Abstract
Based on the ion chromatography method, the chemical characterization of rainwater (RW) samples collected over Srinagar (a location in central Himalaya) has been done during monsoon 2016 (MON-2016). The rainwater shows near acidic pH values ranging from 5.1 to 6.2 (average, 5.7 ± 0.6) during the study. The average ionic concentrations of 97 ± 10 μeq/1 were reported during MON-2016. Ca2+ has significantly high contribution of 24% as compared to NH4+ (18%), Na+ (9%), K+ (4%), and Mg2+ (3%) among cations, whereas Cl−, SO42−, and NO3− have contribution of ~ 15, 11, and 7%, respectively, among anions during chemical analysis. We have reported SO42−/NO3− ratio as 1.49, which shows contribution of 60 and 40% from SO42− and NO3− ions within the predicted limit of RW (H2SO4, 60–70%, and HNO3, 30–40%). Ca2+, Mg2+, and NH4+ have neutralization factors as 2.51, 0.37, and 2.01, respectively, due to the neutralization of acidic species in RW. The non-sea salt (NSS) contribution to total Ca2+, K+, and Mg2+ indicates the major contribution from crustal origin, whereas the NSS contribution to the total Cl− and SO42− was from the anthropogenic source. The principle component analysis (PCA) indicates that the first factor (i.e., natural sources, mainly dust and sea salts) has only ~ 9% variance. In contrast, the second factor (i.e., fossil fuel and biomass burning) has ~ 17% variance, and the third factor has 27% variance may be due to soil, agricultural, and biomass burning origin. The rest of the contributions are from mixed emission sources as well as by the transport of polluted air mass from the Indo-Gangetic Plain (IGP) and Punjab Rajasthan, Pakistan, and Afghanistan. This manuscript helps to understand the impact of crustal and anthropogenic sources in rainwater over the central Himalaya region of Uttarakhand.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The atmospheric aerosols are usually originated from natural and anthropogenic sources (Gautam et al., 2021a, b & 2022). However, aerosols can travel longer distances, diluted, and further removed from the atmosphere by the dry and wet depositions (Bisht et al., 2015, 2017; Budhavant et al., 2009; Das et al., 2010; Tiwari et al., 2012, 2016). Generally, rain, dew, fog, hail, and snow are the important method for wet deposition of air pollutants (APs), which has significant importance for the ecological, hydrological, and biogeochemical cycle, etc. The chemistry of rainwater (RW) indicates the quantity of natural and anthropogenic emissions and the addition of APs in the atmosphere (Obaidy & Joshi, 2006). It also helps to understand the acidification buffering nature of ions (Choi et al., 2008). The RW quality is derived from the components of anionic and cationic (Kulshrestha et al., 2003). RW samples contain cations, which have a vital role in the acidification below cloud scavenging activity in India (Bisht et al., 2017; Tiwari et al., 2012, 2016). RW samples are also rich in metals to influence the physicochemical and microbial behavior in the soils (Pandey, 2008). The presence of metals in the air can lead to serious health issues like carcinogenesis-induced tumor promotion as well as environmental issue degradation of groundwater quality and soil via wet deposition (Pandey & Pandey, 2009). The European and North American countries have witnessed the adverse impact of acid rain (Balasubramanian et al., 1999; Rodhe et al., 2002). The exponential growth in the emission of APs was observed in developing countries such as China, Pakistan, and India (Granat et al., 1996, 2001; Rodhe et al., 2002). The Himalayas are the youngest mountains with the pristine environmental conditions in the world. However, some studies have been conducted to evaluate the chemical composition of aerosol, size distribution, aerosol optical depth, and trace gas concentration, along with snow/ice chemical compositions (Liu et al., 2010; Srivastava et al., 2015). But RW’s chemical analyses are very few over the central, western, and eastern Himalaya, Himalayan region of India (Bisht et al., 2017; Roy et al., 2016; Tiwari et al., 2012). Recently, Ahmed et al. (2022) have analyzed the monsoonal RW samples based on major chemical parameters, viz., pH, electrical conductivity (EC), and ion (SO42−, NO3−, Br−, Cl−, F−, Mg2+, Ca2+, K+, NH4+, Na+, and Li+) analysis. Now to identify the potential source, they have performed principal component analysis, airmass back trajectory clusters, and potential source contribution function (PSCF) over mid-Brahmaputra plain during 2012 to 2018. Kumar et al. (2022) have collected the RW samples in the western Himachal Himalaya, India, and reported major cations were Ca2+ (43.10%) and Na+ (31.97%) and anions were Cl− (37.68%), SO42− (28.71%), and NO3− (23.85%) dominant species in rainwater.
According to the Indian Meteorological Department, the months of June, July, August, and September are usually considered as monsoon season (Attri et al., 2015). However, due to the availability of collected RW samples, we have considered July to September 2016 months’ data. In the various studies, researchers have used PCA and correlation matrix as power full tool to determine the potential contribution from aging sea salt, fossil fuel combustion, agriculture, dust, and biomass burning at various locations (Cerqueira et al., 2014; Cheng & You, 2010; Li et al., 2022; Moreda-Piñeiro et al., 2014; Pandey & Singh, 2012; Salve et al., 2011). In Uttarakhand, very few regular studies have been published so far (Bisht et al., 2017; Gautam et al., 2018).
The main objectives of the paper are to understand the chemical composition of RW at Srinagar, located in Alaknanda valley in the central Himalaya and to assess the impact of anthropogenic and natural emissions in RW samples. Further, this is to evaluate the potential sources by using air mass back trajectory and statistical analysis.
2 Material and Methods
2.1 Site Description and Background Meteorological Conditions
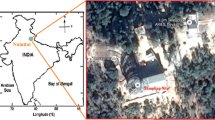
The Hemwati Nandan Bahuguna Garhwal University (HNBGU) Chauras campus (30.22°N 78.78°E, 560 amsl), Srinagar Garhwal, Uttarakhand, had been selected for observation site and having different climates as compared to plains. This observation site is situated on the national highway (NH)-58, and it is 132 km away from the main industrial site Haridwar. The NH-58 connects main tourist hotspots like Chamoli, Badrinath, and Gopeshwar. The vehicle movements are increased during the tourist season (summer and winter). The Srinagar is located on the bank of the Alaknanda River, which is approximately 560 m above sea level (Fig. 1). The Srinagar Garhwal valley is one of the widest valleys in the Garhwal Himalayan region. The observation location far from the major industries and only a small hydropower plant is located; therefore, the APs are transported to the sampling. Gautam et al. (2018) reported the significant contribution of mineral dust, and APs contained air mass from Thar Desert, Gulf countries specially pre-monsoon season. The regional transport activities also contribute to transport the pollutants from the Indo-Gangetic Plain (IGP) and have a potential impact on RW Chemistry (Ram et al., 2008, 2010; Srivastava et al., 2011, 2014). We have collected RW samples from July to September 2016 over the monitoring site. The organization of different work steps is also provided in Fig. 2.
