Abstract
Silver nanoparticles (Ag NPs) have wide medical and industrial applications; therefore, their release into aquatic environments is a problematic issue. The present study aims to evaluate the removal efficiency of Ag NPs from water using orange peel (OP) and banana peel (BP) to moderate their toxicity on Oreochromis niloticus. Fish were divided into 4 groups: control group (dechlorinated tap water), Ag NPs (4 mg/L) exposed group, Ag NPs (4 mg/L) + OP (40 mg/L) group, and Ag NPs (4 mg/L) + BP (40 mg/L) group for 24 h, 48 h, and 96 h. The adsorptive ability of both peels was confirmed by scanning electron microscope and energy-dispersive X-ray spectroscopy after the exposure processes. The biochemical results revealed a gradual elevation in plasma glucose, total proteins, globulin, liver enzymes (AST, ALT, and ALP), creatinine, and uric acid after Ag NPs exposure, while albumin and total lipid concentrations were significantly decreased. The recorded antioxidant biomarkers in gills, and liver tissues after Ag NPs exposure showed severe oxidative damages (maximally after 96 h) as indicated by marked elevations in thiobarbituric acid reactive substances, glutathione peroxidase, catalase, and superoxide dismutase values, and decreased glutathione reduced content. All studied parameters restored more or less to that of control groups after OP and BP water treatment. The adsorbent abilities of both peels could reduce Ag NPs bioavailability and moderate their toxicological impacts.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The extensive use of nanometals in several applications has resulted in overspreading of these nanoparticles (NPs) in aquatic environments (Zeumer et al. 2020). Therefore, nano-toxicity should be regulated and monitored to reach safe limits for both environmental and occupational safety. The environmental impacts of NPs are still questionable and seem to be very limited compared to other contaminants (Khan et al. 2018). Silver NPs are one of the most common nanometals, with a worldwide production rate of nearly 420 tons per year (Pulit-Prociak and Banach 2016). These NPs are classified as a very useful class of metallic NPs that have wide applications in the medical and industrial fields due to their unique physical, chemical, and biological properties (Khan et al. 2018). As a result of their extensive use, the amount of Ag NPs in the aquatic ecosystems will shortly increase leading to unknown hazardous effects on aquatic organisms (Khosravi-Katuli et al. 2018; Tortella et al. 2020). In aquatic environments, Ag NPs are present as colloidal particles that are easily invading the aquatic biota and accumulate in their tissues (Khan et al. 2018). Once these metallic NPs enter the biological system, they may interfere with the physiological processes of aquatic biota posing potential risks. In many toxicological studies, Oreochromis niloticus were used as an animal model because of their sustained and long-term tolerance to many aquatic stressors (Sarkar et al. 2015; Abdel-Khalek et al. 2018; Ibrahim et al. 2021). Lately, many biomarkers are commonly used in aquatic species to evaluate the nanotoxicological effects of NPs (Abdel-Khalek et al. 2020a). For example, the alteration in biochemical and antioxidant status using enzymatic and non-enzymatic endpoints is considered potential biomarkers for assessing the toxicological impacts of NPs in living organisms (Banaee et al. 2019; Kanwal et al. 2019). Several approaches were confirmed the adverse effects of metallic NPs as silver, nickel, iron, and aluminum on the biochemical status and antioxidant capacity of aquatic organisms (Kanwal et al. 2019; Abdel-Khalek et al. 2020b). Thus, metallic NPs should be handled properly before their release into the aquatic environments to meet the standard values. Several techniques such as precipitation, coagulation, ion exchange, reverse osmosis, membrane filtration, and advanced oxidation processes have revealed different degrees of remediation efficiency towards water contaminants including heavy metals (Zhu et al. 2016), but these methods showed some drawbacks as low removal efficiency, high-cost, high energy consumption, and generation of toxic by-products. To overcome these disadvantages, a bio-adsorbent method using agricultural products was developed (Anastopoulos and Kyzas 2014). The bio-adsorption method is the most effective and economical process because it depends on low-cost and highly available materials (Singh et al. 2018). Moreover, many agricultural wastes serve as strong adsorbents because of their unique chemical properties, lignin, and cellulose richness. Orange peel (OP) and banana peel (BP) showed a strong adsorption ability to many metals in their bulk form due to their specific constituents. Liang et al. (2010) reported that OP contains cellulose (11.1%), pectin (51.1%), hemicellulose (12.6%), lignin (1.0%), protein (7.8%), and other constituents in small quantity. Similarly, Ahmad and Danish (2018) reported that BP is a rich source of pectin (10–21%), lignin (6–12%), cellulose (7.6–9.6%), hemicelluloses (6.4–9.4%), and galacturonic acid. Therefore, the present work aims to (1) assess the toxicological impact of Ag NPs on Oreochromis niloticus and (2) to evaluate (for the first time) OP and BP as a bio-adsorbent to reduce the toxicological impacts of Ag NPs using integrated physicochemical, biochemical, and antioxidant endpoints.
Materials and methods
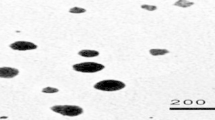
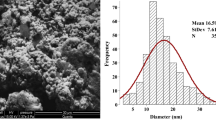
Preparation and characterization of silver nanoparticles
The silver nano-powders (product number of 576832) were purchased from Sigma-Aldrich, St. Louis, MO, USA. As provided by the product specification sheet of the manufacturer, the purity of NPs was 99.5%, the molecular weight was 107.87, and the particle size was less than 100 nm. To determine the actual size range of NPs, structural characterization of Ag NPs was done using field-emission transmission electron microscopy (FETEM; JEM- 2100F, JEOL Inc., Japan) at an accelerating voltage of 200 kV. The average hydrodynamic size of Ag NPs in an aqueous medium was recorded by dynamic light scattering (DLS) (Nano-zeta sizer-HT, Malvern Instrument, UK) and zeta potential was estimated by Malvern Zeta sizer Nano ZS instrument. According to Sarkar et al. (2015), the half lethal concentration (LC50/96 h) of Ag NPs to Oreochromis niloticus is 8 mg/L. In the present work, 1/2 LC50/96 h (4 mg/L) was used by dispersing the dry Ag nano-powders in dechlorinated water with a pH of 7.4, then ultrasonicated for 1 h. (100 W, 40 kHz) using an ultrasonic homogenizer (BioLogics, Inc., Manassas, VA, USA). The selected sub-lethal concentration (4 mg/L) of the present work was according to Sarkar et al. (2015) and Afifi et al. (2016) who reported this concentration as a sub-lethal concentration for O. niloticus with eliciting biological effects. No mortality was recorded during the present study after the exposure process. The nominal concentration of silver in water was 87 ± 2% of the actual concentration as measured by inductively coupled plasma (ICP-AES), Thermo Sci, model: iCAP6000 series. The percentage of released silver ions was also determined according to Navarro et al. (2008) by ultrafiltration and ultracentrifugation of Ag NPs (4 mg/L) for 30 min at 100.000 g; thereafter, the silver content in the supernatant was estimated and related to the total Ag content before filtration (amount of released Ag ions/initial amount of Ag X 100) by inductively coupled plasma (ICP-AES), Thermo Sci, model: iCAP6000 series.
