Abstract
This study investigates chemical composition of rainwater (RW) and its contribution from different sources collected over the period of two years (2016 and 2017) at a high altitude location (1380 m above mean sea level) located at Mahabaleshwar situated in the Western Ghats in Peninsular India. The volume weighted mean pH of RW was found to vary between 4.57 and 7.51 (average 5.95) indicating overall alkaline nature of the RW. Prominent ionic species in the RW were Ca2+ (25%), Na+ (19%), Cl−(23%), SO42− (10%), and Mg2+ (9%) with NH4+, NO3− and K+ together forming about 8% of ionic composition. Moreover, ample presence of dust source (Ca2+) was found that acted as a major neutraliser to the acidic ions. The order of Neutralisation Factor of ions was Ca2+ > Mg2+ > NH4+. In addition, a strong correlation between Na+ and Cl− (r ≈ 0.99) further suggested substantial supplement of marine (NaCl) component to the RW. The impact of local anthropogenic activities such as fossil fuel/biomass burning was observed apart from some contribution from the long-range transport. The high contribution of non-sea salt fractions to Ca2+, SO42−, Mg2+ and K+ showed a substantial effect of crustal and continental air masses. Results of source apportionment for the RW composition by using the Positive Matrix Factorization technique indicated four factors i.e. Marine and long range transport (Na+, Cl−), crustal (Ca2+, Mg2+), emissions from the fossil fuel and biomass burning (NO3−, SO42−) and the agriculture/farming activities (NH4+).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With booming growth in population, economy and energy consumption, atmospheric pollutants are increasing to drastic levels (Mouli et al. 2005; Lelieveld et al. 2019; Keresztesi et al. 2020) in the recent few decades worldwide (Vet et al. 2014). Atmospheric pollutants such as SO2 and NOX are the strong precursors for formation of secondary aerosols SO42− and NO3+, respectively. These pollutants originate from anthropogenic as well as natural activities such as emissions from industries, biomass burning, fossil fuel burning and other commercial/residential activities (Ghude et al. 2008; Mahajan et al. 2015). Past studies have reported acidity of RW turning into extensive environmental hazard affecting the texture of soil, ground water quality, growth of vegetation and plants, surfaces of buildings and monuments, human and animal health, etc. (Fujita et al. 2000; Singh et al. 2007; Alves et al. 2018). Therefore, the WMO (World Meteorological Organisation) initiated GAW (Global Atmospheric Watch) program to keep a close guard on changing RW chemistry via several stations located in different parts of the globe (Bhaskar and Rao 2017).
Chemical composition of RW is an indicator of atmospheric pollution (Calvo et al. 2010), which helps in assessing its broad impact on the ecosystems and ascertains in apprehending the major sources (Mphepya et al. 2004; Li et al. 2007) impacting the environment. RW composition highly varies depending upon the atmospheric gaseous and aerosol composition emitted by various sources and their scavenging by rainfall is the major process that facilitates the encapsulation of these pollutants in RW. This happens by below cloud (wash out) and in cloud (condensation/ incorporation of water vapours on/with particles/gases during the formation of clouds during nucleation process) scavenging (Migliavacca et al. 2010). The falling rain not only removes atmospheric pollutants (Santos et al. 2011; Yang et al. 2019a) by encapsulating the particles and making the atmosphere clean and pristine, but this phenomenon changes the chemical characteristics of the RW. Therefore the chemical composition of RW affected by natural as well as anthropogenic pollutants, indirectly furnishes the information regarding the air quality of the atmosphere (Sakihama et al. 2008).
Earlier studies have reported that the size and chemical composition of the scavenged particles immensely influence the pH and the chemical composition of the RW (Chate et al. 2011; Al-Khashman 2005a, b). In recent times, few studies have suggested that drastic increase in anthropogenic activities have led to increase in acidic ions thereby decreasing the pH of RW (Losno et al. 1991; Chester et al. 1993; Migliavacca et al. 2005). While few studies indicate these changes in pH are mostly due to locally driven aerosol sources, others have shown the influence of long-range transport of pollutants over the locally emitted sources that impact the regional RW chemistry (Yu et al. 1998; Fujita et al. 2000). Acidic/alkaline nature of RW largely depends on the source strength and neutralization extent, produced by different chemical constituents available in the atmosphere such as ammonium, calcium and magnesium carbonates and hydroxides ((Flues et al. 2002; Migliavacca et al. 2005; Al-Khashman 2009).
