Abstract
Open biomass burning in Peninsula Malaysia, Sumatra, and parts of the Indochinese region is a major source of transboundary haze pollution in the Southeast Asia. To study the influence of haze on rainwater chemistry, a short-term investigation was carried out during the occurrence of a severe haze episode from March to April 2014. Rainwater samples were collected after a prolonged drought and analyzed for heavy metals and major ion concentrations using inductively coupled plasma mass spectroscopy (ICP-MS) and ion chromatography (IC), respectively. The chemical composition and morphology of the solid particulates suspended in rainwater were examined using a scanning electron microscope coupled with energy-dispersive X-ray spectroscopy (SEM-EDS). The dataset was further interpreted using enrichment factors (EF), statistical analysis, and a back trajectory (BT) model to find the possible sources of the particulates and pollutants. The results show a drop in rainwater pH from near neutral (pH 6.54) to acidic (<pH 4.00) during the haze to non-haze transitional period, suggesting that the deposition rate of sulfate and nitrate in the atmosphere via the precipitation process was relatively lower compared to the mineral components. Zinc, nitrate, and calcium, which were the predominant elements in the first rainwater samples. Besides, the results of the SEM-EDS indicated that the zinc was possibly originated from anthropogenic activities which are consistent with the results obtained from EF. The BT model showed that pollutants transported from the mainland of Indo-China and the marine region in the South China Sea were responsible for the high pollution event in the study area. These findings can be useful in identifying contributions of pollutants from single or multiple sources in rainwater samples during haze episodes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomass burning is a common issue and received deep concern among countries in tropical Asia. Smoke haze related to biomass burning is a recurring environmental problem which affects air quality not only in the source regions but also in the surrounding areas. In fact, biomass burning may have an acidifying effect on rainwater which can cause problems to human health, buildings, and crops (Crutzen and Andreae 1990; Cachier and Ducret 1991). South East Asia (SEA) is a region that has been severely affected by haze from biomass burning in recent years (Norela et al. 2013; Othman et al. 2014; Radojevic 2003). Thus, air quality study in SEA is important to understand the sources and impacts on earth’s ecosystem.
Tropical Asia has been recognized to be the highest biomass burning region in Asia and has experienced some of the most severe wild land fire events under extreme climatic conditions (Page et al. 2002). Chang and Song (2010) estimated the average annual burned area in tropical Asia to be 85,277 km2 year−1, releasing 122 Tg year−1 of CO2, and 0.63 Tg year−1 of CH4. In SEA, haze events normally occur during the southwest monsoon (June to August) (Mahmud 2009) and from January to February (springtime haze) every year. Fire hotspots in the Indo-China peninsula, including mainland territory of Thailand, Laos, Cambodia, Myanmar, Vietnam, and Malaysia, are known to contribute substantial haze to the region (Chang and Song 2010; Khan et al., 2015a,b Khan et al. 2016; Lin et al. 2009). The occurrence of these hotspots in the region is primarily attributed to slash and burn agricultural practices and land clearing activities, especially during dry weather conditions.
Wet precipitation after a haze event plays an important role in scavenging soluble components and suspended particulates from the atmosphere. Depending on the source and ambient atmospheric chemistry, rainwater after a haze event often displays an acidic character (Balasubramanian et al. 1999; Radojevic and Tan 2000). Previous published works have been focused on the August–September haze, but few studies have discussed the effects of the March–April haze on rainwater chemistry in the SEA (Budiwati et al. 2016). Therefore, it is the purpose of this study to investigate the effects of haze during the northeast monsoon on the rainwater chemistry in Malaysia.
Methodology
Sample collection
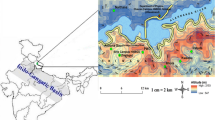
Wet-only deposition (rainwater) samples were collected from 16 March to 15 April 2014 on the rooftop (height ∼10 m above ground) of the Faculty of Science and Technology, National University of Malaysia (UKM) (2°55′20.23″N, 101°46′48.80″E). Samples were collected after a prolonged drought and haze period. The details of the sampling station are described in a previous air study by Khan et al. (2015c). The sampling station is located in Bangi, approximately 25 km south of Kuala Lumpur city center and 35 km east of heavy industrial districts such as Shah Alam and Klang. The Titiwangsa mountain range and a large area of forest reserve are located to the eastern side of the sampling point. Rainwater samples were collected using a 20-cm diameter polyethylene funnel connected to a 2-L high-density polyethylene (HDPE) receiving bottle during each rain event. The rainwater samples were collected from the beginning of rain until the end for each event. After each precipitation event, the funnels and collection bottles were removed and replaced with others that had previously undergone the washing procedure (Al-Momani 2003) (Fig. 1).
