Abstract
Mercury contamination in the water bodies of developing countries is a serious concern due to its toxicity, persistence, and bioaccumulation. Vembanad, a tropical backwater lake situated at the southwest coast of India, is the largest Ramsar site in southern India. The lake supports thousands of people directly and indirectly through its resources and ecosystem services. It is highly polluted with toxic pollutants such as heavy metals, as it receives effluent discharges from Kerala’s major industrial zone. In the present study, water, pore water, sediment, and fish samples collected from Vembanad Lake were analysed for total mercury (THg) and methyl mercury (MHg) contents. The maximum concentrations of THg and MHg in surface water samples were31.8 and 0.21 ng/L, respectively, and those in bottom water samples were 206 and 1.22 ng/L, respectively. Maximum concentration of THg in surface sediment was observed during monsoon season (2850 ng/g) followed by that in the pre-monsoon season (2730 ng/g) and the post-monsoon season (2140 ng/g). The highest sediment concentration of MHg (202.02 ng/g) was obtained during monsoon season. The spatial variation in the mercury contamination clearly indicates that the industrial discharge into the Periyar River is a major reason for pollution in the lake. The mercury pollution was found to be much higher in Vembanad Lake than in other wetlands in India. The bioaccumulation was high in carnivorous fishes, followed by benthic carnivores. The THg limit in fish for human consumption (0.5 mg/kg dry wt.) was exceeded for all fish species, except for Glossogobius guiris and Synaptura orientalis. The concentration of THg was five times higher in Megalops cyprinoides and four times higher in Gazza minuta. Significant variation was observed among species with different habits and habitats. Overall, risk assessment factors showed that the mercury levels in the edible fishes of Vembanad Lake can pose serious health impacts to the human population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury is an extremely toxic trace metal, naturally occurring in air, water, and soil (Lin et al. 2007; Li et al. 2009) and is one of the most studied pollutants (Geen Ruiz et al. 2005). Because of its transboundary nature, mercury is depositing in remote places where no specific sources present (Azevedo-Silva et al. 2016; Kang et al. 2016), with burdens in sediments and other non-biological materials estimated to have increased up to five times pre-human levels, primarily as a result of anthropogenic activities (Daga et al. 2016; Ramasamy et al. 2012; Brim et al. 1994). Earlier studies estimated a global natural mercury emission of 1800–5800 tons/year (Li et al. 2009). The global anthropogenic mercury emission to the atmosphere in 2000 was 2190 tons (Pacyna et al. 2006) and 54% of that was contributed by Asian countries (Li et al. 2009). Therefore, a number of studies have been undertaken on mercury contamination (Kang et al. 2016; Yin et al. 2016; Mason et al. 2006; Bhattacharya et al. 2014; Subramanian et al. 2003), speciation (Gabriel and Williamson 2004), bioavailability (Boszke et al. 2007; Bower et al. 2008; Davis et al. 1997), and bioaccumulation (Lawson and Mason 1998; Sunderland 2007) in various ecosystems.
Earlier studies estimated a global natural mercury emission of 1920.6 tons/year (Pacyna and Pacyna 2002; Wilson et al. 2010). Asian countries are the highest emitter (two thirds of the total emission) of anthropogenic mercury into the atmosphere (Pacyna et al. 2011). India is one among the top three emitters of mercury. According to Ryzhkov et al. (2011) mercury deposition has been significantly increased during the period of 1995–2005 in East and South Asia at 26 and 8% respectively.
The major sink of mercury in aquatic ecosystems is sediments. In sediments, it can stay for a long period and be transformed to more toxic, water-soluble organic forms (mainly methyl mercury) through biotic or abiotic processes (Tomiyasu et al. 2000). Mercury is very hazardous to aquatic ecosystems, especially for brackish water systems because of their high biological productivity (Geen Ruiz et al. 2005). Methyl mercury (MHg) accounts for the major portion of total mercury (THg) accumulated in fish (Lawrence and Mason 2001), posing a threat to human health because of its large half-life for elimination (UNEP 2002; Wiener and Spry 1996) and its biomagnification capability (Mieiro et al. 2009; Cizdziel et al. 2003). Mercury can also cause significant health impacts on fishes and other wildlife (Wiener et al. 2003; Scheuhammer et al. 2007).
Tropical regions (except the Florida Everglades and the Amazon basin) are less studied compared to temperate regions (Watras et al. 1994; Bowles et al. 2001). Therefore, it is very important to study the mercury pollution in developing countries such as India, which has become one of the hotspots of mercury pollution (Sharma 2003). There was little data in the United Nations Environment Program’s (UNEP) report (2002, 2008, and 2013) on mercury monitoring from India, which has been the largest user of mercury in processes and products releasing large amounts of mercury into the environment (Omana and Mahesh 2008; Sharma 2003; Down to Earth 2003; BSCB 2003).
Very few studies have been carried out on mercury pollution along the coastal regions of India (Mohan et al. 2014; Ramasamy et al. 2012; Omana and Mahesh 2008; Ram et al. 2003; Krishnamoorthy and Nambi 1999; Ouseph 1992; Mahajan and Srinivasan 1988; Srinivasan and Mahajan 1989). Studies on contamination of mercury in the lakes and rivers of India are equally meagre (Das et al. 2015; Karunasagar et al. 2006; Mohan and Omana 2004; Agarwal et al. 2007). The rise in mercury resistant bacteria (MRB) also indicates increased mercury pollution in these areas (Ramaiah and De 2003). Vembanad backwater, situated on the southern part of the western coast of India, is highly polluted due to industrial effluents, insecticide usage in agricultural fields, urban sewage, and soil erosion caused by the deforestation in the highland areas. More than 60% of industries in Kerala, including the chlor-alkali industry, are situated on the banks of the Vembanad backwaters (Ouseph 1996). Mercury cell process was used in the chlor-alkali industry and has been releasing three times of the national average. However, the industry decommissioned the mercury cell process and adopted membrane filter technology in 2004. Earlier studies (Li et al. 2009; Karunasagar et al. 2006; Lindeström 2001) report that even after elimination of such sources, mercury remains in the environmental matrices and may take a long period to return to background concentration levels.
In this context, it is very important to study the present state of mercury pollution in the Vembanad backwaters, particularly since a large number of people for their livelihood are using it. There are about 20,000 people engaged in fishing, with an annual catch of 72,000 tons. Furthermore, Vembanad is a Ramsar site. The present study is therefore significant in terms of the knowledge acquired on the distribution patterns of and factors influencing mercury concentrations in the water and sediment. The present study also investigates the accumulation of mercury in selected edible fishes of this backwater, and analyses the potential hazards of contaminated fish to the human population living around Vembanad.
