Abstract
Silver nanoparticles (Ag-NPs) have wide applications in the medical field; however, the toxicological effects are still poorly studied. The study was aimed to determine the effects of 15.78 nm spherical and amine-coated Ag-NPs on hematology and histology of gills and liver tissues in 28 days treated Labeo rohita (L. rohita). It was found that Ag-NPs induced alterations in the hematological parameters in a dose dependent manner. The Ag-NPs also induced histological alterations in a dose-dependent manner. In gill tissues, it induced fusion of secondary lamellae, separation of gill epithelium, fusion and necrosis of lamellar cells, hyperplasia, deformed cartilaginous skeleton, separation and lifting of epithelium, and curling of lamellae in a dose dependent manner. In the liver, Ag-NPs produced abnormalities in hepatic tissues by reducing the size of hepatocytes and nuclei, and stimulated the production of necrotic and apoptotic bodies. It was concluded that Ag-NPs are toxic to aquatic organisms and induce hematotoxicity and histopathological conditions in exposed fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ag-NPs (1–100 nm) are a very important class of nanoparticles with higher surface area to volume ratio [1]. These particles are most commonly used in medical and industrial appliances due to physical, chemical, and biological properties [2,3,4]. The biological properties include antibacterial activity, toxicity to vertebrate’s and invertebrate’s cell lines. The chemical and physical properties include nano-scale sensors for the detection of various compounds, optical properties, electronic properties, and catalytic activities. Their extensive use increases the discharge of these particles in the aquatic habitat through different anthropogenic and industrial activities [5, 6] where they exists in Ag and Ag+ oxidation states [3, 7]. The salts of silver (AgNO3, AgCl) are soluble but silver in metallic form is insoluble [8]. The Ag-NPs also exist in the form of colloidal particles, hence readily absorbed and more toxic to aquatic organisms [3, 9]. Furthermore, various characteristics, including size of particles, composition, surface area and surface chemistry, coagulation and aggregation state, vapor pressure, and lipid and water solubility, also influenced particle properties [8, 10]. The toxicity is also affected by the size of nanoparticles [11,12,13]. However, the relationship between biological effects and particle size of Ag-NPs is still unclear. The particles enter the body of an organism by inhalation, oral absorption, or through damage skin using sliver burns or antiseptic creams [14, 15]. Larese et al. [16] also found Ag-NPs can cross the stratum corneum and the blood brain barrier.
In aquatic environment, the Ag-NPs induce toxicity to invertebrate’s and vertebrate’s cell lines by production of oxidative stress [17, 18], depletion of oxidative stress marker [19], increase lipid peroxidation [17, 20], and reduce mitochondrial function [21,22,23] and apoptosis [18, 24]. They also change the membrane integrity, damage to the skin, olfactory bulbs, lungs and liver [25,26,27,28]. Among all, the liver and gills are most liable sites for toxicity of Ag-NPs [3]. Recent studies show that Ag-NPs cause toxicity in gills of zebra fish [29] and liver of common carp [30]. These particles were directly taken by the gills or absorbed through the digestive tract and reach the liver via blood circulation [31]. Long circulation may increase the chance to penetrate into deeper tissue and enhance cellular uptake [32]. In this study, 15.78 nm amine-coated spherical Ag-NPs were used to study the hemotoxicity and histotoxicity in the L. rohita exposed toAg-NPs for 28 days. It was hypothesized that amine-coated Ag-NPs cause tissue and hematological alterations in fish upon the exposure.
Materials and Methods
Synthesis and Characterization of Particles
Amine-coated and spherical particles were synthesized through the chemical reduction method as explained in our previous studies [17] with some modifications. The 0.05 M AgNO3 solution was prepared in deionized water and stirred over magnetic stirrer (ARE VELP) for 30 min. The solution was colorless at this point. Then, 5 ml of formaldehyde was added as reducing agent and stirred for 5 min. Finally, 4 ml triethylamine was added as protecting and capping agent. The color of the solution was changed to black due to reduction of silver salts into Ag-NPs. The solution was stirred for 2 h at room temperature and for 2 h at 150 °C. The precipitates formed were washed with deionized water, then with ethanol and distilled water, filtered, and dried in the oven at 85 °C overnight. The formed particles were grained in piston mortal to fine powder.
The particles were characterized through SEM, XRD analysis, FT-IR spectroscopy (Thermo Nicolet Avatar, 380) and DLS (Malvern Zetasizer, Nano ZS).