Sampling location at UKM campus, Bangi (Khan et al. 2015c)
Laboratory analysis
After collection, the rainwater samples were immediately measured for pH. pH values of the rainwater samples were measured using a Thermo Fisher Scientific ORION (USA) pH probe (9157BNMD). Rainwater samples were acidified using analytical-grade concentrated nitric acid as a preservation measure, and then the heavy metals (Al, Cu, Fe, Mn, Ni, Pb and Zn) and major elements (Ca, Mg, Na, and K) were analyzed using ICP-MS (Perkin Elmer ELAN 9000, USA). Un-acidified samples were used to determine the major ions using ion chromatography (ICP) (Metrohm Pro 850, Switzerland). Metrosep C4 100 and Metrosep A Supp 5 columns were used to detect the anions (Cl−, NO3 −, and SO4 2−). To better understand the contribution of suspended particles in the atmosphere on rainwater chemistry, we collected solid phase particles associated in the first and final rain events and analyzed for its morphology and chemical composition. For the examination of suspended particles in rainwater, 100 ml of the sample was filtered (using a vacuum pump) through a 0.22-μm Whatman cellulose membrane. The solid phase materials with particle size >0.22 μm that were retained on the surface of filter paper were then air-dried and pre-coated with a thin layer of Au before examination by scanning electron microscopy (SEM) (LEO 1450VP, Germany) fitted with energy-dispersive X-ray spectroscopy (EDS) (Oxford, UK) for visual and chemical validation.
Quality control and quality assurance (QA/QC)
As part of the QA/QC procedures, the chemical analysis of all samples was carried out in duplicates. The pH meter was calibrated with standard solutions of pH 4.0 and 7.0 prior to sample measurement. All collection bottles and glassware involved in the rainwater sampling were acid-soaked (0.1 M HNO3) and rinsed with deionized water prior to oven-drying. Subsequently, the bottles were sealed and stored in a dry and clean environment to avoid potential contamination. A sample blank was also measured during the elemental analysis. Perkin Elmer Quality control standard 3 was used as a multi-element standard solution. Instrumental calibration was carried out prior to each analysis. Other precautionary measures for water analysis were adopted based on standard methods described in APHA (1995). The method detection limits (MDL) for ICP-MS were 1.51 (Al), 0.21 (Cu), 0.31 (Mn), 0.09 (Ni), 0.12 (Pb), 0.12 (Zn), 0.17 (Fe), 60 (Ca), 10 (Mg), 40 (Na), and 10 μg L−1 (K). For ion chromatography, the MDL were 30 (NO3 −), 20 (Cl−), and 110 μg L−1 (SO4 2−). The MDL was calculated by dividing the standard deviation (SD) of the response by the calibration slope (S) and multiplied by 3.3 as shown in Eq. (1):
where MDL = method detection limit
SD = standard deviation of the result values
T = student’s t value appropriate for 99% confidence interval which has the value of 3.3
Statistical analysis
Two types of statistical analyses, one-way analysis of variance (ANOVA) and hierarchical agglomerative clustering analysis (HACA), were used in this study. ANOVA was used to determine the significant difference among the heavy metals and major ions. HACA was used to classify and determine the possible sources of pollutants deposited in the rainwater. These methods have been widely used in classifying and determining the sources for major ions and heavy metals in the atmosphere (Alahmr et al. 2012; Báez et al. 2007; Han et al. 2014; Kazi et al. 2009).
Enrichment factors
Enrichment factors (EFs) were used to determine whether the pollutants in the rainwater originated from natural or anthropogenic sources. This indicator has been widely used for similar purposes by Al-Momani (2003), Hu et al. (2003), Latif et al. (2015), and Razak et al. (2015). EFs were calculated using Eq. (2):
where C x is the concentration of the pollutant of interest, and C ref is the concentration of the reference pollutant (Taylor 1964). Aluminum (Al) was used as the reference pollutant in this study, as the primary source of Al is from soil (i.e., it is crustal in nature). The reason behind this is because it has low spatial variability and stable chemical properties as mentioned in Hu et al. 2014. However, Fe also has been reference pollutant used in previous studies (Baptista et al. 2000; Taylor and McLennan 1995). Parallel to Fe, Al is also widely cited as the reference pollutant used in calculation of EFs (Huang and Lin 2003; Hong et al. 2011). EF values can be classified into several categories: (i) an EF approaching 1 shows the element is of crustal origin, (ii) an EF <2 shows a low enrichment, (iii) an EF = 2–5 shows a moderate enrichment, (iv) an EF = 5–20 shows a significant enrichment, and (v) an EF = 20–40 shows an extremely high enrichment (Khan et al. 2010; Lu et al. 2009; Yongming et al. 2006).
Effect of biomass fire hotspots and HYSPLIT back trajectories
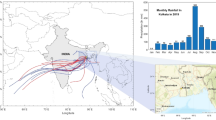
Figure 3 shows the locations of biomass fire hotspots coupled with the synoptic scale wind vector on the 15, 16, and 17 March 2014. The concentrations of the pollutants in rainwater were relatively high or maximum on those dates. Similarly, the highest value of the air pollutants index (API) measured during the sampling period was recorded on 16 March 2014. API is an indicator air pollution level in Malaysia based on the highest sub-indexes of main criteria air pollutants (PM10, CO, SO2, NO2, and O3). During haze event, high API, which is usually represented by PM10 sub-index (Amil et al. 2016), indicates high concentration of particulate matter in the atmosphere, which may contribute to the high level of heavy metals in the rain water. The wind speed was relatively higher over the South China Sea and transported the air mass towards the inland of Peninsular Malaysia. The locations of the fire hotspots were observed more in the Indo-China region compared to the Sumatra and Kalimantan regions of Indonesia. Thus, the wind sector and number of hotspots affect the concentration of pollutants in ambient air. Other studies also reported higher numbers of biomass fire hotspots in the Indo-China region during the northeast monsoon in Malaysia (Chang and Song 2010; Khan et al., 2015a, b, c; Mustaffa et al. 2014). Air mass back trajectories (BTs) were calculated using the online version of the hybrid single particle Lagrangian trajectory model (HYSPLIT) ( http://ready.arl.noaa.gov/HYSPLIT.php ) (Stein et al. 2015). During the estimation of back trajectories, the main input parameters were 120 h total transport of air mass, 500 m as releasing height, and 6 h as trajectory intervals. The results of the BT showed that the air mass was mainly originated from the South China Sea and inland of Indo-China region (Fig. 4).
