and urban. Retrieved October 9, 2022, from https://www.indiagrowing.com/Bihar/Vaishali_District
Aslam A, Parthasarathy P, Ranjan RK (2021) Ecological and societal importance of wetlands: a case study of north Bihar (India). In: Singh P (ed) Sharma S. Wetlands Conservation: Current Challenges and Future Strategies, pp 55–86
Ashraf M, Nawaz R (2015) A comparison of change detection analyses using different band algebras for Baraila wetland with NASA’s multi-temporal Landsat dataset. J Geogr Inf Syst 7:1e19
Bharati KA (2018) indicative flora of eco-sensitive zone of Baraila Lake Salim Ali Jubba Sahni Bird Sanctuary, Vaishali District, Bihar. Central National Herbarium, Botanical Survey of India, Howrah. Retrieved from https://bsi.gov.in/uploads/userfiles/file/ESZ/Indicative%20Flora%20of%20ESZ%20of%20Baraila%20Lake%20BS_Vashali_compressed.pdf
Gazette of India (2015) Gazette notifications for Baraila lake Salim Ali Jubba Sahni bird sanctuary Vaishali district. Controller of Publications, Delhi, Bihar, p 110054
State Action Plan on Climate Change (SAPCC) (2015) Patna, India: Government of Bihar. Retrieved from http://moef.gov.in/wp-content/uploads/2017/08/Bihar-State-Action-Plan-on-Climate-Change-2.pdf
Comptroller and Auditor General of India (CAG) (2017) Audit report. (General, social, and economic sectors) for the year ended March 2017. Government of Bihar, Patna, India. Retrieved from https://calm.cag.gov.in//storage/media/eZc1C8ZLswWzemUsAS7CrdWlE2DorDzIBA3WnEUW.pdf
APHA (2017) Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association and Water Environment Federation
Zwart D de, Trivedi RC, De Kruijf HAM (1995) Manual on integrated water quality evaluation. Retrieved from https://rivm.openrepository.com/handle/10029/10572
Sulaiman MA, Kumari A (2024) Unveiling the rising threat of cadmium pollution and alarming health risks associated with the consumption of 15 commercially important fish species in the middle stretch of River Ganga, at Patna. Biological Trace Element Research, India. https://doi.org/10.1007/s12011-024-04164-x
Mallongi A, Rauf AU, Astuti RDP, Palutturi S, Ishak H (2023) Ecological and human health implications of mercury contamination in the coastal water. Global J Environ Sci Manag 9(2):261–274
Mazurek R, Kowalska J, Gqsiorek M, Zadrozny P, Jozefowska A, Zaleski T, Kepka W, Tymczuk M, Orlowska K (2017) Assessment of heavy metals contamination in surface layers of Roztocze National Park forest soils (SE Poland) by indices of pollution. Chemosphere 168:839–850. https://doi.org/10.1016/j.chemosphere.2016.10.126
Müller G (1979) Heavy metals in the sediment of the rhine-changes seity. Umsch Wiss Tech 79:778–783
Hakanson L (1980) An ecological risk index for aquatic pollution control. a sedimentological approach. Water Res 14:975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Ntakirutimana T, Du G, Guo JS, Gao X, Huang L (2013) Pollution and potential ecological risk assessment of heavy metals in a lake. Pol J Environ Stud 22(4):1129–1134
Song B, Lei M, Chen T, Zheng Y, Xie Y, Li X et al (2009) Assessing the health risk of heavy metals in vegetables to the general population in Beijing, China. J Environ Sci 21:1702–1709
Speedy AW (2003) Global production and consumption of animal source foods. J Nutr 133:4048S-4053S
US EPA (2011) Exposure Factors Handbook 2011 Edition (Final) EPA/600/R-09/052F. US Environmental Protection Agency: Washington, DC, USA. Available online: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=20563