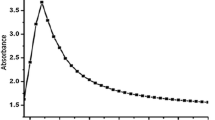
The SEM image showed agglomeration of grain like and spherical Ag-NPs (Fig. 1). The particles were 15.78 ± 5.56 nm with maximum in range of 10–20 nm. The hydrodynamic size was measured through a Malvern Zetasizer (Nano ZS) with backscattering detector. As Ag-NPs were agglomerates, the sample stock solution was diluted 10 folds with distilled water. The hydrodynamic size was represented as intensity (%) of the overall size of particles. The TEM image shows spherical Ag-NPs. The image was only taken for sol and separated Ag-NPs. Along growth direction, the ring patterns of electron diffraction shows (110), (200), (220) and (311) which revealed crystalline, spherical and face-centered cubic nature of growing particles (Fig. 2) The Fig. 2 also shows DLS-measured size distribution of amine coated Ag-NPs. There was no significant difference of DLS-measured and the histogram size of Ag-NPs. However, the DLS showed the maximum frequency of particles between 10 and 45 mm in diameter. The DLS suggested that there was less agglomerations on dilutions with distilled water.
The XRD analysis showed distinct peaks at 38.20 θ (111), 44.40 θ (200), 64.81 θ (220), and 77.90 θ (311) which confirmed the face-centered cubic and crystalline nature of particles (Fig. 3). The FT-IR analysis showed interactions of molecules with Ag-NPs. The broad spectrum at 3441.2 cm−1 was due to –N–H and O–H stretch vibrations. The second absorption band at 2842.23 cm−1 explained C–H group vibrations. The third absorption band at 1631.24 cm−1 showed C = C stretching. The absorption spectrum at 1391.26 cm−1 explained the presence of an amine group (C–N). The absorption bands at 1236.16 and 1055.22 cm−1 show C–N stretch vibrations. The spectra of FT-IR at 2381.25 cm−1 represent stretching and bending vibrations of H–O–H which indicates the free or absorbed water. Overall, the FT-IR spectra analysis confirmed the presence of amine interaction with the Ag-NPs (Fig. 4)
Experimental Conditions and Sample Collection
The experimental L. rohita (50 ± 5 g weight and 24 ± 4 cm in length) were procured from the Punjab Fish Hatchery Department, Faisalabad (Pakistan), acclimatized for 2 weeks at 28 ± 2 °C and 12:12 light and dark period in 40 L glass aquaria. The fish were divided into six groups in triplicate with five fish in each group. The first group acted as control and others were treated with 10, 20, 30, 45, and 55 mg L−1 Ag-NPs for 28 days. The blood samples were collected through cardiac punctured with 2 mL heparinized EDTA needle in EDTA tubes on 14th and 28th day.
Hematological Analysis
All hematological tests were performed on automatic hematology analyzer (M-20GP from MEDONIC Sweden) according to the manufacturer’s instructions. Mean corpuscular volume (MCV) was expressed in fl (femtoliters) and calculated with the following formula:
Mean corpuscular hemoglobin concentration (MCHC) was expressed in g/dl (grams/deciliter) and calculated with the following formula:
Mean corpuscular hemoglobin (MCH) was expressed with Pg (picograms) and calculated with the following formula:
All the samples and readings were taken in five replicates. The data were represented in mean ± SD and compared with post hoc Tukey’s test using IBM statistics v.20.
Histological Studies
The gills and liver tissues were sampled on 28th day and fixed in 10% formalin solution. Fixation was done by series of dehydration steps. First, tissues were placed in 80% ethanol, then 90%, and finally in 100% ethanol for a period of 2 h in each dilution. The tissues were then placed in cedar wood oil until clear. The clear tissues were placed in paraplast for 30 min at 60 °C in the incubator. The paraplast was changed after 30 min and tissues again placed in an incubator for 12 h at 60 °C. The paraplast was third time changed and placed in an incubator for 12 h at 60 °C. The box blocks of each tissue were made and mounted into plastic casters. The embedded tissue was fixed in rotatory microtone and 3–5-μm-thick sections were cut for each tissue. Each section was transferred to a clean slide and stretched on Fisher slides, warmed and remained it on slide for 24 h.
Hematoxyline-Eosin Staining
De-parafinization was done with xylene and rehydration with 50 to 100% dilution of ethanol. Slides were washed with tap water, stain with hematoxyline, dipped again in water for bright coloration, and stained with eosin. The slides were then moved to absolute alcohol for complete dehydration. Two drops of DPX (histology mountant) were put on each slide and covered with a cover slip for complete spreading.
