Abstract
Metal oxide nanoparticles (NPs) have different industrial applications so it is unavoidable that NPs products could find their way into aquatic habitats. Therefore, toxic NPs must be treated sufficiently to reach the standard values before their discharge into the aquatic ecosystems. Our study aimed to investigate the adsorptive capacity of rice husk to iron and aluminum oxides from water and reducing their potential toxic effects. Fish were classified into eight groups for 7 days: Fe2O3 NPs (10 mg/l)-exposed group; Al2O3 NPs (10 mg/l)-exposed group; combined group (same concentrations of Fe2O3 and Al2O3NPs), and control group (dechlorinated water). The other four groups were the same as the above groups but with 50 mg/l rice husk in each group. Compared with control groups, our results showed a significant (p < 0.05) increase in plasma total proteins, globulin, glucose, liver enzymes, and kidney function biomarkers (creatinine and uric acid). While the recorded albumin and total lipids were significantly decreased. The oxidative biomarkers in liver and gill tissues of NPs-exposed fish showed significant (p < 0.05) reduction in glutathione-reduced content and elevation in thiobarbituric acid reactive substances, glutathione peroxidase, catalase, and superoxide dismutase. Based on our results, Fe2O3 NPs were more toxic than Al2O3 NPs. The combined doses of both NPs showed more or less toxicity compared to single doses. Therefore, this point needs more studies to show the mode of interaction. Finally, rice husk was a good adsorber to both NPs as it could improve the biochemical and antioxidant status of the studied fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing exploration of nanotechnology has resulted in the identification of many properties of NPs such as enhanced magnetic, catalytic, optical, and mechanical properties compared to their conventional formulations of the same materials (Rekulapally et al. 2019). The worldwide NPs consumption is expected to grow from 225,060 metric tons in 2014 to nearly 584,984 metric tons in 2019, which represents an annual growth rate of 21.1% (Murali et al. 2017). Therefore, the potential adverse effects of NPs are continuing to be more prominent as the discharging of new NPs is progressively affecting the aquatic ecosystems and its biota. Metal oxide NPs were represented as a fundamental cornerstone of nanoscience and nanotechnology due to their various properties and potential applications (Pinna and Niederberger 2008). For example, Fe2O3 NPs possess unique properties for biomedical, technological, and environmental applications (Shipley et al. 2011). Besides, Al2O3 NPs have been used also in optical polishing, cosmetics, clothing, catalysis, polymer modification, biosensors, and drug delivery (Francis et al. 2011). Therefore, metal oxide NPs could enter the aquatic ecosystem through hospital, municipal, and industrial residues that are discharging into the water without any treatment, resulting in the presence of high NPs concentrations in the aquatic environment (Saravanan et al. 2015; Abdel-Khalek 2016). To investigate the putative ecological and biological impairments caused by those metal oxide NPs, it was necessary to assess their toxicological behavior in the aquatic environment and biota. Recently, several biomarkers have widely been used to study the impacts of NPs on aquatic organisms (Saravanan et al. 2015; Abdel-Khalek et al. 2016). For example, measurement of biochemical parameters, antioxidant enzymatic activities, and concentrations of non-enzymatic antioxidant biomarkers in fish are broadly utilized to characterize the impact of environmental stressors in many laboratory and field studies (Abdel-Khalek et al. 2015; Abdel-Khalek et al. 2018). There are several studies aimed to understand the potential effects of NPs on aquatic life, but till now these studies are not sufficient to understand their exact effects on the aquatic environment (Elsaesser and Howard 2012). Therefore, NPs must be treated sufficiently to reach the standard values before their discharge into the aquatic system. Several methods have been examined for the removal of metals from water such as precipitation, coagulation, adsorption, ultra-filtration, reverse osmosis, and membrane separation (Zhang et al. 2013). One of the most valuable methods is bio-adsorption, which is described as an effective and economical method for metals removal (Cheng et al. 2014). Many methods relayed on the usage of renewable and biodegradable lingo-cellulosic agricultural or industrial by-products to develop low-cost bio-adsorbents for water purification and to reduce environmental problems (Krishnani and Ayyappan 2006). Some of these adsorbents were rice husk, peanut shells, corncobs, sawdust, coir dust, tree leaves, and wheat bran (Sobhanardakani et al. 2013; Lata and Samadder 2014). The current study is the first trial to relay on rice husk (RH) as a bio-adsorbent for the treatment of Fe2O3 and Al2O3 NPs. The RH is low cost, available, and eco-friendly bio-adsorbent which has been studied intensively for the removal of various metals (Pb, Cd, Zn, Cu, Ni, and As) in their conventional sizes from both groundwater and surface water (Lata and Samadder 2014). Therefore, the present study aimed to investigate the RH adsorptive treatment on NPs contaminated water to reduce their potential toxicity in the aquatic environment.
Materials and methods
Preparation and characterization of metal oxides NPs
The two studied NPs (Fe2O3 and Al2O3) were purchased from Sigma-Aldrich, St. Louis, MO, USA, and all information provided by Sigma-Aldrich are presented in Table 1. The suspension concentrations of both metal oxides (10 mg/l) were prepared by weighing dry metal oxide powder into the dechlorinated water (pH 7.4), then ultrasonicated (100 W, 40 kHz) for 1 h to increase their dispersion. Structural studies of both metal oxides (Fig. 1) were done by Field Emission Transmission Electron Microscopy (FETEM, JEM-2100F, JEOL Inc., Japan) at accelerating voltage of 200 kV. The dynamic light scattering (DLS) determination was done using Nano-zeta sizer-HT, Malvern Instrument, UK, to estimate the average hydrodynamic size of metal oxides NPs in water. The zeta potential values of both oxides were recorded using a Malvern Zetasizer Nano ZS instrument.
