Abstract
The present study investigates the nephrotoxic effects of two acute doses of silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on the African catfish, Clarias gariepinus, using biochemical, histochemical, and histopathological changes as biomarkers. AgNP-induced impacts were recorded in some of these characteristics on the bases of their size (20 and 40 nm) and concentration (10 and 100 μg/L) but no significant interaction between size and concentration. AgNO3 had low significant adverse effects on some parameters in comparison with those impacts of AgNPs. The concentrations of creatinine and uric acid exhibited different significant variations under stress in all exposed groups compared with those in the control group. On the tissue and cell levels, histopathological changes were observed. These changes include hypertrophies of glomeruli, proliferation in the haemopoietic tissue, dissociation in renal tubules, shrinkage of glomerulus, hydropic degeneration, dilatation of renal tubules, aggregation of melanomacrophages, rupture of Bowman’s capsule, and the glomerular tuft and dilatation of Bowman’s space. In more severe cases, the degenerative process leads to tissue necrosis in the kidney of AgNP-exposed fish as well as carbohydrate depletion; a faint coloration was also observed in the brush borders and basement membrane with a large amount of connective tissue fibers around the blood vessels and the renal tubules. Recovery period for 15 days led to improvement of most of the alterations in biochemical, histopathological, and histochemical parameters induced by AgNPs and AgNO3. In conclusion, one can postulate on the sensitivity of the kidney of C. gariepinus to AgNPs and recovery strategy is a must.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotoxicology refers to the study of the interactions of nanostructures (1–100 nm) with biological systems on the bases of the relationship between their physical and chemical properties and the toxic biological responses (Asharani et al. 2009; Yang et al. 2009). These responses include growth and reproduction impairment, mortality, and physiological, biochemical, and histological functions in both adult and embryos (Abarghoei et al. 2016; Abdel-Khalek et al. 2015; Al-Bairuty et al. 2013; Alkaladi et al. 2015; Mekkawy et al. 2019; Pournori et al. 2017; Sayed 2016). The small size, chemical composition, surface structure, solubility, shape, agglomeration, and aggregation of these particles may be associated with NP-induced toxicity (Nel et al. 2006; Skebo et al. 2007; Wallace et al. 2007). In spite of their toxicity which must be controlled and managed, nanoparticles have a wide increased spectrum of society benefits in different fields (Attia 2014; Mekkawy et al. 2019; Sayed 2016; Sayed and Soliman 2017; Sayed and Younes 2017). Due to their unique optical, electrical, and thermal properties, silver nanoparticles (AgNPs) are one of the most common types of nanoparticles in use serving various industrial and domestic purposes (Farkas et al. 2010; Morones et al. 2005; Skebo et al. 2007). Such economic success led to their presence in the aquatic environment with the increasing risk of potential adverse effects on aquatic animals including fishes (Mekkawy et al. 2019). Different studies focused on the adverse impacts of AgNPs on fishes identifying a wide spectrum of biomarkers (Asharani et al. 2008; Bar-Ilan et al. 2009; Bilberg et al. 2011; Chae et al. 2009; Choi et al. 2009; Griffitt et al. 2009; Mekkawy et al. 2019; Powers et al. 2010; Sayed 2016; Sayed and Soliman 2017; Wu et al. 2010; Yeo and Kang 2008.). The AgNPs are taken through the skin, gills, and alimentary tracts and are translocated to different organs through the circulatory and lymphatic systems (Panyala et al. 2008) exhibiting variable degrees of toxicity especially in the liver and kidney. On the other hand, silver ions were reported to have dose-dependent effects on fish (Sayed and Soliman 2017).
Biochemical parameters are fundamental in the physio-pathological evaluation of animals (Juneja and Mahajan 1983; Ranzani-Paiva et al. 1999; Sayed and Authman 2018) with identification of the target organs of toxicity (Dube et al. 2014; Sayed et al. 2011; Sayed and Hamed 2017). For example, nephrotoxicity manifests as renal failure with a rise in serum creatinine and uric acid levels (Kotb et al. 2018; Uboh et al. 2009). Serum creatinine and uric acid are some waste metabolic products excreted exclusively via the kidneys so they provide useful information about the health status of the kidney. High levels of such chemicals indicate malfunction of the kidney (Abdel-Khalek et al. 2015; Ajeniyi and Solomon 2014; Alkaladi et al. 2015; Giordano et al. 2015; Noureen et al. 2017; Panda 1999) and histopathological alterations which can be used also as indicators for the effects of various pollutants (Abarghoei et al. 2016; Johari et al. 2015; Mansouri and Johari 2016; Pournori et al. 2017; Ramesh and Nagarajan 2013; Yazdanparast et al. 2016).