Acclimatization of the experimental fish
Experimental fish (adult male Oreochromis niloticus) with a bodyweight range of 41.91–50.17 g and a body length range of 10–15 cm were obtained from an unpolluted fish farm located at Kafr El-Sheikh governorate, Egypt. Fish were transported to the ecology laboratory of the Faculty of Science, Cairo University, with good aeration conditions in large plastic containers. In glass aquaria (40 × 70 × 26 cm), fish were kept for 14 days in 50 L of aerated and dechlorinated tap water with 7 fish per aquarium. The physicochemical characteristics of water were monitored daily and maintained in a stable range of 25±1 °C for temperature, 7.1–7.8 mg/L for dissolved oxygen, and 7.2–7.4 for pH. During this period, fish were fed once daily with commercial food pellets (20% crude protein, 4% crude fat, 5% crude fiber, 12% crude ash, and 10% crude moisture). About 20 L of water was renewed daily using a suction pump to remove any debris or food remnants.
Preparation of orange and banana peels
The peels were washed several times with deionized water then cut into small fragments and left in the oven at 80 °C for 1 day. The dry adsorbents (OP and BP) were ground and sieved to obtain particle size between 1 and 5 mm according to the recommended size of Annadurai et al. (2003). A higher dose of adsorbent materials provides more active sites for possible contact with the target pollutants in the solution. Therefore, the used OP and BP concentrations were 40 mg/L (10 times the NPs concentration) as recommended by Akpomie and Conradie (2020). The adsorbents were isolated in the aquaria by a porous net that permitted the passage of Ag NPs to adsorbents without allowing the opposite to avoid the eating of absorbents by fish.
Experimental design
After the acclimatization period, eighty-four of healthy fish (swimming actively, normal skin appearance, and no sign of fungal infection) were divided into 4 groups: control group (dechlorinated tap water), Ag NPs (4 mg/L) exposed group, Ag NPs (4 mg/L) + OP (40 mg/L) group, and Ag NPs (4 mg/L) + BP (40 mg/L) group for 24 h, 48 h, and 96 h. Each experimental group was divided into 3 aquaria (7 fish/aquarium) and each aquarium was supplied by 4 oxygen pumps. At each time interval, 7 fish were sampled and processed from each group. The experimental and feeding conditions were maintained as in the acclimatization period.
Surface study on the surfaces of orange and banana peels
After each time interval, both types of used adsorbents (OP and BP) were collected and scanned to confirm the adsorption of Ag NPs on their surfaces using scanning electron microscopic (SEM) models FEI Inspect S 50-Netherlands and energy-dispersive X-ray spectroscopy (EDS) attachment with Bruker AXS-Flash Detector, 410-M-Germany.
Biosorbents and fish sampling
After each time interval, OP and BP were collected to measure the concentration of silver metals. Blood samples were taken from 7 fish in each group using a heparin-coated syringe for the biochemical analysis; then, the gills and liver tissues were isolated and stored at −80 °C for the antioxidant assay. All procedures performed in the current work involving fish were approved with approval no. CU I F 2019 and were following the ethical standards of the Faculty of Science, Cairo University, Institutional Animal Care and Use Committee (IACUC) at which the studies were conducted.
The concentration of Ag in both OP and BP
The concentrations of Ag metal were estimated in both peels after each time interval according to APHA (2005) using inductively coupled plasma (ICP-AES), Thermo Sci, model: iCAP6000 series. According to the method described by Neugebauer et al. (2000), the samples were left in the oven (80 °C for 8 h.) to dry out completely. A mixture of concentrated nitric acid (HNO3) and perchloric acid (HClO4) (4:1, v-v) was used in an acid digestion process. After complete digestion, the obtained clear solutions were diluted in volumetric flasks to a known volume. To check the accuracy of the measurement process, procedural blanks were aspirated along with the measuring process to correct background absorption. Besides, standard reference material (Lake Superior fish 1946 NIST, National Institute of Standards and Technology, USA) was used and the metal recovery ranges were between 94 and 106%. The concentrations of Ag in OP and BP were expressed as mg/Kg dry weight.
Biochemical biomarkers
Blood samples were centrifuged (3000 rpm for 10 min) to get plasma (using heparin as an anticoagulant) for biochemical endpoints using commercial kits (Biodiagnostic, Dokki, Giza, Egypt) for enzymatic/colorimetric methods.
The concentrations of plasma glucose and total lipids
The glucose level (mg/dL) was determined (at 510 nm) after converting all glucose into colored quinonemine as described by Trinder (1969). According to the method described by Knight et al. (1972), a pink-colored complex (equivalent to the total lipids) was obtained after treating samples with sulfuric acid, phosphoric acid, and vanillin. The obtained complex was determined colorimetrically at 545 nm and total lipids expressed as mg/dL.
Total proteins, albumin, and globulin concentrations
The total proteins were determined by the biuret method based on the conversion of proteins into a violet-colored compound (measured at 550 nm) in the presence of alkaline cupric sulfate as detailed by Gornal et al. (1949). Albumin concentration was measured after converting albumin into albumin/bromocresol-green according to Doumas et al. (1971). The intensity of the produced color was measured at 630 nm. Globulin concentration was calculated as the difference between the total protein and albumin.
Enzymatic and non-enzymatic biomarkers for liver and kidney functions
The liver functions were evaluated based on the activities of three liver enzymes (IU/L). Aspartate transaminase (AST) and alanine transaminase (ALT) activities were evaluated according to the colorimetric method of Reitman and Frankel (1957) while alkaline phosphatase (ALP) activity was estimated according to Belfield and Goldberg (1971). The kidney functions were estimated based on the creatinine concentration (mg/dL) using the colorimetric method of Bartles et al. (1972) and the concentration of uric acid (mg/dL) was according to Prætorius and Poulsen (1953).