Materials and methods
Study area
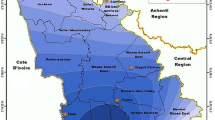
Vembanad Lake, one of three Ramsar sites in Kerala, is a brackish, humid tropical wetland ecosystem having an area of 151,250 ha (Fig. 1), and is of extraordinary importance for its hydrological function, its biodiversity, and its support of fish populations. The lake is connected to the Arabian Sea through Cochin estuary (Jayakumar 2002). The physical settings of Vembanad wetland has been well established by the earlier works of authors and other studies (Mohan et al. 2014; Joseph and Ouseph 2010; Ramasamy et al. 2012; Mohan and Omana 2007). Six major rivers are contributing fresh water into this system. Hence, Vembanad wetland is highly influenced by the fresh water input during the monsoon season as any other wetland systems in south west coast of India (Babu et al. 2010).
Reagents
All samples, reagents, and standards were prepared using ultrapure water (18.2 MΩ). Working standards were prepared from 1000-ppm standard stock solution of mercury (Merck, Germany). Stannous chloride (SnCl2) and sodium borohydride (NaBH4) were used as the reducing agents and were prepared daily. All glassware was cleaned according to the USEPA method 1631 RE (2002).
Instruments
A Cold Vapour Atomic Fluorescence Spectrometer (CVAFS- Model III, Brooks Rand, USA) was used for the determination of Hg concentration in all samples. Hg-free N2 was used as a purging gas, while high-purity A\argon was used as a carrier gas. Cold vapour of elemental mercury formed during aqueous reduction with SnCl2/NaBH4. The resulting Hg vapour was then purged with nitrogen and trapped in a gold trap. After 20 min of purging, the gold trap was moved to the trap desorption module, where the amalgamated mercury was thermally desorbed into the carrier gas stream and detected in the CVAFS detector.
An ultrasonic processor with 130 W power and 20 kHz frequency (Cole-Parmer Instruments, USA) was used for ultrasound-assisted extraction. Organic forms of mercury were converted to inorganic form using UV irradiation in a photochemical reactor (Scientific Aids and Instruments Corporation, India).
Sample collection and preservation
Water and sediment samples (250 g) were collected from 30 locations of the Vembanad Lake, near-shore areas and adjoining river systems during the post-monsoon, pre-monsoon, and monsoon seasons (Fig. 1). Surface and bottom water samples were collected and preserved as per standard procedures for analysis of total mercury and methyl mercury (Parker and Bloom 2005; USEPA 2001, 2002). Sediment samples were collected using a grab sampler, transferred to plastic bags, and kept in a deep freezer at 0 °C.
Fish samples were collected from the study area using traditional fishing methods, such as Chinese dipnets, with the help of local fishermen. Three specimens of each species were captured for the analysis. The fish samples were sealed in polyurethane bags and kept in a freezer at 0 °C. The length and weight of each fish were measured and were sealed in polythene bags and kept in freeze until analysis. The collected fish species form a major part of the human diet for populations living on the banks of Vembanad Lake.
THg and MHg in water
Total mercury (THg) was determined with CVAFS following bromine monochloride (BrCl) oxidation (USEPA–Method 1631, revision E, 2002).Organic mercury was extracted from water samples by selective extraction with dichloromethane (CH2Cl2) and HCl followed by UV irradiation (Logar et al. 2001 and Karunasagar et al. 2006). After the selective extraction, the organic layer was evaporated and mercury was extracted back into water. One portion of the solution was analysed for inorganic mercury. The second portion undergone UV irradiation in a photochemical reactor after acidification with 2%HCl for converting organic to inorganic forms of mercury. The UV irradiated samples were reduced with NaBH4 and detected inorganic mercury with CVAFS. The difference between inorganic and total mercury from the split sample was taken as the methyl mercury as the other forms of organic mercury are less stable, (Karunasagar et al. 2006).
THg and MHg in surface sediments
Sediment samples for THg analysis were dried at 40 °C. The dried samples were subsequently ground in an agate mortar and sieved to <63 μm fraction. Samples for THg analysis (0.1–0.25 g) were subjected to hot re-fluxing HNO3/H2SO4 digestion followed by bromine monochloride (BrCl) oxidation (USEPA 2001).
Alkali-leaching solvent extraction method (25% solution KOH-methanol), proposed by Liang et al. (1996) and later modified by Karunasagar et al. (2006), was used for the extraction of methyl mercury (MHg) in sediments. The results were presented in wet weight basis. After the solvent extraction, MHg was determined by the difference of total and inorganic Hg, as described above for water samples.
THg and MHg in fish
The fish samples were defrosted and rinsed with de-ionized water. Muscle tissue was extracted with stainless-steel instruments. Each tissue sample was dried at 50 °C to a constant weight. Hot re-fluxing with HNO3/H2SO4 digestion was followed by bromine chloride oxidation (USEPA 2001) for the extraction of mercury. MHg was extracted by rapid ultrasonic extraction (Krishna et al. 2005) followed by UV irradiation (Karunasagar et al. 2006). Extracted solutions were then analysed in the way described for THg and MHg in water samples.
Quality assurance
In order to check procedure validity, Certified Reference Materials (from the European Reference Materials of the Institute of Reference Materials and Measurements) ERM CC580 (Estuarine sediment) and ERM CE464 (Tuna fish) were used (Table 1). Replicates of the samples were analysed regularly. The detection limit of Hg was 0.08 ng/L. Pearson’s correlation (SPSS-11) was used for establishing the relationship between background Hg concentrations and the concentrations in fish.
Results and discussion
THg in water
The minimum and maximum concentrations of THg in surface water samples of post-monsoon were 5 and 31.8 ng/L, respectively (Table 2). During the pre-monsoon and monsoon seasons, THg varied from 6.7 to 28.4 and 8.3 to 31.5 ng/L, respectively. In the case of bottom water, the THg concentration was observed to be the maximum during the post-monsoon season (206 ng/L) and the minimum during the pre-monsoon season (2.4 ng/L).
The mean THg of surface and bottom water samples was calculated and graphically represented (Fig. 2). The mean concentration of THg in surface water samples from the different seasons did not show much variation (16.5–17.0 ng/L).Seasonal variation was observed, however, in bottom water THg concentrations (21.6–44.4 ng/L). The maximum mean THg concentration occurred in the bottom water collected during the post-monsoon season (44.4 ng/L). The maximum mean THg content was observed in the post-monsoon season (206 ng/L) whereas during the pre-monsoon and monsoon seasons, the mean THg concentrations varied between 43.7 and 58.1 ng/L. The minimum water concentration (2.4 ng/L) was observed during the pre-monsoon season. THg was high in northern parts of the lake and near-shore areas.