Results
Hematological Analysis
The hemoglobin level increased with increase in concentration of Ag-NPs due to elevated levels of metabolic rate under stress condition. However, this increase in level of hemoglobin was not smooth and decrease in level observed. The lowest level of hemoglobin was found at 55 mg L−1 concentration on the 14th day of sampling and again increased after 28 days (Tables 1 and 2). The level of packed cell volume (PCV) was sharply increased at lowest concentration (10 mg L−1) and then regular decrease at each concentration in both blood samplings at 14th and 28th day (Fig. 5). This study also showed that Ag-NPs stimulated or decreased the production of WBCs in all treatment compared to control. There was a decrease in the number of lymphocytes which were significantly different at each concentration (Tables 3 and 4). This decrease indicated the stress condition. However, numbers of neutrophils increased in number along fluctuations in monocytes and eosinophil at each concentration. The number of monocytes first decreased slightly at 10 mg L−1 treatments and then increased again A similar trend was observed in 28 days sampling (Table 4). The eosinophil showed the same decrease in number at 10 mg L−1 and then increases.
The platelet count was also increased in all concentrations compared to control group. However, the decrease in the platelet counts was found at 45 and 55 mg L−1 concentration. The other parameters of the blood including MCH and MCHC were found to decrease with an increase in the concentrations. Unlike MCH and MCHC, the level of MCV was found to increase. All the treatment showed a significant difference from the control group (Fig. 5).
Histological Alterations in Gills and Liver Tissues
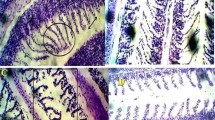
In aquatic environments, gills are the main absorption site for toxicants. The liver is a second most susceptible site for absorption and actively detoxifies the toxic xenobiotic and hence gets damaged. In the present study, there were no recognizable changes in gill tissues of control. The treated group showed proliferation of branchial chloride cells, fused secondary lamellae, separation of gill epithelium, deformation of lamellar cells, fusion and necrosis of lamellae, accumulation of apoptotic bodies, accumulation of macrophages along blood clot, and the formation of aneurism. This type of deformation increases the risk of rupture of gill tissue and result in severe hemorrhage, other complications, or death. The alterations in the gills of the freshwater fish were mostly related to the circulatory disturbances which induce regressive and progressive changes in the gill tissues. In this study, 10 mg L−1 caused the fusion of the secondary and separation of gill epithelium tissue, whereas the treatment of 20 to 55 mg L−1 showed deformation of lamellar cells, fusion and necrosis of lamellae, hyperplasia, deformed cartilaginous skeleton, separation and lifting of epithelium, curling of lamellae inflammation, and deformation of the cartilaginous skeleton (Fig. 6).
Histological changes in gill section treated with Ag-NPs (H and E; ×400). a Control group. b 10 mg L−1 (black arrow fused secondary lamellae; white arrow separated epithelium). c 20 mg L−1 (black arrow = clotted blood; white arrow = deformed lamellar cells; black bent arrow = fused and necrotic lamellae; white bent arrow = fused lamellae). d 30 mg L−1 (black arrow = fusion of secondary lamellae; white arrow = deformed cartilage with macrophages; black bent arrow = separated epithelium. e 45 mg L−1 (black arrow = deformed cartilage; black bent arrow = fusion of lamellae; white arrow = accumulation of apopotic bodies; white bent arrow = accumulation of microphage along blood clot. f 55 mg L−1 (black arrow = hyperplasia lamella fusion; white arrow = fused secondary lamellae; bent black arrow = curled lamellae; white bent arrow = inflamed cartilage; black arrow head = fused and necrotic lamellae)
In the liver sections, normal hepatocytes were recorded in control. The Ag-NPs caused cognitive enlargement of lysosomes leading to degeneration in the liver tissue. The necrosis were recorded at high level in liver tissues. The treated fish also showed abnormalities in hepatic tissues, reducing the size of cells and nuclei. At lowest concentration, the hepatocytes began to swell. The higher concentrations of Ag-NPs caused accumulation of condensed nuclear, pycnotic, necrotic, and apoptotic bodies (Fig. 7b, c). Necrotic condition was very severe in the liver tissue of treated L. rhoita. Histological alterations in the liver of treated fish indicated that the Ag-NPs entered into the liver tissue through the circulatory system and produce the damage. In Fig. 8 first photograph, black arrows showing focal necrosis and inflammation of hepatic tissue forming the vascular dilatation, reduced nuclei, and congestion. In the second photograph, vacuolization of the hepatic cells (black arrow), cells with reduced nuclei (white arrow) accumulation of the colored pigmentation in the hepatic tissue (bent black arrow), damaged tissue due to necrosis (bent white arrow), blood conjunction (arrow head), congestion (red star), and edema were recorded.
