Abstract
This study aims to determine the source apportionment of surfactants in marine aerosols at two selected stations along the Malacca Straits. The aerosol samples were collected using a high volume sampler equipped with an impactor to separate coarse- and fine-mode aerosols. The concentrations of surfactants, as methylene blue active substance and disulphine blue active substance, were analysed using colorimetric method. Ion chromatography was employed to determine the ionic compositions. Principal component analysis combined with multiple linear regression was used to identify and quantify the sources of atmospheric surfactants. The results showed that the surfactants in tropical coastal environments are actively generated from natural and anthropogenic origins. Sea spray (generated from sea-surface microlayers) was found to be a major contributor to surfactants in both aerosol sizes. Meanwhile, the anthropogenic sources (motor vehicles/biomass burning) were predominant contributors to atmospheric surfactants in fine-mode aerosols.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine aerosols contribute significantly to the global aerosol scale and consequently influence the Earth’s radiative budget, scattering incoming solar radiation and modifying cloud properties (Murphy et al. 1998; O'dowd et al. 2004). Early research by Blanchard and Woodcock (1957) described the primary marine aerosols as a suspension of air particles produced as a result of the bubble bursting process created by waves breaking on the sea surface (Ryu et al. 2007). The bubble bursting process, which is influenced by wind speed (Blanchard 1964), is believed to be responsible for the redistribution of trace-level oxidised substances known as marine secondary aerosols (O'dowd and Hoffmann 2005). This process also contributes to the mobilisation of micropollutants, including organic substances from the sea-surface microlayer (SML) to the global atmosphere (Oppo et al. 1999; Marcomini et al. 2001). Surfactants are an important subgroup of dissolved organic substances found in the marine environment. Surfactants may influence the biogeochemical cycle (Wurl et al. 2009, 2011; Cunliffe et al. 2013) and seem to be responsible for the enrichment of microfloatable components in marine aerosols (Mcmurdo et al. 2008).
The accumulation of surfactants in the marine environment presents a risk to the environment of the surrounding area and to living organisms. Surfactants generated by marine aerosols in coastal regions have been reported to have an adverse impact on wildlife and to lead to a decline in coastal vegetation (Guidi et al. 1988; Becagli et al. 2011). Surfactants influence the toxicity of aerosol particles at the air–water interface and have a negative impact on the respiratory system, resulting in asthma, impaired cardiovascular function and allergic reactions (Cserháti et al. 2002; Paur et al. 2011). The effect of surfactants on global climate change is also well recognised. Surfactants reduce the surface tension of aerosols, enhancing the growth of cloud droplets (Shulman et al. 1996; Facchini et al. 1999). The hygroscopic behaviour of aerosol particles could control cloud condensation nuclei activity, supporting the formation of cloud albedo (Albrecht 1989; Sukhapan and Brimblecombe 2002; Udisti et al. 2012).
Surfactants in atmospheric aerosols can be generated from natural and anthropogenic sources. With respect to natural sources, surfactants can originate from the degradation of humic-like substances, while combustion processes and biomass burning represent anthropogenic sources (Latif and Brimblecombe 2004). A surfactant originating from the SML distribute as one of sea spray components was found to be related to the natural sources (Becagli et al. 2011). A previous study showed that atmospheric surfactants in coastal areas are more related to anthropogenic sources (Roslan et al. 2010). Identification of the sources of surfactants, especially in the marine atmosphere, is therefore important. The source apportionment analysis to determine the composition of surfactants in atmospheric aerosols, particularly in tropical areas, has been done by Wahid et al. (2013). Principal component analysis (PCA) in combination with multiple linear regression (MLR) has been chosen to determine the source apportionment of surfactants in fine- and coarse-mode aerosol studies. According to Abdul-Wahab et al. (2005), PCA has been employed in air quality studies to separate inter-relationships into statistically independent components, reduce the number of predictive variables and transform data into new variables. Meanwhile, MLR is applied to explain the dependent variables, as it allows formation of explicit equations that are less complex (Ul-Saufie et al. 2013).
The information on possible sources of atmospheric surfactants in the tropical areas with different backgrounds and monsoonal seasons are important due to the influence of surfactants in the global biogeochemical cycles and climate change. This study aims to determine the composition and source apportionment of surfactants in atmospheric aerosols at two different stations in a tropical coastal area of the Malacca Straits. The concentrations of surfactants in atmospheric aerosols in this study are associated with surfactants in the SML collected at different coastal background areas. The measurements of surfactants were conducted in different monsoons to determine the influence of regional wind direction on the composition of surfactants in the coastal area. Source apportionment of surfactants was performed using a combination of multivariate techniques (PCA-MLR) to indice the main sources of surfactants analysed in this study.
Materials and method
Sampling site
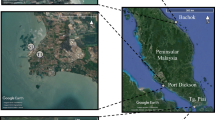
Two sampling stations (Fig. 1) near the Straits of Malacca, peninsular Malaysia, were chosen. The sampling activities were carried out between 2 February and 10 October, 2012. Bayan Lepas, a key population and industrial centre, was selected as the first sampling station. In addition to factories and industrial areas, Bayan Lepas is home to the island’s international airport and Penang’s biggest shopping mall, also built along the coast. Numerous potential sources of contamination, such as urban and industrial waste, and atmospheric deposition from transportation and harbour activities, are well recognised. The sampling was conducted at 5° 18″ 48′ N, 100° 17″ 58′ E, which is located in the southern part of Penang Island, and where extensive industrial activities occur. Furthermore, Penang’s second bridge is under construction and is located near the sampling area.
Further sampling activity was carried out at Port Dickson (2° 28″ 58′ N, 101° 50″ 51′ E) in the state of Negeri Sembilan, which is a centre for tourism. Port Dickson is a popular tourist area along the west coast of the Malaysian Peninsula and is known for water sports. Most of its hotels have been built near the coast, with direct run-off of wastewater to the sea. Anthropogenic sources from shipping and oil refinery activities are another possible source of surfactants in this area (Roslan et al. 2010).
