Abstract
A short-term investigation on the chemical composition of rainwater was carried out at five selected sampling stations in Kuantan district, Pahang, Malaysia. Sampling of rainwater was conducted by event basis between September and November 2011. Rainwater samples were collected using polyethylene containers and the parameters measured were cations (sodium, potassium, ammonium, calcium and magnesium) and anions (chlorides, nitrates and sulphates). The average pH value for rainwater samples was 6.0 ± 0.57 in which most of the sampling sites exhibited pH values >5.6. Calcium and sulphate were the most abundant cation and anion, respectively, whilst the concentrations of other major ions varied according to sampling location.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Wet deposition plays a vital role in removing gases and aerosols from the atmosphere. Chemical composition and pH of wet deposition actually reflects the composition of the atmosphere through which it falls. Rainwater is a form of wet deposition which formed when separate drops of water fall to the Earth’s surface from clouds, generally with a pH slightly under 6. Its composition is influenced by both natural and anthropogenic sources, and is a result of the incorporation of particles and air pollutants into cloud droplets (Hu et al. 2003). Since last several decades, the issue of acid rain has received great attention in the international community due to its notable direct adverse effects on ecosystem and indirect effects on human health. Anthropogenic SO2, NOx and other acid precursors are the primary cause of acidic rain although the final acidity of the wet deposition is also influenced by neutralizing components like ammonia, carbonates and bicarbonates (Das et al. 2005).
Systematic investigations on the chemical composition of rainwater has been carried out extensively in both rural-continental and urban areas, especially in Europe and North America. Acid deposition has been reported in urban cities within the Asia region including Singapore, Hong Kong and South Korea due to the rapid growth in population, industrialization, transportation and energy consumption (Tanner 1999; Lee et al. 2000; Kulshrestha et al. 2003). Several studies in Malaysia have provided assessment of rainwater composition mainly on the west coast of Peninsular Malaysia (Ayers et al. 2002; EANET 2013) as well as East Malaysia (Sumari et al. 2009; EANET 2013). So far, there has been little investigation about rainwater composition in the east coast region. The opening of East Coast Expressway in 2004 and the planned development corridor under the East Coast Economic Region (ECER) has accelerated the growth of east coast states of the Peninsular Malaysia, especially Kuantan city, the largest city in ECER and act as the capital of the Pahang state. The study area focused on a major road connecting the urban and rural area of Kuantan city, with interchange to East Coast Expressway and Tun Razak Highway. An attempt has been made to characterize the ionic compounds in the rainwater at selected sites along the major trunk road, in order to gain an initial understanding of its rainwater composition.
Materials and Methods
Kuantan (3°49′00″N 103°20′00″E) is one of the rapid growing cities in east coast of Peninsular Malaysia, located 250 km away from the federal capital of Kuala Lumpur. It has population approximately 608,000, making it the 9th largest city in Malaysia. Rapid industrialization and urbanization in the last 10 years has created fast economic growth in Kuantan and resulted in tremendous increase of populations and vehicles. Currently Kuantan comprises a wide range of land uses such as traffic, industry, business, residence, garden and tourism, implying different patterns of activities and their possible impacts on air quality.
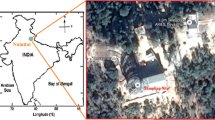
Rainwater was sampled at 5 different locations in Kuantan ( Fig. 1). The detail of each sampling locations were showed in Table 1. A total of twenty-five rainwater samples (average 5 samples per site) were collected from September to November 2011 on event basis. Rainwater was collected manually from the beginning of each rain event using polyethylene open container with 26 cm diameter and 14 cm depth. Prior to be used, polyethylene open container was rinsed with deionized water. The open container was placed at least 4 feet from the ground at an open area without obstructions. After each rainfall, rainwater was transferred into polyethylene bottles and sent to laboratory as soon as possible to prevent contaminations from dry deposition.
The pH values of the collected samples were measured using Hanna Instrument HI98129 pH meter equipped with a combination glass electrode. Major anions were analyzed by METROHM 881 Compact Ion Chromatograph (IC) Pro with 858 autosampler, equipped with 100 mm/4.0 mm Metrosep Dual 3 Suppresed anion column with Na2CO3 and NaHCO2 as eluent. The injection volume was 10.0 μL and flow rate was 0.80 mL/min. The detection limits of Cl−, NO3 − and SO4 2− ions were found to be 0.07, 0.07 and 0.04 ppm, respectively. Major cations were analyzed by IC equipped with 100 mm/4.0 mm Metrosep C2 column with 1.7 mM Nitric acid and 0.7 mM Pyridine dicarboxylic acid as eluent. The injection volume was 20.0 μL and flow rate was 0.90 mL/min. The detection limits of Na+, NH4 +, K+, Ca2+ and Mg2+ ions were found to be 0.02, 0.09, 0.01, 0.06 and 0.09 ppm, respectively. Analysis of blanks showed that contamination during the sampling procedure, transportation and analysis was negligible.