Acclimatization of the experimental fish
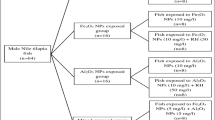
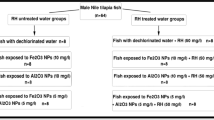
The experimental fish (n = 64) in the present study were Nile Tilapia, Oreochromis niloticus. They were purchased from an unpolluted fish farm located in El-Ismailia Governorate, Egypt. The body length and weight of the studied fish ranged from 10.5–13 cm and 30–39.2 g, respectively. All fish were transported in a plastic container to the ecology laboratory, Faculty of Science, Cairo University, with continuous aeration. Fish were distributed randomly (8 fish/aquarium) and maintained for 2 weeks in glass aquaria (40 × 70 × 26 cm) with 40 L aerated, dechlorinated tap water. The water temperature was maintained at 25 ± 1 °C, while dissolved oxygen and pH were 6.5–7.8 mg l−1 and 7.1–7.3, respectively. During the acclimatization period, fish were fed once daily with commercial pellet food (20% crude protein, 4% crude fat, 5% crude fiber, 12% crude ash, and 10% crude moisture).
Preparation of rice husk
The rice husk (RH) from rice mills in Kafr El-Sheikh were cut into small pieces, washed with deionized distilled water and dried in the oven at 80 °C for 24 h before use. The RH was ground then passed through 70 mesh sieve (< 210 μm) before use. Rice husk has entered the water and isolated by a porous net that allows passage of metal oxide NPs without escaping of RH into the water to avoid eating of RH by fish. The selected concentration of RH was 50 mg/l (5 times the NPs concentration).
Experimental design
After the acclimatization period, fish were randomly allocated in glass aquaria (40 × 70 × 26 cm) with 8 fish per aquarium. Fish were exposed to sub-lethal concentration of our selected NPs according to Saravanan et al. (2015); Murali et al. (2017); Canli et al. (2018) for 7 days. The experimental groups were classified into 8 groups: dechlorinated tap water (control group); dechlorinated tap water + RH (50 mg/l); Fe2O3 NPs (10 mg/l)-exposed group; Fe2O3 NPs (10 mg/l)-exposed group + RH (50 mg/l); Al2O3 NPs (10 mg/l)-exposed group; Al2O3 NPs (10 mg/l)-exposed group + RH (50 mg/l); mixture of same concentrations of Fe2O3 NPs and Al2O3 NPs; mixture of same concentrations of Fe2O3 NPs and Al2O3 NPs + RH (50 mg/l). The conditions of the experiment were similar to the acclimatization period, and water was daily checked for pH, temperature, and dissolved oxygen.
RH removal efficiency
After the end of the experiment, RH was collected from all studied aquaria then the concentrations of Fe and Al were determined in RH using inductively coupled plasma (ICP-AES), Thermo Sci, model: iCAP6000 series. The RH removal efficiency to the studied metals was calculated as follows according to Haris et al. (2011):
where % R is the removal efficiency, C0 is the initial concentration of metal NPs in water, and C is the concentration of the metal NPs in RH.
Fish sampling
After 7 days, blood samples were withdrawn from the caudal vein of the studied fish using heparin as an anticoagulant, then the vital organs (liver and gills) were isolated and stored frozen for further investigations.
Biochemical analyses
Blood samples were centrifuged at 3000 r.p.m. for 10 min to get the plasma for biochemical analyses using enzymatic-colorimetric methods of commercial Biodiagnostic kits (Biodiagnostic, Dokki, Giza, Egypt).
Plasma glucose, total lipids, and proteins concentrations
Plasma glucose was determined according to the method described by Trinder (1969). Total lipid was determined according to Zollner and Kirsch (1962). In addition, total protein measurement was according to biuret method (Gornal et al. 1949). While albumin concentration was measured according to Doumas et al. (1971). Globulin concentration was calculated as the difference between the total protein and albumin according to the method described by Coles (1986).
Indicative biomarkers of liver and kidney functions
Plasma AST and ALT activities were assessed according to Reitman and Frankel (1957). While, ALP activity was estimated according to Belfield and Goldberg (1971). The creatinine concentration was measured using the colorimetric method described by Bartles et al. (1972), while uric acid was measured using enzymatic reaction according to Barham and Trinder (1972).
Determination of antioxidant biomarkers
For the evaluation of the oxidative damage, liver and gill tissues were homogenized in 5-ml cold buffer solution (specific for each kit) per gram tissue. Then the homogenates centrifuged at 4000 r.p.m. for 15 min and the supernatants were obtained to assess all oxidative stress biomarkers using biodiagnostic kits (Biodiagnostic Dokki, Giza, Egypt).
The determination of superoxide dismutase (SOD) activity relied on the ability of this enzyme to inhibit the phenazinemethosulphate mediated reduction of nitro blue tetrazolium dye (Nishikimi et al. 1972) The change in absorbance at 560 nm over 5 min was related with the inhibition rate which is directly proportional to SOD activity. As detailed by Aebi (1984), catalase (CAT) enzyme reacts with a known quantity of H2O2 and the reaction is stopped after 1 min with a catalase inhibitor. In the presence of peroxidase, the remaining H2O2 reacts with 3, 5-dichloro-2-hydroxybenzene sulfonic acid and 4-aminophenazone to form a chromophore with a color intensity inversely proportional to the amount of catalase in the sample. The absorbance was measured at 510 nm. Both SOD and CAT are expressed as U/mg protein. Glutathione reduced (GSH) determination depends on the reduction of 5, 5′-dithiobis 2-nitrobenzoic acid with glutathione produce a yellow color whose absorbance is measured at 405 nm according to Beutler et al. (1963) and expressed as mg/mg protein. The determination of glutathione peroxidase (GPx) activity is an indirect measurement depends on the ability of GPx to reduce organic peroxide to oxidized glutathione (GSSG) which recycled to its reduced state by glutathione reductase (GR). The oxidation of NADPH to NADP+ is accompanied by a decrease in absorbance at 340 nm (A340) providing a spectrophotometric means for monitoring GPx enzyme activity (expressed as U/mg protein) as described by Paglia and Valentine (1967). The lipid peroxidation as indicated by thiobarbituric acid reactive substances (TBARS) level was estimated using the method of Ohkawa et al. (1979), in which the TBARS reacts with thiobarbituric acid forming a colored product. The color intensity of resultant reactive species (at 534 nm) was directly proportional to TBARS level and expressed as nmole/g tissue.