Enzymatic and non-enzymatic antioxidant biomarkers
According to the instructions of the used kits (Biodiagnostic Dokki, Giza, Egypt), the isolated gills and liver tissues (1 gm) were homogenized in 5 ml cold buffer solution (specific for each biomarker). The obtained homogenates were centrifuged at 4000 rpm (15 min) at 4 °C and the resulted supernatants were used in all studied antioxidant biomarkers.
The determination of catalase (CAT) activity was based on stopping the enzymatic reaction between CAT and a known amount of H2O2 after one minute with a catalase inhibitor. The remaining H2O2 reacts with 3, 5-dichloro-2-hydroxybenzene sulfonic acid, and 4-aminophenazone (in the presence of peroxidase) to form a chromophore. The color intensity at 510 nm is inversely proportional to the amount of catalase (Aebi 1984). The used cold buffer solution for CAT was 50 mM potassium phosphate, pH 7.4. 1 mM EDTA, and 1 mL/L Triton X100. As described by Nishikimi et al. (1972), superoxide dismutase (SOD) activity could be estimated depending on its inhibition ability to the phenazine methosulphate-mediated reduction of nitroblue tetrazolium dye. The change in absorbance at 560 nm was directly proportional to the inhibition rate of SOD. The used cold buffer solution for SOD was 100 mM potassium phosphate, pH 7.0, containing 2 mM EDTA. Depending on the ability of glutathione peroxidase (GPx) to reduce organic peroxide to oxidized glutathione (which is recycled to its reduced state by glutathione reductase), GPx activity was indirectly determined as described by Paglia and Valentine (1967). The oxidation of NADPH to NADP+ is combined with a reduction in absorption (at 340 nm) providing a spectrophotometric means for estimating GPx activity. The used buffer for GPx determination was consistent from 50 mM phosphate buffer, pH 7.0, containing 5 mM EDTA and 1 mM 2-mercaptoethanol. All the above enzymes are expressed as U/g protein. According to Beutler et al. (1963), glutathione reduced (GSH) could be determined colorimetrically (at 405 nm) depending on its ability to reduce 5, 5′-dithiobis 2-nitrobenzoic acid into a yellow color product. The used buffer for GSH was 50 mM potassium phosphate, pH 7.5, 1 mM EDTA and the measurement unit is mmol/g protein. The level of lipid peroxidation indicated by thiobarbituric acid reactive substances (TBARS) concentration was evaluated as described by Ohkawa et al. (1979). The reaction between TBARS with thiobarbituric acid forming a colored product with a color intensity is directly proportional to the TBARS level (expressed as nmol/g tissue). The used buffer was 50 mM potassium phosphate, pH 7.5.
Statistical analysis
Data were statistically analyzed using the Statistical Processor Systems Support, SPSS software, version 16.0, IBM, Chicago, IL, USA. The results were expressed as the mean ± SE. Data were statistically analyzed with analyses of variance ANOVA test and Duncan’s multiple ranges to measure the comparability between all studied groups as indicated by different letters and symbols at a significant level of p < 0.05.
Results
Characterization of Ag NPs
As represented in Table 1, the characterization results of Ag NPs revealed that these NPs had spherical shapes with an average size of 41.3 ± 5.2 nm. Moreover, these NPs showed a good distribution pattern and a low tendency for aggregation in water as confirmed by DLS (84.23 nm) and zeta potential (−13.8 mV) results.
The percentage of Ag ions released
As shown in Table 2, approximately 45%, 52%, and 62% of the silver were in the form of silver ions after 24 h, 48 h, and 96 h respectively. The percentage of Ag ions was decreased after water treatment with OP to be 40%, 48%, and 55% and with BP to be 41%, 47%, and 53% at 24 h, 48 h, and 96 h respectively confirming that both OP and BP could decrease the dissolution rate.
Surface study on orange and banana peels using SEM/EDS spectra analysis
Figure 1 shows representative SEM images of OP and BP before and after the adsorption process of Ag NPs. The original peels (Fig. 1a and d) showed highly porous and clear surfaces. Peels after the exposure to Ag NPs (Fig. 1b and e) had surfaces that were partially covered with circular NPs. The data obtained from energy-dispersive X-ray spectroscopy (EDS) confirmed the existence of Ag NPs on the surfaces of both peels (Fig. 1c and f).
Representitive SEM/EDS spectra analysis of orange and banana peels. a Original orange peel; b orange peel after adsorption of Ag NPs; c EDS image of orange peel after adsorption of Ag NPs; d original banana peel; e banana peel after adsorption of Ag NPs; c EDS image of banana peel after adsorption of Ag NPs
The concentration of Ag metal in OP and BP
Both OP and BP unloaded with silver NPs showed undetected concentrations of Ag metal. The concentrations of silver metal in both OP and BP after the exposure to Ag NPs at each studied time interval were represented in Fig. 2. According to the recorded results, the adsorptive capacity of BP toward Ag NPs was higher than OP at all time intervals. Also, the removal efficiency of OP and BP toward Ag NPs was maximized after 96 h compared to the other intervals. According to silver concentrations, the efficiency of Ag NPs removal was arranged as BP (96 h) > BP (48 h) > BP (24 h) > OP (96 h) > OP (48 h) > OP (24 h).
The concentrations of silver metal in OP and BP after 24 h, 48 h, and 96 h. Data are represented as means of three samples at each time interval ± SE. The capital letters represent Duncan’s test (p < 0.05) between OP and BP at the same time interval. Means with the same capital letters are not significantly different; otherwise, they do. The small letters represent Duncan’s test (p < 0.05) between the different time intervals of the same biosorbent (OP or BP). Means with the same small letters are not significantly different; otherwise, they do
Biochemical biomarkers
The concentrations of plasma glucose and total lipids
As shown in Fig. 3, there is a significant elevation in the glucose level in all studied groups compared with the control group. The maximum elevation was observed in the Ag NPs exposed group followed by Ag NPs with OP group in all studied periods. Comparing the elevation level within the same group, the glucose level showed insignificant changes in Ag NPs with OP group through the studied intervals, while the other groups showed marked elevation at the end of the experiment. Regarding the total lipid, a significant decrease in all studied groups was observed except in Ag NPs with BP group after 48 and 96 h of exposure. The maximum decrease was recorded in the Ag NPs exposed group (after 48 and 96 h of exposure) followed by Ag NPs with the OP group.