The Vembanad backwater system is being influenced by six major rivers of Kerala originated from the southern Western Ghats. The system is fully fresh water dominated with high rate of flow during the monsoon time. These rivers may carry a huge amount of surface runoff water from the catchment area. High fresh water flow may influence the sedimentary environment too as the depth of the lake is shallow and most of the places are 1.5 to 4 m deep except water ways where it is varied from 8 to 10 m. The high bottom water concentration obtained in near-shore regions during the post-monsoon season might be due to the dissolution of mercury from dredged sediments and to particulate matter carried by rivers during the end of the monsoon season. That the highest concentrations occur mainly in the bottom waters of the lower reaches of the Periyar River indicates dissolution of mercury (inorganic or organic) from bottom sediments. Furthermore, volatilization of Hg2+ due to photo-reduction can also happen during the pre-monsoon season, which would lower surface water concentrations.
MHg in water
The results showed that MHg content in water samples of all seasons was very low (Table 3). MHg was observed only at a few sites. The maximum MHg concentration in surface water observed during the post-monsoon season was 0.18 ng/L, while in the pre-monsoon and monsoon seasons, it was 0.21 ng/L. Among bottom water samples, the highest concentrations were observed in post-monsoon samples (1.22 ng/L). The minimum MHg concentration in bottom water varied from 0.1 ng/L (monsoon) to 0.3 ng/L (pre-monsoon).
As in the case of THg, variation in MHg concentration along different sites was observed only for bottom water (0.22–0.49 ng/L) and very little difference was observed in the surface water (0.13–0.16 ng/L). The maximum mean MHg was observed during the post-monsoon season (0.49 ng/L).
The mean values of surface and bottom water MHg showed that high concentrations were obtained during the post-monsoon season (Fig. 3). However, at the lower reaches of the Periyar River, post-monsoon samples showed a lower concentration than the other seasons. The sites in the southern part of Vembanad Lake showed maximum concentrations during the post-monsoon season, while in the pre-monsoon season MHg was below detection level.
The Lake Kodaikanal, which was polluted with the effluent from a thermometer factory, showed a higher concentration (34.24–57.36 ng/L) than the Vembanad (0.11–0.52 ng/L) (Karunasagar et al. 2006). The Cochin near-shore region contained a much lower THg than the Kalpakam near-shore (20,420 ng/L) on the southwestern coast of India (Satpathy et al. 2008). The results obtained by Kannan et al. (1998) showed that the concentration of methyl mercury accounted for 0.03–52% of the total mercury. In the case of Kodaikanal Lake, around 10% of the total mercury accounted for MHg, where the total mercury concentration of 356–465 ng/L and methyl mercury concentration of 50 ng/L were observed (Karunasagar et al. 2006). In this study, MHg content was 1.09% of THg.
THg and MHg in surface sediment
THg and MHg in sediments during various seasons are given in Figs. 4 and 5. 13.3% of the samples are below the detection limit for THg. The maximum concentration of THg during the post-monsoon period was observed at Site 9 (2140 ng/g), and the mean THg concentration obtained during this season was 387.49 ng/g (Fig. 4). The THg varied between 382 and 2140 ng/g along the northern limb of Cochin estuary (sites 8–13), whereas the southern part of Vembanad Lake (south of Thanneermukkom bund—sites 27–30) had very low concentrations (17–74.5 ng/g).
THg concentration was ranged from 47 ng/g (site 29) to 2730 ng/g (site 11) with a mean concentration of 755.72 ng/g during pre-monsoon (Fig. 4). Thirty percent of the samples were below detection limit. THg at the confluence point of Periyar River with Cochin estuary (sites 8–13) ranged from 550 to2730ng/g. Maximum THg obtained during monsoon season were 2850 ng/g (site 8) and the mean THg observed was 507.07 ng/g. The concentration was found higher at the near-shore regions.
Very few (13.3%) samples had detectable MHg, and the concentration varied from 0.38 to 9 ng/g during the post-monsoon season. The mean MHg was 6.35 ng/g during the pre-monsoon season. Seventy-seven percent of samples were below detection limit for MHg. Maximum MHg, obtained during monsoon season was 202.02 ng/g (site 11) with a mean of 22.57 ng/g. The mean concentration for THg obtained in this study was higher than the background concentration (200 ng/g) suggested by earlier studies (Craig 1986; Lindqvist et al. 1984).
The sediments of northern limb of the lake have high organic mercury content (sites 8–13) (Fig. 5). All other samples except few in this region and central part of the Lake were below the background concentration (<2 ng/g) as suggested by Davis et al. (1997). The distribution pattern of total mercury in the lake sediment showed high concentration at sites8–15. All of these points are located near the industrial area downstream to the bar mouth. The industrial effluent discharge into the river is a major source of this pollution. Slightly higher concentrations of THg were also recorded from the middle portion of the lake (sites 22 to 25). This may be due to the contribution of effluents from a public sector newsprint-manufacturing unit, which discharges its effluents to the Muvattupuzha River. High concentrations of THg in the comparatively unpolluted southern region of the lake are also an example of indiscriminate disposal of municipal solid wastes from the developing regions of central Kerala. Moreover, the unique water current pattern existing in the lake prevents the mixing and distribution of the Periyar River water from the northern limb to the southern portion of the lake, which is in turn nourished by the Muvattupuzha River. Since the flow from the Periyar River is directly discharged into the sea, the industrial pollution in the lake is confined to a small portion of the northern limb. However, the human population and the fishing activity in the area is very high, posing great risk to human beings even though the actual polluted area is small.
Comparison with Indian sites
Studies on mercury contamination in Indian aquatic ecosystems are rare, and hence, a comparison of THg concentrations in sediments with the concentrations reported for other lakes, rivers, and estuaries in India was very difficult. Still, the comparison (Table 4) indicated that the mean THg content in Vembanad Lake was higher than that in the Kodaikanal Lake (freshwater). The latter one was polluted with the effluent from a thermometer factory. However, the organic mercury (MHg) content was higher in Kodaikanal Lake. Not many studies were reported methyl mercury concentration in the aquatic systems of India except Karunasagar et al. (2006). Pristine lake like Berijam and Kukkal showed low THg content than the Vembanad.