However, chemical composition of RW varies from region to region and also seasonally (Kulshrestha et al. 2003). Dusty soil (Ca2+, Mg2+) constitutes a major fraction of total aerosols over the Indian region (Khemani et al. 1985). Some studies have reported the chemical composition of RW to be more on alkaline side due to the higher concentrations of soil dust and a lower concentration of secondary acidic aerosols (SO42− and NO3−) after the first washout event (Saxena et al. 1996). The concomitant presence of different sources together makes it difficult to apportion and classify the major sources of RW composition. PMF (Environmental Protection Agency −5) is a strong tool that factorizes chemical species into various contributing sources. Rao et al. (2016) have implemented this technique in understanding RW composition-based source apportionment. A detailed discussion about PMF analysis is given in the methodology section. Several studies have been reported on the chemical composition of RW from different locations in India (Khemani et al. 1985, 1987, 1989; Varma, 1989; Rao 1997; Momin 1990; Mukhopadhyay et al. 1992; Naik 2001; Pillai et al. 2001; Safai et al. 2004; Kulshrestha et al. 2003; Khare et al. 2004; Chandra Mouli et al. 2005; Rastogi and Sarin 2005; Singh et al. 2007; Hegde 2007; Nagendra Prasad et al. 2008; Tiwari et al. 2008; Gobre et al. 2010; Gobre et al. 2010; Prathibha et al. 2010; Budhavant 2011). Recently Bhaskar and Rao (2017) have given a very comprehensive review of long term trends in RW chemistry over the Indian region which shows the increasing acidification of RW over almost all the ten GAW stations in India. However, studies on chemical composition of RW from high altitude locations are very few (Safai 2000; Praveen 2008; Tiwari et al. 2012, 2015; Bisht et al. 2017). Especially, such studies in the eco-sensitive region of Western Ghats are very scarce (Budhavant et al. 2009; 2014) and the present study aims to fill this gap to some extent by assessing major ionic species, pH and source apportionment of different constituents of RW.
2 Data & Methodology
2.1 Location and details of sampling site
The mountainous region of Western Ghats runs parallel to the west coast of Indian Peninsula with an average elevation of 1200 m. It is one of the eight biodiversity hotspots in the globe and the mountainous eroded edge of the Deccan Plateau is around 150 Ma old. The rock consists mostly of a Basaltic composition (45–55% wt SiO2, 2–6% wt total alkalis, 0.5–2.0% wt TiO2, 5–14% wt FeO and 14% wt Al2O3. 10% wt CaO, 5–12% wt MgO). During south-west monsoon, these mountains intercept moist-laden clouds and strong winds with an average precipitation of ~300 cm of rainfall and about 80% of it is received during the south west monsoon from June to September. Few major rivers have their origin from Western Ghats such as River Krishna that flows across four states in Peninsular India i.e. Maharashtra, Karnataka, Telangana and Andhra Pradesh.
Mahabaleshwar is about 117 km south to the Pune Metropolis and is one of the famous high altitude tourist spots in India surrounded by natural vegetation that expands to a radius of about 25 km and has an aerial distance of about 60 km from the Arabian Sea to the west. 2011 census reports 12,737 populations for the town; and people livelihoods are mostly driven by tourism. The annual average rainfall at Mahabaleshwar is ≥ 5000 mm. A few chemical and pharmaceutical industries (under the Maharashtra state Industrial Development Cooperation zone) are located in clusters in the vicinity of 60 km distance from the Mahabaleshwar. Due to the prevailing ambient climatic conditions, different varieties of cash crops such as strawberry, mulberry, broccoli, carrots and other are farmed in the surrounding region.