Results and discussion
Variation of pH
The pH of rainwater collected in Klang Valley from 16 March 2014 to 15 April 2014 varied from 3.67 to 6.54 as shown in Table 1. The pH of rainwater on the first raining event was less acidic with a value of 6.54. The neutralization effect largely explains the relatively higher rainwater pH during the first rain, which mainly arises from biomass burning and soil mineral particles suspended in the atmosphere. Biomass burning and soil mineral particles are commonly known to contribute a substantial amount of Ca and Mg that may act to neutralize the rainwater acidity (Balasubramanian et al. 1999; Radojevic 2003; Sanhueza et al. 1989). This explanation is consistent with the high Ca and Mg concentrations observed in the first rain. However, it should be mentioned that besides alkaline mineral dusts (CaCO3), ammonia (NH3) might also play a considerable role in the neutralizing of rainwater (Balasubramanian et al. 1999; Das et al. 2005). To distinguish the relative neutralization of rainwater by crustal components and ammonia, the neutralization factors (NFs) were calculated. NFs were calculated as explained by Saxena et al. (1996) and Possanzini et al. (1988):
From the calculation, the NFNH4 + was less than both NFCa 2+ and NFMg 2+, where the factors were calculated as 0.10 for NH4 +, 3.56 for Ca2+, and 0.31 for Mg2+. This suggests that the crustal components (soil mineral particles) can neutralize a larger fraction of the available acid in the first rain. The presence of soil mineral dusts and its geochemical composition in the first rainwater samples were further visualized under a scanning electron microscope which will be further explained later in the text.
To assess the balance between acidity and alkalinity, the ratio of acidifying potential (AP) to neutralizing potential (NP) was calculated using Eq. (6):
A ratio of less than one and ranging from 0.04 to 0.60 indicates that the NP dominates the AP, in which the dominance of alkaline constituents prevents the acidification of rainwater as explained by Tay et al. (2014). The mean concentrations of these ionic components generally increase with increasing pH value. Such increase is especially notable for Ca2+. This trend is usually expected for the alkaline cations but not for the anions. Based on the assumption that the acidity of the precipitation arises from the presence of H2SO4 and HNO3, one would expect the concentrations of sulfate and nitrate ions to be highest, leading to the lowest pH. This could partially be explained by the extremely high concentrations of calcium which appear to have a neutralizing effect on acidity. This neutralizing effect had also been observed earlier (Gorham 1976; Saxena et al. 1996; Xu and Han 2009). Acidic precursors such as sulfate and nitrate ions can be partially neutralized by alkaline substances.
In the majority of the rain events at our sampling site, most of the sulfate and nitrate were not associated with hydrogen ions. Instead, they occurred as salts associated with other elements (e.g., MgSO4, CaSO4). While some of the measured free hydrogen ions were no doubt donated by strong acids of sulfate and nitrate, weak and organic acids could have accounted for the major portion. Possible explanations are that (i) the sampling site may be receiving alkaline dust such as from limestone quarries in the region and, thus, neutralization could have taken place and (ii) as the sampling site is not in the vicinity of severe pollution sources, air masses appear to have a long atmospheric residence time in which to undergo chemical reactions before deposition in a rain event (Lee and Low 1984).
The Malaysian API ranges from good (0–50) to moderate (51–100), unhealthy (101–200), very unhealthy (201–300), and hazardous (greater than 300) (Othman et al. 2014).
Main compositions in the collected rainwater
Ions and heavy metals in rainwater
The first sample of rainwater was collected on the first day of rain during the haze episode in Klang Valley. Table 2 shows the descriptive statistics of the ions, heavy metals, and major element compositions in 12 samples of rainwater collected during the 2014 haze episode in Klang Valley. The ionic average concentrations in rainwater were in the order of NO3 − (1500 ± 1090 μg L−1) > SO4 2− (1940 ± 1150 μg L−1) > Cl− (380 ± 190 μg L−1). The concentrations of SO4 2− ranged from 510 to 4230 μg L−1. For Cl− and NO3 −, the concentrations ranged from 50 to 620 μg L−1 and from 630 to 3890 μg L−1, respectively (Table 2). High concentrations of heavy metals were measured in rainfall events after period of dryness in March 2014 and experienced a gradual drop in the subsequent rainfall events (Fig. 2). Previous studies conducted in Malaysia by Norela et al. (2013), Latif et al. (2015), and Razak et al. (2015) showed that SO4 2− was the dominant anion in rainfall. These findings are similar to those of Fujii et al. (2016). They reported SO4 2− was at 23.0 to 55.9 wt% of total ions in PM10 over UKM campus which is similar location to this study. SO4 2− can originate from various possible sources including agricultural activities (Tay et al. 2014; Fujii et al. 2016, Das et al. 2005). Previous studies reported that SO4 2− found in sugar marker such as levoglucosan was largely originated from peat fires (Kehrwald et al. 2012; Zhang et al. 2010; Fujii et al. 2016).