THg content in Cochin estuary (<0.001–1.88 μg/g) observed in the present study is higher than the values obtained by Ouseph (1992) for the same region. This might be due to the release of industrial mercury as discussed earlier. However, the sediments of Cochin estuary have shown lower concentrations compared to other estuarine systems of India (Table 4). THg concentration of 0.38–2.85 μg/g as observed in the present study in the industrial region of study area was less compared with the earlier study (5.5–11.5 μg/g) carried out in the same region by Ouseph (1992). Also, he has demonstrated that the upstream regions of industrial area got very low values (0.04–0.05 μg/g) when compared with the lower reaches of Periyar river (Table 4).
Smith et al. (1996) suggested threshold effect level (TEL) (0.13 μg/g) and probable effect level (PEL) (0.70 μg/g) for total mercury in sediments. The mean concentration observed for THg in Vembanad Lake (0.395 μg/g) was between the observed TEL and PEL and this could be associated with adverse biological effects. In the present study, 16% of the sampling sites had Hg content higher than PEL while those of rest of the sites fell between TEL and PEL; hence, adverse effects on biota are expected to occur frequently.
The mercury concentration in sediments was high in the northern part of the lake, which may be the remnants of the once deposited mercury from the chlor-alkali industry. Even though the discharge of mercury has been ceased, the remnant of the mercury remains in the environment for a long time (Alonso et al. 2000; Karunasagar et al. 2006).
THg in pore water
Seasonal THg variation of pore water samples was analysed and the results were graphically represented (Fig. 6). During the post-monsoon season, site 12 showed a maximum concentration of 85.2 ng/L whereas site 29 was observed with the lowest value of 12.9 ng/L. Maximum THg in the pore water samples of the pre-monsoon season was observed at Site 13 (47.1 ng/L). The minimum concentration during this period was 7.4 ng/L. THg content of pore water samples varied from 0.075 to 22.96 ng/L. A larger number of samples had detectable THg content during pre-monsoon (84%) followed by post-monsoon (60%) and monsoon (53.4%).
THg in pore water samples was high during the post-monsoon season, except for the samples from the southern part of the lake and in near-shore areas where it was high during the pre-monsoon season (Fig. 6). The THg content in pore water samples from near-shore areas was detected only during the pre-monsoon season. During the monsoon season, mercury concentration was very low. High pore water mercury concentrations were observed in the northern part of the Cochin estuary followed by the central part of the lake (Fig. 6). During monsoon season, THg in pore water (THgPW) was not detected in the southern part of the lake. The Muvattupuzha River samples also did not have detectable THgPW content during any of the seasons.
THg and MHg in fish muscle
The length and weight of collected fish were measured (Table 5). Previous studies indicate that in non-tropical environments, seasonal variations have no significant effect on mercury bioaccumulation. According to Lewis and Chancy (2007), natural, physical, chemical, and biological factors of habitat, along with life stage, life mode, and feeding category are important factors in bioaccumulation of Hg. The fish samples were studied for total and organic mercury accumulation in the muscle tissue. Samples were analysed for each fish and the mean values are given in Table 6. The THg was calculated based on a dry weight (d.wt.) basis whereas MHg was based on a wet weight (w.wt.) basis. The fish were classified according to feeding habitat as benthic carnivorous, carnivorous (feeding fishes and zooplanktons), or omnivorous. Of the two benthic carnivorous fishes sampled, Arius arius showed higher THg (0.977 mg/kg) and MHg content (0.648 mg/kg w wt.) than Synaptura orientalis (Table 6). Of the carnivorous fishes, the maximum THg concentrations were observed in the muscles of Caranx affinis (3 mg/kg), and the minimum in Glossogobius giuris (0.118 mg/kg). Among the carnivorous fishes, MHg was not detectable in Thryssa mystax and varied from 0.46 mg/kg w.wt (Gazza minuta) to 2.26 mg/kg w.wt. (C. affinis) (Table 6). Cynoglossus semifasciatus showed the maximum THg (2.85 mg/kg d.wt.) and MHg (1.743 mg/kg w.wt.) content compared to other omnivorous fishes. Neither form of mercury was detectable in the muscles of Parambasis dayi.
As per the standards, total mercury in fish meat for human consumption should be below 0.5 mg/kg dry wt., but in the current study, this value was exceeded for all fishes except Glossogobius guiris and S. orientalis. The concentration of THg was measured at five times higher than the safe consumption level in Megalops cyprinoides and four times higher in G. minuta.
THg and MHg content in omnivorous, benthic carnivorous, and carnivorous fishes were graphically plotted (Fig. 7) to understand the variation in accumulation of mercury in relation with their diet. Higher THg and MHg content was observed in carnivorous fishes, followed by omnivorous and benthic carnivorous fishes.
The percentage of MHg to THg was calculated on a dry weight basis (Fig. 8), showing the efficiency of carnivorous fishes in accumulating more toxic organic mercury through their diet. The MHg accounts for 16.68 to 90.25% of THg and varied between species (Fig. 8). A higher percentage of MHg was obtained for pelagic carnivorous fishes (C. affinis and Stolephorus commersonnii), carnivorous bottom feeder fishes (A. arius, C. semifasciatus and Platycephalus indicus), and benthopelagic carnivorous fishes (Lutjanus johnii). High concentrations of mercury in the omnivorous and carnivorous fishes obtained in the current study can be compared with earlier reports (Durrieu et al. 2005). The concentration of mercury in carnivorous fishes was found to be exceeding the permissible limits in several studies (Durrieu et al. 2005).
The THg accumulation in the muscle tissue of carnivorous fishes in other regions of the world showed a similar pattern. Benthic carnivores of the present study showed slightly lower values, however (Table 7). A higher concentration of THg was observed for omnivorous fishes when compared to others (Table 8). The total mean concentration for Vembanad backwater fish was higher than those reported in earlier works, except for those at a Northeast Brazilian estuary (Table 8). The proportion of MHg in THg for carnivorous (max. 90.25%) and benthic carnivorous (max. 80.22%) fishes were found to be less than those reported from around the world (Table 7). Kehrig et al. (2009) observed 7–49% of MHg in THg content for benthic carnivorous fishes of Guanabara Bay, while the present study showed comparatively higher values (31.46–80.22%). Omnivorous fishes of other regions of the world, however, showed a lower percentage of MHg content than in the present study (Table 8). For instance, Agarwal et al. (2007) observed only 14% of THg present as MHg in bottom feeder fishes of the Gomti river (Table 8).