Histological changes in liver section treated with Ag-NPs. a Control group showing the normal structure of the liver, b 10 mg L−1 treatment (black arrow = hepatocytes with normal nuclei; red arrow = hepatocytes with pycnotic nuclei; white arrow necrosis in the tissues). b 20 mg L−1 (white arrow = deformed blood vessel; bent arrow necrotic cells; black arrows; white arrow = deformed blood vessel). d 30 mg L−1 (bent arrow necrotic cells; black arrows = damaged hepatic tissue due to focal necrosis and inflammation of hepatic parenchyma tissue)
Histological changes in liver section treated with Ag-NPs (H and E; ×400). a 45 mg L−1 (white arrow = accumulation of yellow pigmentation; black arrow = congestion and edema; bent black arrow = accumulation of macrophages; the white bend arrow = necrosis of hepatic parenchyma tissue). b 55 mg L−1 (white bent arrow = damaged hepatic tissue due to necrosis; bent black arrow = accumulation of color pigmentation; black arrow head = blood conjunction; a white arrow = cell with reduced nuclei; red star = congestion and edema)
Discussion
Silver nanoparticles attract much attention due to expected toxicity. It can cause damage to the brain, liver, and stem cells in the human body. Thus, instead of using human, it is preferable to use animal models in toxicological studies. Among all models, fish is most dominantly used in toxicological studies. The review of published literature by Khan et al. [3] showed that Ag-NPs pose toxicity to all the life stages of fish. Variation in toxicity due to size, form, and condition of target model, the researchers are encouraged to further investigate different aspects of Ag-NPs toxicity. Efforts are being made for developing standards and environment friendly use of Ag nanoproducts for cleaning of fish parasites.
In chronic toxicity studies regarding hematological analysis, level of hemoglobin initially increased significantly and then decreased. Similar trends were recorded in RBC count. It was probably due to elevated levels of metabolic rate under stress condition [33, 34]. Secondly, the flow of RBCs also increases in blood stream due to hypoxia and dehydration. As Ag-NPs produces hypoxic condition and increase the alkalinity, the kidney sensors detect this condition and increase RBCs movement in the blood flow [35, 36]. Therefore, during the stress condition, the carrying capacity of blood increases to increase the level of hemoglobin and meet the metabolic demands [37].
However, this increase was up to certain concentrations of Ag-NPs. Beyond this level, the animal becomes anemic and decrease in level of hemoglobin was recorded along RBC counts. Further, MCH and MCHC values were significantly different from control and decreased with increase in concentrations of Ag-NPs. This lowering of values were due to RBCs counts and hematocrit reduction, which itself was reduced due to deformation or damage to RBCs [36, 38], bleeding, hemolysis, or decreased RBC generation [35, 38]. Many investigators reported decrease in the level of MCH and MCHC in freshwater fish exposed to metals and nanoparticles [36, 39]. Overall changes in blood parameters were due to the reaction of defense against toxicity through the mechanism of the erythropoiesis [40].
Fluctuations in WBC count were also seen due to non-specific response of the immune system against stress and indicating the stress [41, 42]. The normal value of WBC count represents the normal physiological condition where the changes in quantitative and qualitative characteristics of blood cells are the response of anomalies that interfere with normal functions. This situation usually occurs in inflammation, bacterial, or parasitic infections. The reduction in number of WBC is the suppression of immune response and could be due to hematopoietic system malfunctioning of Ag-NP-treated animals [43, 44].
Lymphocytes control most of immune response of an organism’s body. The decrease in lymphocytes occurs when the fish is subjected to stress [45]. The heavy metals reduce lymphocytes [38, 46]. Many researchers also found a decrease in lymphocyte count when exposed to metal and its salts [47, 48]. There was also a significant difference in neutrophils of all treatments compared to control. Neutrophils increased in number because Ag-NPs increased the infection and damage in tissues [49]. This leads to neutrophila [47, 50, 51]. The degree of elevation of neutrophils represents the infection severity. Many fluctuations were also seen in a number of monocytes relatively in short response of respiratory burst [52, 53]. This change in monocytes might be due to disease condition or hematological tissue dysfunction [54].