Statistical analyses
The results were expressed as means ± SE. Data were statistically analyzed using t test, analyses of variance (ANOVA), and Duncan’s multiple range test to determine the difference in means using Statistical processor Systems support “SPSS” for windows software. A value of p < 0.05 was considered significant.
Results
Characterization of Fe2O3 and Al2O3NPs
As shown in Fig. 1 the sizes of Fe2O3 NPs were ranged from 17.3 to 34.8 nm while the sizes of Al2O3 NPs were ranged from 21.4 to 44.5 nm. Furthermore, the average hydrodynamic size and zeta potential in water were 1547 nm and 1.24 mV for Fe2O3 NPs versus 80.8 nm and 38.2 mV for Al2O3 NPs, respectively.
RH removal efficiency
The removal efficiency of RH for Fe and Al NPs after 7 days of exposure was recorded in Table 2. According to the RH removal efficiency of iron from water, the percentage of removal from the mixture exposed group was higher than the single exposed group. Also, the RH removal efficiency of Al was higher in the mixture group compared to the single exposed group. According to the RH removal efficiency, the percentage of removal of metals with RH was:
Fe NPs of the mixture group > Fe NPs of the single exposed group > Al NPs of the mixture group > Al NPs of the single exposed group.
Biochemical blood constituents
The single and combined effect of Fe2O3 and Al2O3 NPs on plasma glucose, total lipid, and total protein level of Oreochromis niloticus after water treatment with rice husk were recorded in Figs. 2 and 3.
Single and combined effects of Fe2O3 and Al2O3 NPs on plasma total protein level of Oreochromis niloticus with and without rice husk water treatment. Data are represented as means of eight samples in each group ± S.E. The small letters represent the t test (p < 0.05) between rice husk (RH)-treated and -untreated water within the same group. Columns with same letters are not significantly different; otherwise, they do. The capital letters represent the Duncan’s test (p < 0.05) between different metal oxide NPs-exposed groups compared with control groups of each rice husk (RH)-treated and-untreated water groups. Columns with same letters are not significantly different; otherwise, they do. The letters are arranged in a descending order as A, B, C, and D
Plasma glucose concentrations
The one-way analysis of variance (ANOVA) showed a significant difference at p < 0.05. Compared to the control group, the different metal oxide NPs-exposed groups showed significant increases in plasma glucose concentrations in both RH-treated and -untreated water groups (Duncan’s test capital letters). The highest values were recorded significantly in Fe2O3 NPs (RH untreated water) while the lowest values were recorded in Al2O3 NPs-exposed fish (RH-treated water). Comparing the RH-untreated and -treated water, groups exposed to the same metal oxide NPs (t test) showed that glucose levels were always significantly higher in untreated water groups.
Total lipid concentrations
In case of RH-untreated water groups, there were significant differences among the different exposed NPs. The Fe2O3 and combined NPs-exposed groups showed a sharp significant decrease in the total lipid concentrations compared to the control group. While in the case of RH-treated water groups, the decline in total lipid values was recorded only in Fe2O3 NPs and combined NPs groups without any significant change in Al2O3 NPs-exposed group. Comparing the RH-untreated and -treated water groups that are exposed to the same metal oxide NPs (t test), the total lipid levels were always lower in untreated groups except for combined NPs-exposed group.
Plasma total protein level
Comparing with the control group, the total protein levels were significantly increased in the Fe2O3 NPs-xposed group without any significant changes in the other metal oxide NPs-exposed groups (in case of RH-treated and -untreated water groups). Also, the significant difference between RH-untreated and treated water groups (t test) was recorded only in the Fe2O3 NPs-exposed groups. The albumin concentrations were significantly decreased in all studied groups except for Fe2O3 NPs-exposed group (RH treated water).While the RH effect was obvious as all studied fish groups restored albumin values more or less to that of the control groups in RH-treated water compared to untreated water groups that exposed to the same metal oxide NPs (t test). In comparison to the control group, the globulin levels were increased significantly in all studied metal oxides-exposed groups with the highest values in Fe2O3 NPs-exposed groups (RH untreated and treated water groups).There was no significant difference between RH untreated and treated water groups that were exposed to the same metal oxides (t test) except for Fe2O3 NPs-exposed groups.
Indicative biomarkers of liver and kidney functions
In comparison with control groups, the AST, ALT, and ALP activities in the studied fish were significantly higher in all studied metal oxide NPs-exposed groups (Fig. 4). Besides, the concentrations of plasma creatinine and uric acid showed the same results (Fig. 5). The recorded biomarkers showed the evident effect of RH water treatment as they were always significantly lower in RH-treated groups compared with untreated ones.
Single and combined effects of Fe2O3 and Al2O3 NPs on liver functions (AST, ALT, ALP) of Oreochromis niloticus with and without rice husk water treatment. Data are represented as means of eight samples in each group ± S.E. The small letters represent the t test (p < 0.05) between rice husk (RH)-treated and -untreated water within the same group. Columns with same letters are not significantly different; otherwise, they do. The capital letters represent the Duncan’s test (p < 0.05) between different metal oxide NPs-exposed groups compared with control groups of each rice husk (RH)-treated and -untreated water groups. Columns with same letters are not significantly different; otherwise, they do. The letters are arranged in a descending order as A,B, C, and D
Single and combined effects of Fe2O3 and Al2O3 NPs on kidney functions (creatinine and uric acid) of Oreochromis niloticus with and without rice husk water treatment. Data are represented as means of eight samples in each group ± S.E. The small letters represent the t test (p < 0.05) between rice husk (RH)-treated and -untreated water within the same group. Columns with same letters are not significantly different; otherwise, they do. The capital letters represent the Duncan’s test (p < 0.05) between different metal oxide NPs-exposed groups compared with control groups of each rice husk (RH)-treated and -untreated water groups. Columns with same letters are not significantly different; otherwise, they do. The letters are arranged in a descending order as A,B, C, and D
Oxidative stress biomarker
Oxidative stress biomarkers in liver
The activities of all studied enzymatic biomarkers (GPx, CAT, and SOD) showed a significant increase after all metal oxides NPs exposure, while the non-enzymatic biomarkers (TBARS and GSH) showed a sharp increase in the TBARS levels versus a significant decline in GSH content in all studied groups (Figs. 6 and 7).