According to the aforementioned findings, the present work was suggested and aimed to study the nephrotoxicity of the kidney of the African catfish, Clarias gariepinus (Burchell, 1822) induced by AgNPs and AgNO3 in an attempt to determine the histopathological and histochemical biomarkers in corresponding with the biochemical biomarkers of the kidney (creatinine and uric acid).
Materials and methods
Silver nanoparticle characterization
Silver nanoparticles with a size of 20 and 40 nm were purchased from Nanostructured and Amorphous Materials Inc. (Houston). The characterization of these silver nanoparticles was studied and identified in detail by Mekkawy et al. (2019) with confirmation of their crystalline nature in Assiut University Labs. X-ray diffraction (four Bragg reflections at 38.114°, 44.298°, 64.441°, and 77.395° corresponding to 111, 200, 220, and 311 sets of lattice planes, respectively) and stability of AgNPs as well as their average sizes estimated by transmission electron microscope (average particle size and SD of 11.21 ± 4.13 and 32.62 ± 13.48 for 20 nm and 40 nm of AgNPs, respectively) were considered in this concern.
Fish
Juveniles of the African catfish, C. gariepinus, were collected from private fish farm in May 2015 then transported to the Fish Biology and Pollution laboratory, Zoology Department, Faculty of Science, Assiut University. Fish were fed with a commercial fish food (5% body weight) twice a day and kept at approximately in 28 °C with 12 h:12 h light-dark cycle in many tanks (100 L each) for 3 months to acclimatize to laboratory conditions prior to experiments. During the acclimation period, about 20% of the water in each tank was replaced daily. Fish ranged between 23.5 and 32 cm in total length and 70 and 110 g in weight. The water used to raise the fish was dechlorinated and continuously aerated tap water. The water temperature, pH, and dissolved oxygen concentrations (DO) were measured daily (29.17 ± 0.27 °C, 8.5 ± 0.03 pH, and 34.47 ± 11.99 mg/L DO).
Experimental design and AgNP exposure
The acclimatized fishes were randomly divided into 6 groups, control and five exposed ones. Each group contains 12 specimens in a glass tank measuring 100 × 35 × 50 cm (L × W × H) with a total volume of 100 L. These fish groups are the control, 20 nm/10 μg/L AgNPs, 20 nm/100 μg/L AgNPs, 40 nm/10 μg/L AgNPs, 40 nm/100 μg/L AgNPs, and 100 μ/L silver nitrate (AgNO3). Exposure period was 15 days followed by a 15-day recovery period. To minimize the decrease in the nominal concentration of AgNPs, which potentially adsorb onto residual food and feces in the test water, each experimental aquarium was supplied with food for 1 h prior to dosing.
Dosing stocks of the silver particles were made by suspending 1.5 mg of each particle in 1-L ultrapure water and sonicating for 30 min and diluting as required. The silver nitrate dosing stock (1.5 mg/L) was also made up in ultrapure water and sonicated similarly. Experimental tanks were filled with dechlorinated water and dosed by chemicals with immediate addition of the fish to minimize reduction of nominal dosing concentrations through adhesion of the particles/chemicals to the glass. Then, every 48 h, 75% of tank water was changed and immediately re-dosed.
Biochemical analysis
At the end of the experimental periods, six fish from each group were collected and anesthetized using a 200-ppm solution of clove powder (Hedayati and Jahanbakhshi 2012). Blood samples were collected from the caudal veins of fish and allowed to clot in clean, dry centrifuge tubes at room temperature then centrifuged at 5000 rpm, at 4 °C for 20 min, and the serums were separated for the analysis of biochemical parameters (creatinine and uric acid). Biochemical kit of creatinine and uric acid was purchased from Bio-Merieux Co., France.