Sampling procedure
Aerosol samples
A high volume sampler (HVS) (Thermo Scientific Model GS2313-105) in combination with a two-stage cascade impactor (Staplex) and a flow rate of 1.13 m3 min−1 were employed throughout the sampling process. Staplex slotted glass-fiber filters (14.3 × 13.7 cm) (Westech Instrument) were used to collect coarse-mode aerosols (aerodynamic diameter > 1.5 μm) and backup borosilicate glass filters (Whatman EPM 2000) (20.3 × 25.4 cm) were used to collect fine-mode aerosols (aerodynamic diameter < 1.5 μm). Both types of filters were wrapped carefully in aluminium foil and pre-heated in a muffle furnace at 500 °C for 4 h prior to use. This procedure was carried out to remove any possible organic matter on the filter papers. The filters were then conditioned in the desiccator for at least 24 h before being weighed with an electronic balance (Shimadzu) and placed in the cascade impactor and HVS. After installation, the HVS was placed in the open field near the sea at the SML sampling site to avoid any nearby material disturbance to the flow of aerosols entering the instrument. Aerosol samples (both coarse and fine mode) were collected within an average time of 24 h. Two samples were collected at each station for replication. Once sampling was finished, the filters were wrapped in aluminium foil and conditioned in a desiccator (24 h) before weighing. The blank filter papers were prepared in the same way as filters for sampling but without exposure to air.
Sea-surface microlayer
A total of 48 SML samples were collected during high tide conditions at the nearest aerosol sampling area using a rotation drum, as suggested by Harvey (1996). Volumes of the 50–500 mL samples collected were then kept in Schott bottles at 4 °C prior to analysis, to prevent the evaporation of volatile components.
Sample preparation
A quarter of the filter containing aerosol particles was cut into small pieces (1 × 1 cm) in a laminar flow before ultrasonic extraction in 40 mL de-ionised water for 45 min (Roslan et al. 2010; Wahid et al. 2013). The samples were then filtered through Whatman 0.45 μm GF/C glass microfiber filters using a vacuum pump. A total of 64 filtered sample solutions were diluted with de-ionised water and kept at 4 °C before analysis. To extract the SML samples, 20 mL samples were filtered through a GF/C glass microfiber filter (Whatman, 47 mm diameter, 0.45 μm pore size) using a vacuum pump (850 Air Compressor) before being put into 40-mL vials equipped with screw caps and Teflon liners.
Surfactant analysis
Both anionic and cationic surfactants were determined using the colorimetric method, as applied in several studies (Oppo et al. 1999; Roslan et al. 2010; Latif et al. 2012). In this study, anionic surfactant was analysed as methylene blue active substance (MBAS), while cationic surfactant was analysed as disulphine blue active substance (DBAS). The principle of the method is based on the formation of an ionic complex between anionic surfactants and cationic dye, and vice versa, before spectrophotometric measurement of the intensity of the extracted compound.
For MBAS, a 20-mL sample was put into a 40-mL vial (vial A) equipped with a screw cap and a Teflon liner. The alkaline buffer (2 mL) and neutral methylene blue solution (1 mL), followed by 5 mL chloroform, were added to the vial (Vial A) in that order, before shaking it vigorously with a vortex mixture for at least 2 min. Once the two phases were separated, a Pasteur pipette was used to transfer the chloroform layers into the new vial (Vial B), which contained 22 mL of ultrapure water and 1 mL acid methylene blue. Vial B was then shaken for 2 min for mixing purposes. After complete separation, the chloroform layer was collected using a Pasteur pipette and put into a 10 mm quartz cell. The absorbance of the chloroform extract was measured using a UV-Vis spectrophotometer at a wavelength of 650 nm.
Meanwhile, for DBAS, 20 mL of the sample in the vial was mixed with 2 mL acetate buffer, 1 mL of disulphine blue solution and 5 mL of chloroform as an organic solvent. The vial containing the solution was shaken vigorously using a vortex mixture in a similar manner to the MBAS method. After separation was complete, the chloroform phase was collected in a 10 mm quartz cell before being subjected to a UV-Vis spectrophotometer at a wavelength of 628 nm.
The calibration curves were linear from 0.05 to 2.00 μM for both MBAS and DBAS using sodium dodecyl sulphate (SDS) and benzyl-dimethyl-tetradecyl-ammonium chloride dehydrade (Zephiramine), respectively. In this analysis, the limit of detection value of 0.05 μM was established using the standard solutions, and the t value and the standard deviation of both SDS and Zephiramine were found. The recoveries for MBAS and DBAS were recorded as 87 % and 89 %, respectively. Two types of blanks, a field blank (the blank filter papers were prepared in the same way as the other filters and placed on the HVS without turning the sampler for 24 h) and a laboratory blank (the filter paper was left in the laboratory environment for 24 h), were analysed for control purposes. All results were corrected using the average blank concentrations.
Ionic compositions
The concentrations of anions (F−, Cl−, NO3 − and SO4 2−) and cations (Na+, K+, Mg2+ and Ca2+) were measured in aqueous extracts of the sample filters. An ion chromatography (Methrom, 881 Compact IC Pro) was equipped with seven Methrosep A-supp 5-150/4.0 columns and a flow rate of 0.7 mL min−1 was used for the determination of anions concentrations. The ion chromatographic system was calibrated daily using seven Merck-certified standard solutions in the range of the analysed samples. Method detection limits of this IC were 0.005 μg m−3 for F− and Cl− and 0.001 μg m−3 for NO3 − and SO4 2−. The recoveries for all anions determined were recorded between 84 % and 118 %.
The concentrations of cations were also determined using ion chromatography (Metrohm, 850 Professional IC 1). The analysis of cations was completed using column type Metrosep C4-100/4.0 and a flow rate of 0.9 mL min−1. In this study, four elements (Na+, K+, Mg2+ and Ca2+) were analysed. Method detection limits were 0.003 μg m−3 for Na+ and K+, 0.004 μg m−3 for Mg 2+ and 0.002 μg m−3 for Ca 2+. The recoveries for all cations determined varied in the range of 98–118 %. The overall blank concentrations obtained were 0.00 μg m−3 for F−, Cl−, NO3 −, SO4 2− and K+, 0.01 μg m−3 for Na+, 0.13 μg m−3 for Mg2+ and 0.01 for Ca2+.
