Abstract
Two sites of collection along river Nile, nearby metal-related factories (site2) and 7 km downstream (site3) were compared to unpolluted reference fish farm (site1). Metals concentration (Cu, Zn, Pb, Fe, Mn and Cd) in water and sediment samples showed highly significant (p < 0.01) differences among sites. According to contamination factor and pollution load index values, overall pollution was ordered as site2 > site3 > site1. Compared with Oreochromis niloticus of site1, activities of superoxide dismutase, catalase and glutathione-S-transferase as well as malondialdehyde formation were significantly (p < 0.01) increased in both liver and gills of fish collected from metal contaminated sites. This increment showed a tissue-specific pattern with higher rate of increment in liver than in gills. While reduced glutathione level was sharply decreased in site2 and site3. Micronucleus test was assessed as an environmental genotoxic endpoint in erythrocytes. Assessment of eight nuclear deformations showed gradient frequencies related to the distance from the industrial discharges.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Among aquatic pollutants, heavy metals are considered as critical contaminants due to their strong impact on the stability of aquatic bodies, bioaccumulation in living organisms, toxicity persistence and tendency to accumulate in water (Has-Schon et al. 2006). During the assessment of environmental metals, both sediments and water should be considered as metals are circulated between bed sediments and aqueous phase. In fact, it is impossible to evaluate the interactive influences of environmental contaminants on the biota only by chemical analyses. Thus, usage of biomarkers has become a valuable tool during monitoring the environmental quality and the health of fish inhabiting polluted ecosystems (Turkmen et al. 2006). Oxidative stress is an intricate process, starting with reactive oxygen species (ROS) production that elicits adaptive responses of antioxidant defense components and finally may lead to oxidative cellular damage (Cao et al. 2010). Superoxide dismutases (SOD) are metalloenzymes that transform superoxide anions (O2 −) into less reactive species, the molecular oxygen (O2) and H2O2. The formed H2O2 is decomposed to H2O and O2 by catalase (CAT) (Sampaio et al. 2008). Detoxification enzymes, especially glutathione-S-transferase (GST) helps in reducing reactive compounds by forming their conjugates with glutathione and subsequently eliminating them thereby protecting cells against ROS (Baysoy et al. 2012). Also, sulfhydryl-rich tripeptide reduced glutathione (GSH) can interfere with toxic metals by altering the rates of metal uptake and metal elimination (Burton et al. 1995) and/or by protecting against oxidative stress resulting from metal-catalyzed redox reactions (Cao et al. 2012). Lipid peroxidation (LPO) leads to malondialdehyde (MDA) formation, which is the major contributor to the loss of cell function and DNA damage so it was used to express severe oxidative damage (Ruas et al. 2008). Moreover, metals can bind to DNA causing the formation of DNA adducts, single and double strand breakages as well as modifications in DNA repair pattern so, genotoxicity of metals have to be evaluated (Omar et al. 2012). Micronuclei (MN) counts have been widely employed to biomonitor genotoxcity in wild areas with different levels of contamination. So, this work aimed to investigate the environmental health of two important fishery sites along river Nile via chemical analysis of water and sediments as well as evaluation of enzymatic and non-enzymatic biomarkers of oxidative stress in liver and gill tissues of the most abundant fish, Oreochromis niloticus. Also, assess the genotoxicity using MN test in erythrocytes of O. niloticus that can persist in severe conditions as a potential biomarker of environmental pollution.
Materials and Methods
A preliminary survey was done before this current work during the summer season (2012), where the rate of evaporation and accumulation of metals reach maximum values. This survey showed that the selected study sites (site2 and site3) had elevated aqueous metal concentrations and the common metals were Cu, Zn, Pb, Fe, Mn and Cd. All metal concentrations in site1 were within the safe guideline values for both water and sediments as proposed by Persaud et al. (1993), CCME (1999) and MacDonald et al. (2000) so it was selected as a reference site.
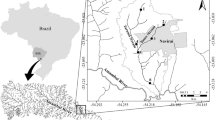
Site1: Mixed fish farm of the Faculty of Agriculture, El-Fayoum University; irrigated with unpolluted branch of the river Nile. Therefore, this site was the reference site (29°17′ 45.19″N and 30°45′ 57.52″E). The studied fish farm use commercial formulated diet (25–35 % crude protein) and with fish stocking density 5–8 fish/m3 for Nile tilapia.