Single and combined effects of Fe2O3 and Al2O3 NPs on some antioxidant enzymes (GPx, CAT, and SOD) in liver of Oreochromis niloticus with and without rice husk water treatment. Data are represented as means of eight samples in each group ± S.E. The small letters represent the t test (p < 0.05) between rice husk (RH)-treated and -untreated water within the same group. Columns with same letters are not significantly different; otherwise, they do. The capital letters represent the Duncan’s test (p < 0.05) between different metal oxide NPs-exposed groups compared with control groups of each rice husk (RH)-treated and -untreated water groups. Columns with same letters are not significantly different; otherwise, they do. The letters are arranged in a descending order as A,B, C, and D
Single and combined effects of Fe2O3 and Al2O3 NPs on some antioxidant biomarkers (TBARS and GSH contents) in liver of Oreochromis niloticus with and without rice husk water treatment. Data are represented as means of eight samples in each group ± S.E. The small letters represent the t test (p < 0.05) between rice husk (RH)-treated and -untreated water within the same group. Columns with same letters are not significantly different; otherwise, they do. The capital letters represent the Duncan’s test (p < 0.05) between different metal oxide NPs-exposed groups compared with control groups of each rice husk (RH)-treated and -untreated water groups. Columns with same letters are not significantly different; otherwise, they do. The letters are arranged in a descending order as A,B, C, and D
Oxidative stress biomarkers in gills
The studied enzymatic and non-enzymatic biomarkers in gills (Figs. 8 and 9) followed the same trend as in liver tissues but the level of TBARS production (an indicator of the lipid peroxidation process) was higher in gills than that of liver indicated the delicate capability of the antioxidant system to resist strong oxidative stress in gills. Comparing the RH-untreated and -treated water groups that are exposed to the same metal NPs, the measured antioxidant status showed obvious amelioration through all studied antioxidant biomarkers after RH treatment.
Single and combined effects of Fe2O3 and Al2O3 NPs on some antioxidant enzymes (GPx, CAT, and SOD) in gills of Oreochromis niloticus with and without rice husk water treatment. Data are represented as means of eight samples in each group ± S.E. The small letters represent the t test (p < 0.05) between rice husk (RH)-treated and -untreated water within the same group. Columns with same letters are not significantly different; otherwise, they do. The capital letters represent the Duncan’s test (p < 0.05) between different metal oxide NPs-exposed groups compared with control groups of each rice husk (RH)-treated and -untreated water groups. Columns with same letters are not significantly different; otherwise, they do. The letters are arranged in a descending order as A,B, C, and D
Single and combined effects of Fe2O3 and Al2O3 NPs on some antioxidant biomarkers (TBARS and GSH contents) in gills of Oreochromis niloticus with and without rice husk water treatment. Data are represented as means of eight samples in each group ± S.E. The small letters represent the t test (p < 0.05) between rice husk (RH)-treated and -untreated water within the same group. Columns with same letters are not significantly different; otherwise, they do. The capital letters represent the Duncan’s test (p < 0.05) between different metal oxide NPs-exposed groups compared with control groups of each rice husk (RH)-treated and -untreated water groups. Columns with same letters are not significantly different; otherwise, they do. The letters are arranged in a descending order as A,B, C, and D
Discussion
With the complexity of NPs behavior in the aquatic environments, it is necessary to fully characterize NPs to understand their effects on different fish and biota (Benavides et al. 2016). Also, particle size is the key factor that influences the toxic effects of NPs (Rossetto et al. 2014). Transmission electron microscope (TEM) imaging in the present study showed that the Fe2O3and Al2O3 NPs sizes were less than 50 nm, which agreed with the information provided by the manufacturer (Sigma Aldrich). While the average hydrodynamic diameters of Fe2O3 and Al2O3 NPs in water measured by DLS were larger than the sizes measured by TEM especially in case of Fe2O3 NPs. These different sizes may be due to the aggregation and hydration of metal oxide NPs. The aggregation of these metal oxides was shown also by the DLS results of Zhu et al. (2012) for Fe2O3 NPs and Benavides et al. (2016) for Al2O3 NPs. The present DLS results indicated that Fe2O3 NPs have a higher ability to aggregate than Al2O3 NPs. Moreover, the measured zeta potential of Fe2O3 NPs was near to zero compared to that of Al2O3 NPs which give Fe2O3 NPs a high ability to aggregate in water. This observation was confirmed by Ghosh et al. (2008) who reported that NPs tend to aggregate as the surface charge became neutral or near to zero.
The scientific base for using RH in the removal of NPs from water is due to its granular structure, chemical stability in addition to its high cellulose, hemicellulose, lignin, and silica contents that can easily bind metals (Ding et al. 2012). The high clearance efficiency of RH toward iron NPs may be due to Fe2O3 NPs has adsorption and magnetic characterizations that can be enabling their adsorption on the surface of rice husk and aggregate around them easier than Al2O3 NPs. Moreover, the adsorbed Fe2O3 NPs on rice husk is considered as an excellent complex that can adsorb other metals from water. This observation was said by Jang and Dempsey (2008) who showed that oxides of iron have an affinity for anionic pollutant and this remark was confirmed in our work as we found that the concentrations of metal oxide NPs was decreased from water in mixture groups (Al2O3 NPs and Fe2O3 NPs) than single NPs contain groups.