Quality control
Quality control measures were taken to reduce the likelihood of errors in the results. All glassware was washed with hexane, followed by acetone and de-ionised water before being used. The vials were dipped in a 20 % nitric acid bath overnight before being heated in a furnace (500 °C, 3 h). During the experiment and in handling filter papers, gloves were worn and care was taken to avoid any exposure to contamination from the hands. In addition, detergent was prohibited for cleaning of glassware as this could contaminate and influence the results.
Statistical analysis
Statistical analyses of data (analysis of variance [ANOVA], t test and Pearson correlation) were performed using the Student’s Version of the Statistical Package for the Social Sciences (SPSS). Meanwhile, a preliminary source identification study of surfactants in ionic compositions was carried out through PCA-MLR using XLSTAT 2013 Software. Thurston and Spengler (1985) explained that the primary objective in applying PCA analysis is to develop a small number of components that can explain the maximum variance possible in the dataset. Prior to analysis, the outlier values were eliminated and reassigned with concentration values of one half detection limits (Zaghden et al. 2007). All data were normalised using the procedure explained by Thurston and Spengler (1985) and Khan et al. (2010a), and the number of principal components was decided based on eigenvalues >1 (Hogarh et al. 2012; Bingöl et al. 2013). A strong factor loading (>0.75) was chosen for classification purposes (Liu et al. 2003).
Trajectory analysis
Air mass backward trajectory analysis for 72 h wind direction was determined using the Internet-based Hybrid Single Particle Lagrangian Trajectory Model (HYSPLIT) (http://ready.arl.noaa.gov/HYSPLIT.php). The trajectory endpoints file was downloaded to generate a new trajectory plot using IGOR Pro 6 Software. The backward trajectory of wind direction was plotted on a map with pressure level, together with fire hotspot locations, which were downloaded from NASA’s Earth Observing System Data and Information System (EOSDIS) website (http://earthdata.nasa.gov).
Result and discussion
Surfactants in atmospheric aerosols
In this study, 64 aerosol samples were analysed to determine the concentration of surfactants in two different sizes of aerosol samples. The results are presented in Table 1. Overall, no significant correlation (p > 0.05) was found between anionic surfactants, as MBAS, and cationic surfactants, as DBAS, in both fine- and coarse-mode aerosols. The range concentration of surfactants, as MBAS, in fine-mode (PM<1.5) and coarse-mode (PM>1.5) aerosols varied from 63.7 to 174.8 and 27.9 to 62.3 pmol m−3, respectively. Meanwhile, the concentration of surfactants as DBAS ranged from 42.3 to 119.6 pmol m−3 for fine-mode aerosols and 23.8 to 39.9 pmol m−3 for coarse-mode aerosols. Fine-mode aerosols showed the highest concentration for both anionic and cationic surfactants. This was proportional to surfactant concentrations analysed by Roslan et al. (2010) in a similar coastal and marine environment, where the concentration of surfactants in aerosols was dominated by anionic surfactants, particularly in fine-mode aerosols. Humic-like substances, as well as abundant use of anionic surfactants in detergent formulations, could significantly contribute to the surface activity of aerosols, and hence influence the concentration of surfactants in the atmosphere (Latif and Brimblecombe 2004). Various fatty acid compounds acting as surface-active substances, such as myristic, lauric and stearic acid, could also potentially modify the surface tension of sea-spray droplets (Becagli et al. 2011).
Higher concentrations of anionic surfactants in Bayan Lepas are likely to be governed by anthropogenic sources, such as nearby industry. As mentioned earlier, Bayan Lepas is an industrial centre with extensive manufacturing and related economic activity. Non-polar compounds emitted from industrial sources could transform into polar functional groups via photochemical reactions (Frka et al. 2012). These compounds, having both polar and non-polar parts, can act as surfactants. On the other hand, the sample was also expected to be influenced by traffic-related particulate emissions enriched with trace elements released from vehicles (Kuzu et al. 2013). Local domestic activities, such as biomass burning and cleaning routines, could also contribute to a higher level of anionic surfactants in this area, the latter due to the wide application of anionic surfactants in detergent formulations (Cirelli et al. 2008).
Conversely, concentrations of cationic surfactants as DBAS in aerosol samples were not as we expected. Concentrations of cationic surfactants were found predominantly in Port Dickson, particularly in fine-mode aerosols at an average of 76.7 ± 19.5 pmol m−3. As a tourist centre, Port Dickson is greatly exposed to anthropogenic sources of waste water intrusion that may contribute to the high levels of cationic surfactants in this area. Work by Roslan et al. (2010) on surfactant concentration in the coastal areas of the Malaysian peninsula discussed the high concentration of cationic surfactants as DBAS, noting that the levels were affected by organic substances originating from local agricultural activity and soil composition. Meteorological factors such as wind direction could also explain these high concentrations. A more detailed explanation is provided below.
Surfactants in sea-surface microlayer
As supporting data, we have summarised statistical results relating to concentration of surfactants in the SML, with three replications from both locations (Table 2). The statistical approach using ANOVA between surfactants (as MBAS and DBAS) in the SML samples collected from both sampling stations exhibited a significant difference (p < 0.05). Average concentrations of surfactants generally ranged from 0.11 to 0.45 μmol L−1 for MBAS and undetected to 0.29 μmol L−1 for DBAS. The high load of anionic surfactants found in the SML sample reflects the results of previous studies (Latif et al. 2005; Roslan et al. 2010), which showed that the SML is notably enriched with anionic surfactants.