In histological studies, there were no recognizable changes in both gills and liver tissues of control. The treatment of Ag-NPs caused proliferation of bronchial chloride cells, lamellae fusion, and formation of aneurism in L. rohita. This type of deformation increases the risk of rupture of gill tissues and result in severe hemorrhage, other complications, or death. Similar deformities were seen in the study of Rajkumar et al. [55] in case of L. rohita and Al-Ghanbousi et al. [56] in Aphanius dispar freshwater fish challenged with deltamethrin, showing vacuolization, fusion of secondary lamellae, and lifting of the lamellar epithelium. The alterations in gill of freshwater fish are mostly related to circulatory disturbances which induce regressive and progressive changes in gill tissue [57]. In the present study, 10 mg L−1 treatment caused a fusion of secondary lamellae and necrosis of gill tissue where the treatment of 20 to 55 mg L−1 showed hyperplasia, deformed cartilaginous skeleton, separation and lifting of epithelium, curling of lamellae inflammation, and deformation of the cartilaginous skeleton (Figs. 5 and 6). Similar histological changes were seen in the case of Nile Tilapia after the exposure of TiO2-NPs [58] and carbon nanotubes [59]. Further Ag-NP treatment also caused hepatocyte enlargement and overfilling of blood vessels due to hemocyte as recorded by Wu and Zhou [60] in the case of Oryzias latipes, and hemorrhage of gills in case of Ag-NP-treated Caspian roach [61].
The liver sections showed normal hepatocytes with no visible alterations in the control. The treatment of Ag-NPs showed congestive enlargement of lysosomes, which create vacuolar degenerations in the liver. The 10 mg L−1 treatment produced pycnotic nuclei in the hepatic cells along with the necrosis. These pycnotic nuclei were similar as recorded by Perera and Pathiratne [58] in Oreochromis niloticus. The necrosis were seen at higher levels in liver tissues of L. rohita treated with Ag-NPs. Further, this study showed congestion in hepatic parenchyma, which decreased the size of hepatic cells. These alterations could be due to excess metabolism and detoxification of toxic particles in the liver [62]. Similar alterations were recorded in the liver of rainbow trout treated with Ag-NPs in the study of Monfared and Soltani [63]. Further 20 to 55 mg L−1 Ag-NP treatment showed deformation of blood vessel, necrosis, focal necrosis, inflammation of hepatic parenchyma tissue, accumulation of the color pigmentation in hepatic tissue, congestion, and edema as recorded in the study by Rajkumar et al. [55] for L. rohita and Lee et al. [64] for Common Carp in case of citrate-capped Ag-NPs.
Conclusions
The amine-coated Ag-NPs with average size of 15.78 nm caused significant changes of hematological parameters in a dose-dependent manner. The higher dose created significantly higher alterations in the production or synthesis of hematological contents. The histopathology of liver showed reduction in the size of hepatocytes, synthesis of necrotic and apoptotic bodies. In gill tissues, the particles caused proliferation of bronchial chloride cells, fusion of lamellae, and formation of aneurism. This study concluded that Ag-NPs cause hematoxicity and histotoxicity in aquatic organisms at an elevated level.
Change history
27 February 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s12011-024-04124-5
References
Völker C, Oetken M, Oehlmann J (2013) The biological effects and possible modes of action of nanosilver, in reviews of environmental contamination and toxicology volume 223, Springer. p. 81–106. doi: 10.1007/978-1-4614-5577-6_4
Woodrow Wilson dWoodrow Wilson d (2016) Nanotechnology consumer product inventory [cited 2016 28 Aprthe case; Available from: http://www.nanotechproject.org/cpi/about/analysis.