Islam MS, Ahmed MK, Habibullah-Al-Mamun M, Raknuzzaman M (2015) The concentration, source, and potential human health risk of heavy metals in the commonly consumed foods in Bangladesh. Ecotoxicol Environ Saf 122:462–469
USEPA (2012) EPA region III risk-based concentration (RBC) table 2008 region III. 1650 Arch Street, Pennsylvania, Philadelphia, pp 19103
Wang XL, Sato T, Xing BS, Tao S (2005) Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci Total Environ 350:28–37
Hallenbeck WH (1993) Quantitative risk assessment for environmental and occupational health. CRC Press
Fairbrother A, Wenstel R, Sappington K, Wood W (2007) Framework for metals risk assessment. Ecotoxicol Environ Saf 68:145–227
BIS (2012) Indian standards drinking water specifications IS 10500:2012. Bureau of Indian Standards Drinking Water Specifications. Retrieved from https://cpcb.nic.in/wqm/BIS_Drinking_Water_Specification.pdf
WHO (2011) Joint FAO/WHO food standards programme codex committee on contaminants in food. Fifth session. The Hague, the Netherlands 90:21–25
Battelle (2006) Management of mercury-contaminated sediments: research, observations, and lessons learned. United States Environmental Protection Agency. Retrieved from https://clu-in.org/download/contaminantfocus/mercury/Hg-in-Sediment-2006DR.pdf
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ Monit Assess 136:227–238
Hakanson L (1988) Metal monitoring in coastal environments. Metals in coastal environments of Latin America. Springer, Berlin Heidelberg, pp 239–257
Hora SL, Pillay TVR (1962) Handbook on fish culture in the Indo-Pacific Region. FAO Fish Biol Tech Pap 14:204
Day F (1889) The fauna of British India including Ceylon and Burma. Fishes. The London, Taylor, and Francis 1:1–548
Jayaram KC (1981) The freshwater fishes of India. Zoological Survey of India, pp 1–438
Talwar PK, Jhingran A (1991) Inland fishes of India and adjacent countries. Oxford and IBH Publishing Co. New Delhi 1 & 2: 1158
FAO, WHO (1983) Compilation of legal limits for hazardous substances in fish and fishery products. Fish Circ 465:5-100
FAO, WHO (1989) National research council recommended dietary allowances, 10th edn. National Academy Press, Washington, DC. Retrieved from http://www.nap.edu/catalog/1349.html
Commission Regulation (EC) (2006) Setting maximum levels for certain contaminants in foodstuffs. Off J European Union. Retrieved from http://data.europa.eu/eli/reg/2006/1881/oj
Sophia S, John MMC, Prakash M (2017) Analysis and seasonal variation of heavy metals in water and sediment from Adyar Estuary. Environ Risk Assess Remediat 1(2):2–7
Khattabi H, Belle E, Servais P, Aleya L (2007) Temporal and Spatial fluctuations in bacterial abundances in 4 basins of landfill leachate treatment (Etueffont, France). Comptes Rendus Biologie 330:429–438
Salem ZB, Capelli N, Laffray X, Elise G, Ayadi H, Aleya L (2014) Seasonal variation of heavy metals in water sediment and rock tissues in landfill draining system pond (Etueffont, France). Ecol Eng 69:25–37
Rajeshkumar S, Liu Y, Zhang X, Ravikumar B, Ge BG, Li X (2018) Studies on seasonal pollution of heavy metals in water, sediment, fish, and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 191:626–638
Farsani NM, Haghparast JR, Naserabad SS, Moghadas F, Bagheri T, Gerami H (2019) Seasonal heavy metals monitoring of water, sediment and common carp (Cyprinus carpio) in Aras Dam Lake of Iran. Int J Aquat Biol 7(3):123–131