The effect of exposure to Ag NPs, Ag NPs with OP, and Ag NPs with BPs on plasma glucose and total lipids of Oreochromis niloticus after 24 h, 48 h, and 96 h. Data are represented as means of seven samples in each time interval of each group ± SE. The capital letters represent Duncan’s test (p < 0.05) between the same time interval among different groups. Columns with the same capital letters are not significantly different; otherwise, they do. The letters are arranged in descending order as A, B, C, and D. Symbol asterisk means that values are significantly different (p < 0.05) from different time intervals within the same group
Total plasma proteins, albumin, and globulin concentrations
As represented in Fig. 4, the total proteins and globulin levels were sharply increased in the Ag NPs group after 96 h of exposure and in Ag NPs with the OP group after 24 h of exposure. Also, a significant decrease in the total protein concentrations was observed after 24 h of exposure to Ag NPs only and Ag NPs with BP. The albumin concentrations were significantly decreased in all studied periods of the Ag NPs-exposed group. While the OP and BP effects were noticeable as all studied fish groups showed insignificant differences with the control groups at all studied periods.
The effect of exposure to Ag NPs, Ag NPs with OP, and Ag NPs with BP on the plasma protein concentrations of Oreochromis niloticus after 24 h, 48 h, and 96 h. Data are represented as means of seven samples in each time interval of each group ± SE. The capital letters represent Duncan’s test (p < 0.05) between the same time interval among different groups. Columns with the same capital letters are not significantly different; otherwise, they do. The letters are arranged in descending order as A, B, C, and D. Symbol asterisk means that values are significantly different (p < 0.05) from different time intervals within the same group
Enzymatic and non-enzymatic biomarkers for liver and kidney functions
The activities of all studied enzymatic biomarkers for evaluating the liver functions (Fig. 5) showed a sharp increase after Ag NPs exposure at all studied periods compared to the control groups. While the recorded liver function biomarkers in OP and BP water-treated groups showed lower increment at all studied periods and in some cases (ex: AST after 24 h of exposure to Ag NPs with BP; ALP of Ag NPs with OP; and Ag NPs with BP groups after 24 and 48 h of exposure), these biomarkers became insignificant with the control groups. Besides, the levels of plasma creatinine and uric acid showed the same trend as liver enzymes (Fig. 6). The recorded biomarkers showed the evident effect of OP and BP water treatment in lowering these studied biomarkers.
The effect of exposure to Ag NPs, Ag NPs with OP, and Ag NPs with BP on liver function biomarkers (AST, ALT, and ALP) of Oreochromis niloticus after 24 h, 48 h, and 96 h. Data are represented as means of seven samples in each time interval of each group ± SE. The capital letters represent Duncan’s test (p < 0.05) between the same time interval among different groups. Columns with the same capital letters are not significantly different; otherwise, they do. The letters are arranged in descending order as A, B, C, and D. Symbol asterisk means that values are significantly different (p < 0.05) from different time intervals within the same group
The effect of exposure to Ag NPs, Ag NPs with OP, and Ag NPs with BP on kidney function biomarkers (plasma creatinine and uric acid) of Oreochromis niloticus after 24 h, 48 h, and 96 h. Data are represented as means of seven samples in each time interval of each group ± SE. The capital letters represent Duncan’s test (p < 0.05) between the same time interval among different groups. Columns with the same capital letters are not significantly different; otherwise, they do. The letters are arranged in descending order as A, B, C, and D. Symbol asterisk means that values are significantly different (p < 0.05) from different time intervals within the same group
Enzymatic and non-enzymatic oxidative stress endpoints
Oxidative stress endpoints in gills
The activities of CAT, SOD, and GPx showed marked and gradual increases in the Ag NPs-exposed group reaching their maximum values after 96 h of exposure. The non-enzymatic endpoints (TBARS and GSH) showed significant elevations in the TBARS content versus significant declines in the GSH level of the Ag NPs-exposed group, while the effects of water treatment with OP and BP were obvious as all studied fish groups restored those studied biomarkers more or less to that of the control groups (Figs. 7 and 8).
The effect of exposure to Ag NPs, Ag NPs with OP, and Ag NPs with BP on some antioxidant enzymes (CAT, SOD, and GPx) in the gills of Oreochromis niloticus after 24 h, 48 h, and 96 h. Data are represented as means of seven samples in each time interval of each group ± SE. The capital letters represent Duncan’s test (p < 0.05) between the same time interval among different groups. Columns with the same capital letters are not significantly different; otherwise, they do. The letters are arranged in descending order as A, B, C, and D. Symbol asterisk means that values are significantly different (p < 0.05) from different time intervals within the same group
The effect of exposure to Ag NPs, Ag NPs with OP, and Ag NPs with BP on some antioxidant biomarkers (GSH and TBARS contents) in the gills of Oreochromis niloticus after 24 h, 48 h, and 96 h. Data are represented as means of seven samples in each time interval of each group ± SE. The capital letters represent Duncan’s test (p < 0.05) between the same time interval among different groups. Columns with the same capital letters are not significantly different; otherwise, they do. The letters are arranged in descending order as A, B, C, and D. Symbol asterisk means that values are significantly different (p < 0.05) from different time intervals within the same group
Oxidative stress endpoints in hepatic tissues
The measured enzymatic and non-enzymatic biomarkers in liver tissues (Figs. 9 and 10) followed the same trend as in gills with an obvious amelioration in the antioxidant status after OP and BP water treatment.