The relationship between THg and MHg accumulation and fish size was statistically tested (ANOVA) for various fish species. Significant difference was observed for THg and MHg in all the tested fishes, which were A. arius (F = 11.195, p < 0.05), Etroplussuratensis (F = 19.064 and 19.178, p < 0.05), and Gerres setifer (F = 19.38 and 19.79, p < 0.05). These results are in agreement with the principle of MHg bioaccumulation in organisms of aquatic food webs, which says that Hg concentrations increase with increasing size (Kainz et al. 2006; Barbosa et al. 2003). The trophic level of the organism based on the feeding habit is also an important factor for this variation as A. arius and others are benthic carnivore and ominivore respectively (Azevedo-Silva et al. 2016). Both positive and negative correlation between body size (age) and mercury bioaccumulation have been reported. Dorea et al. 2006 found no significant relationship between fish size and mercury bioaccumulation from fishes of the RioNegro. Lima et al. (2000) reported a negative correlation between fish size and bioaccumulation of Hg in fishes collected from Santarem, Brazil. Furthermore, the correlation depends on the sampling size and availability of Hg at the sampling station (Alonso et al. 2000). The species that showed positive correlation with size in the current study prefer strictly estuarine or fresh water habitats, where the probability of exposure to mercury from the lake is high.
Correlation was also carried out for mercury concentration in carnivorous, benthic carnivorous and omnivorous fishes with that in water and sediment (Table 9). THg and MHg in omnivorous fishes were positively correlated with sediment MHg and sediment THg. Other types of fishes did not show any positive correlation with THg and MHg in sediment, indicating that most of the mercury transport was through the food web. The feeding habit of omnivorous fishes (detritus feeding) exposes them to the sediment THg and MHg concentration. A significant positive correlation for THg and MHg content between carnivorous and benthic carnivorous fishes shows that their route of exposure is entirely different from omnivorous fishes. This was also confirmed by the negative correlation obtained for omnivorous fishes compared with carnivorous and benthic carnivorous fishes (Table 9). MHg of benthic carnivorous fishes and THg of omnivorous fishes are negatively correlated with water THg and MHg. The positive correlation ofMHg in omnivorous fishes with THg and MHg in water shows the presence of active diffusion of MHg and THg from sediments of the lake. The feeding habits are reported to be the most influencing factor in determining the bioaccumulation of mercury in fishes (Evans et al. 2000; Wiener et al. 2006). More studies are needed to confirm the role of mercury methylation rate in this part of the world.
THg content in fishes was negatively correlated with sediment THg (Table 10). A positive but not significant correlation was obtained between MHg in fishes and MHg in sediment (Table 10). The sediment showed a positive correlation (p = 0.01) between THg and MHg.
Bioaccumulation factor
Bioaccumulation factors were calculated using wet mass data as follows:
A mean water column MHg concentration of 0.1388 ng/L was used in these calculations (Bowles et al. 2001). Bioaccumulation factors were calculated based on the ratio of MHg concentrations in fish muscle tissues to that measured in water. Bioaccumulation factor (BAF) values were presented as log values (Table 11). Among the fish, a maximum BAF value was obtained for pelagic carnivorous species C. affinis (7.21) and a minimum was obtained for benthopelagic carnivorous fish G. giuris (5.54). Both maximum and minimum log BAF values were observed for carnivorous fish. Among the omnivorous fish species, C. semifasciatus showed the maximum BAF (7.10). In the benthic carnivorous group, the maximum BAF was observed for A. arius (6.67). Carnivorous (6.54) and omnivorous (6.51) fishes had almost same BAF values as the benthic carnivorous fishes (6.22). This further indicates that bioaccumulation was mainly through food. The BAF values obtained for carnivorous fishes were comparable with the result obtained by Bowles et al. (2001). He observed log BAF values of 5.84 to 7.25 for carnivorous fishes, while the values obtained in the present study were only slightly less (5.54–7.21). The omnivorous fishes also had similar values (6.045–7.099) as compared with the work done in Lake Murray (6.08–7.09) (Bowles et al. 2001).
Conclusions
The total and methyl mercury content in the water and sediments of Vembanad back water indicated that the mercury contamination is greatest the northern part of the system and is comparable to mercury contamination reported for other regions. The mercury content showed seasonal variation as the system is influenced by the fresh water input from six major rivers. The sediment mercury content is higher than that in water, and they do not positively correlate with each other. More than 16% of the samples have Hg content higher than the PEL. The high mercury content in the pore and bottom water indicates the formation of bioavailable forms of mercury in the sediments. The concentrations of MHg in sediments indicate varying physico-chemical conditions favouring the chemical transformation of mercury in the lake, which is further confirmed by the results obtained from the fish muscle analysis. The MHg content ranged from 16.68 to 90.25% of THg in fish muscle tissues. The statistical analysis showed that mercury accumulation is related to the feeding habits of the fish. High MHg concentrations were found in carnivorous fishes.
Frequent consumption of mercury contaminated muscle tissues can lead to higher accumulation in humans. More than 90% of the THg was in its most toxic organic form in fish, which increases concerns about THg concentration in the common edible fishes observed in the present study.
Because fish is a staple food for humans, the accumulation of metals exceeding permissible limits is a serious health concern. The present study highlighted the mercury bioaccumulation potential in fish, which constitute an important group of animals in this brackish water ecosystem. Since the high concentration of mercury in the backwaters is attributed to anthropogenic origins, adequate strategies are to be adopted in order to control the content so that the possible health hazards to different life forms—including humans—can be prevented.
Reference
Agarwal, R., Kumar, R., & Behari, J. R. (2007). Mercury and lead content in the fish species from the River Gomti, Lucknow, India. Bulletin of Environmental Contamination and Toxicology, 78, 118–122.
Alonso, D., Pineda, P., Olivero, J., Gonzalez, H., & Campos, N. (2000). Mercury levels in muscle of two fish species and sediments from the Cartagena Bay and the Ciénagas Grande de Santa Marta, Colômbia. Environmental Pollution, 109, 157-163.
Azevedo-Silva, C. E., Almeida, R., Carvalho, D., Ometto, J. P. H. B., de Camargo, P. B., Dorneles, P. R., Azeredo, A., Bastos, W. R., Malm, O., & Torres, J. P. M. (2016). Mercury biomagnification and the trophic structure of the ichthyofauna from a remote lake in the Brazilian Amazon. Environmental Research, 151, 286–296.
Babu, N. K., Omana, P. K., & Mohan, M. (2010). Water and sediment quality of Ashtamudi estuary, a Ramsar site, southwest coast of India—a statistical appraisal. Environmental Monitoring and Assessment, 165(1), 307–319.
Barbosa, A. C., Souza, J. D., Dorea, J. G., Jardim, W. F., & Fadini, P. S. (2003). Mercury bio magnification in tropical black water, Rio Negro, Brazil. Archives of Environmental Contamination and Toxicology, 45, 235–246.