Long-range transport of aerosols from industrial activities, motor vehicles, and biomass burning can also contribute to the amount of SO4 2− in this study area. Cl− is expected to be present in the rainwater mainly due to the transport of sea spray from the marine region towards the land (Budhavant et al. 2012), especially at the time of sampling due to the movement of wind from the northeast (South China Sea) direction. The low concentration of NO3 − may be attributed to the lower contribution of vehicle emissions to the study area, and/or the uptake of nitrate aerosol was lower in this rain event.
The average concentrations of heavy metals in the rainwater followed the decreasing order of Zn > Fe > Al > Mn > Cu > Pb > Ni (Table 2). The highest average concentration observed was Zn with an average value of 28.12 μg L−1 (±22.87 μg L−1). The lowest heavy metal in the rainwater was Ni with an average value of 1.56 μg L−1 (±1.17 μg L−1). Such a trend is similar to the order of heavy metal concentrations reported in Razak et al. (2015). In this study area, Zn, Pb, and Ni could be potentially originated from biomass burning emissions and anthropogenic sources such as heavy industrial emissions and traffic emissions nearby UKM campus. These metals can also be derived from traffic emission source (Bove et al. 2014). The anthropogenic source could include fuel combustion from motor vehicles on the UKM campus, Bangi, or pollutants originated from cities in the east coast such as Terengganu (based on the HYSPLIT output). In addition, Amodio et al. (2014) summarized previous studies on heavy metals deposited in the rainwater at different regions of the world.
The elements Mn, Ni, Cu, and Pb were moderately enriched compared to the mean crustal elements indicating the presence of other sources contributing to these elements. The potential sources could be particles from automobile emissions, vehicle wear, and road dust as these sources can contribute to significant Cu, Pb, and Zn in rainwater (Wetang’ula and Wamalwa 2015). Behera et al. (2015) observed that the concentration of Zn was enriched when there is a contribution from biomass burning or wood combustion. Previous studies conducted by Gautam et al. (2013), Khan et al. (2015a), Khan et al. (2015b), and Khan et al. (2016) concluded that during March to April 2014, the Indo-China peninsular regions in SEA experienced wild land forest fires and agricultural crop burning and that forest conversion fires may be responsible for the intense haze episodes in Malaysia.
The major element average concentrations followed the decreasing order of Ca > K > Na > Mg. Previous studies conducted in Malaysia by Norela et al. (2013) and Norela et al. (2009) showed that the major elements that dominated the rainfall composition were K and Ca, respectively. The highest average concentrations of the major elements observed was Ca with a range of 120 to 1180 μg L−1 (±0.28) and the lowest observed was K with a range of 30 to 470 μg L−1 (±0.12). Huang et al. (2013), Khan et al. (2016), Mustaffa et al. (2014), and Tsai et al. (2013) also suggested that water-soluble K is a good indicator of biomass burning emission. The biomass burning emissions can be accounted for both local and regional sources as indicated by the number of hot spots and the trajectory analyses within the selected days (Fig. 3a–c). The trajectory analysis clearly showed the air mass passing through a hotspot area at Terengganu (east of the peninsular) before arriving at the Bangi area on 16 March 2014 (Fig. 4a–c).
The composition of suspended particles in rainwater determined by SEM-EDS analysis
All samples collected during the haze event were analyzed using SEM-EDS. The images of suspended particles in different rainwater events are shown in Fig. 5a, b. These particles were insoluble fraction composed of geogenic particles: mineral fragments; agglomerates of various compositions formed by resuspended dust derived from soils and rocks; and anthropogenic particles that include particles of varied forms and compositions, originating mainly from combustion such as Zn and Mg. The chemical composition of the suspended particles collected on the first rain was in the order of Ca > Zn > Al > Na > K (Fig. 5a). We believe that the dust particles obtained from the first rain have been suspended in the atmosphere for an extended time period during the drought. The dusts were composed of soil particles, with high calcium content which are able to neutralize rain acidity. Such neutralization effect is well observed in the pH on the first day of rain event with a value of pH as 6.54 (Table 1). Figure 5b shows the 12th rainwater sample, where the chemical composition of particles collected was mainly carbon and quartz. Other elements such as Mg and Zn are expected to have washed away (scavenged) during the previous rain events. Therefore, the results obtained in this analysis suggest that Zn is likely to be derived from anthropogenic sources rather than from soil dust. Besides, it is worth mentioning that both solid particles collected from the 1st and 12th rainwater events comprised distinct morphology, confirming that these solid particles are of different origins.