Results and Discussion
Generally pH value for rainwater ranged from 5.35 to 6.65, with a mean value of 6.00. Stations with pH values above 6.0 suggested certain inputs of alkaline species into the precipitation. Ca2+ was the most abundant ion in most of the sampling sites with an average concentration of 50.3 μeq/L, which might come from the natural source through crusting processes and wind-blown soil (Kulshrestha et al. 2003; Okay et al. 2002). For anion species, SO4 2− has the highest proportion (2 %–15 %) while NO3 − is the second highest anion species accounting for 2 %–13 % of total ions. This might due to emission from fossil fuel combustion.
The mean concentrations of major ions in rainwater at each station are showed in Table 2. The concentrations of Ca2+ measured in this study are higher than those reported for Korea (Lee et al. 2000), Hong Kong (Tanner 1999), Singapore (Hu et al. 2003) and a few cities in Malaysia (EANET 2013; Sumari et al. 2009) and lower than those reported for Guangzhou (Cao et al. 2009), Chengdu (Wang and Han 2011) and Jordan (Al-Khashman 2009). For marine elements (Na+ and Cl−), concentrations were much lower than Singapore (Hu et al. 2003), Hong Kong (Tanner 1999) and Jordan (Al-Khashman 2009), due to the close proximity of their sampling site to the sea. The relatively low concentration of NH4 + observed might be due to the low levels of fertilizers uses in the studied area. Anionic composition was found to be statistically different between stations (p < 0.05), in which the inland stations (Station 1 and 2) exhibited relatively higher concentration of Cl−, NO3 − and SO4 2−. However the values were still in the range of those reported by EANET (2013) in Malaysia. Higher concentrations of NO3 − and SO4 2− at Station 1 and 2 are probably due to their geographic location near the main interchange to Tun Razak Highway (Segamat-Kuantan Highway) and East Coast Expressway.
Relationship between ionic species was determined by correlation analysis (Table 3). Na+ ion shows negative correlation with other ionic species, which is contradict with literature (Hu et al. 2003; Cao et al. 2009; Wang and Han 2011; Al-Khashman 2009). However, negative correlation coefficient value (−0.004) between Na+ and Cl− ions had been reported by Ramírez Lara et al. (2010). Chlorine ions that showed negative correlation with Na+, are better correlated with NH4 +, K+, SO4 2− and NO3 − (r = 0.77, 0.86, 0.86, 0.74), respectively. This suggested that the origin of Cl− might be from mixed natural and industrial sources (Samara et al. 1992). Strong correlation between NO3 − and SO4 2− (r = 0.98) might be due to anthropogenic sources and agricultural activities of the studied area, resulting in accumulation of these ions in the upper atmosphere being washed down with rains during the rainy season. NH4 + has relatively higher correlation coefficients with Cl−, NO3 − and SO4 2− (r = 0.77, 0.76, 0.83), respectively. This phenomenon can be explained by reaction of ammonia with sulphuric acid and nitric acid to form (NH4)2SO4 and NH4NO3 aerosols in the atmosphere (Okay et al. 2002).
In order to estimate the marine and non-marine contributions of ionic species into rainwater, enrichment factors (EFs) have been calculated (Table 4). In this case, Na+ has been taken as reference element by assuming that all sodium is of marine origin (Kulshrestha et al. 2003). An EF value much smaller than 1 or much greater than 1 is considered concentrated or diluted relative to the reference source (Okay et al. 2002). Results showed that most of the components are enriched except sea salt. EFsea values for Cl− less than 1 indicated the presence of sodium of terrigenic origin (Sanusi et al. 1996). EFsea values for SO4 2− closed to 1 at Station 3 and 5 suggested significant marine contribution. However at rural site (Station 1), Station 2 and Station 4, the EFsea values were larger and thus the marine contribution is lower in these sites. Higher levels of SO4 2− at Station 1 and 2 could be explained by their geographic location nearby East Coast Expressway and Tun Razak Highway that connects the three eastern states to Kuala Lumpur in the west and Johor in the south, respectively. As expected, EFsea of Ca2+ in all stations ranges from 64.9 to 441, suggesting that most of the calcium came from soil. Highest concentration of Ca2+ is found in Station 4 which is located near to airport and the Royal Air Force base. Construction activities at Jaya Gading area and large turbulences generated by departure and landing of aircraft enhanced the spreading of aerosols in the atmosphere.
The role of NH4 +, Ca2+ and Mg2+ in neutralizing rainwater pH has been validated by calculating the neutralization factors using the equations as follows:
where [X] = concentration of ion (x) of interest.