The effect of exposure to Ag NPs, Ag NPs with OP, and Ag NPs with BP on some antioxidant enzymes (CAT, SOD, and GPx) in the liver of Oreochromis niloticus after 24 h, 48 h, and 96 h. Data are represented as means of seven samples in each time interval of each group ± SE. The capital letters represent Duncan’s test (p < 0.05) between the same time interval among different groups. Columns with the same capital letters are not significantly different; otherwise, they do. The letters are arranged in descending order as A, B, C, and D. Symbol asterisk means that values are significantly different (p < 0.05) from different time intervals within the same group
The effect of exposure to Ag NPs, Ag NPs with OP, and Ag NPs with BP on some antioxidant biomarkers (GSH and TBARS contents) in the liver of Oreochromis niloticus after 24 h, 48 h, and 96 h. Data are represented as means of seven samples in each time interval of each group ± SE. The capital letters represent Duncan’s test (p < 0.05) between the same time interval among different groups. Columns with the same capital letters are not significantly different; otherwise, they do. The letters are arranged in descending order as A, B, C, and D. Symbol asterisk means that values are significantly different (p < 0.05) from different time intervals within the same group
Discussion
The present data showed the toxicological impacts of Ag NPs on the biochemical and antioxidant status of the studied fish species. Therefore, Ag NPs should be reduced or effectively treated before their release into aquatic habitats. The adsorption behavior of several agricultural products has been established as the most effective solution for toxic metals removal from water. Within this context, the present work was designed to be the first approach that evaluates the adsorption efficiency of OP and BP toward Ag NPs to reduce their nano-toxic properties. The observed improvement in all studied biochemical and oxidative stress biomarkers after OP and BP water treatment is consistent with the hypothesis of the present study indicating the effectiveness of both peels in removing Ag NPs from water.
The widespread usage of silver NPs acts like a double-edged sword because their current application in many industries increases the likelihood of their discharge into aquatic bodies (Taju et al. 2014; Naguib et al. 2020). Therefore, it is necessary to understand the actual behavior of Ag NPs in the aquatic environment to determine their toxic effects on different aquatic biota (Ale et al. 2018). The particulate size is the key factor affecting the toxicity of several nanoparticles (Abdel-Khalek et al. 2016). The TEM imaging showed that the sizes of Ag NPs were less than 50 nm which facilitate their entrance into living organisms through different size-dependent mechanisms (Abdel-Khalek 2016). The average hydrodynamic diameter of these NPs in water (recorded by DLS) was larger than 50 nm. The aggregation and hydration of Ag NPs can cause these different sizes. Such unique characterizations of Ag NPs appear remarkably to cause exceptional toxic effects, which is supported by many authors (Ale et al. 2018; Banan et al. 2020). During the toxicity evaluation of Ag NPs, the effects of solid nanoparticles and the metal ions emitted from the nanoparticles should be considered. Kittler et al. (2010) recorded a significant increase in nano-toxicity of Ag NPs that had been stored in dispersion for several weeks due to high concentrations of Ag ions. Chae et al. (2009) reported that the toxicological impacts of Ag nanoparticles on Japanese medaka (Oryzias latipes) were higher than Ag ions after 24 h of exposure and similar in toxicity after 96 h. Therefore, the aim of the present approach was not only to evaluate the toxicity of Ag NPs but also to evaluate a new adsorptive technique to reduce their potential toxicological impacts. Based on their chemical composition and physical properties, the residues of OP and BP are suitable to be used as adsorbents since they have wide surface areas, high swelling abilities, and excellent mechanical strengths (Annadurai et al. 2003). The surface morphology of OP and BP showed the ability of both peels to adsorb Ag NPs on their active sites. The biosorption of BP toward Ag NPs showed higher capacities than that of OP as represented by the higher density of dense spots on the BP surfaces and higher Ag concentrations after the exposure periods. This observation may be due to the presence of additional amino groups in BP that can improve its ability to adsorb metal ions compared to other agricultural products (Oyewo et al. 2018). Moreover, this efficient adsorption ability may be due to the greater number of pores in BP compared to OP. As reported by Safa and Bhatti (2011), biosorption relies on the number of pores of the adsorbents as the greater the number of pores, the greater the biosorption efficiency.
To give a real picture of the role of OP and BP in reducing the Ag NPs nanotoxicity, integrated biochemical and antioxidant biomarkers were applied to various tissues of Oreochromis niloticus. In many toxicological works, the high level of plasma glucose was used as a good sign of exposure to many toxicants (Eslamloo et al. 2014). In the present study, the maximum elevation in glucose levels was observed in Ag NPs-exposed groups. This observation agrees with Farmen et al. (2012) who documented a concentration-dependent elevation in blood glucose of juvenile Atlantic salmon after the exposure to silver NPs and this elevation was related to the accumulation level of Ag NPs in gills. The continuous release of stress-induced hormones like epinephrine and norepinephrine into the blood circulation increases the glucose level via eliciting glycogenolysis which is the key mechanism that compensates the energy shortage during and after stress circumstance (Eslamloo et al. 2014; Abdel-Khalek et al. 2020b; Ibrahim et al. 2021). Furthermore, Bacchetta et al. (2017) showed that Ag NPs exposure may cause excessive production of ROS within the tissues which has catabolic effects on the carbohydrate molecules. In addition to being an essential constituent in many cellular structures and tissues, lipid plays an important role in the physiology and energy regulation processes of fish (Javed et al. 2017). The sharp decrease in total lipids induced by Ag NPs is in line with Zeumer et al. (2020) who reported a significant depletion in total lipids of rainbow trout after 28 days of exposure to Ag NPs. This lipid depletion may be due to the stress-induced lipolysis to manage the stress metabolically and/or due to overproduction of ROS and high lipid oxidation following metallic NPs exposure (Kanwal et al. 2019; Abdel-Khalek et al. 2020b). The current data generally showed that the total proteins were decreased after 24 h of exposure to Ag NPs then started to show a significant increase at the end of the experiment. This trend of elevation was also observed with the globulin levels. Ale et al. (2018) recorded the same observation in Prochilodus lineatus fish after 5 days of exposure to Ag NPs as an inflammatory response because Ag NPs can bind to various tissues inducing cell activation, production of ROS, and finally severe inflammation. Also, NPs-protein corona (NPs coated with proteins) increases the synthesis of several stress proteins as reported by Besnaci et al. (2016) so both reasons may elevate the level of total proteins. The degree of elevation may be attributed to different factors like the concentration of NPs, inter-species responses, and time of exposure, while the recorded albumin depletion after Ag NPs exposure at all studied periods in the present work coincides with Naguib et al. (2020) who suggested that the hypo-albuminemia in Clarias gariepinus fish may be due to albumin use to meet the direct energy requirements to face stress induced by Ag NPs.