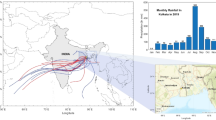
The sampling site is situated at the High Altitude Cloud Physics Laboratory (HACPL) located in Mahabaleshwar (17.92 ON, 73.66 °E) at an altitude of 1380 m above the mean sea level (AMSL) (Fig. 1). The yearly average maximum temperature is ~26 °C and average minimum is ~16 °C (Source: India Meteorological Department). Due to the high altitude site, the average ambient pressure is ~850 hPa and is directly influenced by strong south-westerly low-level jets during the monsoon period. The average wind speed during monsoon is ~ 5 m/s during active spells. The region features an amalgamation of various active sources of aerosols present during the south-west monsoon period, such as dust (Ca2+) transport from across the Arabian Sea, sea salt (NaCl) from the ocean and secondary aerosols (SO42− and NO3−) from local anthropogenic activities and partial transport from the distant sources.
a) Location of Mahabaleshwar in India map with the accumulated TRMM rainfall pattern for 2016 and 2017 overlapped by ERA 850 hPa wind vectors during SW monsoon rain (small window left image shows automatic precipitation collector installed at HACPL), b) ionic balance of RW for 2016 and 2017 monsoon rainfall
2.2 Rainwater sampling and chemical analysis
RW samples were collected during south-west monsoon period (June – September) for two consecutive years 2016 and 2017. The total rainfall during the monsoon period was 6665 mm and 5527 mm respectively in 2016 and 2017. RW samples were collected by an automatic precipitation collector (Eigenbrodt GmbH and Co, KG, NMO191/E) which is wet only bulk collector. The instrument (Fig. 1a.) is installed onto the terrace of the HACPL which elevates about 10 m above the ground surface. The collector stands 2 m above the terrace surface to avoid hindrances and unwanted splashes of rain from the nearby collocated instruments and parapet wall. This instrument has a lid on top of collecting funnel that opens only during rainy events and automatically closes in non-rainy duration. This auto shut helps in efficient collection of wet deposition by inhibiting dry deposition during non-rainy events. The inner funnel has a nylon mesh which avoids entry of any coarse unwanted solid particles carried by wind during the open lid periods. The instrument collects rainwater samples in the pre-cleaned high-density polyethylene (HDPE) bottle for the span of 24 h. The samples were collected daily and immediately transferred from the collection jar to a small aliquot sample of 100 ml in HDPE bottles after being filtered through 0.45 μm pore size nylon filters. The electrical conductivity (EC) and pH of every sample were measured immediately after the sample is collected and filtered to avert any contamination in the chemical composition due to absorption of the gases and the samples were stored in refrigerator at 4 °C to avoid any decomposition or volatilization of ions. The chemical constituents of RW were further analysed by using ion chromatography (IC) technique. Major cations (NH4+, Na+, K+, Ca2+, and Mg2+) and anions (Cl−, NO3− and SO42−) were quantified by analysis done with an Ion Chromatograph (Metrohm IC, 850). The samples were injected through two different 20 μl sample loops for anion and cation columns. 3.2 mMol/L Na2CO3 and 1.0 mMol/L NaHCO3 eluent solution (mobile phase) with a flow rate of 0.7 ml/Min was used with Metrosep A Supp 5–150/4.0 column for determination of anions (Metrosep A Supp 5–150/4.0 column is 150 mm in length and 4 mm in diameter and have Polyvinyl alcohol with quaternary ammonium groups as substrate or stationary phase) For cations, 0.7 mMol/L Dipicolinic acid and 1.7 mMol/L HNO3 eluent solution (mobile phase), maintained with a flow rate of 0.9 ml/min was passed through Metrosep C 4–150/4.0 column (same dimensions as anion column except substrate or stationary phase which is silica gel with carboxyl groups). The precision of relative standard deviation of repeated samples was found to be ~ 4% for both the cations and anions. The HCO3− concentrations were estimated using the pH of the sample (Das et al. 2005).