Port Dickson, known as an area of high tourism, demonstrated the highest concentration for both types of surfactants (MBAS = 0.29 ± 0.11 μmol L−1, DBAS = 0.20 ± 0.08 μmol L−1). It is suggested that the high concentrations of surfactants in this area were strongly influenced by anthropogenic activities, due to high levels of tourism before and during the sampling campaign. The sampling area also includes a large oil refinery and extensive shipping activities. We expected the results to be influenced by nearby harbour activity, where surfactants are used as dispersants for fuel oil spillage from boats and ships (Scott and Jones 2000). Wastewater and run-off from neighbouring hotels and restaurants has been identified as the primary source of commercial surfactants released into the sea (Ying 2006). The combination of domestic and industrial discharge into sea water could result in deterioration of water quality (Aydin-Onen et al. 2012) and increase the presence of anthropogenic surfactants in the SML. However, the natural sources of surfactants in the marine environment should be noted. Phytoplankton excretion and bio-degradation of organic substances may be correlated with the production of biogenic surfactants in this area (Vojvodic and Ćosović 1996; Gašparović and Ćosović 2003; Gašparović et al. 2007).
Impact of trajectory on surfactant concentrations
It is important to understand the influence of trajectory on transportation and formation of surfactants in atmospheric aerosols. The regional pressure gap governs the transport of surfactants from their sources. Therefore, we have shown the pressure difference and the subsequent transport pattern of surfactants using a backward trajectory. The backward air mass trajectory for both sampling stations was calculated during the period studied using HYSPLIT Model. The 72-h air mass backward trajectories were plotted together with fire hotspot locations (shown as dotted areas) and trajectory presented in pressure levels mode.
The air mass backward trajectories calculated at both sampling stations showed that the air masses came from southwest Sumatra and travelled from a high pressure level to a lower pressure level (0–3,000 hPa) (Fig. 2). Referring to the air mass backward trajectory for Bayan Lepas (Fig. 2a), we noted several fire hotspots located near the sampling area during the period studied; other points were observed in the north of Sumatra (96°E, 4°N). This observation suggests that the concentration of surfactants in Bayan Lepas is strongly affected by anthropogenic sources arising from nearby industry and from biomass burning composed of high amounts of humic-like substances which enriched the surfactants (Latif et al. 2005; Lin et al. 2010; Song et al. 2012). A study conducted by Wahid et al. (2013) on the effect of the monsoon on surfactant concentrations in tropical areas suggested that southwest wind contributed to the highest level of anionic surfactants, particularly in fine-mode aerosols. This finding strongly supports our discussion in “Surfactants in atmospheric aerosols”, where we found that a higher concentration of anionic surfactant was detected in Bayan Lepas. As for natural sources, sea spray compositions (Cl−, Na+, Mg2+) produced by the bubble scavenging process from the SML (Despiau et al. 1996) and which travels from ocean to land could have significantly influenced the surfactant concentrations in atmospheric aerosols (Roslan et al. 2010; Becagli et al. 2011).
The air mass backward trajectory plot for Port Dickson (Fig. 2b) showed a different pattern from that of Bayan Lepas. The length of the trajectory was shorter, and localised winds within −1°N–3.5°N and 100°E–103°E were observed. The trajectory seems to indicate that sea salts were blown from the ocean to the sampling area, with a small amount of the sea salt being washed out by rainfall (Masiol et al. 2012). Apart from that, several biomass hotspots also revealed during the sampling period represent anthropogenic sources. Internal mixing between sea salt and anthropogenic particles might occur in the coastal atmosphere (Roth and Okada 1998) owing to the high temperature and relative humidity in tropical areas. However, further investigation is needed to verify this speculation.
Ionic composition of aerosols
The ionic compositions of atmospheric aerosol samples at both stations are reported in Table 3. Sea-salt sulphate (ss-SO4 2−) were indirectly calculated from non-sea-salt sulphate (nss-SO4 2−) and Na+ concentration (assuming that the soluble Na+ in aerosol samples comes solely from sea salts) using the seawater ratio, according to the Eq. 1 (Wang and Shooter 2001; Kang et al. 2010; Masiol et al. 2012).
Significant correlations (p < 0.05) were calculated between elements. The major aerosol components at Bayan Lepas, fine- and coarse-mode aerosols, were found in this order: SO4 2− > Na+ > NO3 − > K+ > Cl−, meanwhile the trend of ionic compositions for both aerosol sizes in Port Dickson were: K+ > SO4 2− > Na+ > NO3 − > Cl−. The high concentrations of SO4 2− and NO3 − accounted for the fine-mode aerosol, demonstrating that temporary and localised air pollution does occur in the study areas (Wang and Shooter 2001). At Bayan Lepas station, the high concentrations of SO4 2− and NO3 − presented could have been derived from the formation of secondary aerosols through oxidative processes of SO2 and NOx, emitted most from industrialised regions (Kim and Hopke 2008). The high concentration of K+ in both sampling stations could suggest a biomass burning factor, where K+ is recognised as a biomass tracer (Calvo et al. 2013; Cusack et al. 2013). The presence of hotspot locations during the sampling campaign, as discussed in “Impact of trajectory on surfactant concentrations”, strongly supported this finding. Meanwhile, the sea spray associated with high concentrations of Na+ and Cl− (Cheng et al. 2000; Wu et al. 2007; Karnae and John 2011) was presented at the highest concentrations in Bayan Lepas station; this might be related to its geographical position as a coastal island. The same finding was also observed by Wang and Shooter (2001) in three New Zealand cities, whereby Auckland and Christchurch (coastal cities) demonstrated high levels of Na+ and Cl− compared with Hamilton city (an inland city). However, the ratio of Na+ and Cl− found in both aerosol sizes did not reflect the mean seawater composition. According to Finlayson-Pitts and Pitts (2000), the Cl− depletion process may be the reason for the drop in Cl− to Na+ ratio. Sulfuric and nitric acids have the tendency to react rapidly with NaCl, which is known as the major component of sea-salt particles to produce gaseous HCl under high relative humidity conditions (50–100 %). A similar observation was made by Khan et al. (2010b).
Principal component analysis
In this analysis, the original dataset of ionic compositions were each normalised by using the statistical formula suggested by Thurston and Spengler (1985), before being subjected to the PCA tool in varimax rotation mode using XLSTAT Software. According to these authors, each of the datasets should be transformed into a dimensionless standard form, and an ‘absolute zero’ principle component score should then be calculated by adding an extra day wherein all elemental concentrations are zero. The formula can be expressed as Eq. 2:
where Z is the normalised value of elements, X is the concentration of elements, μ is the mean value of element concentrations and α is the standard deviation. Meanwhile i = 1, 2,…..n, is the number of elements in the analysis and j = 1, 2,….m, is the total number of observations.