Metallic NPs could induce hepatotoxic damages in fish as the liver acts as the major metabolic tissue for several NPs (Abdel-Khalek 2016; Banaee et al. 2019). The significant elevation in all studied liver enzymes in blood after Ag NPs exposure with the maximum level at the end of the experiment indicates the presence of metallic-NPs liver damage. This damage could develop hepatic leaky membranes and permit the escaping of intracellular enzymes into the bloodstream (Banaee 2020; Banaee et al. 2020). This result is in agreement with Hajirezaee et al. (2020) who observed increases in ALT, AST, and ALP activities in common carp exposed to TiO2 NPs. Also, Banan et al. (2020) observed an increase in ALP levels in juvenile Persian sturgeon exposed to Ag NPs due to stress-based tissue damage after a high amount of Ag storage in the liver.
Blood levels of creatinine and uric acid may serve as a useful index for renal filtration and kidney dysfunction. In line with the present observations, Mahmoud et al. (2019) reported an elevation in both creatinine and uric acid of Clarias gariepinus after exposure to different sizes (20 and 40 nm) and concentrations (10 and 100 μg/L) of Ag NPs. These raises in blood levels of uric acid and creatinine indicate that the absorbed Ag NPs could be filtered in the renal system inducing kidney dysfunction and impair renal filtration.
Ag NPs can modify antioxidant defense systems by increasing oxidative damage through the production of reactive oxygen species (Taju et al. 2014). The results of the present work showed a growing induction of all analyzed antioxidant enzymes (CAT, SOD, and GPx) in both the gills and the liver after exposure to Ag NPs. Such activation gradually reached its maximum level after 96 h of exposure. This observation agrees with Govindasamy and Rahuman (2012) who recorded CAT-SOD induction in Oreochromis mossambicus after 8 days of exposure to 50 mg L−1 of Ag NPs. The reported elevation in GPx activities was also observed by Hao and Chen (2012) in gills and liver tissues of carps (Cyprinus carpio) after their exposure to ZnO NPs. In the present study, the growing elevation in GPx activities synchronized with a gradual decrease in the GSH content during the studied periods which indicates that the glutathione antioxidant process progressed through activation of GPx enzymes with ineffectively GSH generation. Jozefczak et al. (2012) reported that the high utilization rate of GSH by ROS and GS-metal complex formations are the major causes of GSH depletion after metal-induced oxidative stress. The TBARS level is an effective biomarker for monitoring the degree of lipid peroxidation and the stability of cell membranes to relatively evaluate the oxidative damage (Abdel-Khalek 2015; Abdel-Khalek et al. 2018). The gradual elevation in TBARS levels in the gills and liver tissues after the exposure to Ag NPs indicated that the mobility of enzymatic and non-enzymatic antioxidant components could not overcome the lipid peroxidation and oxidative membrane damages. Benavides et al. (2016) indicated the ineffective antioxidant actions in the gills and liver of Carassius auratus exposed to Al2O3 NPs by high TBARS levels.
Conclusion
The present findings indicate that Ag NPs can cause significant time-dependent toxicity in Oreochromis niloticus. Ag NPs could alter the biochemical constituents (glucose, total lipid, protein profile), cause hepatotoxic and nephrotoxic effects. Besides, Ag NPs can induce oxidative stress conditions in the gills and liver tissues based on the studied oxidative biomarkers (CAT, SOD, GPx, GSH, and TBARS). Ag NPs toxic behavior may be due to the direct adherence of Ag NPs aggregates on the external surface like gills (observed as grey spots in the present study) or the different internalization mechanisms of the nano-sized particles which facilitate their absorption into the bloodstream. Both OP and BP showed a high adsorptive capacity for Ag NPs and both peels succeeded to decrease the Ag nanotoxicity via adsorption of NPs and decreasing their bioavailability. The adsorptive efficiency of BP was higher than OP; therefore, complementary studies are needed to study more biosorbents with different concentrations and time intervals to improve their removal effectiveness. Applications of different biosorption techniques (on a large scale) are needed to improve the health status of the aquatic habitats and their biota.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdel-Khalek AA (2015) Antioxidant responses and nuclear deformations in freshwater fish, Oreochromis niloticus, facing degraded environmental conditions. Bull Environ Contam Toxicol 94:701–708. https://doi.org/10.1007/s00128-015-1509-5
Abdel-Khalek AA (2016) Comparative evaluation of genotoxic effects induced by CuO bulk and nano-particles in Nile tilapia, Oreochromis niloticus. Water Air Soil Pollut 227:35. https://doi.org/10.1007/s11270-015-2737-3
Abdel-Khalek AA, Badran SR, Marie M-AS (2020a) The efficient role of rice husk in reducing the toxicity of iron and aluminum oxides nanoparticles in Oreochromis niloticus: hematological, bioaccumulation, and histological endpoints. Water Air Soil Pollut 231:53. https://doi.org/10.1007/s11270-020-4424-2
Abdel-Khalek AA, Badran SR, Marie M-AS (2020b) The effective adsorbent capacity of rice husk to iron and aluminum oxides nanoparticles using Oreochromis niloticus as a bioindicator: biochemical and oxidative stress biomarkers. Environ Sci Pollut Res 27:23159–23171. https://doi.org/10.1007/s11356-020-08906-x
Abdel-Khalek AA, Elhaddad E, Mamdouh S, Marie M-AS (2018) The chronic exposure to discharges of Sabal drain induces oxidative stress and histopathological alterations in Oreochromis niloticus. Bull Environ Contam Toxicol 101:92–98. https://doi.org/10.1007/s00128-018-2366-9
Abdel-Khalek AA, Hamed A, Marie M-AS (2016) The accumulation potency of bulk and nano zinc metal and their impacts on the hematological and histological perturbations of Oreochromis niloticus. Water Air Soil Pollut 227:206. https://doi.org/10.1007/s11270-016-2908-x