Site2: Southern part of river Nile at Shoubra El-Khaema before El-Ismailia canal close to the industrial discharges from many metal-related industries as Cement industry, red brick and ceramic industry (30°5′ 8.76″N and 31°13′ 50.94″E).
Site3: About 7 km downstream the industrial discharges (30°7′ 46.42″N and 31°13′ 24.85″E) after El-Ismailia canal.
Water, sediments and fish samples were collected with the help of local fishermen during the summer season (July and August) in 2012. Water samples were taken with a water sampler from different localities (n = 8) in each site between 10:00 and 12:00 a.m. at a depth of 30 cm below the water surface then transferred to the laboratory and stored at 4 °C in clean 1000-ml sampling glass bottles according to Boyd (1990). Eight of core sediment samples up to 20 cm in length were taken from each sampling site using polyvinyl chloride (PVC) corers. The corers were immediately sealed and stored at 4 °C (Cabrera et al. 1992). A total number of 36 adult O. niloticus (n = 12 per site) ranging from 18.5 to 24.8 cm total length and weighing 160–180 g (desirable marketing size) were collected from the same localities of water and sediment collection. Blood was sampled from the caudal vein using heparin as an anticoagulant, then the fish were dissected and required tissues were obtained.

Water temperature was measured at the site of sampling using water thermometer. Dissolved oxygen (mg/l) was measured at the site of sampling, using an oxygen meter (model YSI 58). Salinity was measured by using a salinity-conductivity meter (model YSI 57). Total hardness and total alkalinity were measured as CaCO3 content by titration method according to the American Public Health Association standard methods (APHA 2005). Concentrations of six metals were determined by flam atomic absorption spectrophotometry (Model, PerkinElmer-2280) according to APHA (2005). Sediment samples were dried, acid-digested using 99 % HCl, and diluted with deionized water to known volume using the dry-ashing procedure proposed by Issac and Kerber (1971) and Hseu (2004). The procedural blanks were aspirated along the analytical procedures in order to correct the background absorption. To check measurement accuracy, a known concentration samples of standard solution were measured during the analysis process. The analysis accuracy in sediment was checked by standard reference material (Lake Superior fish 1946 NIST, National Institute of Standards and Technology, USA) and the metal recovery ranges lied between 95 and 110 %.
Levels of sediment contamination are expressed as contamination factor: CF = Metal concentration in the sediments/Background value of the metal where, background value is the value of metal equal to the world surface rock average given by Martin and Meybeck (1979). Contamination levels are estimated according to Hakanson (1980). Also, pollution Load Index (PLI) has been evaluated for each site following the method proposed by Tomlinson et al. (1980): PLI = (CF1 × CF2 × CF3 ×···× CFn)1/n where, n is the number of metals (six in this work).
According to Tomlinson et al. (1980), value of PLI < 1 represents perfection; PLI = 1 displays that only baseline levels of pollutants are presented and PLI > 1 indicates worsening of site quality.
Liver and gills tissues were rinsed in phosphate buffered saline solution (pH 7.4 containing 0.16 mg/ml heparin) to remove any red blood cells and clots. Tissues were homogenized in 5 ml cold buffer (50 mM potassium phosphate, pH 7.5, 1 mM EDTA) per gram tissue then centrifuged at 100,000×g for 15 min at 4 °C. The supernatant was removed for assay and preserved at −80 °C until use. Superoxide dismutase activity was assayed as described by Nishikimi et al. (1972). Briefly, this assay relies on the ability of the enzyme to inhibit the phenazine methosulphate-mediated reduction of nitroblue tetrazolium dye. The change in absorbance at 560 nm over 5 min was related to the inhibition rate that is directly proportional to SOD activity and expressed as U/mg protein. As described by Aebi (1984), Catalase reacts with a known quantity of H2O2 then the reaction is stopped after exactly 1 min with catalase inhibitor. In the presence of peroxidase, remaining H2O2 reacts with 3, 5-Dichloro-2-hydroxybenzene sulfonic acid and 4-aminophenazone to form a chromophore with a color intensity (at 510 nm) inversely proportional to the amount of catalase and expressed as U/mg protein. The evaluation of total Glutathione-S-transferases activity was according to Habig et al. (1974) depends on measuring the conjugation of 1-chloro-2, 4-dinitrobenzene with reduced glutathione which is accompanied by an increase in absorbance. The rate of the absorbance increase is directly proportional to the GST activity in the sample. The OD of reaction product was read at 340 nm and expressed as U/mg protein. The assay of reduced glutathione levels was measured by the method of Beutler et al. (1963) which is based on the reduction of 5,5′dithiobis 2-nitrobenzoic acid with GSH to produce a yellow chromogen whose absorbance (at 405 nm) is directly proportional to GSH concentration and expressed as mg/mg protein. The lipid peroxidation level was assayed according to the method described by Ohkawa et al. (1979), in which the malondialdehyde (index of LPO) reacts with thiobarbituric acid forming thiobarbituric acid reactive species. The absorbance (534 nm) of the resultant pink product was directly proportional to LPO level and expressed as nmole/g tissue.