The normalised factor loadings after Varimax rotation of the chemical species for the eight sources generated by PCA are given in Table 4. In this study, only factors with eigenvalues > 1 were selected for interpretation. Three major factor loadings were identified from the dataset, with the exception of the fine-mode aerosol at Port Dickson (which were only evident in two major factor loadings). In the fine-mode aerosols from Bayan Lepas, Factor 1 explained 39.28 % of the total variance and was dominated by F− (0.81) and Mg2+ (0.90) which indicated that the sources are from sea spray. According to the Pearson correlation coefficient (Table 5), strong correlations between Mg2+ and Na+ at p < 0.01 were generally found in both sizes of aerosols and strongly supported our interpretations. Meanwhile, Factor 2 explained 19.64 % of the total variance and originated from motor vehicles. This source was classified based on high factor loadings of NO3 − (0.79) and Cl− (0.95) (Tsitouridou et al. 2003; Wahid et al. 2013), with a strong correlation at p < 0.01 (r = 0.74) between both elements. Heavy traffic in Bayan Lepas might attribute to the enhancement of NO3 − concentrations in this area (Khan et al. 2010a). On the other hand, the strong factor loading of Cl− presented in this study was not associated with sea spray sources as there was no correlation found between Cl− and Na+. There was also a moderate correlation at p < 0.05 (r = 0.53) between NO3 − and K+, which demonstrated that sources are strongly associated with combustion-related processes. Factor 3 showed a strong loading of Ca2+ (0.74) with 16.70 % of the total variance, indicating that the source was mineral dust (Tsitouridou et al. 2003; Moreno et al. 2013). The high loading of Ca2+ present could originate from soil dust and nearby construction activities, with a huge application of cement (typically containing >80 % calcium materials and 90 % of CaCO3) (Toledo et al. 2008; Moreno et al. 2013) and other construction materials. All sources for both sampling stations were classified in the same way as in previous apportionment studies.
To determine the surfactant contribution of the identified sources, the MLR statistical approach was then applied. In this study, an absolute principal component score value (APCS) was calculated from each of the factor scores and subtracted from the factor score of absolute zero, as explained by Thurston and Spengler (1985). In the linear regression model, APCS values were assigned as independent variables, meanwhile surfactant concentrations were assigned as dependent variables. The coefficient value (R 2) in each factor was then used to calculate the apportionment of each source to the surfactants concentration. Figure 3 shows the overall apportionment results for MBAS and DBAS for both sizes of aerosol using the ‘leave one out’ validation technique (Dominick et al. 2012). The same technique was applied by Wahid et al. (2013) in the source apportionment study of surfactants based on ionic compositions of particulate matter in tropical urban areas. For Bayan Lepas, motor vehicles (52 %) indicated the highest contribution of MBAS in fine-mode aerosols, followed by sea spray (47 %) and mineral dust (1 %). As for coarse-mode aerosols, sea spray accounted for the highest percentage (51 %), followed by biomass burning (34 %) and motor vehicles (15 %). Conversely, for surfactants as DBAS in fine-mode aerosols, sea spray (56 %) was the main contributor. Meanwhile in coarse mode, the combustion process from motor vehicles (52 %) and biomass burning (37 %) were revealed to be the dominant contributors to DBAS. It was noted that source contributions to surfactants at Bayan Lepas station were actively influenced by both natural and anthropogenic sources.
At Port Dickson, particularly in terms of fine mode aerosols, only two major factor loadings were found. Sea spray accounted for the highest percentage of surfactant contributors for MBAS (88 %) but only 39 % for DBAS. Meanwhile, biomass burning accounted for 12 % and 61 % for MBAS and DBAS, respectively. It can be observed that the source contributors for coarse-mode aerosols for MBAS and DBAS were sea spray (87 % MBAS, 33 % DBAS) followed by motor vehicles (10 % MBAS, 64 % DBAS) and waste incinerators (3 % MBAS, 3 % DBAS). Sea spray showed dominancy in coarse-mode aerosols at both sampling stations, indicating the influence of the SML on the concentration of surfactant in atmosphere (Aller et al. 2005; Becagli et al. 2011).
The mass contribution of each source to the surfactants concentration was then calculated using the APCS-MLR technique (Thurston and Spengler 1985). In this method, only positive masses of APCS were considered for calculation such that the negative masses were removed. According to Khan et al. (2010a), regression of the APCS from each factor will provide an estimation of the coefficients which convert the APCS into pollutant source mass contributions from each source for each sample. In this study, the average mass of each source were compared with the average surfactant concentrations. The results are shown in Table 6. For example, at the Bayan Lepas station the MBAS in fine-mode aerosols, Factor 1, indicated that sea spray represented 9.8 pmol m−3 (8 %) of the surfactant mass, while Factor 2 (motor vehicles) represented 29 %, with a mass of 36.3 pmol m−3 and Factor 3 regarded as mineral dust showed 0.8 pmol m−3 (1 %) of the total surfactant mass. Undefined sources yielded the highest amount of mass contribution, with 76.6 pmol m−3 (62 %) of the total surfactant mass. According to Masiol et al. (2012), a high value of undefined sources is likely to be obtained due to carbonaceous and water components in particles that are not calculated. However, in coarse-mode aerosols, sea spray showed the highest amount of mass contribution (2.5 pmol m−3, 5 %), followed by motor vehicles (2.0 pmol m−3, 4 %) and biomass burning (0.1 pmol m−3, 1 %). Meanwhile, the undefined mass accounted for 41.6 pmol m−3 (89 %) of the total surfactant mass.