Annually, Uttarakhand reports 1550 mm rainfall along with temperatures to range between 0 and 43 °C (FSI 2009). According to the Forest Survey of India (FSI) 2009 report, 64.79% of the state’s land is occupied forests (area = 34,691 km2). The background metrological parameters such as wind speed (m/s), wind direction (°), relative humidity (%), and temperature °C were measured using an automatic weather station (AWS), whereas RW samples were simultaneously measured over the station. Monthly variations of metrological parameters are depicted in Fig. 3. January 2016 was reported as the coldest month of winter with a mean temperature of 12.5 °C and starts rising to the month of June 2016 with a mean temperature of (30.5 °C). During summer 2016 and monsoon 2016, we have reported a consistent weather pattern and stats decreasing onward (Fig. 3). The relative humidity and wind speed were observed in the range of 38% (April) to 83% (August) and 5.8 km h−1 (December) to 16.56 km h−1 (June). The wind from the east–west direction is predominately reported during the study.
2.2 Sampling and Analysis
Thrifty seven (37) RW samples (total rainfall in 24 h) were collected during southwest summer monsoon 2016. RW sampling was performed above 10 m from the ground by using a polyethylene funnel of 20 cm diameter (Bisht et al., 2017; Tiwari et al., 2016). Ultrapure water was used to clean the rain collector two times (after and before sampling) to stop contamination of sample. Further, samples were filtered by using Whatman-41 and stored in the polypropylene sampling bottles, and 5 ml thymol solution was added for avid contamination. Further, the pH and EC of RW samples were determined by using the pH meter (Cyber Scan-540) and conductivity meter (Elico CM-183), and samples were stored at 4 °C for next ion chromatography. We have measured the cation and anion concentration as per the guidance of Tiwari et al. (2012) and Bisht et al. (2017). The HCO3− concentration was calculated theoretically based on pH and HCO3− ions (Parashar et al., 2001). If the pH > 5.6 (equilibrium with atmospheric CO2), then the HCO3− concentration (mol/l) can be calculated by [HCO3−] = 10−11.2+pH. The Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT) model, of NOAA Air Resources Laboratory (http://www.arl.noaa.gov/ready/ hysplit4.html), was used to understand the air movement at Srinagar at an altitude of 1000 (Datta et al., 2010; Draxler et al., 2014; Gautam et al., 2021a, b).
2.3 Data Quality
We have discarded the RW sample affected by bird droppings and control the quality of sampling by visual interpretation. The neutralizations of cation and anion are helpful for the first-order checking of a sample. The equivalent ionic ratio (EIR) is defined as the ratio of the sum of anions to the sum of cations, and this ratio is very near to unity (One). Over the current observation site, the EIR of the RW was 0.34 ± 0.26 during observation. All collected samples were within the permissible limits and can be used for further ionic characterization (Saxena et al., 1996). The observed ionic concentration was below the less polluted in which a slightly weak change in ionic balance is permissible (WMO, 1994). Such kinds of variations in ionic ratio are probably due to the presence of weak organic acids (WOA) which may be originated from the green plants over the sampling site. Kulshrestha et al. (2005) observed the presence of organic acids (associated with H+) having a very short life in the non-preserved RW samples. The fluoride ion shows relative concentrations in RW as compared to Indian polluted locations.
3 Results and Discussion
3.1 Day-to-Day Variability of pH in Rainwater
The RW pH is a tool to identify the presence of different ionic species, which are governed by the neutralization of the cations such as Ca2+, NH4+, and Mg2+ and the scavenging process in the atmosphere (Khwaja & Husain, 1990). RW pH > 5.6 is impacted by crustal species or mineral dust and is deemed to be alkaline, whereas if RW pH < 5.6, then SO42+ and NO3− dominate and are regarded as the acidity nature of RW (Bisht et al., 2017; Isikdemir, 2006).
The monthly pH values are depicted in Fig. 3 and individual precipitation events over the monitoring site. In the monsoon (MON) season, the volume-weighted mean (VWM) pH values of RW were 5.77 ± 0.40 (n = 37) reported at Srinagar Garhwal. The pH value of collected RW samples varying in the range of 5.05 to 6.65 indicates contamination/mixing of chemicals originating from anthropogenic and natural sources. RW’s high pH values were observed in arrival of MON (July) which might be due to the neutralization of acidic constituents with the alkaline components. But the low pH values of RW were observed in last MON (September) due to the scavenging process of alkaline particles and buffering capacity of RW during the year 2016 (Bisht et al., 2017; Kulshrestha et al., 2005). In the current study, we have reported the lowest pH value as 4.95 and the difference in free acidity of [H+] is 3.21 μeq/1 to the natural rainfall.
Tiwari et al. (2012) planned a study to understating the rain chemistry over the rural location over northwestern Himalayan, India, and observed most alkaline rainfall events (VWM pH mean = 5.68 ± 0.26) during MON 2006 and 2007. Bisht et al. (2017) followed a pH of RW very close to the acidity level in the range of 4.95 to 6.50 (mean = 5.6 ± 0.3) during MON over the Nainital (a location in the central Himalayan region). The pH of RW was reported in the range of 5.73 to 7.21 over Pune (Budhavant et al., 2009). Rastogi and Sarin (2005) obtained the alkaline nature (mean = 6.7) of RW over Ahmedabad due to the significant nearly ~ 60–80% contribution of mineral dust during the observation period of 2000–2002. Recently, Kumar et al. (2022) observed the volume-weighted mean (VWM) pH value of RW in the range of 4.59 and 6.73, with an average value of 5.47 ± 0.69, indicating the alkaline nature of rainfall over Baspa basin, Himalayan region in Himachal Pradesh. Zhang et al. (2018) obtained pH in the range of 4.0 to 8.3 (VWM = 5.0) in which approximately 46.9% sample acidity (pH below 5.6) and 11.6% sample in the firm acidity (pH below 4.5) in the RW of Wanzhou, China.
To understand RW’s trend and chemical composition over the Gulf of Mexico, Sosa-echeverría et al. (2018) take an observation from 2003 to 2014 and reported the VWM pH in the acidity range (4.78–5.40). Cereceda-Balic et al. (2020) observed the pH of RW in the range of 5.02 to 5.38 during May to August of 2010 and 2011 over the Puchuncaví Valley, Chile. Similarly, the pH value of RW was reported in the range of 4.0–6.8 over Mt. Fuji, Japan. Watanabe et al. (2006) and Aiuppa et al. (2003) observed pH values in the ranges of 3.8 to 7.22 over Mount Etna, Italy. In South China, Li et al. (2022) have evaluated the annual VWM pH of rainwater of 5.8 based on RW data of year 2018–2020 at Mt. Lushan location.