Assessment of biochemical parameters acts as a monitoring tool for measuring the adverse effects of NPs and it could help to identify the potential toxicity of target organs as well as the general health status of animals (Chupani et al. 2017). Significant elevation of plasma glucose has been used in many toxicological works as a good indicator of many environmental stresses (El Basuini et al. 2016). In the present work, a significant increase in plasma glucose was recorded among the different NPs-exposed groups. This observation was in accordance with Saravanan et al. (2015) who reported an increase in plasma glucose level after the exposure of Indian major carp (Labeo rohita) to two different concentrations of Fe2O3 NPs. In addition, Farmen et al. (2012) recorded a concentration-dependent increase in plasma glucose of juvenile Atlantic salmon when exposed to Ag NPs and this increment was parallel to the gill accumulation of Ag NPs. According to our characterization results, Fe2O3 NPs are more aggregated in water and showed high adherence tendency to gills. Therefore, the sharp glucose elevation in Fe2O3 NPs-exposed group might be due to respiratory insufficiency, which agreed with Saravanan et al. (2015) who suggested that the elevation of blood glucose level may be a response to respiratory disturbances caused by Fe2O3 NPs. The increased plasma glucose level may generally occur in response to the release of stress-induced hormones (e.g., epinephrine and norepinephrine) into the circulation triggering glycogenolysis to compensate the increased energy demands during and after stress condition (Eslamloo et al. 2014). Moreover, the overproduction of the reactive oxygen species (ROS) within the tissues which recorded by many authors after metal NPs exposure have a strong catabolic activity on the carbohydrates and can cause a disturbance in plasma glucose levels (Gupta et al. 2016; Bacchetta et al. 2017).
Lipid plays an important role in the physiology of fish regarding its vital role in energy production in addition to its importance as an essential component of many cellular structures and tissues (Javed et al. 2017). The present results indicated a significant decrease in total lipid among the different metal oxide NPs-exposed groups. This was in agreement with our previous study that showed a decrease in plasma total lipid of Nile Tilapia after 30 days of exposure to CuO NPs when compared with control groups (Abdel-Khalek et al. 2015). This decrease may be resulted from the breakdown of lipid molecules as energetic substrates to cope with stress metabolically (Abdel-Tawwab et al. 2013). Moreover, the decreased lipid content may reflect the overproduction of ROS within the tissue and high lipid oxidation after metal oxide NPs exposure (Abdel-Khalek 2015).
The present results generally indicated that although total protein and the globulin levels were significantly increased, the albumin levels were significantly decreased in the studied groups comparing with the control groups. These results were demonstrated by Javed et al. (2017), as they found the same results in Channa punctatus collected from a metal-contaminated site. Kanwal et al. (2019) recorded an elevation in total protein and globulin in Labeo rohita after their exposure to different NPs of many metals (Ni, Co3O4, Cr3O4) and they referred this increase to the high rate of protein synthesis to realize the necessities of high energy needs and to meet the immunotoxic challenge (Kanwal et al. 2019). Moreover, NPs are always coated with proteins resulting in NPs-protein corona and it could stimulate the synthesis of stress proteins (enzymes of detoxification) therefore both reasons might induce a change in the level of total proteins (Nel et al. 2009; Besnaci et al. 2016). The reduction in albumin concentration in the current study was in agreement with Javed and Usmani (2015) who suggested that the hypo-albuminemia in fish could be due to the utilization of albumin to meet the immediate energy demand to face the exposure of pollutants or other stressful situations.
Liver enzymes (AST, ALT, and ALP) are important biomarkers in many ecotoxicological studies (Nel et al. 2009). Sharma et al. (2012) indicated that the liver is the primary organ of metabolism and might act as a major target organ for nanoparticles after they enter the body through any of the possible routes. The present results indicated a significant increase in all studied enzyme activities among the different NPs-exposed groups. A significant increase in liver enzymes was observed in different fish species as Cyprinus carpio and Labeo rohita when exposed to various metal NPs like TiO2 NPs, Ag NPs, Ni NPs, Co3O4 NPs, and Cr3O4 NPs (Banaee et al. 2019; Kanwal et al. 2019). This elevation in liver enzymes in plasma is assumed to be a result of metallic liver damage and develop leaky membranes that permit the escape of intracellular enzymes into the bloodstream (Paunovic et al. 2017).
Kidney functions measured by creatinine and uric acid in blood could be used as a good index for glomerular filtration rate and kidney dysfunction. The recorded elevation of both creatinine and uric acid were in agreement with Canli et al. (2018) who studied the effect of Al2O3 NPs on Oreochromis niloticus and they indicated that the creatinine (up to 455%) levels increased sharply at the highest concentration (25 mg/L) of Al2O3 NPs in comparison to the control group. Also, NPs that absorbed into the circulation can be filtered by the renal system inducing kidney dysfunction and increases uric acid and creatinine levels in the blood.
Oxidative stress occurs when pro-oxidant forces overwhelm antioxidant defenses, as pollutants go into the body and produce ROS through a series of metabolic conversion. If they are not cleared promptly, the balance will be destroyed and cause oxidative damage (Abdel-Khalek 2018). Oxidative stress has been also proposed as a common mechanism of cell damage induced by many types of NPs (Jayaseelan et al. 2014).