Identification of sources of the rainwater composition
The source identification of the rainwater composition was conducted using enrichment factor (EF), correlation analysis, and hierarchical agglomerative clustering analysis (HACA). Detailed description is given in the following sections:
Enrichment factors
The results of the EF for each element contained in rainwater are shown in Table 3. This study revealed that the heavy metals in the rainwater samples were derived both from anthropogenic and natural sources (the Earth’s crust). Fe, Ca, Mg, Na, and K originated from the Earth’s crust and Cu, Mn, Ni, Pb, and Zn originated from anthropogenic sources. The EF values for elements originating from the Earth’s crust were within the range 0.01 to 1.75. Zn recorded the highest average EF value of 1974, and Mg recorded the lowest value of 0.01. The decreasing pattern of average EF values recorded in this study is Zn > Pb > Mn > Cu > Ni > Fe > K > Ca > Na and Mg.
The EF values for Zn recorded in the study by Latif et al. (2015) were more than 16,000. Studies done by Kim et al. (2012) and Kyllönen et al. (2009) identified that Pb and Zn were originated from anthropogenic sources. A few studies showed different outcomes compared with this study. For example, the results obtained by Başak and Alagha (2004) and Mimura et al. (2016) illustrated that EF values for all the variables measured in the study were approaching 1 which implies that the variables originated from natural Earth’s crust sources. Uygur et al. (2010) also reported that variables such as Mg, K, Ca, Na, and Fe showed low EF values (<1.0). A study by Cheng and You (2010) revealed that the EF values for Pb, Zn, Na, Ni, and K are extremely high: Pb = 10,202,566; Zn = 27,921; Na = 4548; Ni = 3047; and K = 1008. Therefore, the results obtained in this study suggests that there is an extremely high probability of the Zn, Ni, Pb, Mn, and Cu originating from anthropogenic activities rather than desorption from soil dusts.
Correlation between major ions and heavy metals
Correlation analysis was carried out on the major elements and heavy metals to determine the relationship between elements in the collected rainwater (Table 4). The highest correlation was found between Ca and Mg (r 2 = 0.91; p < 0.01), suggesting both elements were derived from a common source. Mn exhibits the strongest positive correlation with SO4 2− (r 2 = 0.87, p < 0.01) while Zn showed the weakest positive correlation (r 2 = 0.10, p < 0.05). Mg and Na showed the strongest correlation with an r 2 value of 0.87 (p < 0.01). This is similar to the results found by Latif et al. (2015) in dust fall (r 2 = 0.96, p < 0.01). For the relationship between heavy metals, it can be clearly seen in Table 3 that Fe and Ni showed the moderate significant correlation (r 2 = 0.75, p < 0.01). Satsangi et al. (1998) found a positive correlation between SO4 2− and NO3 − and suggested these may be derived from soil associated with Ca, Mg, and Na during the monsoon in Gopalpura, India. Such finding contradicts with the results in the present study where the SO4 2− and NO3 − associated with Ca, Mg, and Na were found to be weakly correlated (∼r2 < 0.5, p > 0.01), suggesting that both SO4 2− and NO3 − (anions) and Ca, Mg, and Na (elements) were derived from distinct sources.
Norela et al. (2013) suggested that the main sources of Mg, Mn, and Zn in rainwater could be of natural origin. Strong correlations between Mg, Mn, and Zn were found by Norela et al. (2013) during the July to September haze in Peninsular Malaysia (mostly r 2 > 0.8, p < 0.01). The correlation values between metals in this study with p < 0.01 were Mn and Mg (r 2 = 0.66), Pb and Mg (r 2 = 0.15), Zn and Mg (r 2 = 0.64), Pb and Mn (r 2 = 0.74), Zn and Mn (r 2 = 0.76), and Zn and Pb (r 2 = 0.60), suggesting that haze during the northeast monsoon could be from both natural and anthropogenic sources. Based on Table 4, it can be seen that the heavy metals and major ions in the rainwater was influenced by anthropogenic sources such as vehicle emissions and biomass burning.
HACA clustering patterns
The dendrogram produced by HACA showing the distribution of the variables is depicted in Fig. 6. From the result, group 1 consists of Ca, Mg, and Na, whereas group 2 consists of Pb, NO3 −, Cu, Zn, Mn, and SO4 2− and group 3 includes Ni, Fe, and K. The classification of each of the variables into the same group is most likely because of the similar characteristics in terms of their sources. Variables in group 1 are correlated strongly to each other (r > 0.82) (Table 3). The EF values for these variables range from 0.01 to 0.05 which indicates that the sources of Ca, Mg, and Na are likely to be natural Earth’s crust sources. Variables in group 2 also show a similar pattern as they have strong correlations with each other (Table 3). In terms of EF values, these variables recorded values that were more than 200, suggesting these variables shared a common anthropogenic source. Group 3 is a mixed group where, based on the EF values, the sources of these variables are from both natural Earth’s crust and anthropogenic sources. Only Ni and Fe show strong correlations in this group. Ni and Fe can be originated from industrial emissions. While, K can be originated from both anthropogenic such as fertilizers (Reimann and de Caritat 1998) and natural sources such as clay minerals.
Thus, the results of correlation and HACA reasonably explained the co-existence of Ni and Fe in group 3. However, it should be noted that the outcome of EF differs with the results from correlation analysis and HACA. Further works including analysis of dry precipitation is needed to examine the effects and relationships of dust fall on the chemical composition of rainwater. Long-term data collection and monitoring are needed to arrive at meaningful valuations and correlations between differences in composition over time and the type of meteorological events.