3.2 Major Ionic Composition of Rainwater (RW)
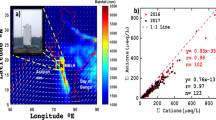
The major ion concentration of RW samples depends on environmental factors such as precipitation, wet and dry scavenging, meteorology, and location of the sampling site. The total sum of chemical species/ions was 97.35 ± 26.46 μeq/1 during MON season (Table 1). The percentage contribution of different chemical species during MON season has also presented in Fig. 4. The anion concentration was 41.54%, which denotes the contribution of long-range transport (LRT) of APs (Roy et al., 2016). The concentration of the cation was 58.46% during the MON season. The calcium was principal chemical species present in 24% of total collected samples; however, the SO42− and NO3− ions have 11% and 7% during the MON season. Now the combined dominance of Na+ and Cl or sea salt was 24% which may be transported from the ocean via LRT of atmospheric particles. The anions have trend as C1− > SO42− > NO3− > HCO3− in the percentage contribution of major ions, whereas cations show the trend as Ca2+ > Na+ > Mg2+ > K+ > NH4+ > H+ during MON (Fig. 4). Higher Ca2+ contribution indicates the emission form stone crusher activities or resuspension of soil particles over Srinagar. A similar trend of major ion concentration was also observed by Bisht et al. (2017) at Nainital.
Figure 5a, b represents the daily variation of measured anion and cation during MON season. The concentrations C1−, NO3−, and SO42− were high in July and last week of September 2016, whereas the lower concentrations were observed due to the wet scavenging process. The HCO3 also exhibited a similar trend of variation ion during the 2nd week and also reduced in September 2016 over the monitoring site. The highest values of C1− > SO42− > NO3− > HCO3 were measured as 25.77 μeq/1, 31.81 μeq/1, 25.84 μeq/1, and 13.49 μeq/1, and lower concentration was also measured as 3.31 μeq/1, 4.19 μeq/1, 1.47 μeq/1, and 10.6 μeq/1, respectively Fig. 6.
Now the daily concentration of cation such as Ca2+ and NH4+ was observed highest during July and last week of August 2016 due to calcareous particles of MON seasons. The Na+, Mg2+, and K+ have almost similar trends, which may be transported from marine aerosols/sea salt. But most of the ionic species were low in August 2016 due to heavy rainfall (22 events) during the 4th week of July to the 5th week of August 2016. The daily concentration of cation such as Ca2+ > Na+ > Mg2+ > K+ > NH4+ > H+ was observed to be varied in the range of 6.40–42.20 μeq/l, 1.04–24.00 μeq/1, 0.08–10.50 μeq/1, 0.56–9.23 μeq/1, 0.06–31.17 μeq/1, and 0.22–0.87 μeq/1, respectively. A comparative study of major ions is described in Fig. 5, which shows the concentration trend of major ions as Ca2+ (23.33 μeq/l) > NH4+ (17.58 μeq/l) > C1− (14.44 μeq/l) > SO42− (10.56 μeq/l) > Na+ (8.84 μeq/l) > NO3− (7.15 μeq/l) > HCO3− (5.47 μeq/l) > K+ (4.09 μeq/l) > Mg2+ (2.81 μeq/l) > H+ (2.80 μeq/l), respectively, over Srinagar. The higher Ca2+ concentrations may be due to emission from the roadside and nearest (within 100 km) cement industry at Roorkee, Uttarakhand (Sanusi et al., 1996). The chemical species analysis of RW samples was also conducted at Pune (Maharashtra), Kothi (Himachal Pradesh), Varanasi (Uttar Pradesh), Nainital (Uttarakhand), and Guwahati (Assam) by Safai et al. (2004); Tiwari et al. (2012); Bisht et al. (2015) and (2017); and Bhaskar and Rao (2017) in India, respectively.
3.3 Evaluation of Ionic Ratios of RW
The estimation of the primary ion ratio depend upon the MON location and transportation of sources from local and regional sources to the observation site during the wet deposition process. We have collected the worldwide SO42−/NO3− ratio during rainfall (MON) season (Table 2). The measurement of the SO42−/NO3− ratio gives brief information regarding the dominance of H2SO4 and HNO3 acids in the RW. The SO42−/NO3− ratio was 1.53 during MON 2016, which indicates the contribution of 60 and 40% in the SO42− and NO3−. The calculated value is slightly similar to previously evaluated values of H2SO4 (60–70%) and HNO3 (30–40%) in the RW (Bisht et al., 2017; Tuncer et al., 2001). The atmospheric removal of NO3− is rapid as compared to SO42− and contributing to the high SO42−/NO3 ratio in the RW samples. The cation such as Ca2+, NH4+, and Mg2+ is neutralized by anions SO42− and NO3− in the atmosphere (Bisht et al., 2017). The H+/(NO3− + SO42−) ratio has great significance to evaluate the quantity of neutralization of ions during rainfall. If the H+/(NO3− + SO42−) ratio is close to zero, then it represents the process of extensive neutralization, whereas if the H+/(NO3− + SO42−) ratio is near to one, then it suggests weak neutralization. We have observed the H+/(NO3− + SO42−) ratio as 0.14, which suggests near-complete neutralization by the neutralizing agents such as Ca2+, Mg2+, and NH4+ ions during rainfall (Isikdemir, 2006).
The impact of HNO3, HCl, and H2SO4 acid is investigated by (NO3− + C1−)/SO42− ratio; we have calculated the value as 1.83, which suggests the significant contribution of nitric and hydrochloric as compared to sulfuric acid RW (Khemani et al., 1994). The NH4+/NO3− ratio (2.40) and NH4+/SO42− (1.57) indicate a significant contribution of NH4NO3 and (NH4)2SO4 compounds in the atmosphere of Srinagar (Seinfeld, 1986). However, the ratio of (SO42− + NO3−)/(Ca2+ + Mg2+) is (0.69), suggesting the alkaline nature of RW. The of (Ca2+ + NH4+)/(NO3− + SO42−) is 2.22, suggesting the neutralization of acid components by Ca2+ and NH4+ cations in RW.