Histological and histopathological examination
For microscopic preparations, after intervals of 15-day exposure and 15-day recovery period, 4 fish of each group were removed and sacrificed. Small pieces of the kidney tissue were taken and immediately fixed in 10% neutral buffered formalin. Fixed tissues were processed routinely for paraffin embedding technique. Embedded tissues were sectioned at 5 μ in thickness and then stained by the following stain: Harris’s hematoxylin and eosin stain (HE) (Bancroft and Stevens 1982). Ten randomly selected sections of four fish from each experimental group were chosen to indicate each histopathological parameter as (control, − no alteration (0–2); mild, + (> 2–10%) area of section; moderate, ++ (> 10–40%) area of section; and severe, +++ (> 40% area of section). Finally, tissues were examined and imaged using an Omax advanced trinocular biological microscope with a 14-MP USB digital camera (A35140U3; China). Measurements were made on images of the kidney tissue. The cross-sectional area of altered tissue as well as the total area of the histological section was estimated according to the Motic Images Plus 2.0 program.
Histochemical preparation
Estimation of general carbohydrates represents the important parameters among the histochemical ones. For the demonstration of the polysaccharides status, the periodic acid Schiff’s (PAS) technique was applied (Mc Manus 1946). In this regard, carbohydrates were first-oxidized with 0.1%periodic acid; aldehyde groups (-HCO-HCO) were liberated from the glycol reagent, producing a compound of magenta coloration.
Statistical analysis
The basic statistics, means, standard errors, and ranges of the measured parameters were estimated. Levene’s test of equality of error variance of the variables was applied, with rejection of the null hypothesis for raw, log-transformed, and SQRT-transformed data. So, the homogeneity of variance was assumed for raw data. The pattern of variations in the parameters considered due to the size and concentration of the AgNPs and size-concentration interaction was studied by the two-way ANOVA. Moreover, in the absence of interactions, the pattern of variations was recorded by the one-way ANOVA in all the exposed and control groups. The Tukey HSD test was considered for multiple comparisons. The IBM-SPSS package version 21 (IBM-SPSS 2012) and Xls sheets were considered at 0.05 significance level.
Results
Kidney functions
The creatinine levels of Clarias gariepinus for 15 days of exposure and 15 days of recovery periods are given in Table 1. The main effects of AgNP size and concentration on creatinine and uric acid levels were significant with no significant size-concentration interaction. In the recovery period, such significant main effects were eliminated except for the main effect of concentration factor which is still significant for uric acid. In comparison with the control and silver nitrate groups, the creatinine and uric acid levels of the nanoparticle-exposed fish exhibited significant variability (Table 1). After 15-day recovery, the pollutant adverse effects on the creatinine level were eliminated in comparison with the control whereas such effects are still recorded to some extent in uric acid (Table 1).
Histopathological and histochemical studies
Control kidney
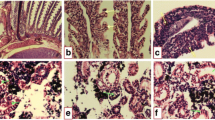
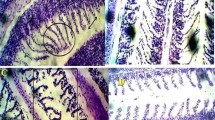
The kidney of the control fish Clarias gariepinus is composed of a large number of nephrons (Fig. 1a). Each nephron is composed of a roughly spherical corpuscle enclosing a tuft of blood capillaries with Bowman’s space, renal tubules, and collecting tubules. These structures are surrounded by haemopoietic tissue. The renal tubules are numerous and their cross sections exhibit a round or oval outline with a narrow lumen. Their lining coat is composed of columnar epithelial cells with markedly eosinophilic cytoplasm and centrally located nuclei. The collecting tubules are lined with cubical or low columnar epithelial cells with basally located nuclei (Fig. 1a). However, in the control kidney of fish, low damage of 4.14% was recorded in kidney sections examined. Histochemically, the PAS technique displayed a positive reaction mainly at the brush border and at the basement membrane of the renal tubules. Renal (Malpighian) corpuscles appeared moderately reacted (Fig. 2a).