Bhattacharya, S., Dubey, S. K., Dash, J. R., Patra, P. H., Das, A. K., et al. (2014). Assemblages of Total mercury in the tropical Macrotidal Bidyadhari estuarine stretches of Indian Sundarban mangrove eco-region. Journal of Environmental Analytical Toxicology, 4, 241. doi:10.4172/2161-0525.1000241.
Bonzongo, J. J., & Lyons, B. W. (2004). Impact of landuse and physiochemical settings on aqueous methyl mercury levels in the mobile-Alabama river system. Ambio, 33(6), 328–333.
Boszke, L., Kowalski, A., Astel, A., Baranski, A., Gworek, B., & Siepak, J. (2007). Mercury mobility and bioavailability in soil from contaminated area. Environmental Geology. doi:10.1007/s00254-007-1056-4.
Bourgoin, L. M., Quiroga, I., Chincheros, J., & Courau, P. (2000). Mercury distribution in waters and fishes of the upper Maderia rivers and mercury exposure in riparian Amazonian populations. Science of the Total Environment, 260, 73–86.
Bowles, K. C., Apte, S. C., Maher, W. A., Kawei, M., & Smith, R. (2001). Bioaccumulation and biomagnification of mercury in Lake Murray, Papua New Guinea. Canadian Journal of Fisheries and Aquatic Science, 58, 888–897.
Bower, J., Savage, K. S., Weinman, B., Barnett, M. O., Hamilton, W. P., & Harper, W. F. (2008). Immobilization of mercury by pyrite (FeS2). Environmental Pollution, 156, 504–514.
Brim, M.S., Bateman, D., Jarvis, R., Carmody, G. (1994). Mercury in fishes of the J. N. Ding Darling national Wildlife Refuge. U. S. Fish and Wildlife Service and Wildlife Enhancement. Publication No. PCFO-EC.94–03.
BSCB. (2003). India replaces US as biggest consumer of mercury. BS Corporate Bureau in New Delhi.
Cizdziel, J., Hinners, T., Crossb, C., & Pollard, J. (2003). Distribution of mercury in the tissues of five species of freshwater fish from Lake Mead, USA. Journal of Environmental Monitoring, 5, 802–807.
Costa, M. F., Barbosa, S. C. T., Barletta, M., Dantas, D. V., Kehrig, H. A., Seixas, T. G., & Malm, O. (2009). Seasonal differences in mercury accumulation in Trichiurus lepturus (Cutlassfish) in relation to length and weight in a Northeast Brazilian estuary. Environmental Science and Pollution Research. doi:10.1007/s11356-009-0120-x.
Craig, P. J. (1986). Organomercury compounds in the environment. In P. J. Craig (Ed.), Organometallic compounds in the environment: principles and reactions (pp. 65–110). Harlow: Longman.
Daga, R., Guevara, S. R., Pavlin, M., Rizzo, A., Lojen, A., Vreca, P., Horvat, M., & Arribere, M. (2016). Historical records of mercury in southern latitudes over 1600 years: Lake Futalaufquen, Northern Patagonia. Science of the Total Environment, 553, 541–550.
Das, T. B., Choudhury, A., & Senapati, R. N. (2015). Mercury emissions from coal fired power plants of India—case study. International Journal of Energy, Sustainability and Environmental Engineering Vol., 2(1), 21–24.
Davis, A., Bloom, N. S., & Hee, S. S. Q. (1997). The environmental geochemistry and bioaccessibility of mercury in soils and sediments: a review. Risk Analysis, 17(5), 557–569.
De Marco, S. G., Botte, S. E., & Marcovecchio, J. E. (2006). Mercury distribution in abiotic and biological compartments within several estuarine systems from Argentina: 1980-2005 period. Chemosphere, 65, 213–223.
Dorea, J. G., Barbosa, A. C., & Silva, G. S. (2006). Fish-mercury bioaccumulation as a function of feeding behavior and hydrological cycles of the Rio Negro, Amazon. Comparative Biochemistry and Physiology, 142, 275–283.
Down to Earth. (2003). Mapping the Scourge, India is a mercury pollution hotspot: Oct3–74.
Durrieu, G., Maury-Brachet, R., & Boudou, A. (2005). Goldmining and mercury contamination of the piscivorous fish hoplias aimara in French Guiana (Amazon Basin). Ecotoxicology and Environmental Safety 60(3), 315–323.
Gabriel, M. C., & Williamson, D. G. (2004). Principal biogeochemical factors affecting the speciation and transport of mercury through the terrestrial environment. Environmental Geochemistry and Health, 26, 421–434.
Geen Ruiz, C., Inzunza, J. R., & Osuna, F. P. (2005). Mercury in surface sediments and benthic organisms from Guaymas Bay, east coast of the Gulf of California. Environmental Geochemistry and Health, 27, 321–329.
Guentzel, J. L., Portilla, E., Keith, K. M., & Keith, E. O. (2007). Mercury transport and bioaccumulation in riverbank communities of the Alvarado Lagoon System,Veracruz State, Mexico. Science of Total Environment, 388, 316–324.
Jayakumar, M. (2002). Wetland conservation and management in Kerala. Government of Kerala: KCSTE.
Joseph, S., & Ouseph, P. P. (2010). Assessment of nutrients using multivariate statistical techniques in estuarine systems and its management implications: a case study from Cochin Estuary, India. Water and Environment, 24, 126–132.
Kainz, M., Telmer, K., & Mazumder, A. (2006). Bioaccumulation patterns of methyl mercury and essential fatty acids in lacustrine planktonic food webs and fish. Science of the Total Environment, 368, 271–282.
Kang, S., Huang, J., Wang, F., Zhang, Q., Zhang, Y., Li, C., Wang, L., Chen, P., Sharma, C. M., Li, Q., Sillanpaa, M., Hou, J., Xu, B., & Guo, J. (2016). Atmospheric mercury depositional chronology reconstructed from lake sediments and ice core in the Himalayas and Tibetan plateau. Environmental Science and Technology, 50(6), 2859–2869.
Kannan, K., Smith, R. G., Lee, R. F., Windom, H. L., Macauley, J. M., & Summers, J. K. (1998). Distribution of Total mercury and methyl mercury in water sediment and fish from South Florida estuaries. Archives of Environmental Contamination and Toxicology, 34, 109–118.
Karunasagar, D., Krishna, M. V. B., Anjaneyulu, Y., & Arunachalam, J. (2006). Studies of mercury pollution in a lake due to a thermometer factory situated in a tourist resort: Kodaikanal, India. Environmental Pollution, 143(1), 153–158.