The IC was calibrated before every analysis. The calibration was cross verified with known concentration of laboratory simulated rainwater samples provided by World Meteorological Organisation under Laboratory Inter-comparison Study (LIS) program. A detailed validation using LIS sample for the IC is reported in different study for the same region (Yang et al. 2019b). Also, the data quality of analysis is assured for each batch by ionic balance approach. For EPA PMF 5.0 model run, Samples having missing data were excluded, and sample having concentrations lower than Method Detection Limit (MDL) was replaced by the species MDL/2 (Polissar et al. 1998; Reff et al. 2007). Ionic balance method was applied on data gained by the chemical analysis of samples using the guidelines provided by World Meteorological Organisation - Global Atmosphere Watch quality criteria (2004). As a quality control measure of the data, linear regression analysis was used to assess the net charge balance between the rainwater sample cation (NH4+, Na+, K+, Ca2+, and Mg2+) versus anion (Cl−, NO3−, SO42−, and HCO3−) (Rastogi and Sarin 2005). The slope value > 0.9 of the regression analysis in 2016 and 0.73 for 2017 depicted considerable agreement between the ionic (cations and anions) charges (Fig. 1 b.), and was also found to be in a coherent range as earlier reported (Rastogi and Sarin 2005). Considering the above criteria, a complete data set of 225 samples was confirmed and considered for further analysis.
2.3 Neutralization and enrichment factor calculation
The Volume Weighted Average (VWA) of the ionic species in RW was calculated as:
Here, Xi stands for the concentration of ionic species (μeq/L) in RW collected over 24 h. Ti represents total rainfall amount of the event (ml) and n is the total of number of rainfall events (Akpo et al. 2015). In addition, to understand the dominant neutralising ion that may counter acidic ion such as SO42−and NO3−, the neutralization factors (NF) were computed for dominant cations (Ca2+, Mg2+, NH4+) using the formula (Possanzini et al. 1988; Prathibha et al. 2010) shown as per Eq.2, 3 and 4.
The extent of enrichment of ionic species in RW in terms of sea salt and non-sea salt fractions was assessed by computing the Enrichment Factor (EF) (Kulshrestha et al. 1996) as shown in Eq.5. Na+ is considered as a reference element having absolute sea origin (Samara et al. 1992). And, ‘X’ is the desired ion of interest in the RW sample.
(X/Na+)Rain is ratio of desired ion to Na+ in RW sample, and (X/Na+)sea is standard ratio of desired ion to Na+ in seawater. Sea water standard reference ratios have been obtained from Keene et al. (1986). EF has been broadly used for identifying the source of different chemical species in RW and its extent of contribution (Bisht et al. 2017). EF value near or equal to 1 signals the dominance of marine sources, whereas EF values greater than 1 suggest enrichment from non-marine sources (Alves et al. 2018). Sea Salt Fraction (SSF) and Non-Sea Salt Fraction (NSSF) of ions to the total concentration can be calculated by considering Na+ as reference element, which has marine origin only (Keene et al. 1986). Percentage contribution of sea salt and non-sea salt to the total concentration of SO42−, K+, Ca2+, Mg2+ were calculated by using Eq.6 and 7 (Kulshrestha et al. 1996).
2.4 Use of EPA PMF 5.0 model for rainwater source apportionment
One of the widely used techniques for assessing the magnitude of contribution of various sources towards composition of the RW is the receptor models based on information of the chemical constituents of RW. In this work, Windows based Environmental Protection Agency-Positive Matrix Factorization (EPA - PMF V.5.0.14) technique was employed to segregate the emission sources of chemical species found in RW. This can be done using measured source profile information and emissions inventories. However, such source profiles information for the RW is yet not fully accomplished. And this pertains to utilize PMF model that decomposes the input concentration matrix X of sample data in (a) factor contribution - G and (b) factor profiles –F matrix. The concentration data matrix X of the samples is of dimensions i by j, in which i is number of samples and j is the column representing various chemical species in RW, with uncertainties u for the chemical components of interest. The aim of receptor models is to solve the chemical mass balance (CMB) between measured species concentrations and source profiles, with number of factors p, the species profile fk of each source or factor K, and the amount of mass gk contributed by each factor k to each individual sample and can be represented by the following eq. (8).
Here, eij is the residual part of individual sample and cij denotes the modelled part of individual sample (Paatero et al. 2014). In case of notations, capital case letters denote whole matrices, gk denotes columns of the factor contribution matrix G, and fk denotes rows of factor profile matrix F (Paatero et al. 2014; Paatero 1997).