3.4 Neutralization Factor (NF)
The Ca2+ and NH4+ ions have a well-defined neutralization impact on H2SO4 and HNO3 acids and can control the acidity rainfall (Rodhe et al., 2002). The neutralization factor (NF) is defined as a potential to neutralize the acidic components of RW by alkaline (Bisht et al., 2017; Safai et al., 2004; Tiwari et al., 2012). Mathematically, the NF may be expressed as follows:
where Xi is the concentration of major alkaline species (Ca2+, Mg2+, and NH4+). The NF shows the trend as Ca2+ (1.27) > NH4+ (0.97) > and Mg2+ (0.17) and have contribution of NF(Ca) = 53.07%, NF(NH4) = 39.70%, and NF(Mg) = 7.15% in the RW over Srinagar. Many researchers (Das et al., 2005; Kulshrestha et al., 2003; Parashar et al., 2001; Rastogi & Sarin, 2005) observed the predominant neutralization capacity due to Ca2+ and NH4+ in the different parts of India. Bisht et al. (2017) observed the predominant contribution of Ca2+ and NH4+ over Nainital, India. Singh et al. (2007) suggest the neutralization of acid components of RW by Ca2+ and Mg2+ below the cloud and NH4+ in-cloud, respectively.
3.5 Sea Salt (SS) and Non-Sea Salt (NSS) Contribution
To understand the contribution of SS, NSS, and enrichment factor (EF), the Cl−/Na+ ratio was calculated for RW as well as seawater (Table 3) separately (Moody et al., 1991). The dominance of NSS was calculated by simply differentiating SS from the total measured ion in the collected sample. The C1−/Na+ ratio (1.37), Ca2+/Na+(1.34), SO42−/Na+ (1.14), Mg2+/Na+ (0.32), and K+/Na+ (0.44) of RW was higher than the ratio of ion in seawater (SW) C1−/Na+ (1.16), Ca2+/Na+(10.04), SO42−/Na+ (0.12), Mg2+/Na+ (0.22), and K+/Na+ (0.02) during the MON. This clearly indicates the significant contribution of SS as well as that originating from soil. Based on the relative ratio of ions, we can estimate the possible compounds such as NaCl, CaSO4, MgSO4, MgCl2, HNO3, NH4NO3, NH4SO4, and (NH4)2SO4 (Bisht et al., 2017; Chandra Mouli et al., 2005), which the correlations in Section 3.6.2 will also verify.
EF of major ions can be calculated by the ratio of significant ion (Xi) concerning Na+ that was presented in the RW and SW by using Eq. 2 (Bisht et al., 2017; Cereceda-Balic et al., 2020; Tiwari et al., 2012):
where Xi = major ion, if EF < 1, then SW is a major contributor or if EF > 1, then NSS is the source.
EF of C1−, Mg2+, K+, Ca2+, and SO42− and sea salt fraction (SSF) and a non-sea salt fraction (NSSF) have been listed in Table 3, which indicates the dominance of anthropogenic source or NSS origin over Srinagar. The principal source of C1− and Mg2+ was from the sea and contributed 86.57% and 74.94% and 13.43% and 29.06% from the non-ocean source, respectively.
It is noticed that the K+ (95.05%), Ca2+ (98.17%), and SO42− (89.04%) have a contribution from the anthropogenic or non-ocean salt sources (Bisht et al., 2017; Safai et al., 2004; Singh et al., 2007; Tiwari et al., 2012, 2016). The result suggests the significant contribution of anthropogenic and natural sources.
3.6 Identification of Source
To track and identify the different sources of major ionic species, the air mass back trajectory (AMBT) analysis and factor analysis (FA) were also conducted for Srinagar during 2016.
3.6.1 Air Mass Back Trajectory (AMBT) Analysis
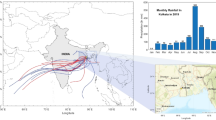
A 5-day AMBT data was extracted from HYSPLIT and plotted by MeteoInfo software during MON-2016 (Gautam et al., 2021a, b; Hernández-Ceballos et al., 2011; Rico-Ramirez et al., 2007). We have calculated AMBTs at the elevation of 500 m, 1000 m, and 1500 m above mean sea level (Fig. 7). The air mass is coming mainly from two branches, i.e., it is one from the Indian Gangetic plain (IGP) from the Bay of Bengal. In contrast, the second one originates from the Arabian Gulf Sea at an altitude of 500 m. However, at high levels of air mass such as 1000 m and 1500 m, the air mass is originating from Iran, Afghanistan, and Pakistan to Punjab Haryana to Srinagar Garhwal. The AMBT analysis gives evidence for the transportation of sea salt and APs from the IGP region during MON season. Bisht et al. (2017); Gautam et al. (2018); and Panicker et al. (2019) observed the clear evidence of transportation of APs from IGP and sea salt contribution from the Arabian and Bay of Bengal side.
3.6.2 Principle Component and Correlation Matrix Analysis
The principle component analysis (PCA) is a powerful tool for the investigation of all possible sources in the RW sample, and it was estimated by using R (Statistical Software) as given by Table 4 (Ahmed et al., 2022; Jeong et al., 2009; Jombart et al., 2008; Sidou & Borges, 2020). The Pearson correlation coefficient (PCC) of major ions of RW samples is also presented in Table 5. Factor 1 gives the idea for the 9% of data variance and has a positive relation link for loading of NO3−, SO42−, Ca2+, HCO3−, and F−, which indicate the origin of ions emitted from the process of burning fuel and soil. The mutual PCC of Na+ and K+ with Cl− ion are 0.75 and 0.66, respectively (Table 5), suggesting transport from the marine background (Bisht et al., 2017; Norman et al., 2001). The SO42− shows good correlation with Ca2+ (0.48), Mg2+ (0.75), and NO3−(0.45), indicating the transportation from biofuel and soil. A moderate correlation is also observed between Ca2+ and carbonate ions due to the contribution of CaCO3 from the ground. Ammonium ion has a good PCC with NO3− and SO42− attributed from the secondary aerosol particle as (NH4)2SO4 and NH4NO3 emitted from fertilizer agricultural activities (Al-Momani et al., 1995; Kaya & Tuncel, 1997). The primary source of SO42− may be due to incomplete burning of bio-fuel, pesticides, thermal power plants, oil refineries workshops, and transportation from IGP and Gulf countries.
Factor 2 suggests a contribution of about 18% as variance and showing loading of F−, NH4+, Mg2+, and H+ during MON 2016. Factor 3 has a variance of 27% with the loading of Ca2+, F−, Na+, Cl−, NH4+, and K+ from the soil, agricultural, and biomass burning activities.