Kehrig, H. A., Costa, M., Moreira, I., & Maim, O. (2001). Methyl mercury and total mercury in estuarine organisms from Rio de Janeiro Brazil. Environmental Science and Pollution Research International, 8(4), 275–279.
Kehrig, H. A., Seixas, T. G., Palermo, E. A., Baêta, A. P., Castelo-Branco, C. W., Malm, O., & Moreira, I. (2009). The relationships between mercury and selenium in plankton and fish from a tropical food web. Environmental Science and Pollution Research, 6, 10–24.
Krishna, M. V., Ranjit, M., Karunasagar, D., & Arunachalam, J. (2005). A rapid ultrasound assisted thiourea extraction method for the determination of inorganic and methyl mercury in biological and environmental samples by CVAAS. Talanta, 67(1), 70–80.
Krishnamoorthy, S., & Nambi, G. (1999). History of accumulation of mercury and nickel in Thane creek, Mumbai, using 210Pb dating technique. Science of the Total Environment, 236, 91–99.
Lawrence, L. A., & Mason, R. P. (2001). Factors controlling the bioaccumulation of mercury and methyl mercury in the estuarine amphipod Leptocheirus plumulosus. Environmental Pollution, 111, 217–231.
Lawson, N. M., & Mason, R. P. (1998). Accumulation of mercury in estuarine food chains. Biogeochemistry, 40, 235–247.
Lewis, M., & Chancy, C. (2007). A summary of total mercury concentration in flora and fauna near common contaminant sources in the Gulf of Mexico. Chemosphere. doi:10.1016/j.chemosphere.2007.09.020.
Li, P., Feng, X. B., Qiu, G. L., Shang, L. H., & Li, Z. G. (2009). Mercury pollution in Asia: a review of the contaminated sites. Journal of Hazardous Materials, 168, 591–601.
Liang, L., Horvat, M., Cerichiari, E., & Balogh, S. (1996). Simple solvent extraction technique for elmination of matrix interferences in the determination of methyl mercury in environmental and biological samples by ethylation-gas chromatography-cold vapour atomic flourescence spectrometry. Talanta, 43, 1883.
Lima, L. H. C., Návia, D., Inglis, P. W., & Oliveira, M. R. V. (2000). Survey of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) biotypes in Brazil using RAPD markers. Genetics and Molecular Biology, 23, 1-5.
Lin, C., He, M., Zhou, Y., Hu, L., Guo, W., Quan, X., & Yang, Z. (2007). Mercury contamination and dynamics in the sediment of the second Songhua River, China. Soil and Sediment Contamination, 16, 397–411.
Lindeström, L. (2001). Mercury in sediment and fish communities of Lake Vänern, Sweden: recovery from contamination, Ambio. The Large Lakes of Sweden, 30(8), 538–544.
Lindqvist, O., Jernelov, A., Johansson, K., Rohde, H. (1984). Mercury in the Swedish environment, Global and Local sources. National Swedish Environmental Protection Board. SNV report PM 1816.
Logar, M., Horvat, M., Akagi, H., Ando, T., Tomiyasu, T., & Fajon, V. (2001). Determination of total mercury and monomethyl mercury compounds in water samples from Minamata Bay, Japan: an interlaboratory comparative study of different analytical techniques. Applied Organometalic Chemistry, 15, 515.
Mahajan, B. A., & Srinivasan, M. (1988). Mercury pollution in the estuarine region around Bombay Island. Environmental Technology, 9, 331–336.
Marrugo-Negrete, J., Verbel, J. O., Ceballos, E. L., & Benitez, L. N. (2008). Total mercury and methylmercury concentrations in fish from the Mojana region of Colombia. Environmental Geochemistry and Health, 30, 21–30.
Mason, R. P., Kim, E., Cornwell, J., & Heyes, D. (2006). An examination of the factors influencing the flux of mercury, methylmercury and other constituents from estuarine sediment. Marine Chemistry, 102, 96–110.
Mieiro, C. L., Pacheco, M., Pereiraa, M. E., & Duartea, A. C. (2009). Mercury distribution in key tissues of fish (Liza aurata) inhabiting a contaminated estuary—implications for human and ecosystem health risk assessment. Journal of Environmental Monitoring, 11, 1004–1012.
Mohan, M., Omana, P.K. (2004).Mercury pollution in Vembanad Lake, Kerala, India presented in the International Conference on Conservation, Restoration and Management of Lakes and Coastal Wetlands, 9–13 December 2004 at Bhubaneswar, Orissa.
Mohan, M., & Omana, P. K. (2007). Statistical analysis of water quality data from a Ramsar site, Vembanad backwaters, southwest coast of India. Asian Journal of Microbiology Biotechnology and Environmental Science, Vol., 9(1).
Mohan, M., Shylesh, C. M. S., Jayasooryan, K. K., & Ramasamy, E. V. (2014). Mercury in the sediments of Vembanad Lake. Western Coast of India. Environmental Monitoring and Assessment., 186, 3321–3336. doi:10.1007/s10661-014-3620-1.
Mol, J. H., Ramlal, J. S., Lietar, C., & Verloo, M. (2001). Mercury contamination in fresh water estuarine, and marine fishes in relation to small scale gold mining in Suriname, South America. Environmental Research, 86(A), 183–197.
Nair, M.N.M. (1994) Residual mercury in the sediments of Beypore estuary. Proceedings of the 6th Kerala Science Congress. 60–61.
Omana, P. K., & Mahesh, M. (2008). The new mercury pollution threat to aquatic ecosystems of India—an example from Kerala. Ecology and Environment Conservation, 14(2–3), 1–4.
Ouseph, P. P. (1992). Dissolved, particulate and sedimentary mercury in the cochin estuary, south west coast of India. In W. Michales (Ed.), coastal and Estuarine studies (pp. 461–465). Berlin: Springer-Verlag.
Ouseph, P.P. (1996). Distribution of mercury, copper, zinc, cadmium, lead and chromium in the sediments of River Periyar and cochin harbour. Report submitted to Kerala State Council for Science, Technology and Environment.
Pacyna, E. G., & Pacyna, J. M. (2002). Global emission of mercury from anthropogenic sources in 1995. Water Air and Soil Pollution, 137, 149–165.
Pacyna, E. G., Pacyna, J. M., & Steenhuisen, F. (2006). Global anthropogenic mercury emission inventory for 2000. Atmospheric Environment, 40, 4048–4063.
Pacyna, J. M., Sundseth, K., Pacyna, E. G., Munthe, J., Kindbom, K., & Wilson, S. (2011). Mercury emissions on a global scale and their control options, the 10th International Conference on Mercury as a Global Pollutant (ICMGP). Halifax.