Conclusions
The monitoring results of pH demonstrated that the rainwater was acidic in nature, except the first rain after a long period of dry spell. The anthropogenic input of trace gases such as SOx and NOx might be the main reason to explain the acidic rain as the location of the sampling site was in close proximity to urbanized areas. Further, the results of pH and NF in the rainwater samples showed that the pH value switch considerably from near neutral (pH 6.54) to acidic (<pH 4.00) during the haze to non-haze transitional period, suggesting that the removal of sulfate and nitrate via the precipitation process was relatively higher than the removal of the mineral components. The NF suggests that the crustal components (soil mineral particles) can neutralize a larger fraction of the available acid in the first rain. The results of the rainwater compositions showed that the most dominant anion recorded was NO3 − followed by SO4 2− and Cl−. The trend in the average concentrations of heavy metals in the rainwater was Zn > Fe > Al > Mn > Cu > Pb > Ni. The highest average concentration reported for Zn was 28.12 μg L−1 (±22.87 μg L−1) while the major elements followed an order of Ca > K > Na > Mg. The results of the scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDS) analysis indicated that Zn, Al, and Mg were possibly emitted from a similar anthropogenic origin. The source identification by EF, correlation, and HACA demonstrated nearly similar characteristics of Ca, Mg, and Na. The BT model and number of biomass fire locations showed that pollutants were transported from the mainland of Indo-China and marine region in the South China Sea. This study recommends a need for long-term monitoring of rainwater chemistry in the region especially during haze event.
References
Alahmr FOM, Othman M, Wahid NBA, Halim AA, Latif MT (2012) Compositions of dust fall around semi-urban areas in Malaysia. Aerosol Air Qual Res 12:629–642
Al-Momani IF (2003) Trace elements in atmospheric precipitation at northern Jordan measured by ICP-MS: acidity and possible sources. Atmos Environ 37:4507–4515
Amil N, Latif M T, Khan M F, Mohamad M (2016) Seasonal variability of PM2.5 composition and sources in the Klang Valley urban-industrial environment. Atmos Chem Phys 16:5357–5381
Amodio M, Catino S, Dambruoso PR, de Gennaro G, Di Gilio A, Giungato P, Laiola E, Marzocca A, Mazzone A, Sardaro A, Tutino M (2014) Atmospheric deposition: sampling procedures, analytical methods, and main recent findings from the scientific literature. Adv Meteorol 2014:161730, 27 pages. doi:10.1155/2014/161730
APHA (1995) Standard methods for the examination of water and wastewater. American Public Health Association, 19th edn. American Public Health Association, Washington, D.C
Báez A, Belmont R, García R, Padilla H, Torres MC (2007) Chemical composition of rainwater collected at a southwest site of Mexico City, Mexico. Atmos Res 86:61–75
Balasubramanian R, Victor T, Begum R (1999) Impact of biomass burning on rainwater acidity and composition in Singapore. Journal of Geophysical Research: Atmospheres 104:26881–26890
Baptista NA, Smith BJ, Mcallister JJ (2000) Heavy metal concentrations in surface sediments in a nearshore environment, Jurujuba sound, SE Brazil. Environ Pol 109:1–9
Başak B, Alagha O (2004) The chemical composition of rainwater over Büyükçekmece Lake, Istanbul. Atmos Res 71:275–288
Behera SN, Betha R, Huang X, Balasubramanian R (2015) Characterization and estimation of human airway deposition of size-resolved particulate-bound trace elements during a recent haze episode in Southeast Asia. Environ Sci Pollut Res 22:4265–4280
Bove MC, Brotto P, Cassola F, Cuccia E, Massabò D, Mazzino A, Piazzalunga A, Prati P (2014) An integrated PM2.5 source apportionment study: positive matrix factorisation vs. the chemical transport model CAMx. Atmos Environ 94:274–286. doi:10.1016/j.atmosenv.2014.05.039
Budhavant K, Rao P, Safai P, Gawhane R, Raju M, Mahajan C, Satsangi P (2012) Atmospheric wet and dry depositions of ions over an urban location in south-West India. Aerosol Air Qual Res 12:561–570
Budiwati T, Setyawati W, Tanti DA (2016) Chemical characteristics of rainwater in Sumatera, Indonesia, during 2001–2010. Int Journal of Atm Sciences 2016:1876046, 11 pages doi:10.1155/2016/1876046
Cachier, H., Ducret, J., 1991. Influence of biomass burning on equatorial rains. Nature 352: 228 to 230.