The GPx is an efficient enzyme for H2O2 and organic peroxide detoxification (Abdel-Khalek 2018). The present study showed a general significant increase in GPx activity in the liver and gill tissues. This observation is in agreement with Hao and Chen (2012) who reported that exposure of carps (C. carpio) to ZnO NPs resulted in an increase in GPX in the gills and liver tissues. This elevation in GPx activities was occurring at the same time with decreasing GSH content, signposted that the antioxidant progression occurred by GPx enzyme was activated along with an ineffective GSH production. Moreover, CAT and SOD are involved in a defense mechanism against the deleterious effects of ROS in cells by metabolizing toxic oxidative intermediates (Abdel-Khalek 2015). The observed elevation in the activities of both enzymes in our study was shown also by Benavides et al. (2016) who observed an increase in CAT and SOD activity in the gills and livers of Carassius auratus after 14 days of exposure to single and combined Al2O3 NPs and ZnO NPs. The elevation in these enzymes was to decrease the toxicity that occurs by ROS due to metals stress. The TBARS level is an important biomarker for monitoring lipid peroxidation and the health condition of biological membranes and its measurement provides a relative measure of oxidative injury (Saddick et al. 2017). The present investigation showed a significant increase in TBARS contents in both liver and gill tissues of O. niloticus after the exposure to different metal oxides NPs when compared to control groups. This was similar to Li et al. (2009) who observed that lipid peroxidation and the production of TBARS were elevated in embryonic medaka Oryzias latipes after iron NPs exposure. Also, according to Benavides et al. (2016), Al2O3 NPs induced an increase in TBARS levels in gill and liver of Carassius auratus. The entrance of metals could initiate ROS production that extracts hydrogen atom from unsaturated bonds and damages the lipids in the membrane (Javed et al. 2017). Glutathione reduced is an effective protectant as it can quench oxyradicals induced by chemical contaminants and acts as a reductant in conjugation with xenobiotic (Kanak et al. 2014). The significant decrease in GSH content among the studied groups was in agreement with Yildirim et al. (2011) who found a decline in GSH level in Capoeta trutta fish caught from a contaminated site in the Munzur River. Moreover, Abdel-khalek et al. (2015) observed a decrease in GSH of Oreochromis niloticus exposed to CuO NPs in comparison with that of the control group. The decreased GSH content confirmed its high utilization by ROS. Moreover, Jozefczak et al. (2012) revealed the cause of GSH inadequacy to the binding of metals to GSH forming GS-metal complex.
In general, our results showed that both Fe2O3 and Al2O3NPs could induce oxidative damage to both studied tissues. The severity of the oxidative damage and lipid peroxidation level was different depending on the tissue and the exposed metal NPs. For example, the gills of Fe2O3 NPs-exposed group showed high-antioxidant enzyme activities with elevated lipid peroxidation rate compared to Al2O3NPs-exposed group. This may be due to direct adherence of Fe2O3 aggregates on the surface of the gills (observed as red aggregates during our work) leads to high levels of free ions resulting in the accumulation of Fe2O3 NPs. This observation was confirmed by the high concentration of iron in gills (Abdel-Khalek et al. 2020). The toxicity of Fe2O3 NPs to the internal tissues may also come from high adhesion tendency of iron nanoparticles to the surfaces of the gills which could damage the epithelial cell and facilitate the entry of NPs into the fish body as shown by Chen et al. (2012). While the high oxidative damage in the liver of Al2O3NPs-exposed group may be due to the low adhesion tendency of Al2O3NPs and its small particle size which facilitates the internalization mechanism of these particles to accumulate in hepatic tissues and induce severe oxidative damage. The combined doses of our studied NPs showed more or less toxicity compared to single doses. Therefore, this point needs more studies on the mode of interaction of the combined doses of our studied NPs and their toxicity on the aquatic environment.
This is the first study done to show the efficiency of RH to decrease the toxic properties of metal NPs. The recorded improvement in all recorded biochemical and oxidative stress parameters after RH water treatment proved that using RH is an efficient tool for the removal of both NPs from water. The clearance efficiency of RH is due to the good adsorptive properties of its components such as cellulose, hemicellulose, lignin, and silica (Srivastava et al. 2006). Treatment of water with RH succeeded to decrease the toxicological impacts of the studied NPs especially Fe2O3. This may be due to Fe2O3 NPs’ adsorption and magnetic characterizations, enabling their adsorption on the surface of rice husk and aggregate around them easier than Al2O3 NPs. But this aggregation of NPs on the RH surfaces may cause a decrease in the total surface area available for adsorption and the RH becomes saturated with NPs after time. Therefore, if this experiment was practically applied, we recommend to collect, wash, and reuse RH periodically. The adsorbed Fe2O3 NPs on rice husk is considered as an excellent complex that can adsorb other metals from water. This observation was said by Jang and Dempsey (2008) who showed that oxides of iron have an affinity for anionic pollutant and this remark was confirmed in our experiment as we found that the efficiency of RH to remove Al2O3 NPs was increased in water especially in mixture groups (Al2O3 NPs and Fe2O3 NPs) than single Al2O3 NPs-containing groups.
Conclusions
It can be concluded that (1) Fe2O3 NPs and Al2O3 NPs in single and combined doses could induce biochemical alterations and oxidative stress to Oreochromis niloticus. (2) This is the first study done to show the efficiency of RH to decrease the toxic properties of metal NPs and the recorded results proved that RH had clearance potency to both metal oxides NPs and could reduce their toxic effect on fish. (3) Rice husk is safe and efficient to use in metal NPs treatment but we need more studies to improve RH removal efficiency.