Paller, M. H., Bowers, J. A., Littrel, J. W., & Guanlao, A. V. (2004). Influence of mercury bioaccumulation factors for the Savannah River. Archives of Environmental Contamination and Toxicology, 46, 236–243.
Parker, J. L., & Bloom, N. S. (2005). Preservation and storage techniques for low level aqueous mercury speciation. Science of the Total Environment, 337, 253–263.
Ram, A., Rokade, M. A., Borole, D. V., & Zingde, M. D. (2003). Mercury in sediments of Ulhas estuary. Marine Pollution Bulletin, 46, 846–857.
Ramaiah, N., & De, J. (2003). Unusual rise in mercury-resistant bacteria in coastal environs. Microbial Ecology, 45(4), 444–454.
Ramasamy, E. V., Toms, A., Shylesh, C. M. S., Jayasooryan, K. K., & Mahesh, M. (2012). Mercury fractionation in the sediments of Vembanad wetland, west coast of India. Environmental Geochemistry and Health, 34(5), 575–586.
Roy, D. V., Chandrashekar, N., & Kumaresan, S. (2004). Mercury pollution in Tambraparni estuary, Tamilnadu, India. Pollution Research, 23(2), 251–258.
Ryzhkov, A., Dastoor, A., Figueras, D., Durnford, D., & Dugas, B. (2011). Atmospheric mercury trends from 1990 to 2005: a modelling analysis, the 10th International Conference on Mercury as a Global Pollutant (ICMGP). Halifax.
Satpathy, K. K., Natesan, U., Sarguru, S., Mohanty, A. K., Prasad, M. V. R., & Sarkar, S. K. (2008). Seasonal variation in mercury concentrations in the coastal waters of Kalpakkam, southeast coast of India. Current Science, 95(3), 374–381.
Scheuhammer, A. M., Meyer, M. W., Sandheinrich, M. B., & Murray, M. W. (2007). Effects of environmental methylmercury on the health of wild birds, mammals, and fish. AMBIO A Journal of the Human Environment, 36(1), 12–18.
Sharma, D. C. (2003). India:a hotspot for mercury pollution. Frontiers of Ecology and Environment, 1(9), 458.
Sinha, R. K., Sinha, S. K., Kedia, D. K., Kumari, A., Rani, N., Sharma, G., & Prasad, K. (2007). A holistic study on mercury pollution in the Ganga River system at Varanasi, India. Current Science, 92(9), 1223.
Smith, S. L., MacDonald, D. D., Keenleyside, K. A., & Gaudet, C. L. (1996). The development and implementation of Canadian sediment quality guidelines. In M. Munawar & G. Dave (Eds.), Development and progress in sediment quality assessment: rationale, challenges, techniques and strategies (pp. 233–249). Amsterdam: SPB Academic Publishing.
Srinivasan, M., & Mahajan, B. A. (1989). Mercury pollution in an estuarine region and its effects on a coastal population. International Journal of Environmental Studies., 35, 63–69.
Subramanian, V., Madhavan, N., Saxenab, R., & Lundin, L. (2003). Nature of distribution of mercury in the sediments of the River Yamuna (tributary of the Ganges), India. Journal Environmental Monitoring, 5, 427–434.
Sunderland, E. M. (2007). Mercury exposure from domestic and imported estuarine and marine fish in the U.S. seafood market. Environmental Health Perspectives, 115(2), 235–242.
Tomiyasu, T., Nagano, A., Yonehara, N., et al. (2000). Mercury contamination in the Yatsushiro Sea, south-western Japan: spatial variations of mercury in sediment. Science of the Total Environment, 257(2–3), 121–132.
UNEP. (2002). Global Mercury Assessment Report, draft1, 25, United Nations Environmental Program.
United States Environmental Protection Agency (2002). Method 1631, revision E. USEPA, Office of Water, Office of Science and Technology, Engineering and Analysis Division (4303), 1200 Pennsylvania Avenue NW, Washington, DC, 20460.
USEPA. (2001). Appendix to method 1631: Total mercury in tissue, sludge, sediment and soil by acid digestion and BrCl oxidation, United States Environmental Protection Agency.
Watras, C. J., Bloom, N. S., Hudson, R. J. M., Gherini, S., Munson, R., Class, S. A., et al. (1994). Sources and fates of mercury and methyl mercury in Wisconsin lakes. In C. J. Watras & J. W. Huckabee (Eds.), Mercury pollution: integration and synthesis (pp. 153–179). Boca Raton: Lewis Publishers.
Wiener, J.G., Spry, D.J. (1996). Toxicological significance of mercury in freshwater fish. In: Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations. Beyer W N, Heinz G H, Redman-Norwood AW (ed) Special Publication of the Society of Environmental Toxicology and Chemistry. Lewis Publishers, Boca Raton, FL, USA. pp 297–339.
Wiener, J.G., Gilmour, C.C., and Krabbenhoft, D.P. (2003). Mercury Strategy for the Bay-Delta Ecosystem: A Unifying Framework for Science, Adaptive Management, and Ecological Restoration. Final Report to the California Bay Delta Authority.
Wiener, J. G. K., Nights, B. C., Sandheinrich, M. B., Jeremiason, J. D., Brigham, M. E., Engstrom, D. R., Woodruff, L. G., Cannon, W. F., & Balogh, S. J. (2006). Mercury in soils, lakes, and fish in voyageurs National Park (Minnesota): importance of atmospheric deposition and ecosystem factors. Environmental Science and Technology, 40, 6261–6268.
Wilson S, Munthe J, Sundseth K, Kindbom K, Maxson P, Pacyna J, Steenhuisen F (2010) Updating Historical Global Inventories of Anthropogenic Mercury Emissions to Air. AMAP Technical Report No. 3, Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway.
Yin, R., Lepak, R. F., Krabbenhoft, D. P., & Hurley, J. P. (2016). Sedimentary records of mercury stable isotopes in Lake Michigan. Elementa, 4, 86.
Acknowledgements
The financial support from Ministry of Earth Sciences (MoES), Government of India, through their project (No. MoES/8/PC/2(3)/2007-PC-IV) is gratefully acknowledged. We also acknowledge the financial support from UGC and DST, Government of India, and KSCSTE, Government of Kerala, for setting up the lab facility. Authors also acknowledges the anonymus reviewers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramasamy, E.V., Jayasooryan, K.K., Chandran, M.S.S. et al. Total and methyl mercury in the water, sediment, and fishes of Vembanad, a tropical backwater system in India. Environ Monit Assess 189, 130 (2017). https://doi.org/10.1007/s10661-017-5845-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-5845-2