The frequency of MN in erythrocytes was evaluated according to Fenech (1993). A drop of blood was smeared on clean slides (two slides per fish), which were dried at room temperature and fixed in 100 % methanol for 10 min. Samples were stained with 10 % Giemsa solution for 15 min, air dried and then prepared for permanent use. A total number of 2000 erythrocytes were examined for each specimen under a light microscope, with oil immersion at 1000× magnification. To minimize the technical variation, the blind scoring of micronuclei was performed on randomized and coded slides. The criteria described by Fenech et al. (2003) were considered: the diameter of the MN should be less than one-third of that of the main nucleus, MN should be separated from or marginally overlapping with the main nucleus as long as there is clear identification of the nuclear boundary, and MN should have similar staining as the main nucleus. Other nuclear anomalies (NA) such as notched nuclei, blebbed nuclei, erythrocytes bearing more than a single micronucleus, bi-nucleated erythrocytes, poly-nucleated erythrocytes, karyolysis and nuclear retraction were recorded separately, on the basis of the criteria described by Da Silva Souza and Fontanetti (2006).
The results were expressed as mean ± SE. Data were statistically analyzed with analyses of variance (F test, t test), and Duncan’s multiple-range test to evaluate the comparability between means (p < 0.05 and p < 0.01) by Statistical Analysis System (SAS), Version 9.1, 2006.
Results and Discussion
The industrial effluents not only alter aquatic inhabitants but also deteriorate the physico-chemical equilibrium of the aquatic bodies. The analysis of variance of the water quality indices (Table 1) showed highly significant differences among the values of pH, dissolved oxygen (DO) and salinity of the studied sites. In fact, the low dissolved oxygen values in the vicinity of the industrial discharges still within the permissible level as recorded in water quality guidelines for the protection of aquatic life (AEP 1997) but the continuous decrease in DO may threaten the aquatic biota in the near future. It is well known that organisms exposed to low oxygen level are subjected to marked oxyradical burst, which can be countered by elevated antioxidants level to prevent oxidative damage (Melegaria et al. 2013). Robert et al. (1986) reported that fish are significantly affected by metals in water with high pH as metals precipitate from water column to the sediment under slightly high pH. Thus, metals in water may undergo rapid changes affecting the rate of uptake or release by sediments which has a harmful effect on living organisms throughout the water–sediment interaction chain. Moreover, relative salinity increase in the site2 compared to the other sites led to accelerate the metal accumulation by altering both permeability of fish and metals availability.
As showed by Harabawy and Mosleh (2014), metals in a mixture have much additive toxic properties compared to their individual effects. So, evaluation of individual metal toxicity does not offer a realistic environmental model. Table 2 displayed highly significant differences (p < 0.01) in all metal concentrations of water and sediment samples among sites. The concentrations of all metals collected from site2 were significantly higher than those of other sites and exceeded the guideline values of aquatic life. The results affirmed that all metals of the reference site had significantly lower concentrations within the guideline values of aquatic life. The recorded metals in water and sediment samples collected from site3 downstream to the source of pollution were within the guideline values except for Pb, Cd and Fe.