In PMF sample concentration the uncertainty matrix related to the sample data ensures the trust in the measurement. PMF tool gives an advantage to include data below detection limit to be retained but with the associated uncertainty so that such data have less influence on the solution than the measurements which are above detection limit (Paatero et al. 2014). The eq. (9) is next step where the Q (objective function) is minimized for best solution, which is also based on uncertainties within the measurement, factor matrix elements gik and fkj to non-negativity constraints (EPA 2014; Alves et al. 2018).
In PMF analysis, finding the uncertainties is of critical importance. For this reason, sample data was categorised in two sections. Uncertainties of the ionic species having concentration values less than Method detection limit (MDL) are calculated by (5/6)*MDL (Polissar et al. 1998; EPA 2014). Whereas the uncertainties of the ionic species with concentration greater than MDL are calculated as per Eq. 10.
Prior to run PMF a signal to noise(S/N) ratio was used to categorise the chemical species into good or bad data classification. The species which showed S/N less than 0.5 were considered as ‘Bad’ data whereas the species between 0.5–1 were considered as ‘Weak’ and greater than 1 as ‘Strong’ (EPA 2014). Majority of the species had S/N ratio greater than 1 and were categorised as ‘Strong’ for PMF analysis.
3 Results and discussions
3.1 Chemical characterisation of rainwater
The Minimum, Maximum, Average with Standard deviation and the volume weighted averages (VWA) in μeq/L of concentration of ionic species and pH for the samples of RW collected in 2016 and 2017 at Mahabaleshwar are shown in Table 1. The descending order of VWA concentration in μeq/L of the major ionic species in RW follows the order Ca2+ (45.71) > Cl−(39.32) > Na+ (35.86) > Mg2+ (20.06) > SO42− (18.10) > NH4+ (10.27) > HCO3− (9.29) > NO3−(5.04)> K+ (4.59) > H+ (2.11). Figure 2 depicts the percentage contribution of measured ionic species of RW. It can be seen that maximum fraction was from Ca2+ (24%) and minimum from H+ (0.6%). The significant input from marine source in the form of NaCl sea salt (Rastogi and Sarin 2005; Alves et al. 2018) and from the crustal source in the form of Ca2+and Mg2+ can be observed. In addition to it, contribution from anthropogenic sources (SO42−, NO3−, K+ and NH4+) is also found in RW composition during the monsoon rains, which had been earlier associated with local wood burning activities (Mukherjee et al. 2018). However, another major source for the observed monsoonal RW characteristics could be transported dust and sea salt from other regions. Similar results are also reported by earlier studies (Ramaswamy et al. 2017; Yang et al. 2019a). In this study, the sum of average values of cations was about 151μeq/L and that of anions was ~104 μeq/L. This clearly shows the dominance of cations over anions. The average values of concentrations in the ionic species shows higher than that of the median values, which indicates the uneven distribution of the species, suggesting skewness to the left. However, the median values and the average concentration follow the similar pattern. Thus, to understand the higher concentration during short spells and to avoid clean rain dilution affecting the rainwater concentration, VWA is utilized (Xiao 2016). Surprisingly, it is noticed that first four highest VWA species for the monsoon rainfall were Ca2+, Cl−, Na+ and Mg2+. An VWM pH was found to be 5.95 and reached as high to 7.51 during increase in Ca2+ (478.5 μeq/L) in RW. This indicates Ca2+ to be the major neutralizing agent in rainwater. However, it is also good to mention that Mg2+, NH4+, and K+ can also neutralize acidity generated due to SO42− and NO3− to control the pH of RW in the alkaline range.
VWA of major ions and pH of RW reported from the studies conducted from different high altitude locations in India have been compared with the present study (Table 2). The pH of RW observed at Mahabaleshwar was slightly more alkaline than that reported for the high altitude locations in north (Kothi, Nainital), east (Darjeeling) and south (Silent Valley) Indian region. However, it was comparatively less alkaline as compared to the other high altitude site, Sinhagad in the Westerh Ghat ranges. The concentration of acidic components (NO3+ and SO42−) and at alkaline ion NH4+ at Mahabaleshwar was comparable with the other locations but was much less than that at Darjeeling. Concentration of K+ was also comparable with the other locations while it was less than that at Darjeeling and Nainital. Sea salt component (Na+ and Cl−) was comparable with the other locations but was comparatively more than that at Kothi. Dust component (Ca2+ and Mg2+) was much less that that at Darjeeling and Nainital. The observed differences between ionic composition and subsequently that between the pH values of Mahabaleshwar with the other reported high altitude sites from India are attributed to the differences in the surrounding topography, altitude variation and varying local activities/sources over these locations.