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Afifi M, Saddick S, Abu Zinada OA (2016) Toxicity of silver nanoparticles on the brain of Oreochromis niloticus and Tilapia zillii. Saudi J Biol Sci 23(6):754–760. https://doi.org/10.1016/j.sjbs.2016.06.008
Ahmad T, Danish M (2018) Prospects of banana waste utilization in wastewater treatment: a review. J Environ Man 206:330–348. https://doi.org/10.1016/j.jenvman.2017.10.061
Akpomie KG, Conradie J (2020) Banana peel as a biosorbent for the decontamination of water pollutants. A review. Environ Chem Lett 18:1085–1112. https://doi.org/10.1007/s10311-020-00995-x
Ale A, Rossi AS, Bacchetta C, Gervasio S, de la Torre FR, Cazenave J (2018) Integrative assessment of silver nanoparticles toxicity in Prochilodus lineatus fish. Ecol Indic 93:1190–1198. https://doi.org/10.1016/j.ecolind.2018.06.023
Anastopoulos I, Kyzas GZ (2014) Agricultural peels for dye adsorption: a review of recent literature. J Mol Liq 200:381–389. https://doi.org/10.1016/j.molliq.2014.11.006
Annadurai G, Juang RS, Lee DJ (2003) Adsorption of heavy metals from water using banana and orange peels. Water Sci Technol 47:185–190. https://doi.org/10.2166/wst.2003.0049
APHA (2005) American Water Works Association. Standard methods for the examination of water and wastewater. American Public Health Association, New York
Bacchetta C, Ale A, Simoniello MF, Gervasio S, Davico C, Rossi AS, Desimone MF, Poletta G, Lopez G, Monserrat JM, Cazenave J (2017) Genotoxicity and oxidative stress in fish after a short-term exposure to silver nanoparticles. Ecol Indic 76:230–239. https://doi.org/10.1016/j.ecolind.2017.01.018
Banaee M (2020) Alkaline phosphatase activity as a biochemical biomarker in aqua-toxicological studies. Int J Aquat Biol 8(2):143–147. https://doi.org/10.22034/ijab.v8i2.880
Banaee M, Akhlaghi M, Soltanian S, Sureda A, Gholamhosseini A, Rakhshaninejad M (2020) Combined effects of exposure to sub-lethal concentration of the insecticide chlorpyrifos and the herbicide glyphosate on the biochemical changes in the freshwater crayfish Pontastacus leptodactylus. Ecotoxicology 29(9):1500–1515. https://doi.org/10.1007/s10646-020-02233-0
Banaee M, Tahery S, Nematdoost Haghi B, Shahafve S, Vaziriyan M (2019) Blood biochemical changes in common carp (Cyprinus carpio) upon co-exposure to titanium dioxide nanoparticles and paraquat. Iran J Fish Sci 18(2):242–255 http://jifro.ir/article-1-3844-en.html
Banan A, Kalbassi MR, Bahmani M, Sotoudeh E, Johari SA, Ali JM, Kolok AS (2020) Salinity modulates biochemical and histopathological changes caused by silver nanoparticles in juvenile Persian sturgeon (Acipenser persicus). Environ Sci Pollut Res 27:10658–10671. https://doi.org/10.1007/s11356-020-07687-7
Bartles H, Bohmer M, Heirli C (1972) Serum creatinine determination without protein precipitation. Clin Chim Acta 37:193–197. https://doi.org/10.1016/0009-8981(72)90432-9
Belfield A, Goldberg DM (1971) Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzyme 12:561–573. https://doi.org/10.1159/000459586
Benavides M, Fernández-Lodeiro J, Coelho P, Lodeiro C, Diniz MS (2016) Single and combined effects of aluminum (Al2O3) and zinc (ZnO) oxide nanoparticles in a freshwater fish, Carassius auratus. Environ Sci Pollut Res 23:24578–24591. https://doi.org/10.1007/s11356-016-7915-3
Besnaci S, Bensoltane S, Zerari L, Samia C, Hamlet SA, Berrebbah H (2016) Impact of nanometric iron oxide in the hepatopancreas of terrestrial gastropod Helix Aspersa: histological changes and biochemical parameters. Int J Pharm Sci Rev Res 36:234–241. https://doi.org/10.13140/RG.2.2.10749.92644
Beutler E, Duron O, Kelly MB (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Chae YJ, Pham CH, Lee J, Bae E, Yi J, Gu MB (2009) Evaluation of the toxic impact of silver nanoparticles on Japanese medaka (Oryzias latipes). Aquat Toxicol 94:320–327. https://doi.org/10.1016/j.aquatox.2009.07.019
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chim Acta 31:87–96. https://doi.org/10.1016/0009-8981(71)90365-2
Eslamloo K, Akhavan SR, Fallah FJ, Henry MA (2014) Variations of physiological and innate immunological responses in goldfish (Carassius auratus) subjected to recurrent acute stress. Fish Shellfish Immunol 37:147–153. https://doi.org/10.1016/j.fsi.2014.01.014
Farmen E, Mikkelsen HN, Evensen Ø, Einset J, Heier LS, Rosseland BO, Salbu B, Tollefsen KE, Oughton DH (2012) Acute and sub-lethal effects in juvenile Atlantic salmon exposed to low μg/L concentrations of Ag nanoparticles. Aquat Toxicol 108:78–84. https://doi.org/10.1016/j.aquatox.2011.07.007
Gornal AC, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the Biuret reaction. J Biol Chem 177:751–766
Govindasamy R, Rahuman AA (2012) Histopathological studies and oxidative stress of synthesized silver nanoparticles in Mozambique tilapia (Oreochromis mossambicus). J Environ Sci 24:1091–1098. https://doi.org/10.1016/S1001-0742(11)60845-0
Hajirezaee S, Mohammadi G, Naserabad SS (2020) The protective effects of vitamin C on common carp (Cyprinus carpio) exposed to titanium oxide nanoparticles (TiO2-NPs). Aquaculture 518:734734. https://doi.org/10.1016/j.aquaculture.2019.734734
Hao L, Chen L (2012) Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol Environ Saf 80:103–110. https://doi.org/10.1016/j.ecoenv.2012.02.017
Ibrahim ATA, Banaee M, Sureda A (2021) Genotoxicity, oxidative stress, and biochemical biomarkers of exposure to green synthesized cadmium nanoparticles in Oreochromis niloticus (L.). Comp Biochem Physiol C Toxicol Pharmacol 242:108942. https://doi.org/10.1016/j.cbpc.2020.108942
Javed M, Ahmad MI, Usmani N, Ahmad M (2017) Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste water. Sci Rep 7:1675–1685. https://doi.org/10.1038/s41598-017-01749-6
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13:3145–3175. https://doi.org/10.3390/ijms13033145
Kanwal Z, Raza MA, Manzoor F, Riaz S, Jabeen G, Fatima S, Naseem S (2019) A comparative assessment of nanotoxicity induced by metal (Silver, Nickel) and metal oxide (Cobalt, Chromium) nanoparticles in Labeo rohita. Nanomaterials 9:309–328. https://doi.org/10.3390/nano9020309