The degree of contamination and considered CF for various metals in sediments is showed in Tables 3 and 4. On this basis, site2 has very high CF values for Pb and Cd; moderate CF for Cu and Zn and low CF for Mn and Fe. Site3 has very high CF values for Cd; considerable CF for Pb and low CF for the rest of the studied metals. While the CF values of all metals in the reference site were <1 and showed low degree of contamination. Moreover, PLI values (Table 5) of the reference site showed no overall pollution whereas site2 and site3 showed signs of sites quality deterioration but with various degrees of contamination. Greater values of CF and PLI signify extensive anthropogenic inputs in the aquatic environment around site2. Whereas, the decrease in these values downstream the industrial site indicating dilution and dispersion of metals content with increasing distance from industrial discharge point.
Table 6 showed the profile of different enzyme activities in liver (organ of detoxification) and gill (in continuous contact with water) tissues from the studied sites. The recorded F-value showed highly significant differences among the sites of concern. Raised levels of metals persuade oxidative stress by generating ROS via Haber–Weiss and Fenton’s reactions. ROS production leads to increased antioxidants that neutralize free radicals or their toxic effects (Melegaria et al. 2013). Site2 witnessed a significant increase over the reference fish in SOD activities of both liver and gills (355 and 122 %, respectively), and also CAT activities of both hepatic and gill tissues (350 and 177 %, respectively) was increased. There were no statistically relevant differences in these enzyme activities between liver and gills at the reference site. The SOD-CAT system represent the first line of defense against oxidative stress (Qu et al. 2014), varied based on the response of the antioxidant system to cope with induced oxidative stress. The increase in SOD and CAT activities are usually observed in the face of prolonged exposure of metals (Sampaio et al. 2008). The enhancement of this system in liver may be due to the production of superoxide anion radicals which led to the SOD induction to convert them to H2O2. The increase in CAT activity is a common result to convert H2O2 produced by SOD activity into water. This study showed also a lesser increase in the activities of SOD and CAT of gills which chronically exposed to a combination of metals. The continual exposure to metals and flux of superoxide radicals can induce severe disturbance of CAT activity in gill tissues by binding of these metal ions to –SH groups of enzyme. Consequently, this lead to overproduction of H2O2 and/or superoxide radical followed by SOD activity alteration (Atli et al. 2006). Also, Table 6 depicted the GST activity and GSH levels as glutathione metabolism indices. The recorded GST activities in site2 increased significantly in liver (by 740 %) and gills (by 405 %). A similar increasing trend was seen at site3, but with lower increment percentage than that of site2. The levels of GSH showed sharp decrease in both hepatic and gills tissues sampled from site2 and site3. The resultant variation of GST activity and GSH level confirmed that GST catalysis the transformation of a wide variety of electrophilic compounds to less toxic substances by conjugating them to GSH (Baysoy et al. 2012). Previous studies showed that exposure to metals can lead to an increase of GST in liver (Guilherme et al. 2008) and to a lesser extent in the gills (Dautremepuits et al. 2009). The slightly increase of antioxidant components in gills compared to liver facing same level of metal pollution indicated the feeble ability of antioxidant system to resist the prolonged oxidative stress in gills. Cao et al. (2012) showed that persistent metals contact has ability to convert GSH to GS–metal complexes with various metals through its thiolate sulfur atom results in decrease availability of GSH. The variations in MDA formation (Table 6) was significantly elevated in liver and gills tissues from site2 by 290 % compared to those of reference fish. A comparable rising was gotten at the site3, but with slighter increase with 117 % increase in liver and 60 % gills. The raised MDA in liver and gills signifying that the mobilization of enzymatic and non-enzymatic antioxidant components could not prevent LPO (Souid et al. 2014).