Manikandan R, Ezhili N, Muthulakshmiandal N, Venkatachalam P (2016) Assessment of physicochemical characteristics and the level of nutrient contents as well as heavy metal ions in waters of three lakes at Coimbatore. Tamil Nadu, India
Duffus JH (2002) Heavy metals" a meaningless term? (IUPAC Technical Report). Pure Appl Chem 74(5):793–807
Abadin H, Ashizawa A, Llados F, Stevens YW (2007) Toxicological profile for lead. US department of health and human services, agency for toxic substances and disease registry. Retrieved from https://stacks.cdc.gov/view/cdc/37676
Meili M (1991) The coupling of mercury and organic matter in the biogeochemical cycle—towards a mechanistic model for the boreal forest zone. Water Air Soil Pollut 56:333–347
Beiras R, Fernandez N, González JJ, Besada V, Schultze F (2002) Mercury concentrations in seawater, sediments and wild mussels from the coast of Galicia (NW Spain). Mar Pollut Bull 44(4):345–349
Carreón-Martínez LB, Huerta-Diaz MA, Nava-Lopez C, Siqueiros-Valencia A (2002) Levels of reactive mercury and silver in sediments from the port of Ensenada, Baja California, Mexico. Bull Environ Contam Toxicol 68:138–147
Bashir I, Lone FA, Bhat RA, Mir SA, Dar ZA, Dar SA (2020) Concerns and threats of contamination on aquatic ecosystems. Bioremediation and biotechnology: sustainable approaches to pollution degradation, pp 1–26. https://doi.org/10.1007/2F978-3-030-35691-0_1
Karouna-Renier NK, Sparling DW (2001) Relationships between ambient geochemistry, watershed land-use, and trace metal concentrations in aquatic invertebrates living in stormwater treatment ponds. Environ Pollut 112(2):183–192
Aljohani AS (2023) Heavy metal toxicity in poultry: a comprehensive review. Front Veterinary Sci 10. https://doi.org/10.3389/2Ffvets.2023.1161354
Counter SA, Buchanan LH (2004) Mercury exposure in children: a review. Toxicol Appl Pharmacol 198(2):209–230
Shah SB (2021) Heavy metals in the marine environment—an overview. Heavy metals in scleractinian corals, pp 1–26. Retrieved from https://springerlink.bibliotecabuap.elogim.com/chapter/10.1007/978-3-030-73613-2_1
Desforges JP, Mikkelsen B, Dam M, Rigét F, Sveegaard S, Sonne C, ... & Basu N (2021) Mercury and neurochemical biomarkers in multiple brain regions of five Arctic marine mammals. Neurotoxicology 84:136–145
Mohmmed AM, Ali MM, Islam MS, Rahaman MZ (2016) Preliminary assessment of heavy metals in water and sediments of Karnaphuli River Bangladesh. Environ Nanotechnol Monit Manag 5:27–35. https://doi.org/10.1016/j.enmm.2016.01.002
Kumar A, Kumar A, Jha SK (2020) Seasonal pollution of heavy metals in water, sediment, and tissues of catfish (Hetropneustes fossilis) from Gogabil Lake of north Bihar, India. Int J Fish Aquat Stud 8(2):163–175
Kwokal Z, Franciskovic-Bilinski S, Bilinski H, Branica M (2002) A comparison of anthropogenic mercury pollution in Kastela Bay (Croatia) with pristine eustaries in Ore (Sweden) and Krka (Croatia). Mar Pollut Bull 44:1152–1169
Kwokal Z, Sarkar SK, Chatterjee M, Franciskovic-Bilinski S, Bilinski H, Bhattacharya A, Bhattacharya BD, Aftab MA (2008) An assessment of mercury loading in core sediments of Sunderban mangrove wetland, India (a preliminary report). Bull Environ Contam Toxicol 81:105–112
Ramasamy EV, Jayasooryan KK, Chandran MSS, Mohan M (2017) Total and methyl mercury in the water, sediment, and fishes of Vembanad, a tropical backwater system in India. Environ Monit Assess 189:130
Rasmussen PE (1994) Current methods of estimating atmospheric mercury fluxes in remote areas. Environ Sci Technol 28:2233–2241
Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller CL (2003) Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. Biogeochemistry of Environmentally important trace elements. ACS Symp Ser 835:262–297
Birkett JW, Noreng JMK, Lester JN (2002) Spatial distribution of mercury in sediments and riparian environment of River Yare, Norfolk, UK. Environ Pollut 126:425–433
Pradit S, Noppradit P, Jitkaew P, Sengloyluan K, Kobkeatthawin T, Laerosa A, Sirivithayapakom, (2022) Heavy metal contamination and ecological risk assessment in the sediment cores of the wetlands in Southern Thailand. J Mar Sci Eng 10(12):1921. https://doi.org/10.3390/jmse10121921
Zhang Z, Wei S, Liu J (2021) Pollution characteristics and risk assessment of heavy metal elements in sediment in the West Lake of Hengshui Lake. Adv Mater Sci Eng 6:8178966
Esmaeilzadeh M, Mahmodpor E, Haghighat S, Esmaeilzadeh S, Aliani H, Yazdanfar N (2021) Contamination and ecological risk assessment of trace elements in sediments of the Anzali Wetland, Northern Iran. Water Sci Technol 84(9):2578–2590. https://doi.org/10.2166/wst.2021.455
Ranjbar Jafarabadi A, Mitra S, Raudonyte-Svirbutavičiene E, Riyahi Bakhtiari A (2020) Large-scale evaluation of deposition, bioavailability and ecological risks of the potentially toxic metals in the sediment cores of the hotspot coral reef ecosystems (Persian Gulf, Iran). J Hazard Mater 400:122988
Wang L, Hou D, Cao Y, Ok YS, Tack FMG, Rinklebe J, O’Connor D (2020) Remediation of mercury contaminated soil, water, and air: a review of emerging materials and innovative technologies. Environ Int 134:105281
Aguilar G, Valdés A, Cabré A, Galdames F (2021) Flash floods controlling Cu, Pb, As and Hg variations in fluvial sediments of a river impacted by metal mining in the Atacama Desert. J South Am Earth Sci 109:103290
Elsagh A, Jalilian H, Ghaderi Aslshabestari M (2021) Evaluation of heavy metal pollution in coastal sediments of Bandar Abbas, the Persian Gulf, Iran: mercury pollution and environmental geochemical indices. Mar Pollut Bull 167:112314
Huang WY, Huang CW, Li YL, Huang TP, Lin C, Ngo HH, Bui XT (2023) Reduced pollution level and ecological risk of mercury-polluted sediment in an alkali-chlorine factory’s brine water storage pond after corrective actions: a case study in Southern Taiwan. Environ Technol Innov 29. https://doi.org/10.1016/j.eti.2022.103003
Ben Mna H, Helali MA, Oueslati W, Amri S, Aleya L (2021) Spatial distribution, contamination assessment and potential ecological risk of some trace metals in the surface sediments of the Gulf of Tunis, North Tunisia. Mar Pollut Bull 170:112608
El Zrelli R, Yacoubi L, Wakkaf T, Castet S, Grégoire M, Mansour L, Rabaoui L (2021) Surface sediment enrichment with trace metals in a heavily human-impacted lagoon (Bizerte Lagoon, Southern Mediterranean Sea): spatial distribution, ecological risk assessment, and implications for environmental protection. Mar Pollut Bull 169:112512
Vahidipour M, Raeisi E, van der Zee SEATM (2022) Potentially toxic metals in sediments, lake water and groundwater of the Ramsar wetlands Bakhtegan-Tashk, South Iran: distribution and source assessment. Environ Technol Inno 28
Anwar MA, Elbekai HR, El-Kadi AO (2009) Regulation of CYP1A1 by heavy metals and consequences for drug metabolism. Expert Opin Drug Metab Toxicol 5:501–521
Rashed MN (2001) Cadmium and Lead levels in fish (Tilapia Nilotica) tissues as a biological indicator for lake water pollution. Environ Monit Assess 68(1):75–89