Chang D, Song Y (2010) Estimates of biomass burning emissions in tropical Asia based on satellite-derived data. Atmos Chem Phys 10:2335–2351
Cheng M-C, You C-F (2010) Sources of major ions and heavy metals in rainwater associated with typhoon events in southwestern Taiwan. J Geochem Explor 105:106–116
Crutzen PJ, Andreae MO (1990) Biomass burning in the tropics: impacts on atmospheric chemistry and biogeochemical cycles. Science 250:1669–1678
Das R, Das SN, Misra VN (2005) Chemical composition of rainwater and dustfall at Bhubaneswar in the east coast of India. Atmos Environ 39:5908–5916
Fujii Y, Mahmud M, Oda M, Tohno S, Matsumoto J, Mizohata A (2016) A key indicator of transboundary particulate matter pollution derived from Indonesian peatland fires in Malaysia. Aerosol Air Qual Res 16:69–78
Gautam R, Hsu NC, Eck TF, Holben BN, Janjai S, Jantarach T, Tsay S-C, Lau WK (2013) Characterization of aerosols over the Indochina peninsula from satellite-surface observations during biomass burning pre-monsoon season. Atmos Environ 78:51–59
Gorham E (1976) Acid precipitation and its influence upon aquatic ecosystems—an overview. Water Air Soil Pollut 6:457–481
Han NMiM, Latif MT, Othman M, Dominick D, Mohamad N, Juahir H, Tahir NM (2014) Composition of selected heavy metals in road dust from Kuala Lumpur city centre. Environmental Earth Sciences 72:849–859
Hong Y, Ma Y, Li C, Liu N, Gao S, Zhang Y (2011) Elemental size distribution characteristics of atmospheric particles on hazy days during winter in Shenyang. Res Environ Sci 24:637–644
Hu GP, Balasubramanian R, Wu CD (2003) Chemical characterization of rainwater at Singapore. Chemosphere 51:747–755
Hu Z, Wang J, Chen Y, Chen Z, Xu S (2014) Concentrations and source apportionment of particulate matter in different functional areas of Shanghai, China. Atmos Pollut Research 5:138–144
Huang K, Lin S (2003) Consequences and implication of heavy metal spatial variations in sediments of Keelung River drainage basin, Taiwan. Chemosphere 53:1113–1121
Huang K, Fu JS, Hsu NC, Gao Y, Dong X, Tsay S-C, Lam YF (2013) Impact assessment of biomass burning on air quality in Southeast and East Asia during BASE-ASIA. Atmos Environ 78:291–302
Kazi TG, Arain MB, Jamali MK, Jalbani N, Afridi HI, Sarfraz RA, Baig JA, Shah AQ (2009) Assessment of water quality of polluted lake using multivariate statistical techniques: a case study. Ecotoxicol Environ Saf 72:301–309
Kehrwald N, Zangrando R, Gabrielli P, Barbante C, Gambaro A (2012) Levoglucosan as a specific marker of fire events in Greenland snow. Tellus B 64:1–9
Khan MF, Shirasuna Y, Hirano K, Masunaga S (2010) Urban and suburban aerosol in Yokohama, Japan: a comprehensive chemical characterization. Environ Monit Assess 171:441–456
Khan F, Latif MT, Juneng L, Amil N, Mohd Nadzir MS, Syedul Hoque HM (2015a) Physicochemical factors and sources of particulate matter at residential urban environment in Kuala Lumpur. J Air Waste Manage Assoc 65:958–969
Khan MF, Latif MT, Amil N, Juneng L, Mohamad N, Nadzir MSM, Hoque HMS (2015b) Characterization and source apportionment of particle number concentration at a semi-urban tropical environment. Environ Sci Pollut Res 22:13111–13126
Khan MF, Latif MT, Lim CH, Amil N, Jaafar SA, Dominick D, Mohd Nadzir MS, Sahani M, Tahir NM (2015c) Seasonal effect and source apportionment of polycyclic aromatic hydrocarbons in PM2.5. Atmos Environ 106:178–190
Khan MF, Latif MT, Saw WH, Amil N, Nadzir MSM, Sahani M, Tahir NM, Chung JX (2016) Fine particulate matter in the tropical environment: monsoonal effects, source apportionment, and health risk assessment. Atmos Chem Phys 16:597–617
Kim J-E, Han Y-J, Kim P-R, Holsen TM (2012) Factors influencing atmospheric wet deposition of trace elements in rural Korea. Atmos Res 116:185–194
Kyllönen K, Karlsson V, Ruoho-Airola T (2009) Trace element deposition and trends during a ten year period in Finland. Sci Total Environ 407:2260–2269
Latif MT, Ngah SA, Dominick D, Razak IS, Guo X, Srithawirat T, Mushrifah I (2015) Composition and source apportionment of dust fall around a natural lake. J Environ Sci 33:143–155
Lee CK, Low KS (1984) An investigation of the chemical composition of precipitation in the and around Kuala Lumpur. Pertanika 7:43–51
Lin CY, Hsu HM, Lee YH, Kuo CH, Sheng YF, Chu DA (2009) A new transport mechanism of biomass burning from Indochina as identified by modeling studies. Atmos Chem Phys 9:7901–7911
Lu X, Wang L, Lei K, Huang J, Zhai Y (2009) Contamination assessment of copper, lead, zinc, manganese and nickel in street dust of Baoji, NW China. J Hazard Mater 161:1058–1062
Mahmud M (2009) Mesoscale model simulation of low level equatorial winds over Borneo during the haze episode of September 1997. Journal of Earth System Science 118:295–307
Mimura AMS, Almeida JM, Vaz FAS, de Oliveira MAL, Ferreira CM, Silva JCJ (2016) Chemical composition monitoring of tropical rainwater during an atypical dry year. Atmos Res 169:391–399