4 Conclusions
The major chemical species of RW collected over Srinagar, Garhwal, during the year 2016 gives significant result of influencing by various anthropogenic and natural sources, which was found to have potential change in RW chemistry. The pH value of RW was observed in the range of 5.05 to 6.65 with an average value of 5.77 ± 0.40 during MON season, which is a clear indication of significant contribution of natural as well as from the anthropogenic background in the RW. The total sum of the concentration of major ions of RW was 97.35 ± 26.46 μeq/1 during MON season. The percentage contribution of anions has the trend as C1− > SO42− > NO3− > HCO3−, and percentage contribution of cations has as Ca2+ > Na+ > Mg2+ > K+ > NH4+ > H+ in the collected sample of RW at Srinagar in MON season. The SO42−/NO3− ratio (1.53) clearly indicates 60% and 40% contribution from the SO42− and NO3 and have strong evidence of significant neutralization (H+/(NO3− + SO42−) = 0.14) of Ca2+, NH4+, and Mg2+ by anions SO42− and NO3−. The NF has a trend as Ca2+ (1.27) > NH4+ (0.97) > Mg2+ (0.17) for cations and significantly contributing with about 53%, 40%, and 7%, respectively, as neutralization process in the atmosphere. We observed the ratio of C1−/Na+ (1.37), Ca2+/Na+ (1.34), SO42−/Na+ (1.14), Mg2+/Na+ (0.32), and K+/Na+ (0.44) ions of RW is slightly higher than the ionic ratio C1−/Na+ (1.16), Ca2+/Na+ (10.04), SO42−/Na+ (0.12), and Mg2+/Na+ (0.22) of seawater (SW), which indicates the sampling site is significantly affected by SS and soil driven dust. The C1− and Mg2+ shows 86.57% and 74.94% contribution from the marine source, whereas K+ (95.05%), Ca2+ (98.17%), and SO42− (89.04%) have anthropogenic or non-ocean salt background. PCA shows that the SO42− has a good correlation with Ca2+ (0.48), Mg2+ (0.75), and NO3− (0.45). This may be emitted from soil dust, agriculture, secondary aerosols, and combustion of biofuel from the IGP and further transported to the monitoring site via regional and LRT of air masses. These findings could be helpful in establishing the emission control policies to protect and manage pollution level in the eco-environmental system in Uttarakhand. This can further be helpful through deployment of more such monitoring stations in and around the Himalayan region.
Data Availability
Not applicable.
Abbreviations
- AMBT:
-
Air mass back trajectory
- APs:
-
Air pollutants
- AWS:
-
Automatic weather station
- EC:
-
Electrical conductivity
- EF:
-
Enrichment factor
- EIR:
-
Equivalent ionic ratio
- FSI:
-
Forest Survey of India
- HNBGU:
-
Hemwati Nandan Bahuguna Garhwal University
- HYSPLIT:
-
Hybrid Single-Particle Lagrangian Integrated Trajectory
- IGP:
-
Indo-Gangetic Plain
- LRT:
-
Long-range transport
- MON:
-
Monsoon
- NF:
-
Neutralization factor
- NH:
-
National highway
- NOAA:
-
National Oceanic and Atmospheric Administration
- NSS:
-
Non-sea salt
- NSSF:
-
Non-sea salt fraction
- PCA:
-
Principle component analysis
- PCC:
-
Pearson correlation coefficient
- PSCF:
-
Potential source contribution function
- RW:
-
Rainwater
- SS:
-
Sea salt
- SSF:
-
Sea salt fraction
- SW:
-
Seawater
- VWM:
-
Volume-weighted mean
- WMO:
-
World Meteorological Organization
- WOA:
-
Weak organic acids
References
Ahmed, M., Bhuyan, P., Sarkar, S., & Hoque, R. R. (2022). Seven-year study of monsoonal rainwater chemistry over the mid-Brahmaputra plain, India: Assessment of trends and source regions of soluble ions. Environmental Science and Pollution Research, 29(17), 25276–25295.
Aiuppa, A., Bonfanti, P., & D’Alessandro, W. (2003). Rainwater chemistry at Mt Etna (Italy) Natural and anthropogenic sources of major ions. Journal of Atmospheric Chemistry, 46(1), 89–102.
Al-Momani, I. F., Tuncel, S., Eler, Ü., Örtel, E., Sirin, G., & Tuncel, G. (1995). Major ion composition of wet and dry deposition in the eastern Mediterranean basin. Science of the Total Environment, 164(1), 75–85. https://doi.org/10.1016/0048-9697(95)04468-G
Attri, SD., Laskar, SI., Singh, UP., M.K. B (2015) Annual Report 2015. India Meteorol Dep 1–108. https://doi.org/10.1353/bmc.2015.0008
Avila, A., & Alarcón, M. (1999). Relationship between precipitation chemistry and meteorological situations at a rural site in NE Spain. Atmospheric Environment, 33(11), 1663–1677. https://doi.org/10.1016/S1352-2310(98)00341-0
Báez, A. P., Belmont, R. D., García, R. M., Torres, M. C. B., & Padilla, H. G. (2006). Rainwater chemical composition at two sites in Central Mexico. Atmospheric Research, 80(1), 67–85. https://doi.org/10.1016/j.atmosres.2005.06.008
Balasubramanian, R., Victor, T., & Begum, R. (1999). Impact of biomass burning on rainwater acidity and composition in Singapore. Journal of Geophysical Research (Vol. 104) Blackwell Publishing Ltd. https://doi.org/10.1029/1999JD900247
Bayraktar, H., & Turalioglu, F. S. (2005). Composition of wet and bulk deposition in Erzurum Turkey. Chemosphere, 59(11), 1537–1546. https://doi.org/10.1016/j.chemosphere.2005.02.013
Bhaskar, V. V., & Rao, P. S. P. (2017). Annual and decadal variation in chemical composition of rain water at all the ten GAW stations in India. Journal of Atmospheric Chemistry, 74(1), 23–53. https://doi.org/10.1007/s10874-016-9339-3
Bisht, D. S., Tiwari, S., Srivastava, A. K., Singh, J. V., Singh, B. P., & Srivastava, M. K. (2015). High concentration of acidic species in rainwater at Varanasi in the Indo-Gangetic Plains India. Natural Hazards, 75(3), 2985–3003. https://doi.org/10.1007/s11069-014-1473-0
Bisht, D. S., Srivastava, A. K., Joshi, H., Ram, K., Singh, N., Naja, M., et al. (2017). Chemical characterization of rainwater at a high-altitude site “Nainital” in the central Himalayas India. Environmental Science and Pollution Research, 24(4), 3959–3969. https://doi.org/10.1007/s11356-016-8093-z
Budhavant, K. B., Rao, P. S. P., Safai, P. D., & Ah, K. (2009). Chemistry of monsoon and post-monsoon rains at a high altitude location, Sinhagad India. Aerosol and Air Quality Research, 9(1), 65–79. https://doi.org/10.4209/aaqr.2008.07.0033
Cao, Y. Z., Wang, S., Zhang, G., Luo, J., & Lu, S. (2009). Chemical characteristics of wet precipitation at an urban site of Guangzhou South China. Atmospheric Research, 94(3), 462–469. https://doi.org/10.1016/j.atmosres.2009.07.004
Cereceda-Balic, F., de la Gala-Morales, M., Palomo-Marín, R., Fadic, X., Vidal, V., Funes, M., et al. (2020). Spatial distribution, sources, and risk assessment of major ions ad trace elements in rainwater at Puchuncaví Valley, Chile: The impact of industrial activities. Atmospheric Pollution Research, 11(6), 99–109. https://doi.org/10.1016/j.apr.2020.03.003
Cerqueira, M. R. F., Pinto, M. F., Derossi, I. N., Esteves, W. T., Santos, M. D. R., Matos, M. A. C., & Matos, R. C. (2014). Chemical characteristics of rainwater at a southeastern site of Brazil. Atmospheric Pollution Research, 5(2), 253–261.