Evans R, Bails J, D’Itri F (1972) Mercury levels in muscle tissues of preserved museum fish. Environ Sci Technol 6:901–905
Peterson CL, Klawe WL, Sharp GD (1973) Mercury in tunas: a review. Fish Bull 71:603–613
Esmaili-Sari A, Ghasempouri SM, Ghasemzadeh G, Taheriazad L (2006) Determination of mercury concentration in liver, lien, and muscle of 10 freshwater fishes in Anzali wetland: a simple food chain. Madison, Wisconsin
Cheevaparanapivat V, Menasveta P (1979) Total and organic mercury in marine fish of the Upper Gulf of Thailand. Bull Environ Contam Toxicol 23:229–291
Burger J, Gochfeld M (2007) Risk to consumers from mercury in Pacific cod (Gadus macrocephalus) from the Aleutians: fish age and size effects. Environ Res 105(2):276–284
Kalay M, Ay O, Canli M (1999) Heavy metal concentrations in fish tissues from the Northeast Mediterranean Sea. Bull Environ Contam Toxicol 63:673–681
Kalita S, Kumar S, Sarma HP, Devi A (2021) Total organic carbon, heavy metal content and metal bioaccumulation in a freshwater wetland of Indo-Burmese province, India. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.1928104
Ahmed MK, Shaheen N, Islam MS, Mamun MH, Islam S, Mohiduzzaman M, Bhattacharjee L (2015) Dietary intake of trace elements from highly consumed cultured fish (Labeo rohita, Pangasius pangasius and Oreochromis mossambicus) and human health risk implications in Bangladesh. Chemosphere 128:284–292
Ambreen A, Syed AMASE, Nida H, Syeda MA, Mauro F, Habib B, Ioannis AK, Heqing S (2017) Human exposure to trace metals and arsenic via consumption of fish from river Chenab, Pakistan and associated health risks. Chemosphere 168:1004–1012
Flora SJ (2011) Arsenic-induced oxidative stress and its reversibility. Free Radical Biol Med 51(2):257–281
Rice KM, Walker EM, Wu MJ, Gillette C, Blough ER (2014) Environmental mercury and its toxic effects. J Prev Med Public Health 47:74–83
Yang L, ZhangY WF, Luo Z, Guo S, Strähle U (2020) Toxicity of mercury: MOLECULAR evidence. Chemosphere 245:125586
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12:643972
Renu K, Chakraborty R, Myakala H, Koti R, Famurewa AC, Madhyastha H, Vellingiri B, George A, Gopalakrishnan AV (2021) Molecular mechanism of heavy metals (lead, chromium, arsenic, mercury, nickel and cadmium)-induced hepatotoxicity: a review. Chemosphere 271:129735
Díez S (2009) Human health effects of methylmercury exposure. Rev Environ Contam Toxicol 198:111–132
Zhang L, Wong MH (2007) Environmental mercury contamination in China: sources and impacts. Environ Int 33:108–121
Bjørklund G, Dadar M, Mutter J, Aaseth J (2017) The toxicology of mercury: current research and emerging trends. Environ Res 159:545–554
Acknowledgements
The authors are thankful to all the members of the Environmental Biology Laboratory.
Author information
Authors and Affiliations
Contributions
S.A.: writing original draft, data curation. A.K.: supervision, writing review, and editing.
Corresponding author
Ethics declarations
Ethics Approval
Ethics clearance is not necessary.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A higher THQ value of 1303 was observed in carnivore fsh during the pre-monsoon season s/b A higher THQ value of 1.303 was observed in carnivore fsh during the pre-monsoon season.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anjum, S., Kumari, A. Bioaccumulation of Lead and Mercury in Water, Sediment, and Fish Samples of Baraila Lake, Vaishali, Bihar. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04224-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04224-2