Mustaffa NIH, Latif MT, Ali MM, Khan MF (2014) Source apportionment of surfactants in marine aerosols at different locations along the Malacca Straits. Environ Sci Pollut Res 21:6590–6602
Norela S, Nurfatihah M, Maimon A, Ismail B (2009) Wet deposition in the residential area of the Nilai Industrial Park in Negeri Sembilan, Malaysia. World Applied Sciences Journal 7:170–179
Norela S, Saidah MS, Mahmud M (2013) Chemical composition of the haze in Malaysia 2005. Atmos Environ 77:1005–1010
Othman J, Sahani M, Mahmud M, Sheikh Ahmad MK (2014) Transboundary smoke haze pollution in Malaysia: inpatient health impacts and economic valuation. Environ Pollut 189:194–201
Page SE, Siegert F, Rieley JO, Boehm H-DV, Jaya A, Limin S (2002) The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature 420:61–65
Possanzini M, Buttini P, Dipalo V (1988) Characterization of rural area in terms of dry and wet deposition. Sci Total Environ 74:111–120
Radojevic M (2003) Chemistry of Forest fires and regional haze with emphasis on southeast Asia. Pure Appl Geophys 160:157–187
Radojevic M, Tan KS (2000) Impacts of biomass burning and regional haze on the pH of rainwater in Brunei Darussalam. Atmos Environ 34:2739–2744
Razak IS, Latif MT, Jaafar SA, Khan MF, Mushrifah I (2015) Surfactants in atmospheric aerosols and rainwater around lake ecosystem. Environ Sci Pollut Res 22:6024–6033
Reimann C, de Caritat P (1998) Chemical elements in the environment—factsheets for the geochemist and environmental scientist. Germany7 Springer-Verlag, Berlin ISBN 3-540-63670-6
Sanhueza E, Elbert W, Rondón A, Arias MC, Hermoso M (1989) Organic and inorganic acids in rain from a remote site of the Venezuelan savannah. Tellus B 41B:170–176
Satsangi GS, Lakhani A, Khare P, Singh SP, Kumari KM, Srivastava SS (1998) Composition of rain water at a semi-arid rural site in India. Atmos Environ 32:3783–3793
Saxena A, Kulshrestha UC, Kumar N, Kumari KM, Srivastava SS (1996) Characterization of precipitation at Agra. Atmos Environ 30:3405–3412
Stein AF, Draxler RR, Rolph GD, Stunder BJB, Cohen MD, Ngan F (2015) NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. B Am Meteorol Soc 96:2059–2077
Tay JH, Jaafar S, Mohd Tahir N (2014) Ionic composition of rainwater at selected sites of Kuantan, Pahang, Malaysia: a preliminary study. Bull Environ Contam Toxicol 92:329–333
Taylor SR (1964) Trace element abundances and the chondritic earth model. Geochim Cosmochim Acta 28:1989–1998
Taylor SR, McLennan SM (1995) The geochemical evolution of the continental crust. Rev Geophys 33:241–265
Tsai YI, Sopajaree K, Chotruksa A, Wu H-C, Kuo S-C (2013) Source indicators of biomass burning associated with inorganic salts and carboxylates in dry season ambient aerosol in Chiang Mai Basin, Thailand. Atmos Environ 78:93–104
Uygur N, Karaca F, Alagha O (2010) Prediction of sources of metal pollution in rainwater in Istanbul, Turkey using factor analysis and long-range transport models. Atmos Res 95:55–64
Wetang’ula, G.N., Wamalwa, H. M (2015) Trace elements in rainfall collected around Menengai Area Kenya, Proceedings World Geothermal Congress.
Xu Z, Han G (2009) Chemical and strontium isotope characterization of rainwater in Beijing, China. Atmos Environ 43(12):1954–1961
Yongming H, Peixuan D, Junji C, Posmentier ES (2006) Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci Total Environ 355:176–186
Zhang X, Hecobian A, Zheng M, Frank NH, Weber RJ (2010) Biomass burning impact on PM2.5 over the southeastern US during 2007: integrating chemically speciated FRM filter measurements, MODIS fire counts and PMF analysis. Atmos Chem Phys 10:6839–6853
Acknowledgements
We would like to thank Universiti Kebangsaan Malaysia for the Research University Grants (GUP-2014-041 and GGPM-2013-080) and the Ministry of Education for the Fundamental Research Grant (ERGS/UM grant ER025-2013A). We would like to thank Mr. Ak. Jalaludin Pg. Awang Besar for assistance in the ICPMS analysis and Mr. Jing Xiang Chung for providing the hot spot and wind direction maps. The authors gratefully acknowledge the NOAA Air Resources Laboratory (ARL) for the provision of the HYSPLIT transport and dispersion model and/or READY website (http://www.ready.noaa.gov) used in this publication. Finally, we also would like to thank Dr. Rose Norman and Dr. Andrew Robinson for proofreading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerhard Lammel
Rights and permissions
About this article
Cite this article
Nadzir, M.S.M., Lin, C.Y., Khan, M.F. et al. Characterization of rainwater chemical composition after a Southeast Asia haze event: insight of transboundary pollutant transport during the northeast monsoon. Environ Sci Pollut Res 24, 15278–15290 (2017). https://doi.org/10.1007/s11356-017-9131-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9131-1