Transverse section photomicrographs of the control (a), exposed (b–f), and recovered (g–h) kidney sections of fish Clarias gariepinus (H&E × 400). a Control fish liver showing the renal corpuscle (RC), renal tubules (RT), and haemopoietic tissue (HT). b Exposure to 100 μg/L of silver nitrate (AgNo3) for 15 days showing degeneration (D) and dissociation (DI), hypertrophy in the glomerulus (HYT), proliferation in renal tubules (PR), necrosis (N), and melanomacrophages (Mm). c Exposure to 20 nm/10 μg/L AgNPs for 15 days showing proliferation (PR) in the renal tubular cells and the haemopoietic tissue (HT), dissociation (DI) in renal tubules, shrinkage (SH) of glomerulus, slight dilation of Bowman’s space (BS), and necrosis (N). d Exposure to 20 nm/100 μg/L AgNPs for 15 days showing rupture of Bowman’s capsule (RBC), shrinkage (SH) of glomerulus, slight dilation of Bowman’s space (BS), dilated of renal tubules (DRT), degeneration (D) and dissociation (DI), and necrosis (N). e Exposure to 40 nm/10 μg/L AgNPs for 15 days showing hydropic degeneration (D) and dissociation (DI), hypertrophy in the glomerulus (HYT), proliferation (PR) in the renal tubules, melanomacrophages (Mm), and necrosis (N). f Exposure to 40 nm/100 μg/L AgNPs for 15 days showing hydropic degeneration (D) and dissociation (DI) in renal tubules, proliferation (PR) in haemopoietic tissue, shrinkage (SH) of glomerulus, slight dilation of Bowman’s space (BS), melanomacrophages (Mm) ,and necrosis (N). g, h Recovered fish liver sections showing improvement in the renal corpuscle, glomeruli, and haemopoietic tissue and some of the renal tubules, while proliferation (PR), hyaline degeneration (D) and dissociation (DI) in the renal tubules, and necrotic area (N) were observed
Transverse section photomicrographs of the control (a), exposed (b–f), and recovered (g, h) kidney sections of fish Clarias gariepinus (PAS reaction, × 400). a Control fish kidney showing positive PAS reactivity in brush border (BB) and the basement membrane (BM) of the renal tubules, renal corpuscle (RC), and glomerulus (G). b Exposure to 100 μg/L of silver nitrate (AgNo3) for 15 days showing carbohydrate depletion observed in a faint coloration of the basement membrane (BM) and brush border (BB). c Exposure to 20 nm/10 μg/L AgNPs for 15 days showing a remarkable depletion of carbohydrate materials in brush border (BB) and the basement membrane (BM) of the renal tubules and glomerulus (G). d Exposure to 20 nm/100 μg/L AgNPs for 15 days showing great decline in carbohydrate materials in brush border (BB) and the basement membrane (BM) of the renal tubules and glomerulus (G). e Exposure to 40 nm/10 μg/L AgNPs for 15 days showing carbohydrate depletion observed in a faint coloration of the brush borders (BB), basement membrane (BM), and glomerulus (G). f Exposure to 40 nm/100 μg/L AgNPs for 15 days showing carbohydrate depletion observed in a faint coloration of the brush borders (BB) and basement membrane (BM). g, h Recovered fish kidney sections showing moderate amount of carbohydrate observed in the glomerulus (G), basement membrane (BM), the brush border (BB), and the haemopoietic tissue (HT)
Exposure of Clarias gariepinus to100 μg/L of silver nitrate
The silver nitrate dose used for 15 days of exposure led to adverse impacts on the kidney including hydropic degeneration and dissociation, hypertrophy of glomerulus, and necrosis in some renal tubules and haemopoietic tissue. Proliferation in renal tubules and melanomacrophages were also observed (Fig. 1b). Such impacts are reflected in terms of morphometric data and semiquantitative evaluations of the histopathology of the AgNO3-exposed kidney of C. gariepinus (Tables 2 and 3) with 33.7% of damage.
After 15 days of recovery, healthy glomeruli, structural improvement of some of the renal tubules, and the absence of melanomacrophages were recorded whereas the haemopoietic tissue is still exhibiting necrosis. Marked proliferation and hyaline degeneration in the renal tubules with reduced lumen were also observed (Fig. 1g, h) with 18.83% of damage (Table 2).
PAS reaction revealed carbohydrate depletion observed as faint coloration of the glomerulus and basement membrane after 15 days of exposure compared with those of the control (Fig. 2b). After 15 days of recovery, large amount of carbohydrate was observed in the glomerulus, basement membrane, and the brush border (Fig. 2g, h).
Exposure of Clarias gariepinus to 10 μg/L of silver nanoparticles (20 nm)
The kidney of fish exposed to 10 μg/L of silver nanoparticles for 15 days showed severe damage in the renal tubules with dissociation, necrosis, and proliferation. The haemopoietic tissue also exhibited proliferated under stress. Glomerulus showed shrinkage and hence increasing in the Bowman’s space (Fig. 1c). In terms of the morphometric data and semiquantitative evaluations of the histopathology in exposed kidney of C. gariepinus (Tables 2 and 3), a damage of 51.67% was recorded.
After 15 days of recovery period, improvement in the renal tissue, in glomeruli, and in haemopoietic tissue were observed to some extent. Some of the renal tubules showed normal structures and some showed hydropic degeneration and dissociation (Fig.2g, h) with a damaged percent of 65.51% (Table 2).