Khan MS, Jabeen F, Qureshi NA, Asghar MS, Shakeel M, Noureen A (2015a) Toxicity of silver nanoparticles in fish: a critical review. J Bio Environ Sci 6(5):211–227
Schluesener JK, Schluesener HJ (2013) Nanosilver: application and novel aspects of toxicology. Arch Toxicol 87(4):569–576. doi:10.1007/s00204-012-1007-z
Taju G, Majeed SA, Nambi K, Hameed AS (2014) In vitro assay for the toxicity of silver nanoparticles using heart and gill cell lines of Catla catla and gill cell line of Labeo rohita. Comp Biochem Physiol C Pharmacol Toxicol 161:41–52. doi:10.1016/j.cbpc.2014.01.007
Awasthi KK, Awasthi A, Bhoot N, John P, Sharma SK, Awasthi K (2013) Antimicrobial properties of electro-chemically stabilized organo-metallic thin films. Adv Electrochem 1(1):42–47. doi:10.1166/adel.2013.1013
Smith IC, Carson BL (1977) Trace metals in the environment. Vol. 1. Arbor Science Publishers, USA
Wijnhoven SW, Peijnenburg WJ, Herberts CA, Hagens WI, Oomen AG, Heugens EH, Roszek B, Bisschops J, Gosens I, Van De Meent D (2009) Nano-silver—a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicol 3(2):109–138. doi:10.1080/17435390902725914
Yin L, Cheng Y, Espinasse B, Colman BP, Auffan M, Wiesner M, Rose J, Liu J, Bernhardt ES (2011) More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol 45(6):2360–2367. doi:10.1021/es103995x
Khan MS, Jabeen F, Asghar MS, Qureshi NA, Shakeel M, Noureen A, Shabbir S (2015) Role of nao-ceria in the amelioration of oxidative stress: current and future applications in medicine. Int J Biosci 6(8):89–109. doi:10.12692/ijb/6.8.89-109
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150(1):5–22
Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ (2008) Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B 112(43):13608–13619
K-i I, Takano H, Yanagisawa R, Koike E, Shimada A (2009) Size effects of latex nanomaterials on lung inflammation in mice. Toxicol Appl Pharmacol 234(1):68–76
Parish C (2013) Agency for toxic substances and disease registry doi: 10.1.1.361.6740
Wan AT, Conyers R, Coombs CJ, Masterton JP (1991) Determination of silver in blood, urine, and tissues of volunteers and burn patients. Clin Chem 37(10):1683–1687
Larese FF, D’Agostin F, Crosera M, Adami G, Renzi N, Bovenzi M, Maina G (2009) Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology 255(1):33–37. doi:10.1016/j.tox.2008.09.025
Khan MS, Qureshi NA, Jabeen F, Asghar MS, Shakeel M, Fakhar-E-Alam M (2016) Eco-friendly synthesis of silver nanoparticles through economical methods and assessment of toxicity through oxidative stress analysis in the Labeo Rohita. Biol Trace Elem Res:1–13. doi:10.1007/s12011-016-0838-5
Ali D (2014) Oxidative stress-mediated apoptosis and genotoxicity induced by silver nanoparticles in freshwater snail Lymnea luteola L. Biol Trace Elem Res 162(1–3):333–341. doi:10.1007/s12011-014-0158-6
Arora S, Jain J, Rajwade J, Paknikar K (2009) Interactions of silver nanoparticles with primary mouse fibroblasts and liver cells. Toxicol Appl Pharmacol 236(3):310–318. doi:10.1016/j.taap.2009.02.020
Zhornik E, Baranova L, Drozd E, Sudas M, Chau N, Buu N, Dung T, Chizhik S, Volotovski I (2014) Silver nanoparticles induce lipid peroxidation and morphological changes in human lymphocytes surface. Biophys 59(3):380–386. doi:10.1134/s0006350914030282
Schrand AM, Braydich-Stolle LK, Schlager JJ, Dai L, Hussain SM (2008) Can silver nanoparticles be useful as potential biological labels? Nanotech 19(23):235104. doi:10.1088/0957-4484/19/23/235104
Ahamed M, Alsalhi MS, Siddiqui MK (2010) Silver nanoparticle applications and human health. Clin Chim Acta 411(23–24):1841–1848. doi:10.1016/j.cca.2010.08.016
Zhang T, Wang L, Chen Q, Chen C (2014) Cytotoxic potential of silver nanoparticles. Yonsei Med J 55(2):283–291. doi:10.3349/ymj.2014.55.2.283