3.2 Characteristics of rainwater pH
The average pH of RW at Mahabaleshwar was 6.01 ± 0.51 whereas the VWM pH was 5.95 and it ranged between 4.57 and 7.51 (Table 1). This indicates dominance of alkaline species in the RW at Mahabaleshwar (Wu et al. 2016; Cao et al. 2009). The pH of 5.65 represents balance between atmospheric CO2 and atmospheric water; this value is also called as CO2 equilibrated value and is a threshold pH for natural acidity of RW (Khemani et al. 1989). While deciding this threshold value, only the acidic effect of weak carbonic acid is considered and that of the other acidic components originated from sulphur and nitrogen compounds is not taken into account. In the present study, about 77% of samples fell under the category of alkaline nature. The frequency distribution of pH (Fig. 3) shows maximum frequency in the range between 6.01–6.50 (46%) followed by the range 5.51–6.00 (27%) indicating higher occurrences of pH in the range 5.51–6.50. Samples with pH below 5.65 are all considered as acidic. This pinpoints that lesser contribution by acidic components or their total neutralisation which resulted in their minor influence on pH values. The pH category of 4.5–5.0 is mostly dominated by SO42− and NO3− making 46% of the total ionic contribution to this category. This is followed by pH 5.0 to 5.5 range, having more contribution of cations shifting the pH value towards neutrality. The pH values which fall in alkaline range had shown more presence of cations. Ca2+ contributes 48%, 51%, 52% and 58% as compared to Mg2+ (19%, 18%, 18% and 16%) and NH4+ (8%, 6%, 3% and 3%) for the pH categories of 5.5–6.0, 6.0–6.5, 6.5–7.0 and 7.0–7.5, respectively. This clearly indicates that Ca2+ was the dominant species and played a vital role in deciding the alkaline nature of the RW. The high load of Ca2+ can be justified with higher contribution by local crustal source as well as from the long-range transport from the Arabian Gulf region as discussed in PMF analysis in the section 3.5. A similar study carried out at another location in Western Ghats namely Sinhagad (a high altitude station near Pune which has similar geographical conditions) during summer monsoon showed similar type of pH values of RW, indicating the alkaline nature of RW underlining the dominance of alkaline components (Budhavant et al. 2009). Therefore, it necessitates further investigating the leading neutralizers and their possible enrichment from sea and land originated components to the RW chemistry at Mahabaleshwar.
3.3 Wind back trajectories and impact on pH
Even though the winds were predominantly from south west and west direction during the monsoon at the sampling site; occasionally some winds from other directions were also observed. Figure 4 depicts the variation of pH of RW according to the changes in wind direction. For pH bins 4.5–5.0 and 5.0–5.5, the major contribution (about 40%) was from south westerly winds however winds from north, north west, north east and south also showed presence and these winds were comparatively calm and carried air mass from local sources (vehicular and industrial emissions) with them. The anthropogenic influence from these winds might have reduced the pH due to the probable involvement from certain acidic species. For pH bins 5.5–6.0 and 6.0–6.5, almost 80% of winds were from south west and west bringing in more marine species from the Arabian Sea which are normally neutral in nature. As the occurrences of terrestrial winds were few, they showed lesser impact. This could be the reason the pH lingers towards neutrality in this case. In case of 6.5–7.0 pH bins, westerly wind was mostly observed that brings in marine as well as some terrestrial components. While pH bin 7.0–7.5 had major influence from south-westerly, westerly and north-westerly bringing with them mainly sea salt aerosols from the Arabian Sea and dust particles from the arid areas in north western part of India.