Chandra Mouli, P., Venkata Mohan, S., & Reddy, S. J. (2005). Rainwater chemistry at a regional representative urban site: Influence of terrestrial sources on ionic composition. Atmospheric Environment, 39(6), 999–1008. https://doi.org/10.1016/j.atmosenv.2004.10.036
Cheng, M. C., & You, C. F. (2010). Sources of major ions and heavy metals in rainwater associated with typhoon events in southwestern Taiwan. Journal of Geochemical Exploration, 105(3), 106–116.
Choi, B., Yun, S.-T., Kim, K.-H., & Kim, K.-H. (2008). Spatio-temporal variation of pH and ionic concentrations in precipitation: Interaction between two contrasting stationary sources affecting air quality. Geosciences Journal, 12(3), 205–213. https://doi.org/10.1007/s12303-008-0021-x
Das, R., Das, S. N., & Misra, V. N. (2005). Chemical composition of rainwater and dustfall at Bhubaneswar in the east coast of India. Atmospheric Environment, 39, 5908–5916. https://doi.org/10.1016/j.atmosenv.2005.06.030
Das, N., Das, R., Chaudhury, G. R., & Das, S. N. (2010). Chemical composition of precipitation at background level. Atmospheric Research, 95(1), 108–113. https://doi.org/10.1016/j.atmosres.2009.08.006
Datta, A., Saud, T., Goel, A., Tiwari, S., Sharma, S. K., Saxena, M., & Mandal, T. K. (2010). Variation of ambient SO2 over Delhi. Journal of Atmospheric Chemistry, 65(2–3), 127–143. https://doi.org/10.1007/s10874-011-9185-2
Draxler, R., Stunder, B., Rolph, G., Stein, A., & Taylor, A. (2014). HYSPLIT4 user ’s guide version 4 - Last revision: September 2014
Flues, M., Hama, P., Lemes, M. J. L., Dantas, E. S. K., & Fornaro, A. (2002). Evaluation of the rainwater acidity of a rural region due to a coal-fired power plant in Brazil. Atmospheric Environment, 36(14), 2397–2404. https://doi.org/10.1016/S1352-2310(01)00563-5
Gautam, S., Sammuel, C., Bhardwaj, A., Esfandabadi, Z. C., Santosh, M., Gautam, A. S., Joshi, A., Justin, A., Wessley, J. W., & James, E. J. (2021). Vertical profiling of atmospheric air pollutants in rural India A case study on particulate matter (PM10/PM2.5/PM1) carbon dioxide and formaldehyde. Measurement, 185, 110061.
Gautam, A. S., Kumar, S., Gautam, S., Anand, A., Kumar, R., Joshi, A., et al. (2021b). Pandemic induced lockdown as a boon to the environment: Trends in air pollution concentration across India Asia-Pacific. Journal of Atmospheric Sciences, 2020, 1–16. https://doi.org/10.1007/s13143-021-00232-7
Gautam, A. S., Negi, R. S., Singh, S., Srivastava, A. K., Tiwari, S., & Bisht, D. S. (2018). Chemical characteristics of atmospheric aerosol at Alaknanda Valley (Srinagar) in the Central Himalaya Regio, India. International Journal of Environmental Research, (Vol. 12). .https://doi.org/10.1007/s41742-018-0125-8
Gautam S, Elizabeth J, Gautam AS, Singh K, Abhilash P. (2022). Impact assessment of aerosol optical depth on rainfall in Indian rural areas. Aerosol Science and Engineering. (Accepted - https://doi.org/10.1007/s41810-022-00134-9).
Gobre, T., Salve, P. R., Krupadam, R. J., Bansiwal, A., Shastry, S., & Wate, S. R. (2010). Chemical composition of precipitation in the coastal environment of India. Bulletin of Environmental Contamination and Toxicology, 85(1), 48–53. https://doi.org/10.1007/s00128-010-0006-0
Granat, L., Suksomsankh, K., Simachaya, S., Tabucanon, M., & Rodhe, H. (1996). Regional background acidity and chemical composition of precipitation in Thailand. Atmospheric Environment, 30(10–11), 1589–1596. https://doi.org/10.1016/1352-2310(95)00455-6
Granat, L., Das, S. N., Tharkur, R. S., & Rodhe, H. (2001). Atmospheric deposition in a rural area in India Net and potential acidity. Water, Air, and Soil Pollution, 130(1–4), 469–474. https://doi.org/10.1023/A:1013810007438
Hernández-Ceballos, M. A., García-Mozo, H., Adame, J. A., Domínguez-Vilches, E., Bolívar, J. P., De La Morena, B. A., et al. (2011). Determination of potential sources of Quercus airborne pollen in Córdoba city (southern Spain) using back-trajectory analysis. Aerobiologia, 27(3), 261–276. https://doi.org/10.1007/s10453-011-9195-1
Isikdemir, O. (2006). 8-year-long composition record in the Eastern Mediterranean precipitation. middle East Technical University, Ankara,1–128.