References
Abdel-Khalek AA (2015) Antioxidant responses and nuclear deformations in freshwater fish, Oreochromis niloticus, facing degraded environmental conditions. Bull Environ Contam Toxicol 94(6):701–708
Abdel-Khalek AA (2016) Comparative evaluation of genotoxic effects induced by CuO bulk and nano-particles in Nile Tilapia, Oreochromis niloticus. Water Air Soil Pollut 227:35
Abdel-Khalek AA (2018) Chronic exposure to water of lake Qaroun induced metal-related testicular damage and endocrine disruption in male fish. Biol Trace Elem Res 185(1):197–204
Abdel-Khalek AA, Kadry AM, Badran SR, Marie M-AS (2015) Comparative toxicity of copper oxide bulk and nano particles in Nile Tilapia; Oreochromis niloticus: biochemical and oxidative stress. J Basic Appl Zool 72:43–57
Abdel-Khalek AA, Badran SR, Marie M-AS (2016) Toxicity evaluation of copper oxide bulk and nanoparticles in Nile tilapia, Oreochromis niloticus, using hematological, bioaccumulation and histological biomarkers. Fish Physiol Biochem 42:1225–1236
Abdel-Khalek AA, Elhaddad E, Mamdouh S, Marie MS (2018) The chronic exposure to discharges of Sabal drain induces oxidative stress and histopathological alterations in Oreochromis niloticus. Bull Environ Contam Toxicol 101(1):92–98
Abdel-Khalek AA, Badran SR, Marie M-AS (2020) The efficient role of rice husk in reducing the toxicity of iron and aluminum oxides nanoparticles in Oreochromis niloticus: hematological, bioaccumulation, and histological endpoints. Water Air Soil Pollut 231:53
Abdel-Tawwab M, Mousaad MN, Sharafeldin KM, Ismaiel NEM (2013) Changes in growth and biochemical status of common carp, Cyprinus carpio L. exposed to water-born zinc toxicity for different periods. Int Aquat Res 5:11
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Bacchetta C, Ale A, Simoniello MF, Gervasio S, Davico C, Rossi AS, Desimone MF, Poletta G, Lopez G, Monserrat JM, Cazenave J (2017) Genotoxicity and oxidative stress in fish after a short-term exposure to silver nanoparticles. Ecol Indic 76:230–239
Banaee M, Tahery S, Haghi BN, Shahafve S, Vaziriyan M (2019) Blood biochemical changes in common carp (Cyprinus carpio) upon co-exposure to titanium dioxide nanoparticles and paraquat. Iran J Fish Sci 18(2):242–255
Barham D, Trinder P (1972) Enzymatic determination of uric acid. Analyst 97:142–145
Bartles H, Bohmer M, Heirli C (1972) Colorimetric kinetic method for creatinine determination in serum and urine. Clin Chem Acta 37:193
Belfield A, Goldberg DM (1971) Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzymes 12:561–573
Benavides M, Fernández-Lodeiro J, Coelho P, Lodeiro C, Diniz MS (2016) Single and combined effects of aluminum (Al2O3) and zinc (ZnO) oxide nanoparticles in a freshwater fish, Carassius auratus. Environ Sci Pollut Res 23(24):24578–24591
Besnaci S, Bensoltane S, Zerari L, Samia Ch, hamlet SA, Berrebbah H (2016) Impact of nanometric iron oxide in the hepatopancreas of terrestrial gastropod Helix Aspersa: histological changes and biochemical parameters. Int J Pharm Sci Rev Res 36(2):234–241
Beutler E, Duron O, Kelly MB (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Canli EG, Dogan A, Canli M (2018) Serum biomarker levels alter following nanoparticle (Al2O3, CuO, TiO2) exposures in freshwater fish (Oreochromis niloticus). Environ Toxicol Pharmacol 62:181–187
Chen PJ, Tan SW, Wu WL (2012) Stabilization or oxidation of nanoscale zerovalent iron at environmentally relevant exposure changes bioavailability and toxicity in medaka fish. Environ Sci Technol 46(15):8431–8439
Cheng C, Wang J, Yang X, Li A, Philippe C (2014) Adsorption of Ni (II) and Cd (II) from water by novel chelating sponge and the effect of alkali-earth metal ions on the adsorption. J Hazard Mater 264:332–341
Chupani L, Zusková E, Niksirat H, Panáček A, Lünsmann V, Haange SB, von Bergen M, Jehmlich N (2017) Effects of chronic dietary exposure of zinc oxide nanoparticles on the serum protein profile of juvenile common carp (Cyprinus carpio L.). Sci Total Environ 579:1504–1511
Coles EH (1986) Veterinary clinical pathology, 4th edn. W.B. Saunders, Philadelphia, pp 10–42
Ding Y, Jing D, Gong H, Zhou L, Yang X (2012) Biosorption of aquatic cadmium (II) by unmodified rice straw. Bioresour Technol 114:20–25
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chim Acta 31:87–96
El Basuini MF, El-Hais AM, Dawood MAO, Abou-Zeid A E-S, EL-Damrawy SZ, Khalafalla M M E-S, Koshio S, Ishikawa M, Dossou S (2016) Effect of different levels of dietary copper nanoparticles and copper sulfate on growth performance, blood biochemical profiles, antioxidant status and immune response of red sea bream (Pagrus major). Aquaculture 455:32–40
Elsaesser A, Howard CV (2012) Toxicology of nanoparticles. J Adv Drug Deliv Rev 64(2):129–137
Eslamloo K, Akhavan SR, Fallah FJ, Henry MA (2014) Variations of physiological and innate immunological responses in goldfish (Carassius auratus) subjected to recurrent acute stress. Fish Shellfish Immunol 37(1):147–153
Farmen E, Mikkelsen HN, Evensen Ø, Einset J, Heier LS, Rosseland BO, Salbu B, Tollefsen KE, Oughton DH (2012) Acute and sub-lethal effects in juvenile Atlantic salmon exposed to low μg/L concentrations of Ag nanoparticles. Aquat Toxicol 108:78–84
Francis AP, Babu GJD, Lavanya M, Vidhya KS, Devasena T (2011) Toxicity studies of aluminium oxide nanoparticles in cell lines. Int J Nanotechnol Appl 5(2):99–107
Ghosh S, Mashayekhi H, Pan B, Bhowmik P, Xing B (2008) Colloidal behavior of aluminum oxide nanoparticles as affected by pH and natural organic matter. Langmuir 24(21):12385–12391
Gornal AC, Bardawill CJ, David MM (1949) Colorimetris method for determination of total protein. J Biol Chem 177:751
Gupta YR, Sellegounder D, Kannan M, Deepa S, Senthilkumaran B, Basavaraju Y (2016) Effect of copper nanoparticles exposure in the physiology of the common carp (Cyprinus carpio): biochemical, histological and proteomic approaches. Aquac Fish 1:15–23