Under silver nanoparticles, examination of PAS-exposed kidney sections of silver nanoparticles showed great decline in carbohydrate materials as reflected by the feebly stained renal corpuscle after 15 days of exposure. Also, the basement membranes and brush borders of the renal tubules were suffering from great deficiency of carbohydrate materials (Fig. 2c). After 15 days of recovery, moderate carbohydrate material localization was observed in the renal tissue (Fig. 2g, h).
Exposure of Clarias gariepinus to 100 μg/L of silver nanoparticles (20 nm)
Increasing the concentration of AgNPs of 20 nm led to severe alterations including rupture of Bowman’s capsule with disorganization in the glomerular tuft and glomerulus shrinkage with increasing in the Bowman’s space. Dissociation of indistinct lumen and focal tubular necrosis and necrotic area was also recorded. Proliferation and degeneration of the epithelial cells lining the renal tubules and of the parietal cells of renal corpuscles were observed (Fig. 1d); damage was represented by 85.97% (Tables 2 and 3).
After 15 days of recovery, kidney sections showed improvement in the renal tubules, renal corpuscle, and haemopoietic tissue. However, some other tubules showed hydropic degeneration and dissociation (Fig. 1g, h) with a general damage of 58.13% (Table 2).
The application of PAS reaction showed great decline in carbohydrate materials as reflected by the feebly stained renal corpuscle after 15 days of exposure. Also, the basement membranes and brush borders of the renal tubules were suffering from a great deficiency of carbohydrate materials (Fig. 2d). After 15 days of recovery, moderate carbohydrate material localization was revealed in the renal tissue (Fig. 2g, h).
Exposure of Clarias gariepinus to 10 μg/L and 100 μg/L of silver nanoparticles (40 nm)
As regards the two concentrations of AgNPs of 40 nm in size, severe alterations in the fish kidney structures in terms of histopathology and PAS reaction (Figs. 1 and 2) were recorded with variability after 15 days of exposure. These alterations were improved by 15-day recovery period. These patterns of damage (Figs. 1 and 2e, f) and recovery improvement (Figs. 1 and 2g, h) were similar to those considered in the case of the two concentrations of AgNPs of 20 nm in size previously mentioned (Tables 2 and 3) with a variable percent of damage.
Discussion
The evaluation of biochemical characteristics in fish has become an important health indicator (Mekkawy et al. 2019; Saravanan et al. 2011; Sayed and Authman 2018). Serum creatinine and uric acid can be used as a rough index of the glomerular filtration rate and kidney dysfunction (Ajeniyi and Solomon 2014; Maita et al. 1984). The results in the present study indicated a significant increase in serum creatinine and uric acid of C. gariepinus exposed to AgNPs for 15 days. These results were in agreement with (Abdel-Khalek et al. 2015; Alkaladi et al. 2015) who observed a highly significant increase in serum creatinine and uric acid of Nile tilapia, Oreochromis niloticus, after exposure to copper and zinc oxide nanoparticles respectively. This elevation of serum creatinine level may be related to kidney dysfunction. In the present work, a recovery period for 15 days improved creatinine and uric acid levels compared with that of control group of Clarias gariepinus. Different authors referred to the requirement of the recovery period to remove the impacts of pollutants (Gaber 2007; Shaw and Handy 2006).
Histological studies by different authors and the present study were found to be a useful tool for assessing the damage of different tissues caused by nanomaterial (Abarghoei et al. 2016; Johari et al. 2015; Mansouri and Johari 2016; Pournori et al. 2017; Yazdanparast et al. 2016). In the present study, the impact of the AgNPs was observed in the tissue kidney with different degrees of variable impacts and alterations. These impacts and alterations are in agreement with those observed in Zebrafish (Danio rerio) exposed to silver nanoparticles (Yazdanparast et al. 2016), in Cyprinus carpio L. exposed to copper (Al-Tamimi et al. 2015) and in Oreochromis niloticus and Clarias gariepinus exposed to cadmium and atrazine (Mekkawy et al. 2013; Mekkawy et al. 2012), respectively, and in Clarias gariepinus exposed to silver nanoparticles (Sayed and Younes 2017).