Jeong, D. H., Fisher, B. D., Ziemkiewicz, C., Fisher, B., Ribarsky, W., & Chang, R. (2009). IPCA: An interactive system for PCA-based visual analytics Cloud Computing View project attentional tokens in dynamic displays view project iPCA: An interactive system for PCA-based visual analytics. Wiley Online Library, 28(3), 767–774. https://doi.org/10.1111/j.1467-8659.2009.01475.x
Jombart, T., Lyon, D., & Biome, L. D. (2008). adegenet : A R package for the multivariate analysis of genetic markers. Bioinformatics Applications Note, 24(11), 1403–1405. https://doi.org/10.1093/bioinformatics/btn129
Kaya, G., & Tuncel, G. (1997). Trace element and major ion composition of wet and dry deposition in Ankara Turkey. Atmospheric Environment, 31(23), 3985–3998. https://doi.org/10.1016/S1352-2310(97)00221-5
Keene, W. C., Pszenny, A. A. P., Galloway, J. N., & Hawley, M. E. (1986). Sea-salt corrections and interpretation of constituent ratios in marine precipitation. Journal of Geophysical Research, 91(D6), 6647. https://doi.org/10.1029/jd091id06p06647
Khemani, L. T., Momin, G. A., Rao, P. S. P., Pillai, A. G., Safai, P. D., Mohan, K., & Rao, M. G. (1994). Atmospheric pollutants and their influence on acidification of rain water at an industrial location on the West Coast OF India. Atmospheric Environment, 28(19), 3145–3154. https://doi.org/10.1016/1352-2310(94)00148-E
Khwaja, H. A., & Husain, L. (1990). Chemical characterization of acid precipitation in Albany, New York. Atmospheric Environment Part a, General Topics, 24(7), 1869–1882. https://doi.org/10.1016/0960-1686(90)90519-S
Kulshrestha, U. C., Kulshrestha, M. J., Sekar, R., Sastry, G. S. R., & Vairamani, M. (2003). Chemical characteristics of rainwater at an urban site of south-central India. Atmospheric Environment, 37(21), 3019–3026. https://doi.org/10.1016/S1352-2310(03)00266-8
Kulshrestha, U. C., Granat, L., Engardt, M., & Rodhe, H. (2005). Review of precipitation monitoring studies in India - A search for regional patterns. Atmospheric Environment, 39(38), 7403–7419. https://doi.org/10.1016/j.atmosenv.2005.08.035
Kumar, R., Kumar, R., Singh, A., et al. (2022). Chemometric approach to evaluate the chemical behavior of rainwater at high altitude in Shaune Garang catchment Western Himalaya. Sci Rep, 12, 12774. https://doi.org/10.1038/s41598-022-15422-0
Kuniyal, J., Momin, G., … P. R.-92. 60. M. 92. 6. S., & 2005, undefined. (n.d.). Aerosols behaviour in sensitive areas of the northwestern Himalaya—A case of Kullu-Manali tourist complex, India. nopr.niscair.res.in. http://nopr.niscair.res.in/handle/123456789/25668. Accessed 13 June 2021
Li, J., Wu, H., Jiang, P., & Fu, C. (2022). Rainwater chemistry in a subtropical high-altitude mountain site, South China: Seasonality, source apportionment and potential factors. Atmospheric Environment, 268, 118786.
Liu, Y., & ping, Geng, Z. xing, & Hou, S. gui. (2010). Spatial and seasonal variation of major ions in Himalayan snow and ice: A source consideration. Journal of Asian Earth Sciences, 37(2), 195–205. https://doi.org/10.1016/j.jseaes.2009.08.006
Migliavacca, D., Teixeira, E. C., Wiegand, F., Machado, A. C. M., & Sanchez, J. (2005). Atmospheric precipitation and chemical composition of an urban site, Guaíba hydrographic basin Brazil. Atmospheric Environment, 39(10), 1829–1844. https://doi.org/10.1016/j.atmosenv.2004.12.005
Moody, J. L., Pszenny, A. A. P., Gaudry, A., Keene, W. C., Galloway, J. N., & Polian, G. (1991). Precipitation composition and its variability in the southern Indian Ocean: Amsterdam Island, 1980–1987. Journal of Geophysical Research, 96(11). https://doi.org/10.1029/91jd01921
Moreda-Piñeiro, J., Alonso-Rodríguez, E., Moscoso-Pérez, C., Blanco-Heras, G., Turnes-Carou, I., López-Mahía, P., & Prada-Rodríguez, D. (2014). Influence of marine, terrestrial and anthropogenic sources on ionic and metallic composition of rainwater at a suburban site (northwest coast of Spain). Atmospheric Environment, 88, 30–38.
Norman, M., Das, S. N., Pillai, A. G., Granat, L., & Rodhe, H. (2001). Influence of air mass trajectories on the chemical composition of precipitation in India. Atmospheric Environment, 35(25), 4223–4235. https://doi.org/10.1016/S1352-2310(01)00251-5
Obaidy, A. H., & Joshi, H. (2006). Chemical composition of rainwater in a tropical urban area of northern India. Atmospheric Environment, 40(35), 6886–6891. https://doi.org/10.1016/j.atmosenv.2005.01.031
Pandey, J., & Pandey, U. (2009). Accumulation of heavy metals in dietary vegetables and cultivated soil horizon in organic farming system in relation to atmospheric deposition in a seasonally dry tropical region of India. Environmental Monitoring and Assessment, 148(1–4), 61–74. https://doi.org/10.1007/s10661-007-0139-8
Pandey, J., & Singh, A. (2012). Chemical characterization of rain water in a seasonally dry tropical region (Varanasi) India. Journal of Environmental Biology, 33(3), 629.
Pandey, J. (2008). Microbial biomass at land water interface and its role in regulating ecosystem properties of a fresh water dry tropical woodland lake. Journal of Environmental Biology, 29(3), 333–337. http://www.jeb.co.in/journal_issues/200805_may08/paper_11.pdf. Accessed 13 June 2021
Panicker, A. S., Sandeep, K., Negi, R. S., Gautam, A. S., Bisht, D. S., Beig, G., et al. (2019). Estimates of carbonaceous aerosol radiative forcing over a semiurban environment in Garhwal Himalayas. Pure and Applied Geophysics, 176(11), 5069–5078. https://doi.org/10.1007/s00024-019-02248-7
Parashar, D. C., Kulshrestha, U. C., & Jain, M. (2001). Precipitation and aerosol studies in India. Environmental Monitoring and Assessment, 66(1), 47–61. https://doi.org/10.1023/A:1026424013890
Ram, K., Sarin, M. M., & Hegde, P. (2008). Atmospheric abundances of primary and secondary carbonaceous species at two high-altitude sites in India: Sources and temporal variability. Atmospheric Environment, 42(28), 6785–6796. https://doi.org/10.1016/j.atmosenv.2008.05.031
Ram, K., Sarin, M. M., & Hegde, P. (2010). Long-term record of aerosol optical properties and chemical composition from a high-altitude site (Manora Peak) in Central Himalaya. Atmospheric Chemistry and Physics, 10, 11791–11803. https://doi.org/10.5194/acp-10-11791-2010
Rastogi, N., & Sarin, M. M. (2005). Chemical characteristics of individual rain events from a semi-arid region in India: Three-year study. Atmospheric Environment, 39(18), 3313–3323. https://doi.org/10.1016/j.atmosenv.2005.01.053
Rico-Ramirez, M. A., Cluckie, I. D., Shepherd, G., & Pallot, A. (2007). A high-resolution radar experiment on the island of Jersey. Meteorological Applications, 14(2), 117–129. https://doi.org/10.1002/met.13
Rodhe, H., Dentener, F., & Schulz, M. (2002). The global distribution of acidifying wet deposition. Environmental Science and Technology, 36(20), 4382–4388. https://doi.org/10.1021/es020057g
Roy, A., Chatterjee, A., Tiwari, S., Sarkar, C., Das, S. K., Ghosh, S. K., & Raha, S. (2016). Precipitation chemistry over urban, rural and high altitude Himalayan stations in eastern India. Atmospheric Research, 181, 44–53. https://doi.org/10.1016/j.atmosres.2016.06.005
Safai, P. D., Rao, P. S. P., Momin, G. A., Ali, K., Chate, D. M., & Praveen, P. S. (2004). Chemical composition of precipitation during 1984–2002 at Pune India. Atmospheric Environment, 38(12), 1705–1714. https://doi.org/10.1016/j.atmosenv.2003.12.016
Salve, P. R., Gobre, T., Krupadam, R. J., Shastry, S., Bansiwal, A., & Wate, S. R. (2011). Chemical characterization of rainwater at Akkalkuwa India. Mausam, 62(3), 425–432.