To verify the presence of DNA damage, MN test was used to compare MN and other NA frequencies among different sites (Fig. 1). Analysis of MN in fish erythrocytes showed gradient frequencies related to the distance from the industrial discharge. There were highly significant differences in the eight erythrocytic deformations of the studied fish collected from the studied sites (Fig. 2). The potential genotoxic role of metals probably contributed to the increase in genotoxic damage, either through interaction of reactive oxygen intermediates and lipid peroxidation products with DNA or to direct interaction of metal with cellular macromolecules forming adducts, alkaline labile sites and strand breaks (Omar et al. 2012). Micronucleus frequencies vary according to the degree of environmental stress and could be related to the kind and degree of pollution. Moreover, other nuclear abnormalities have been used by various authors as good indicators of genotoxicity in fish (Omar et al. 2012; Harabawy and Mosleh 2014). Therefore, seven additional nuclear abnormalities were accurately recorded to be potent indicators of genotoxic damage and complement the scoring of MN during the monitoring of genotoxic status. These nuclear projections and distortions could be caused by genotoxic metals during the elimination of the amplified DNA from the nucleus, which cause problems in chromosomal attachments or gene amplification (Ergene et al. 2007). The clastogenic effect was represented by the formation of MN and other nuclear deformations in the following order: Industrial site (site2) > downstream site (site3) > reference site (site1).
Representative nuclear alteration recorded in erythrocytes of O. niloticus a micro-nucleated erythrocyte, b notched nucleus, c blebbed nucleus, d erythrocyte bearing more than a single micronucleus, e bi-nucleated erythrocyte, f cariolysis, g polynucleated erythrocyte and h nuclear retraction. 1000× magnification
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
AEP (1997) Alberta water quality guideline for the protection of freshwater aquatic life: Dissolved oxygen. Standards and Guidelines Branch, Alberta Environmental Protection, Edmonton
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association, New York
Atli G, Alptekin O, Tukel S, Canli M (2006) Response of catalase activity to Ag2+, Cd2+, Cr2+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comp Biochem Physiol C 143:218–224
Baysoy E, Atli G, Gürler CÖ, Dogan Z, Eroglu A, Kocalar K, Canli M (2012) The effects of increased freshwater salinity in the biodisponibility of metals (Cr, Pb) and effects on antioxidant systems of Oreochromis niloticus. Ecotoxicol Environ Saf 84:249–253
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Boyd CE (1990) Water quality in ponds for aquaculture. Birmingham Publishing Co., Birmingham, Alabama
Burton CA, Hatlelid K, Divine K, Carter DE, Fernando Q, Brendel K, Gandolfi AJ (1995) Glutathione effects on toxicity and uptake of mercuric chloride and sodium arsenite in rabbit renal cortical slices. Environ Health Perspect 103:81–84
Cabrera F, Conde B, Flores V (1992) Heavy metals in the surface sediments of the tidal river Tinto (SW Spain). Fresenius Environ Bull 1:400–405
Cao L, Huang W, Liu J, Yin X, Dou S (2010) Accumulation and oxidative stress biomarkers in Japanese flounder larvae and juveniles under chronic cadmium exposure. Comp Biochem Physiol C 151:386–392
Cao L, Huang W, Shan X, Ye Z, Dou S (2012) Tissue-specific accumulation of cadmium and its effects on antioxidative responses in Japanese flounder juveniles. Environ Toxicol Pharmacol 33:16–25
CCME (1999) Canadian water quality guidelines for the protection of aquatic life: summary table. In: Canadian environmental quality guidelines. Canadian Council of Ministers of the Environment, Winnipeg, Canada
Da Silva Souza T, Fontanetti CS (2006) Micronucleus test and observation of nuclear alterations in erythrocytes of Nile tilapia exposed to waters affected by refinery effluent. Mutat Res 605(1–2):87–93
Dautremepuits C, Marcogliese DJ, Gendron AD, Fournier M (2009) Gill and kidney antioxidant processes and innate immune system responses of yellow perch (Perca flavescens) exposed to different contaminants in the St. Lawrence River, Canada. Sci Total Environ 407:1055–1064
Ergene S, Avas TC, Celik A, Koleli N, Kaya F, Karahan A (2007) Monitoring of nuclear abnormalities in peripheral erythrocytes of three fish species from the Goksu Delta (Turkey): genotoxic damage in relation to water pollution. Ecotoxicology 16(4):385–391