The damage recorded in the kidney and accordingly in erythropoiesis was confirmed in the first part of this study as erythrocytes alterations and erythrocytes account decrease (Mekkawy et al. 2019). The kidney damages especially in the glomeruli and renal tubules were interpreted by different authors including Bucher and Hofer (1993), Robbins and Angell (1976), Thomson (1984), and Tu (1991). In the present work and those of Khidr et al. (2001), Mekkawy et al. (2013), Mekkawy et al. (2012), Mohamed (2006), and Wassif et al. (2000), these damages were also recorded by PAS reactions in terms of carbohydrate depletion under stress.
Conclusion
The AgNO3 and silver nanoparticles in different concentrations lead to adverse impacts on physio-biochemical parameters and damage the function and histopathology of the kidney of C. gariepinus. So, the use and application of such chemicals should be managed and controlled to protect the aquatic ecosystems. Moreover, the recovery strategy of pollutants is a must for fish species according to their positions in the food web.
References
Abarghoei S, Hedayati A, Ghorbani R, Miandareh HK, Bagheri T (2016) Histopathological effects of waterborne silver nanoparticles and silver salt on the gills and liver of goldfish Carassius auratus. Int J Environ Sci Technol 13:1753–1760
Abdel-Khalek AA, Kadry MAM, Badran SR, Marie MAS (2015) Comparative toxicity of copper oxide bulk and nano particles in Nile tilapia; Oreochromis niloticus: Biochemical and oxidative stress. J Basic Appl Zool 72:43–57
Ajeniyi SA, Solomon RJ (2014) Urea and creatinine of Clarias gariepinus in three different commercial ponds. Nat Sci 12:124–138
Al-Bairuty GA, Shaw BJ, Handy RD, Henry TB (2013) Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 126:104–115
Alkaladi A, Nasr AM, El-Deen N, Afifi M, Abu Zinadah OA (2015) Hematological and biochemical investigations on the effect of vitamin E and C on Oreochromis niloticus exposed to zinc oxide nanoparticles. Saudi J Biol Sci 22:556–563
Al-Tamimi AH, Al-Azzawi AJ, Al-A'dhmi MA (2015) Chronic toxicity assessment of histological changes and micronuclei in fish Cyprinus carpio L. after exposed to copper. Am Sci Res J Eng Technol Sci 13:194–210
Asharani PV, Wu YL, Gong Z, Valiyaveettil S (2008) Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 19:255102
Asharani PV, Hande MP, Valiyaveettil S (2009) Anti-proliferative activity of silver nanoparticles. BMC Cell Biol 10:65
Attia AA (2014) Evaluation of the testicular alterations induced by silver nanoparticles in male mice: biochemical, histological and ultrastructural studies research. J Pharm Biol Chem Sci 5:1558
Bancroft D, Stevens A (1982) Theory and practice of histological techniques. Churchill Livingstone, Edinburgh, London, Melaborne
Bar-Ilan O, Albrecht RM, Fako VE, Furgeson DY (2009) Toxicity assessments of multisized gold and silver nanoparticles in Zebrafish embryos. SmaII 5:1897–1910
Bilberg K, Doving KB, Beedholm K, Baatrup E (2011) Silver nanoparticles disrupt olfaction in Crucian carp (Carassius carassius) and Eurasian perch (Perca fluviatilis). Aquat Toxicol 104:145–152
Bucher F, Hofer R (1993) The effects of treated domestic sewage on three organs (gill, kidney and liver) of brown trout (Salmo trutta). Water Res 27:255–261
Chae YJ, Pham CH, Lee J, Bae E, Yi J, Gu MB (2009) Evaluation of the toxic impact of silver nanoparticles on Japanese medaka (Oryzias latipes). Aquat Toxicol 94:320–327
Choi JE et al (2009) Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat Toxicol 100:151–159
Dube PN, Shwetha A, Hosetti BB (2014) Impact of copper cyanide on the key metabolic enzymes of freshwater fish Catla catla (Hamilton). Biotechnology in Animal Husbandry 30:499–508
Farkas J, Christian P, Urrea JAG, Roos N, Hassellov M (2010) Effects of silver and gold nanoparticles on rainbow trout (Oncorhynchusmykiss) hepatocytes. Aquat Toxicol 96:44–52
Gaber HS (2007) Impact of certain heavy metals on the gill and liver of the Nile tilapia (Oreochromis niloticus). EwpLJAquaLBioL&Fish 11:79–100