Piao MJ, Kang KA, Lee IK, Kim HS, Kim S, Choi JY, Choi J, Hyun JW (2011) Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett 201(1):92–100. doi:10.1016/j.toxlet.2010.12.010
Hussain S, Hess K, Gearhart J, Geiss K, Schlager J (2005) In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol in Vitro 19(7):975–983. doi:10.1016/j.tiv.2005.06.034
Sung JH, Ji JH, Yoon JU, Kim DS, Song MY, Jeong J, Han BS, Han JH, Chung YH, Kim J (2008) Lung function changes in Sprague-Dawley rats after prolonged inhalation exposure to silver nanoparticles. Inhal Toxicol 20(6):567–574. doi:10.1080/08958370701874671
Recordati C, De Maglie M, Bianchessi S, Argentiere S, Cella C, Mattiello S, Cubadda F, Aureli F, D’Amato M, Raggi A (2016) Tissue distribution and acute toxicity of silver after single intravenous administration in mice: nano-specific and size-dependent effects. Part Fibre Toxicol 13(1):1
Kataria N, Kataria AK, Pandey N, Gupta P (2010) Serum biomarkers of physiological defense against reactive oxygen species during environmental stress in Indian dromedaries. HVM Bioflux 2(2):55–60
Govindasamy R, Rahuman AA (2012) Histopathological studies and oxidative stress of synthesized silver nanoparticles in Mozambique tilapia (Oreochromis mossambicus). J Environ Sci 24(6):1091–1098. doi:10.1016/S1001-0742(11)60845-0
Lee B, Duong CN, Cho J, Lee J, Kim K, Seo Y, Kim P, Choi K, Yoon J (2012, 2012) Toxicity of citrate-capped silver nanoparticles in common carp (Cyprinus carpio). Biomed Res Int
Afifi M, Saddick S, Zinada OAA (2016) Toxicity of silver nanoparticles on the brain of Oreochromis niloticus and Tilapia zillii. Saudi J Biol Sci 23(6):754–760. doi:10.1016/j.sjbs.2016.06.008
Li S-D, Huang L (2008) Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm 5(4):496–504. doi:10.1021/mp800049w
Dobšíková R, Svobodová Z, Blahova J, Modrá H, Velíšek J (2006) Stress response to long distance transportation of common carp (Cyprinus carpio L.) Acta Vet Brno 75(3):437–448
Dobšíková R, Svobodova Z, Blahova J, Modra H, Velíšek J (2009) The effect of transport on biochemical and haematological indices of common carp (Cyprinus carpio L.) Czech J Anim Sci 54(11):510–518
Di Giulio RT, Hinton DE (2008) The toxicology of fishes. Crc Press. doi:10.1201/9780203647295
Imani M, Halimi M, Khara H (2015) Effects of silver nanoparticles (AgNPs) on hematological parameters of rainbow trout, Oncorhynchus mykiss. Comp Clin Pathol 24(3):491–495. doi:10.1007/s00580-014-1927-5
Ruane N, Bonga SW, Balm P (1999) Differences between rainbow trout and brown trout in the regulation of the pituitary–interrenal axis and physiological performance during confinement. Gen Comp Endocrinol 115(2):210–219. doi:10.1006/gcen.1999.7292
Witeska M, Kościuk B (2003) The changes in common carp blood after short-term zinc exposure. Environ Sci Pollut R 10(5):284–286. doi:10.1065/espr2003.07.161
Vutukuru S (2005) Acute effects of hexavalent chromium on survival, oxygen consumption, hematological parameters and some biochemical profiles of the Indian major carp, Labeo rohita. Int J Environ Res Publ Health 2(3):456–462. doi:10.3390/ijerph2005030010
Vinodhini R, Narayanan M (2008) Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (common carp). Int J Environ Sci Tech 5(2):179–182. doi:10.1007/bf03326011
Stoskopf KM (1993) Fish medicine, 1st edition. W.B. Saunders Co., Philadelphia
Vandebriel RJ, Tonk EC, de la Fonteyne-Blankestijn LJ, Gremmer ER, Verharen HW, van der Ven LT, van Loveren H, de Jong WH (2014) Immunotoxicity of silver nanoparticles in an intravenous 28-day repeated-dose toxicity study in rats. Part Fibre Toxicol 11(1):1–9. doi:10.1186/1743-8977-11-21
Adams SM (2002) Biological indicators of aquatic ecosystem stress. American Fisheries Society.