On the whole, the different background activities of sampling areas and geographical positions potentially influenced the origin of the surfactants in marine aerosols. In this study, it was revealed that natural sources largely contributed to surfactant concentrations. Sea spray contributed the greatest amount to surfactant concentrations in both aerosol sizes. The SML, enriched with surface-active substances, was strongly involved in the formation of sea spray aerosol (De Leeuw 1999; Cincinelli et al. 2001). Blanchard and Woodcock (1957) and Blanchard (1975) explained that the formation of bubbles bursting from the sea-surface acts as a transport vector of surface-active materials into the atmosphere. The same findings were observed by Becagli et al. (2011), where surfactant, originating from the SML, was distributed in the same size of sea spray components and found to be related to the natural sources. Moreover, motor vehicles and biomass burning were another contributor to the amount of surfactant concentrations particularly in fine-mode aerosols, as predicted by other studies (Pöschl 2005; Wahid et al. 2013). Diesel vehicles in particular emit high amounts of gases and particulate matter, thereby leading to high loads of surfactants (Chow et al. 2002; Latif et al. 2005). Study by Latif et al. (2011) has suggested that there are indications that biomass burning can contribute to a large polar group of molecules which behave like anionic surfactants and are correlated with the amount of surfactants. Another source of surfactants in fine-mode aerosol was found from mineral dust. Meanwhile, waste incinerators were also indicated as a main source of surfactants, mainly in coarse-mode aerosols, characterised by high loadings of SO4 2− and Cl−, which were found predominantly at Port Dickson station.
Conclusion
The main sources of surfactants determined in an industrial (Bayan Lepas) and tourist area (Port Dickson) background stations were identified by using a combination of multivariate analyses (PCA-MLR). This study showed that the anionic surfactants as MBAS dominated both sizes of aerosols. Industrial areas enriched with high loads of anthropogenic sources accounted for the greatest surfactants concentrations of MBAS and DBAS (particularly in fine-mode aerosols). In the SML, however, the highest surfactant levels were found in Port Dickson, owing to the high loads of wastewater released into the sea. Regional wind movement has been recognised as a transport vector for anthropogenic sources (biomass burning) and natural sources (sea spray generated from the SML). Moreover, the high relative humidity observed in tropical areas led to a Cl− depletion process in the atmosphere, which may have caused a lowering in Cl− to Na+ ratio. However, the high amount of secondary aerosols in both aerosols sizes revealed that the influence of localised emissions and short-lived air pollutants is also present at coastal areas of Malacca Straits. The differences in geographical positions and activities occurring around sampling areas have a significant connection to the surfactant concentrations and their origins.
The PCA-MLR and APCS-MLR analyses indicated that sea spray was the main contributor to surfactants as MBAS and DBAS in both aerosol sizes. Meanwhile, motor vehicles and biomass burning were also main contributors to surfactants, particularly in the fine-mode aerosols. Other sources such as mineral dust and waste incinerator were also regarded as another possible source of surfactants in tropical coastal area. The results showed that the surfactants in tropical coastal environments are actively generated from natural and anthropogenic sources. This can affect the biogeochemical cycles and climate in the study areas. In future research, we recommend that wind speed velocity and other meteorological factors should be considered during sampling campaigns. Other organic matter, such as humic-like substances, may be important in predicting the source apportionment of surfactants.
References
Abdul-Wahab SA, Bakheit CS, Al-Alawi SM (2005) Principal component and multiple regression analysis in modelling of ground-level ozone and factors affecting its concentrations. Environ Model Softw 20:1263–1271
Albrecht BA (1989) Aerosols, cloud microphysics, and fractional cloudiness. Science 245:1227–1230
Aller JY, Kuznetsova MR, Jahns CJ, Kemp PF (2005) The sea surface microlayer as a source of viral and bacteria enrichment in marine aerosols. J Aerosol Sci 36:801–812
Aydin-Onen S, Kocak F, Kucuksezgin F (2012) Evaluation of spatial and temporal variations of inorganic nutrient species in the eastern Aegean Sea waters. Mar Pollut Bull 64:2849–2856
Becagli S, Ghedini C, Peeters S, Rottiers A, Traversi R, Udisti R, Chiari M, Jalba A, Despiau S, Dayan U, Temara A (2011) MBAS (methylene blue active substances) and LAS (linear alkylbenzene sulphonates) in Mediterranean coastal aerosols: sources and transport processes. Atmos Environ 45:6788–6801
Bingöl D, Ay Ü, Karayünlü Bozbaş S, Uzgören N (2013) Chemometric evaluation of the heavy metals distribution in waters from the Dilovası region in Kocaeli, Turkey. Mar Pollut Bull 68:134–139
Blanchard DC (1964) Sea-to-air transport of surface active material. Science 146:396–397
Blanchard DC (1975) Bubbles scavenging and the water to air transfer of organic material in the sea. Adv Chem Ser 145:360–387
Blanchard DC, Woodcock AH (1957) Bubble formation and modification in the sea and its meteorological significance. Tellus 9:145–158
Calvo AI, Alves C, Castro A, Pont V, Vicente AM, Fraile R (2013) Research on aerosol sources and chemical composition: past, current and emerging issues. Atmos Res 120–121:1–28