Giordano C, Karasik O, King-Morris K, Asmar A (2015) Uric acid as a marker of kidney disease: review of the current literature. Disease Markers 2015:6 pages
Griffitt RJ, Hyndman K, Denslow ND, Barber DS (2009) Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles. Toxicol Sci 107:404–415
Hedayati A, Jahanbakhshi A (2012) The effect of water-soluble fraction of diesel oil on some hematological indices in the great sturgeon Husohuso. Fish Physiol Biochem 38:1753–1758
IBM-SPSS (2012) IBM-SPSS Statistics version 21
Johari SA, Kalbassi MR, Yu IJ, Lee JH (2015) Chronic effect of waterborne silver nanoparticles on rainbow trout (Oncorhynchus mykiss): histopathology and bioaccumulation. Comp Clin Pathol 24:995–1007
Juneja C, Mahajan C (1983) Hematological and haemopoietic changes in fish Channa punctatus due to mercury pollution in water. Indian J Anim Sci 17:63–71
Khidr BM, Wassif ET, Hussein SY, Mekkawy IAA (2001) Studies on the effect of the herbicide atrazine on the liver and kidney of the fresh water catfish Chrysichys auratus. J Egyptian-German Soc Zool 34(C):283–300
Kotb MA et al (2018) Pilot study of classic galactosemia: neurodevelopmental impact and other complications urge neonatal screening in Egypt. J Adv Res 12:39–45
Maita M, Shiomitsu K, Ikeda Y (1984) Health assessment by the climogram of hemochemical constituents in cultured yellow tail. Bull Jpn Soc Sci Fish 51:205–211
Mansouri B, Johari SA (2016) Effects of short-term exposure to sublethal concentrations of silver nanoparticles on histopathology and electron microscope ultrastructure of zebrafish (Danio rerio) gills. Iran J Toxicol 10:15–20
Mc Manus JPA (1946) Histological demonistration of mucin after periodic acid. Nature 158:2002
Mekkawy IAA, Mahmoud UM, Wassif ET, Naguib M (2012) Protective roles of tomato paste and vitamin E on cadmium- induced histological and histochemical changes of liver of Oreochromis niloticus (Linnaeus,1758). J Fish Aquat Sci 7:240–265
Mekkawy IAA, Mahmoud UM, Wassif ET, Mohammed RH (2013) Protective roles of tomato paste and vitamin E on atrazine- induced histological and histochemical changes of liver of Clarias Gariepinus (Burchell,1822). Glob Adv Res J Environ Sci Toxicol 2:011–021
Mekkawy IA, Mahmoud UM, Hana MN, Sayed AEH (2019) Cytotoxic and hemotoxic effects of silver nanoparticles on the African catfish, Clarias gariepinus (Burchell, 1822). Ecotoxicol Environ Saf 171:438–446
Mohamed SH (2006) Studies on the protective effect of melatonin, vitamin C and vitamin E on lead-induced oxidative stress on Clarias gariepinus
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353
Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622–627
Noureen A, Jabeen F, Yaqub S, Alam MF (2017) Assessment of genotoxicity and nephrotoxicity induced by copper nanoparticles and copper (II) oxide in Cyprinus carpio. Int J Biosci 11:360–371
Panda NC (1999) Kidney in textbook of biochemistery and human biology
Panyala NR, Pena-Mendez EM, Havel J (2008) Silver or silver nanoparticles: a hazardous threat to the environment and human health? J Appl Biomed 6:117–129
Pournori B, Paykan Heyrati F, Dorafshan S (2017) Histopathological changes in various tissues of striped catfish, Pangasianodon hypophthalmus, fed on dietary nucleotides and exposed to water-borne silver nanoparticles or silver nitrate. Iran J Aquat Anim Health 3:36–52
Powers CM, Yen J, Linney EA, Seidler FJ, Slotkin TA (2010) Silver exposure in developing zebrafish (Danio rerio): persistent effects on larval behavior and survival. Neurotoxicol Teratol 32:391–397
Ramesh F, Nagarajan K (2013) Histopathological changes in the muscle tissue of the fish Clarias batrachus exposed to untreated and treated sago effluent. Adv Biosci Bioeng 1:74–80
Ranzani-Paiva MJT, Salles FA, Eiras JC, Eiras AC, Ishikawa CM, Ac A (1999) Hematological de curimbat (Prochilodus scrofa), pacu (Piractus mesopotamicus) e tambaqui (Colossoma macropomum) das estac ̧oes depiscicultura do Instituto de Pesca, Estado de Sao Paulo. Bol Inst Pesca 25:77–83