Cheraghi J, Hosseini E, Hoshmandfar R, Sahraei R (2013) Hematologic parameters study of male and female rats administrated with different concentrations of silver nanoparticles. Intl J Agri Crop Sci 5(7):789
Ellsaesser C, Clem L (1986) Haematological and immunological changes in channel catfish stressed by handling and transport. J Fish Biol 28(4):511–521. doi:10.1016/0145-305x(86)90149-7
Ikramullah A, Salve D, Pai G, Rathore M, Joshi D (2013) In vitro cytotoxicity testing of silver nano-particals in lymphocyte and sperm cells. Ind J Fund Appl Life Sci 3:44–47
Banaee M, Mirvagefei A, Rafei G, Majazi Amiri B (2008) Effect of sub-lethal diazinon concentrations on blood plasma biochemistry. Int J Environ Res 12(2):189–198
Abarghoei S, Hedayati SA, Ghafari Farsani H, Gerami MH (2015) Hematological responses of goldfish (Carassius auratus) to different acute concentrations of silver sulfate as a toxicant. Pollution 1(3):247–256. doi:10.7508/pj.2015.03.001
Williams KM, Gokulan K, Cerniglia CE, Khare S (2016) Size and dose dependent effects of silver nanoparticle exposure on intestinal permeability in an in vitro model of the human gut epithelium. J Nanobiotechnology 14(1):62. doi:10.1186/s12951-016-0214-9
Soares T, Ribeiro D, Proença C, Chisté RC, Fernandes E, Freitas M (2016) Size-dependent cytotoxicity of silver nanoparticles in human neutrophils assessed by multiple analytical approaches. Life Sci 145:247–254. doi:10.1016/j.lfs.2015.12.046
Liz R, Simard J-C, Leonardi LBA, Girard D (2015) Silver nanoparticles rapidly induce atypical human neutrophil cell death by a process involving inflammatory caspases and reactive oxygen species and induce neutrophil extracellular traps release upon cell adhesion. Int Immunopharmacol 28(1):616–625. doi:10.1016/j.intimp.2015.06.030
Neumann NF, Barreda DR, Belosevic M (2000) Generation and functional analysis of distinct macrophage sub-populations from goldfish (Carassius auratus L.) kidney leukocyte cultures. Fish Shellfish Immunol 10(1):1–20. doi:10.1006/fsim.1999.0221
Rieger AM, Hall BE, Barreda DR (2010) Macrophage activation differentially modulates particle binding, phagocytosis and downstream antimicrobial mechanisms. Dev Comp Immunol 34(11):1144–1159. doi:10.1016/j.dci.2010.06.006
Al-Bairuty GA, Shaw BJ, Handy RD, Henry TB (2013) Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 126:104–115. doi:10.1016/j.aquatox.2012.10.005
Rajkumar K, Kanipandian N, Thirumurugan R (2015) Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl Nanosci: 1–11. doi: 10.1007/s13204-015-0417-7
Al-Ghanbousi R, Ba-Omar T, Victor R (2012) Effect of deltamethrin on the gills of Aphanius dispar: a microscopic study. Tissue Cell 44(1):7–14. doi:10.1016/j.tice.2011.09.003
Van Dyk J, Marchand M, Pieterse G, Barnhoorn IE, Bornman M (2009) Histological changes in the gills of Clarias gariepinus (Teleostei: Clariidae) from a polluted south African urban aquatic system. Afr J Aquat Sci 34(3):283–291. doi:10.2989/ajas.2009.34.3.10.986
Perera S, Pathiratne A (2012) Haemato-immunological and histological responses in Nile tilapia, Oreochromis niloticus exposed to titanium dioxide nanoparticles. Sri Lanka J Aquat Sc 17: 1–18. Doi: 0.4038/sljas.v17i0.6852
Salah M, Farghali AA, Azmy H, Khedr MH (2013) Biological compatibility of carbon nanotubes for treatment of pollution of Nile tilapia (Oreochromis niloticus) by lead acetate. Life Sci J 10(2)
Wu Y, Zhou Q (2013) Silver nanoparticles cause oxidative damage and histological changes in medaka (Oryzias latipes) after 14 days of exposure. Environ Toxicol Chem 32(1):165–173. doi:10.1002/etc.2038
Sharifian M, Khani F, Khosravi K, Khalili M, Hedayati A (2013) Sublethal effect of nanosilver on the structure of gill of Caspian roach (Rutilus rutilus caspicus) fingerlings. Intl J Aquat Biol 1(2):55–60
Patel J, Bahadur A (2011) Histopathological manifestations of sub lethal toxicity of copper ions in Catla catla. Am-Eurasian J Toxicol Sci 4(1):01–05
Monfared AL, Soltani S (2013) Effects of silver nanoparticles administration on the liver of rainbow trout (Oncorhynchus mykiss): histological and biochemical studies. Eur J Exp Biol 3(2):285–289
Lee O, Green JM, Tyler CR (2015) Transgenic fish systems and their application in ecotoxicology. Crit Rev Toxicol 45(2):124–141. doi:10.3109/10408444.2014.965805
Author information
Authors and Affiliations
Corresponding author
Additional information
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s12011-024-04124-5
About this article
Cite this article
Khan, M.S., Qureshi, N.A., Jabeen, F. et al. RETRACTED ARTICLE: Assessment of Waterborne Amine-Coated Silver Nanoparticle (Ag-NP)-Induced Toxicity in Labeo rohita by Histological and Hematological Profiles. Biol Trace Elem Res 182, 130–139 (2018). https://doi.org/10.1007/s12011-017-1080-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1080-5