Khan MS, Qureshi NA, Jabeen F, Shakeel M, Asghar MS (2018) Assessment of waterborne amine-coated silver nanoparticle (Ag-NP)-induced toxicity in labeo rohita by histological and hematological profiles. Biol Trace Elem Res 182:130–139. https://doi.org/10.1007/s12011-017-1080-5
Khosravi-Katuli K, Shabani A, Paknejad H, Imanpoor MR (2018) Comparative toxicity of silver nanoparticle and ionic silver in juvenile common carp (Cyprinus carpio): accumulation, physiology and histopathology. J Hazard Mater 359:373–381. https://doi.org/10.1016/j.jhazmat.2018.07.064
Kittler S, Greulich C, Diendorf J, Kӧller M, Epple M (2010) Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater 22:4548–4554. https://doi.org/10.1021/cm100023p
Knight JA, Rawle JM, Anderson S (1972) Chemical basis of sulfo-phospho-vanillin reaction for estimating total serum lipids. Clin Chem 18:199–202
Liang S, Guo X, Feng N, Tian Q (2010) Isotherms, kinetics and thermodynamic studies of adsorption of Cu2+ from aqueous solutions by Mg2+/K+ type orange peel adsorbents. J Hazard Mater 174:756–762. https://doi.org/10.1016/j.jhazmat.2009.09.116
Mahmoud UM, Mekkawy IAA, Naguib M, Sayed AE-DH (2019) Silver nanoparticle–induced nephrotoxicity in Clarias gariepinus: physio-histological biomarkers. Fish Physiol Biochem 45:1895–1905. https://doi.org/10.1007/s10695-019-00686-7
Naguib M, Mahmoud UM, Mekkawy IA, Sayed AE-DH (2020) Hepatotoxic effects of silver nanoparticles on Clarias gariepinus; biochemical, histopathological, and histochemical studies. Toxicol Rep 7:133–141. https://doi.org/10.1016/j.toxrep.2020.01.002
Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R (2008) Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol 42:8959–8964. https://doi.org/10.1021/es801785m
Neugebauer EA, Sans Cartier GL, Wakeford BJ (2000) Methods for the determination of metals in wildlife tissues using various atomic absorption spectrophotometry techniques. Technical Report Series No. 337E. Canadian Wildlife Service, Headquarters, Hull http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.578.2937&rep=rep1&type=pdf
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys Res Common 46:849–854. https://doi.org/10.1016/S0006-291X(72)80218-3
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Oyewo OA, Onyango MS, Wolkersdorfer C (2018) Lanthanides removal from mine water using banana peels nanosorbent. Int J Environ Sci Technol 15:1265–1274. https://doi.org/10.1007/s13762-017-1494-9
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Prætorius E, Poulsen H (1953) Enzymatic determination of uric acid with detailed directions. Scand J Clin Lab Inv 5:273–280. https://doi.org/10.3109/00365515309094197
Pulit-Prociak J, Banach M (2016) Silver nanoparticles - a material of the future...? Open Chem 14:76–91. https://doi.org/10.1515/chem-2016-0005
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63. https://doi.org/10.1093/ajcp/28.1.56
Safa Y, Bhatti HN (2011) Adsorptive removal of direct textile dyes by low cost agricultural waste: application of factorial design analysis. Chem Eng J 167:35–41. https://doi.org/10.1016/j.cej.2010.11.103
Sarkar B, Jaisai M, Mahanty A, Panda P, Sadique M, Nayak BB, Gallardo G, Thakur D, Bhattacharjee S, Dutta J (2015) Optimization of the sublethal dose of silver nanoparticle through evaluating its effect on intestinal physiology of Nile tilapia (Oreochromis niloticus L.). J Environ Sci Health 50:814–823. https://doi.org/10.1080/10934529.2015.1019800
Singh NB, Nagpal G, Agrawal S, Rachna (2018) Water purification by using adsorbents: a review. Environ Technol Innovat 11:187–240. https://doi.org/10.1016/j.eti.2018.05.006
Taju G, Abdul Majeed S, Nambi KSN, Sahul Hameed AS (2014) In vitro assay for the toxicity of silver nanoparticles using heart and gill cell lines of Catla catla and gill cell line of Labeo rohita. Comp Biochem Physiol – part C: Toxicol Pharmacol 161:41–52. https://doi.org/10.1016/j.cbpc.2014.01.007
Tortella GR, Rubilar O, Durán N, Diez MC, Martínez M, Parada J, Seabra AB (2020) Silver nanoparticles: toxicity in model organisms as an overview of its hazard for human health and the environment. J Hazard Mater 390:121974. https://doi.org/10.1016/j.jhazmat.2019.121974
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6:24–27 https://journals.sagepub.com/doi/pdf/10.1177/000456326900600108
Zeumer R, Galhano V, Monteiro MS, Kuehr S, Knopf B, Meisterjahna B, Soares AMVM, Loureiro S, Lopes I, Schlechtriem C (2020) Chronic effects of wastewater-borne silver and titanium dioxide nanoparticles on the rainbow trout (Oncorhynchus mykiss). Sci Tot Environ 723:137974. https://doi.org/10.1016/j.scitotenv.2020.137974
Zhu R, Chen Q, Zhou Q, Xi Y, Zhu J, He H (2016) Adsorbents based on montmorillonite for contaminant removal from water: a review. Appl Clay Sci 123:239–258. https://doi.org/10.1016/j.clay.2015.12.024
Acknowledgements
The authors extend their appreciation to the Zoology department, Faculty of Science, Cairo University, Egypt for supporting the present study.
Funding
Faculty of Science, Cairo University funded the present study through the analysis and interpretation of data.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Amr Adel Abdel-Khalek, Aliaa Hamed, and Wafaa S.F. Hasheesh. The first draft of the manuscript was written by Amr Adel Abdel-Khalek and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in the current work involving fish were approved with approval no. CU I F 2019 and were following the ethical standards of the Faculty of Science, Cairo University, Institutional Animal Care and Use Committee (IACUC) at which the studies were conducted.
Consent to participate
All authors read and approved the final manuscript.
Code availability
Not applicable
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Khalek, A.A., Hamed, A. & Hasheesh, W.S. Does the adsorbent capacity of orange and banana peels toward silver nanoparticles improve the biochemical status of Oreochromis niloticus?. Environ Sci Pollut Res 28, 33445–33460 (2021). https://doi.org/10.1007/s11356-021-13145-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13145-9