Robbins SL, Angell M (1976) Basic pathology. W. B. Saunders Company, Philadelphia, London and Toronto
Saravanan M, Kumar KP, Ramesh M (2011) Haematological and biochemical responses of freshwater teleost fish; Cyprinus carpio (Actinopterygii: Cypriniformes) during acute and chronic sublethal exposure to lindane. Pestic Biochem Physiol 100:206–211
Sayed AH (2016) Genotoxicity detection following exposure to silver nanoparticles in African catfish (Clarias gariepinus). International journal of nanoparticles, Inderscience Enterprises Ltd, NULL 9 41–53
Sayed AH, Authman MMN (2018) The protective role of Spirulina platensis to alleviate the sodium dodecyl sulfate toxic effects in the catfish Clarias gariepinus (Burchell, 1822). Ecotoxicol Environ Saf 163:136–144
Sayed AH, Hamed HS (2017) Induction of apoptosis and DNA damage by 4-nonylphenol in African catfish (Clarias gariepinus) and the antioxidant role of Cydonia oblonga. Ecotoxicol Environ Saf 139:97–101
Sayed AH, Soliman HAM (2017) Developmental toxicity and DNA damaging properties of silver nanoparticles in the catfish (Clarias gariepinus). Mutat Res - Genet Toxicol Environ Mutagen 822:34–40
Sayed AH, Younes HAM (2017) Melanomacrophage centers in Clarias gariepinus as an immunological biomarker for toxicity of silver nanoparticles. J Microsc Ultrastruct 5:97–104
Sayed AH, Mekkawy IAA, Mahmoud U (2011) Effects of 4-nonylphenol on metabolic enzymes, some ions and biochemical blood parameters of the African catfish Clarias gariepinus (Burchell, 1822). African Journal of Biochemistry Research, Academic Journals 5:287–297
Shaw BJ, Handy RD (2006) Dietary copper exposure and recovery in Nile tilapia, Oreochromis niloticus. Aquatic Toxicol 75:111–121
Skebo JE, Grabinski CM, Schrand AM, Schlager JJ, Hussain SM (2007) Assessment of metal nanoparticle agglomeration, uptake, and interaction using high-illuminating system. Int J Toxicol 26:135–141
Thomson RG (1984) General veterinary pathology. W. B. Saunders Company, Philadelphia, London, Toronto
Tu AT (1991) Hand book of natural toxins, reptile venoms and toxins. Marcel Dekker, Inc. New York
Uboh FE, Ebong PE, Umoha IB (2009) Comparative hepatoprotective effect of vitamins A and E against gasoline vapor toxicity in male and female rats. Gastroenterol Res 2:295–302
Wallace WE, Keane MJ, Murray DK, Chisholm WP, Maynard AD, Ong TM (2007) Phospholipid lung surfactant and nanoparticle surface toxicity: lessons from diesel soots and silicate dusts. J Nanopart Res 9:23–38
Wassif ET, Kider BM, Hussein SY, Mekkawy IA, Hassan HI (2000) Effects of the herbicide Atrazine on the structure of some organs of the Nile fish Oreochromis niloticus. Egypt J Aquat Biol Fish 4:197–234
Wu Y, Zhoua Q, Li H, Liua W, Wanga T, Jianga G (2010) Effects of silver nanoparticles on the development and histopathology biomarkers of Japanese medaka (Oryzias latipes) using the partial-life test. Aquat Toxicol 100:160–167
Yang W, Shen C, Ji Q, An H, Wang J, Liu Q, Zhang Z (2009) Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology 085102:1–7
Yazdanparast T, Sharifpour I, Soltani M, Esfahani HK (2016) Evaluation of Silver retention in different organs of zebrafish (Danio rerio) fed diet supplemented with silver nanoparticles. Int J Eng Res 5:269–274
Yeo M, Kang M (2008) Effects of nanometer sized silver materials on biological toxicity during Zebra fish embryogenesis. Bull Kor Chem Soc 29:1179–1184
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• AgNPs were tested in catfish.
• AgNP-induced kidney functions alterations in Clarias gariepinus.
• Histopathological changes were observed in a dose-dependent manner.
• Recovery period improved some alterations after AgNP exposure period.
Rights and permissions
About this article
Cite this article
Mahmoud, U.M., Mekkawy, I.A.A., Naguib, M. et al. Silver nanoparticle–induced nephrotoxicity in Clarias gariepinus: physio-histological biomarkers. Fish Physiol Biochem 45, 1895–1905 (2019). https://doi.org/10.1007/s10695-019-00686-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00686-7