Hao L, Chen L (2012) Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol Environ Saf 80:103–110
Haris MRHM, Wahab NAA, Reng CW, Azahari B, Sathasivam K (2011) The sorption of cadmium (II) ions on mercerized rice husk and activated carbon. Turk J Chem 35:939–950
Jang J-H, Dempsey BA (2008) Coadsorption of arsenic (III) and arsenic(V) onto hydrous ferric oxide: effects on abiotic oxidation of arsenic(III), extraction efficiency, and model accuracy. Environ Sci Technol 42(8):2893–2898
Javed M, Usmani N (2015) Stress response of biomolecules (carbohydrate, protein and lipid profiles) in fish Channa punctatus inhabiting river polluted by thermal power plant effluent. Saudi J Biol Sci 22(2):237–242
Javed M, Ahmad MI, Usmani N, Ahmad M (2017) Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste water. Sci Rep 7:1675–1685
Jayaseelan C, Abdul Rahuman A, Ramkumar R, Perumal P, Rajakumar G, Kirthi AV, Santhoshkumar T, Marimuthu S (2014) Effect of sub-acute exposure to nickel nanoparticles on oxidative stress and histopathological changes in Mozambique tilapia, Oreochromis mossambicus. Ecotoxicol Environ Saf 107:220–228
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13(3):3145–3175
Kanak EG, Dogan Z, Eroglu A, Atli G, Canli M (2014) Effects of fish size on the response of antioxidant systems of Oreochromis niloticus following metal exposures. Fish Physiol Biochem 40(4):1083–1091
Kanwal Z, Raza MA, Manzoor F, Riaz S, Jabeen G, Fatima S, Naseem S (2019) A comparative assessment of nanotoxicity induced by metal (silver, nickel) and metal oxide (cobalt, chromium) nanoparticles in Labeo rohita. Nanomaterials 9:309–328
Krishnani KK, Ayyappan S (2006) Heavy metals remediation of water using plants and lignocellulosic agrowastes. Rev Environ Contam Toxicol 188:59–84
Lata S, Samadder SR (2014) Removal of heavy metals using rice husk: a review. Int J Environ Res Develop 4(2):165–170
Li H, Zhou Q, Wu Y, Fu J, Wang T, Jiang G (2009) Effects of waterborne nano-iron on medaka (Oryzias latipes): antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicol Environ Saf 72(3):684–692
Murali M, Suganthi P, Athif P, Sadiq Bukhari A, Syed Mohamed HE, Basu H, Singhal RK (2017) Histological alterations in the hepatic tissues of Al2O3 nanoparticles exposed freshwater fish Oreochromis mossambicus. J Trace Elem Med Biol 44:125–131
Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M (2009) Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater 8(7):543–557
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Rese Common 46:849–854
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Paunovic J, Vucevic D, Radosavljevic T, Pantic S, Nikolovski D, Dugalic S, Pantic I (2017) Effects of metallic nanoparticles on physiological liver functions. Rev Adv Mater Sci 49:123–128
Pinna N, Niederberger M (2008) Oxide synthesis as cornerstone of nanoscience. Eur J Inorg Chem 6:825
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 2:56–60
Rekulapally R, Murthy Chavali LN, Idris MM, Singh S (2019) Toxicity of TiO2, SiO2, ZnO, CuO, au and Ag engineered nanoparticles on hatching and early nauplii of Artemia sp. Peer J 6:1–16
Rossetto AL, Melegari SP, Ouriques LC, Matias WG (2014) Comparative evaluation of acute and chronic toxicities of CuO nanoparticles and bulk using Daphnia magna and Vibrio fischeri. Sci Total Environ 490:807–814
Saddick S, Afifi M, Abu Zinada OA (2017) Effect of zinc nanoparticles on oxidative stress-related genes and antioxidant enzymes activity in the brain of Oreochromis niloticus and Tilapia zillii. Saudi J Biol Sci 24(7):1672–1678
Saravanan M, Suganya R, Ramesh M, Poopal RK, Gopalan N, Ponpandian N (2015) Iron oxide nanoparticles induced alterations in haematological, biochemical and ionoregulatory responses of an Indian major carp Labeo rohita. J Nanopart Res 17:274–285
Sharma V, Anderson D, Dhawan A (2012) Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 17(8):852–870
Shipley HJ, Engates KE, Guettner AM (2011) Study of iron oxide nanoparticles in soil for remediation of arsenic. J Nanopart Res 13(6):2387–2397
Sobhanardakani S, Parvizimosaed H, Olyaie E (2013) Heavy metals removal from wastewaters using organic solid waste-rice husk. Environ Sci Pollut Res Int 20(8):5265–5271
Srivastava VC, Mall ID, Mishra IM (2006) Characterization of mesoporous rice husk ash (RHA) and adsorption kinetics of metal ions from aqueous solution onto RHA. J Hazard Mater 134(1–3):257–267
Trinder P (1969) Enzymatic colorimetric method of glucose. Ann Clin Biochem 6:24–27
Yildirim NC, Benzer F, Danabas D (2011) Evaluation of environmental pollution at Munzur River of Tunceli applying oxidative stress biomarkers in Capoeta trutta (Heckel, 1843). J Anim Plant Sci 21(1):66–71
Zhang S, Zhang Y, Liu J, Xu Q, Xiao H, Wang X, Xu H, Zhou J (2013) Thiol modified Fe3O4@SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal. Chem Eng J 226:30–38
Zhu X, Tian S, Cai Z (2012) Toxicity assessment of iron oxide nanoparticles in zebra fish (Danio rerio) early life stages. PLoS One 7(9):e46286
Zollner N, Kirsch K (1962) Micro determination of lipids by the sulfo-phospho-vanillin reaction. Zool Gesta Exp Med 135:545–561
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in this study involving animals (fish) were approved (approval no. CUFS F ECO 4615) and were in accordance with the ethical standards of Faculty of Science, Cairo University, Institutional Animal Care and Use Committee (IACUC), at which the studies were conducted.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Thomas Braunbeck
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Khalek, A.A., Badran, S.R. & Marie, MA.S. The effective adsorbent capacity of rice husk to iron and aluminum oxides nanoparticles using Oreochromis niloticus as a bioindicator: biochemical and oxidative stress biomarkers. Environ Sci Pollut Res 27, 23159–23171 (2020). https://doi.org/10.1007/s11356-020-08906-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08906-x