Fenech M (1993) The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res 285:35–44
Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E (2003) HUMN project: detailed description of the scoring criteria for the cytokinesis block micronucleus assay using isolated human lymphocyte cultures. Mutat Res 534(1–2):65–75
Guilherme S, Válega M, Pereira ME, Santos MA, Pacheco M (2008) Antioxidant and biotransformation responses in Liza aurata under environmental mercury exposure–relationship with mercury accumulation and implications for public health. Mar Poll Bull 56:845–859
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hakanson L (1980) An ecological risk index for aquatic pollution control a sedimentological approaches. Water Res 14(8):975–1001
Harabawy AS, Mosleh YY (2014) The role of vitamins A, C, E and selenium as antioxidants against genotoxicity and cytotoxicity of cadmium, copper, lead and zinc on erythrocytes of Nile tilapia, Oreochromis niloticus. Ecotoxicol Environ Saf 104:28–35
Has-Schon E, Bogut I, Strelec I (2006) Heavy metal profile in five fish species included in human diet, domiciled in the end flow of River Neretva (Croatia). Arch Environ Contam Toxicol 50(4):545–551
Hseu ZY (2004) Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour Technol 95:53–59
Issac RA, Kerber JD (1971) Atomic absorption and flame photometry. Techniques and uses in soil, plant and water analysis. In: Walsh LM (ed) Instrumental methods for analysis of soil and plant tissue. Soil Science Society of America-Agronomy Society of America Inc, Madison, WI, pp 17–37
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Martin J, Meybeck M (1979) Elemental mass-balance of material carried by major world rivers. Mar Chem 7(3):178–206
Melegaria SP, Perreault F, Costa RHR, Popovic R, Matias WG (2013) Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii. Aquat Toxicol 142–143:431–440
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Omar WA, Zaghloul KH, Abdel-Khalek AA, Abo-Hegab S (2012) Genotoxic effects of metal pollution in two fish species, Oreochromis niloticus and Mugil cephalus, from highly degraded aquatic habitats. Mutat Res 746:7–14
Persaud D, Jaagumagi R, Hayton A (1993) Guidelines for the protection and management of aquatic sediment quality in Ontario. Ontario Ministry of the Environment, Queen’s Printer of Ontario, Ontario
Qu R, Feng M, Wang X, Qin L, Wang C, Wang Z, Wang L (2014) Metal accumulation and oxidative stress biomarkers in liver of freshwater fish Carassius auratus following in vivo exposure to waterborne zinc under different pH values. Aquat Toxicol 150:9–16
Robert FC, David FB, Gary AC (1986) Effects of pH on the Toxicities of cadmium, copper, and zinc to Steelhead Trout (Salmo gairdneri). Can J Fish Aquat Sci 43(8):1497–1503
Ruas CBG, Carvalho Cd-S, de Araújo HSS, Espíndola ELG, Fernandes MN (2008) Oxidative stress biomarkers of exposure in the blood of cichlid species from a metal-contaminated river. Ecotoxicol Environ Saf 71:86–93
Sampaio FG, Boijink CL, Oba ET, Santos LRB, Kalinin AL, Rantin FT (2008) Antioxidant defenses and biochemical changes in pacu (Piaractus mesopotamicus) in response to single and combined copper and hypoxia exposure. Comp Biochem Physiol C 147:43–51
Souid G, Souayed N, Yaktiti F, Maaroufi K (2014) Lead accumulation pattern and molecular biomarkers of oxidative stress in sea bream (Sparus aurata) under short-term metal treatment. Drug Chem Toxicol 15:1–8
Tomlinson DC, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in assessment of heavy metals in estuaries and the formation of pollution index. Helgol Mar Res 33:566–575
Turkmen A, Turkmen M, Tepe Y, Mazlum Y, Oymael S (2006) Metal concentrations in Blue Crab (Callinectes sapidus) and Mullet (Mugil cephalus) in Iskenderun Bay, Northern East Mediterranean, Turkey. Bull Environ Contam Toxicol 77:186–193
Conflict of interest
Amr Adel Abdel-Khalek declares that he has no conflict of interest.
Compliance with Ethical Standards
This manuscript complies to the Ethical Rules applicable for this journal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Khalek, A.A. Antioxidant Responses and Nuclear Deformations in Freshwater Fish, Oreochromis niloticus, Facing Degraded Environmental Conditions. Bull Environ Contam Toxicol 94, 701–708 (2015). https://doi.org/10.1007/s00128-015-1509-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1509-5