Cheng ZL, Lam KS, Chan LY, Wang T, Cheng KK (2000) Chemical characteristics of aerosols at coastal station in Hong Kong. I. Seasonal variation of major ions, halogens and mineral dusts between 1995 and 1996. Atmos Environ 34:2771–2783
Chow JC, Watson JG, Edgerton SA, Vega E (2002) Chemical composition of PM2.5 and PM10 in Mexico City during winter. Sci Total Environ 287:177–201
Cincinelli A, Stortini AM, Perugini M, Checchini L, Lepri L (2001) Organic pollutants in sea-surface microlayer and aerosol in the coastal environment of Leghorn—(Tyrrhenian Sea). Mar Chem 76:77–98
Cirelli AF, Ojeda C, Castro MJL, Salgot M (2008) Surfactant in sludge-amended agricultural soils: a review. Environ Chem Lett 6:135–148
Cserháti T, Forgács E, Oros G (2002) Biological activity and environmental impact of anionic surfactants. Environ Int 28:337–348
Cunliffe M, Engel A, Frka S, Gašparović B, Guitart C, Murrell JC, Salter M, Stolle C, Upstill-Goddard R, Wurl O (2013) Sea surface microlayers: a unified physicochemical and biological perspective of the air–ocean interface. Prog Oceanogr 109:104–116
Cusack M, Peréz N, Pey J, Alastuey A, Querol X (2013) Source apportionment of fine PM and sub-micron particle number concentrations at a regional background site in the western Mediterranian: 2.5 year study. Atmos Chem Phys 13:5173–5187
De Leeuw G (1999) Sea spray aerosol production from waves breaking in the surf zone. J Aerosol Sci 30:63–64
Despiau S, Cougnenc S, Resch F (1996) Concentrations and size distributions of aerosol particles in coastal zone. J Aerosol Sci 27:403–415
Dominick D, Juahir H, Latif MT, Zain SM, Aris AZ (2012) Spatial assessment of air quality patterns in Malaysia using multivariate analysis. Atmos Environ 60:172–181
Facchini MC, Fuzzi S, Zappoli S, Andracchio A, Gelencsér A, Kiss G, Krivácsy Z, Mészáros E, Hansson H-C, Alsberg T, Zebühr Y (1999) Partitioning of the organic aerosol component between fog droplets and interstitial air. J Geophys Res D 104:26821–26832
Finlayson-Pitts BJ, Pitts JN Jr (2000) Chemistry of the upper and lower atmosphere: theory, experiments, and applications. Wiley, New York
Frka S, Dautovic J, Kozarac Z, Cosovic B, Hoffer A (2012) Surface-active substance in atmospheric aerosol: an electrochemical approach. Tellus B: Chem Phys Meteorol. doi:10.3402/tellusb.v64i0.18490
Gašparović B, Ćosović B (2003) Surface-active properties of organic matter in the North Adriatic Sea. Estuar Coast Shelf Sci 58:555–566
Gašparović B, Plavšić M, Ćosović B, Saliot A (2007) Organic matter characterization in the sea surface microlayers in the surbartic Norwegian Fjords region. Mar Chem 105:1–14
Guidi L, Lorenzini L, Soldatini GF (1988) Phytotoxicity of sea-water aerosols on the forest plants with the special reference to the role of surfactants. Environ Exp Bot 28:85–94
Harvey GW (1996) Microlayer collection form the sea surface: a new method and initial results. Limnol Oceanogr 11:608–613
Hogarh JN, Seike N, Kobara Y, Habib A, Nam J-J, Lee J-S, Li Q, Liu X, Li J, Zhang G, Masunaga S (2012) Passive air monitoring of PCBs and PCNs across East Asia: a comprehensive congener evaluation for source characterization. Chemosphere 86:718–726
Kang J, Cho BC, Lee C-B (2010) Atmospheric transport of water-soluble ions (NO3 −, NH4 + and NSS-SO4 2−) to the southern East Sea (Sea of Japan). Sci Total Environ 408:2369–2377
Karnae S, John K (2011) Source apportionment of fine particulate matter measured in an industrialized coastal urban area of South Texas. Atmos Environ 45:3769–3776
Khan MF, Hirano K, Masunaga S (2010a) Quantifying the sources of hazardous elements of suspended particulate matter aerosol collected in Yokohama, Japan. Atmos Environ 44:2646–2657
Khan MF, Shirasuna Y, Hirano K, Masunaga S (2010b) Characterization of PM2.5, PM2.5–10 and PM10 in ambient air, Yokohama, Japan. Atmos Res 96:159–172
Kim E, Hopke PK (2008) Source characterization of ambient fine particles at multiples sites in the Seattle area. Atmos Environ 42:6047–6056
Kuzu SL, Saral A, Demir S, Summak G, Demir G (2013) A detailed investigation of ambient aerosol composition and size distribution in an urban atmosphere. Environ Sci Pollut Res Int 20:2556–2568
Latif MT, Brimblecombe P (2004) Surfactants in atmospheric aerosol. Environ Sci Technol 38:6501–6505
Latif MT, Brimblecombe P, Ramli NA, Sentian J, Sukhapan J, Sulaiman N (2005) Surfactants in South East Asian aerosols. Environ Chem 2:198–204
Latif MT, Anuwar NY, Srithawirat T, Razak IS, Ramli NA (2011) Composition of levoglucosan and surfactants in atmospheric aerosols from biomass burning. Aerosol Air Qual Res 2011:837–845
Latif MT, Wanfi L, Hanif N, Roslan R, Ali M, Mushrifah I (2012) Composition and distribution of surfactants around Lake Chini, Malaysia. Environ Monit Assess 184:1325–1334
Lin P, Huang X-F, He L-Y, Zhen Yu J (2010) Abundance and size distribution of HULIS in ambient aerosols at a rural site in South China. J Aerosol Sci 41:74–87
Liu CW, Lin KH, Kuo YM (2003) Application of factor analysis in the assesment of ground water quality in Blackforest disease area in Taiwan. Sci Total Environ 313:77–89
Marcomini A, Pojana G, Giacometti A, Oppo C (2001) Aerosolization of an anionic surfactant (LAS) and dissolved organic carbon (DOC) under laboratory conditions. Chemosphere 44:257–262
Masiol M, Squizatto S, Ceccato D, Rampazzo G, Pavoni B (2012) A chemomatric approach to determine local and regional sources of PM10 and its geochemical composition in a coastal area. Atmos Environ 54:127–133
Mcmurdo CJ, Ellis DA, Webster E, Butler J, Christensen RD, Reid LK (2008) Aerosol enrichment of the surfactant PFO and mediation of the water–air transport of gaseous PFOA. Environ Sci Technol 42:3969–3974