Sanusi, A., Wortham, H., Millet, M., & Mirabel, P. (1996). Chemical composition of rainwater in eastern France. Atmospheric Environment, 30(1), 59–71. https://doi.org/10.1016/1352-2310(95)00237-S
Saxena, A., Kulshrestha, U. C., Kumar, N., Kumari, K. M., & Srivastava, S. S. (1996). Characterization of precipitation at Agra. Atmospheric Environment, 30(20), 3405–3412. https://doi.org/10.1016/1352-2310(96)00049-0
Seinfeld, J. H. (1986). ES&T Books: Atmospheric chemistry and physics of air pollution. Environmental Science & Technology, 20(9), 863–863. https://doi.org/10.1021/es00151a602
Sidou, L. F., & Borges, E. M. (2020). Teaching principal component analysis using a free and open source software program and exercises applying PCA to real-world examples. Journal of Chemical Education, 97(6), 1666–1676. https://doi.org/10.1021/acs.jchemed.9b00924
Singh, A. K., Mondal, G. C., Kumar, S., Singh, K. K., Kamal, K. P., & Sinha, A. (2007). Precipitation chemistry and occurrence of acid rain over Dhanbad, coal city of India. Environmental Monitoring and Assessment, 125(1–3), 99–110. https://doi.org/10.1007/s10661-006-9243-4
Sosa-echeverría, R., Bravo-álvarez, H., Alarcón-jiménez, A. L., Torres-barrera, M. C., Jaimes-palomera, M., & Sánchez-álvarez, P. (2018). Acid rain in a Mexican site on the coast of the Gulf of Mexico. Atmosfera, 31(4), 317–330. https://doi.org/10.20937/ATM.2018.31.04.01
Srivastava, A. K., Pant, P., Hegde, P., Singh, S., Dumka, U. C., Naja, M., et al. (2011). The influence of a South Asian dust storm on aerosol radiative forcing at a high-altitude station in central Himalayas. International Journal of Remote Sensing, 32(22), 7827–7845. https://doi.org/10.1080/01431161.2010.531781
Srivastava, A. K., Ram, K., Singh, S., Kumar, S., & Tiwari, S. (2015). Aerosol optical properties and radiative effects over Manora Peak in the Himalayan foothills: Seasonal variability and role of transported aerosols. Science of the Total Environment, 502, 287–295. https://doi.org/10.1016/j.scitotenv.2014.09.015
Tiwari, S., Chate, D. M., Bisht, D. S., Srivastava, M. K., & Padmanabhamurty, B. (2012). Rainwater chemistry in the North Western Himalayan Region, India. Atmospheric Research, 104–105, 128–138. https://doi.org/10.1016/j.atmosres.2011.09.006
TiwariP. K., Thimmaiah, D., Dumka, U. C., Srivastava, A. K., Bisht, D. S., H., et al. (2016). Nature and sources of ionic species in precipitation across the Indo-Gangetic Plains India. Aerosol and Air Quality Research, 16(4), 943–957. https://doi.org/10.4209/aaqr.2015.06.0423
Tuncer, B., Bayar, B., Yeşilyurt, C., Environment, G. T.-A., & 2001, U. (2001). Ionic composition of precipitation at the Central Anatolia (Turkey). Atmospheric Environment, 35(34), 5989–6002. https://www.sciencedirect.com/science/article/pii/S135223100100396X. Accessed 11 February 2021
Wai, K. M., Lin, N.-H., Wang, S.-H., Dokiya, Y., Wai, K. M., Lin, N.-H., et al. (2008). Rainwater chemistry at a high‐altitude station Mt Lulin Taiwan Comparison with a background station Mt Fuji. Geophys. Res, 113(6), 6305. https://doi.org/10.1029/2006JD008248
Watanabe, K., Takebe, Y., Sode, N., Igarashi, Y., Takahashi, H., & Dokiya, Y. (2006). Fog and rain water chemistry at Mt Fuji A case study during the September 2002 campaign. Atmospheric Research, 82(3–4), 652–662. https://doi.org/10.1016/j.atmosres.2006.02.021
WMO (1994) In: Mohnen V, Santroch J, Vet R (Eds.) Report of the workshop on precipitation chemistry laboratory techniques, Hradec Kralove, Czech Republic 17–21 October. WMO Report no 102
Yang, F. M., He, K. Bin, Lei, Y., Ma, Y. L., Yu, X. C., Tanaka, S., et al. (2004). Chemical characters of atmospheric precipitation in Beijing in years of 2001–2003. Zhongguo Huanjing Kexue/China Environmental Science, 24(5), 538. https://en.cnki.com.cn/Article_en/CJFDTotal-ZGHJ200405007.htm. Accessed 13 June 2021
Zhang, L., Qiao, B., Wang, H., Tian, M., Cui, J., Fu, C., et al. (2018). Chemical characteristics of precipitation in a typical urban site of the hinterland in Three Gorges Reservoir, China. Journal of Chemistry, 2018, 1–10. https://doi.org/10.1155/2018/2914313
Acknowledgements
The authors are thankful to the Director, IITM, for their guidance and unstinted support for this study. ASG thanks Prof Annpurna Nautiyal, Vice-Chancellor, HNBGU, Srinagar, Garhwal Uttarakhand, Head, Department of Physics, HNBGU, Srinagar, for encouragement and providing the necessary infrastructure facility for this study.
Funding
The authors are grateful to the Ministry of Earth Sciences (Government of India) for providing financial support for Cloud-Aerosol Interaction and Precipitation Enhancement Experiment (CAIPEEX). ASG is also thankful to the Department of Science and Technology, Government of India, for funding support under Climate Change Programme (CCP), SPLICE Division, Department of Science, New Delhi-110 016 (DST/CCP/Aerosol/83/2017(G).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bisht, D.S., Srivastava, A.K., Singh, V. et al. High-Altitude Air Pollutants Monitored from Rainwater Chemistry in the Central Himalaya. Water Air Soil Pollut 233, 392 (2022). https://doi.org/10.1007/s11270-022-05855-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05855-8