The high values of neutralization factor (NF) obtained for Ca2+ in all stations and NH4 + in Station 5 (Table 5) indicated that major neutralization has occurred due to the presence of Ca2+ and NH4 +, with Mg2+ playing only a minor role. In order to assess the balance between acidity and alkalinity, the ratio of acidifying potential (AP) to neutralizing potential (NP) was calculated by using equation (2) (Table 5). The ratio less than one in all the locations (ranging from 0.04 to 0.60) indicating the NP dominates the AP, in which the dominance of alkaline constituents had prevented acidification of rainwater.
In general, a preliminary study of the chemical composition of wet deposition was carried out in Kuantan, Pahang, Malaysia from September to November 2011. Results showed that the mean pH value for rainwater in the studied area is 6.00 with Ca2+ as the most dominant species in the rainwater. Neutralization of the rainwater acidity by ammonia and calcium occurred during the precipitation process. Average concentrations of ions were within the range of literature whilst the chemical composition is influenced by the geographical location and the main activities in the area.
References
Al-Khashman OA (2009) Chemical characteristics of rainwater collected at a western site of Jordan. Atmos Res 91(1):53–61
Ayers G, Peng L, Gillett R, Fook L (2002) Rainwater composition and acidity at five sites in Malaysia, in 1996. Water Air Soil Pollut 133(1–4):15–30
Báez A, Belmont R, García R, Padilla H, Torres MdC (2007) Chemical composition of rainwater collected at a south-west site of Mexico city, Mexico. Atmos Res 86(1):61–75
Cao YZ, Wang S, Zhang G, Luo J, Lu S (2009) Chemical characteristics of wet precipitation at an urban site of Guangzhou, south China. Atmos Res 94(3):462–469
Das R, Das SN, Misra VN (2005) Chemical composition of rainwater and dustfall at Bhubaneswar in the east coast of India. Atmos Environ 39(32):5908–5916
EANET (2013) Data Report on the Acid Deposition in the East Asian Region 2011. http://www.eanet.asia/product/datarep/datarep11/datarep11.pdf. Accessed 25 October 2013
Hu G, Balasubramanian R, Wu C (2003) Chemical characterization of rainwater at Singapore. Chemosphere 51(8):747–755
Kulshrestha U, Kulshrestha MJ, Sekar R, Sastry G, Vairamani M (2003) Chemical characteristics of rainwater at an urban site of south-central India. Atmos Environ 37(21):3019–3026
Lee BK, Hong SH, Lee DS (2000) Chemical composition of precipitation and wet deposition of major ions on the Korean peninsula. Atmos Environ 34(4):563–575
Okay C, Akkoyunlu BO, Tayanç M (2002) Composition of wet deposition in Kaynarca, Turkey. Environ Pollut 118(3):401–410
Panyakapo M, Onchang R (2008) A four-year investigation on wet deposition in western Thailand. J Environ Sci 20(4):441–448
Ramírez Lara E, Miranda Guardiola R, Gracia Vásquez Y, Balderas Rentería I, Bravo Álvarez H, Sosa Echverría R, Sánchez Álvarez P, Alarcón Jiménez A, Torres M, Kahl J (2010) Chemical composition of rainwater in northeastern México. Atmósfera 23:213–224
Samara C, Tsitouridou R, Balafoutis C (1992) Chemical composition of rain in Thessaloniki, Greece, in relation to meteorological conditions. Atmos Environ B Urban Atmosp 26(3):359–367
Sanusi A, Wortham H, Millet M, Mirabel P (1996) Chemical composition of rainwater in eastern France. Atmos Environ 30(1):59–71
Sumari SM, Darus FM, Kantasamy N, IzzaTaib N (2009) Compositions of rainwater and aerosols at global atmospheric watch in Danum Valley, Sabah. Malays J Anal Sci 13(1):107–119
Tanner PA (1999) Analysis of Hong Kong daily bulk and wet deposition data from 1994 to 1995. Atmos Environ 33(11):1757–1766
Wang H, Han G (2011) Chemical composition of rainwater and anthropogenic influences in Chengdu, southwest China. Atmos Res 99(2):190–196
Acknowledgments
Financial supports from the Faculty of Industrial Sciences and Technology, Universiti Malaysia Pahang through a Grant for final year project and UMP Research Grant (RDU110313) is kindly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tay, J.H., Jaafar, S. & Mohd Tahir, N. Ionic Composition of Rainwater at Selected Sites of Kuantan, Pahang, Malaysia: A Preliminary Study. Bull Environ Contam Toxicol 92, 329–333 (2014). https://doi.org/10.1007/s00128-014-1203-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1203-z