Moreno T, Karanasiou A, Amato F, Lucarelli F, Nava S, Calzolai G, Chiari M, Coz E, Artíñano B, Lumbreras J, Borge R, Boldo E, Linares C, Alastuey A, Querol X, Gibbons W (2013) Daily and hourly sourcing of metallic and mineral dust in urban air contaminated by traffic and coal-burning emissions. Atmos Environ 68:33–44
Murphy DM, Anderson JL, Qulnn PK, Mclnns LM, Brechtel FJ, Kreidenwels SM, Middlebrook AM, Posfai M, Thomson DS, Buseck PR (1998) Influence of sea-salt on aerosol radiative properties in the Southern Ocean boundary layer. Nature 392:62–65
O'dowd CD, Hoffmann T (2005) Coastal new particles formation: a review of the current state-of-art. Environ Chem 2:245–255
O'dowd CD, Facchini MC, Cavalli F, Ceburnis D, Mircea M, Decesari S, Fuzzi S, Young JY, Putaud JP (2004) Biogenically driven organic contribution to marine aerosol. Nature 431:676–680
Oppo C, Bellandi S, Degli IN, Stortini AM, Loglio G, Schiavuta E, Cini R (1999) Surfactant components of marine organic matter as agents for biogeochemical fractionation and pollutant transport via marine aerosols. Mar Chem 63:235–253
Paur HR, Cassee FR, Teeguarden J, Fissan H, Diabate S (2011) In-vitro cell exposure studies for the assessment of nanoparticle toxocity in the lung—a dialog between aerosol science and biology. J Aerosol Sci 42:668–692
Pöschl U (2005) Atmospheric aerosols: composition, transformation, climate and health effects. Angew Chem Int Ed 44:7520–7540
Roslan RN, Hanif NM, Othman MR, Azmi WNFW, Yan XX, Ali M, CaR M, Latif MT (2010) Surfactants in the sea-surface microlayer and their contribution to atmospheric aerosols around coastal areas of the Malaysian peninsula. Mar Pollut Bull 60:1584–1590
Roth B, Okada K (1998) On the modification of sea-salt particles in the coastal atmosphere. Atmos Environ 32:1555–1569
Ryu SY, Kwon BG, Kim YJ, Kim HH, Chun KJ (2007) Characteristics of biomass burning aerosol and its impact on regional air quality in the summer of 2003 at Gwangju, Korea. Atmos Res 84:362–373
Scott MJ, Jones MN (2000) The biodegradation of surfactants in the environment. Biochim Biophys Acta 1508:235–251
Shulman ML, Jacobson MC, Carlson RJ, Synovec RE, Young TE (1996) Dissolution behavior and surface tension effects of organic compounds in nucleating cloud droplets. Geophys Res Lett 23:277–280
Song J, He L, Peng PA, Zhao J, Ma S (2012) Chemical and isotopic composition of humic-like substances (HULIS) in ambient aerosols in Guangzhou, South China. Aerosol Sci Technol 46:533–546
Sukhapan J, Brimblecombe P (2002) Ionic surface active compounds in atmospheric aerosols. Sci World J 2:1138–1146
Thurston GD, Spengler JD (1985) A quantitative assessment of source contributions to inhalable particulate matter pollutant in Metropolitan Boston. Atmos Environ 19:9–25
Toledo V, Almeida Júnior P, Quiterio S, Arbilla G, Moreira A, Escaleira V, Moreira J (2008) Evaluation of levels, sources and distribution of toxic elements in PM10 in a suburban industrial region, Rio de Janeiro, Brazil. Environ Monit Assess 139:49–59
Tsitouridou R, Voutsa D, Kouimtzis T (2003) Ionic composition of PM10 in the area of Thessaloniki, Greece. Chemosphere 52:883–891
Udisti R, Dayan U, Becagli S, Busetto M, Frosini D, Legrand M, Lucarelli F, Preunkert S, Severi M, Traversi R, Vitale V (2012) Sea spray aerosol in central Antarctica. Present atmospheric behavior and implications for paleoclimatic reconstructions. Atmos Environ 52:109–120
Ul-Saufie AZ, Yahaya AS, Ramli NA, Rosaida N, Hamid HA (2013) Future daily PM10 concentrations prediction by combining regression models and feedforward backpropagation models with principal component analysis (PCA). Atmos Environ. doi:10.1016/j.atmosenv.2013.05.07
Vojvodic V, Ćosović B (1996) Fractionation of surface active substances on the XAD-8 resin; Adriatic Sea samples and phytoplankton culture media. Mar Chem 54:119–133
Wahid NBA, Latif MT, Suratman S (2013) Composition and source apportionment of surfactants in atmospheric aerosols of urban and semi-urban areas in Malaysia. Chemosphere 91:1508–1516
Wang H, Shooter D (2001) Water soluble ions of atmospheric aerosols in three New Zealand cities: seasonal changes and sources. Atmos Environ 35:6031–6040
Wu C-F, Larson TV, Wu S-Y, Williamson J, Westberg HH, Liu L-JS (2007) Source apportionment of PM2.5 and selected hazardous air pollutants in Seattle. Sci Total Environ 386:42–52
Wurl O, Miller L, Röttgers R, Vagle S (2009) The distribution and fate of surface-active substances in the sea-surface microlayer and water column. Mar Chem 115:1–9
Wurl O, Wurl E, Miller L, Johnson K, Vagla S (2011) Formation and global distribution of sea-surface microlayer. Biogeosciences 8:121–135
Ying GG (2006) Fate, behavior and effects of surfactants and their degradation products in the environment. Environ Int 32:417–431
Zaghden H, Kallel M, Elleuch B, Oudot J, Saliot A (2007) Source and distribution of aliphatic and polycyclic aromatic hydrocarbons in sediments of Sfax, Tunisia, Mediterranean Sea. Mar Chem 105:70–89
Acknowledgement
This study was funded by the Ministry of Science, Technology and Innovation of Malaysia (MOSTI) research grant, E-Science Fund 04-01-02-SF0752. We would like to thank Mrs. Caroline Brimblecombe, Ms. K Alexander and Ms. Fatimah Ahamad for proof reading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Constantini Samara
Rights and permissions
About this article
Cite this article
Mustaffa, N.I.H., Latif, M.T., Ali, M.M. et al. Source apportionment of surfactants in marine aerosols at different locations along the Malacca Straits. Environ Sci Pollut Res 21, 6590–6602 (2014). https://doi.org/10.1007/s11356-014-2562-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2562-z