3.4 Neutralization and enrichment factors
The neutralization factor (NF) analysis of RW reveals that Ca2+ has maximum efficiency in neutralising the acidity of SO42−and NO3− ions (NF=1.82). Whereas Mg2+was found to be second most considerable contributor in neutralising acidic species showing neutralisation factor near to 0.99. Moreover, NH4+ had least presence to the overall neutralization of acidic anions with NF around 0.59. Similar study conducted at New Delhi and Agra (Saxena et al. 1996; Bisht et al. 2017) that fall sunder IGP and dust transported region as well as that at Pune (Khemani et al. 1985; Rao et al. 2016) showed comparable results and also concluded that Ca2+ is the important species for neutralising acidity of RW.
In addition to it, enrichment factors (EF) of Ca2+, K+, Mg2+and SO42− were calculated along with the sea salt and non-sea salt fractions to RW (Table S 1.). It is identified that, all the aforementioned chemical species have EF values >1which clearly infers towards the enrichment of all the measured species of RW from sources other than seawater. The order of non- sea salt (NSS) contribution is Ca2+ (96%) > K+ (79%) > SO42− (78%) > Mg2+ (62%). There may be dissolution of Ca2+, K+ and Mg2+ from crustal source into the RW that might have led to higher enrichment for them. However, at Mahabaleshwar during the frequent and occasionally incessant spells of rains in monsoon, surface soil always remains wet, thus suppressing local airborne dust and also the soil is mostly covered by vegetation and therefore the enrichment of these components could be attributed to the major contribution from long range transported dust from other sources. But it is good to mention that during break spells in monsoon, local surface is drier and soil eroded dust might also play significant role in enriching these components of RW. Enrichment factor (Table S 1) and Correlation study (Table S 2) point towards the appreciable involvement of sources other than marine salt to higher NSS% of Ca2+ that probably come from the crustal source. Previous studies have indicated that wind transported dust and sea salt particles and local soil were salient sources of many ions in precipitation (Khemani et al. 1985; Al-Momani 2003; Başak and Alagha 2004). This observation shows the crucial role of contribution from crustal source to the RW composition together with the marine source. Cl− originates mainly from sea (Rastogi et al., 2005) and showed good correlation with Na (r=0.90) suggesting sea salt engulfing the observational site due to its close proximity (~100 km aerially) to the Arabian Sea.
Subsequently, contribution from the anthropogenic activities towards the acidic ions such as SO42− and NO3− was noticed due to secondary aerosol formation that originated from local combustion activities involving fossil fuel burning (vehicular exhausts) and biomass burning (domestic and agricultural activities). Similarly, K+ shows NSS% as high as 79%, which was earlier reported from the local wood burning activities used for heating water by local residents during monsoon due to unavailability of solar heater because of persistent dense cloud cover (Singla et al. 2019). Also, NH4+ was poorly correlated with any other species implying the presence of a single source which could be use of fertiliser in local agricultural activities or human/animal excreta. Overall, the presence of vegetation and farming activities in the region strongly implicate in RW composition at Mahabaleshwar apart from the impact from certain tourist related anthropogenic activities.
3.5 Sources of chemical species by PMF analysis
Furthermore, to unravel the impact of different sources of chemical species on RW composition, Positive Matrix Factorisation (EPA PMF 5.0) based assessment was carried out. PMF was run by considering 2 to 6 factors. The optimum 4 factors solution was accepted based on scaled residuals. As seen in Fig. 5, Factor 1 had dominance of NH4+ and K+ that is 100% and 69% respectively. NH4+ ion is produced by variety of sources which involves volatilization of animal remainings, human excretion, decomposition of plants, and other sources such as emissions from fossil fuel and fertilizer industries (Wu et al. 2016). On the other hand, higher presence of K+ can be attributed to biomass burning (Pachon et al. 2013) as also confirmed from NSS% (see Table S 1). Moreover, use of coal and wood burning in the local household results in the higher presence of K+. Agricultural activities could also be a source for the presence of K+ (Yang et al. 2019a). Therefore factor 1 is labelled as from biological sources and biomass burning.
Crustal source which is shown by factor 2 was reported to be completely dominated by Ca2+ as well as with considerable presence of Mg2+. About 55% of the total contribution of Ca2+ was seen in the factor 2 whereas Mg2+ contributed only about 21%. Both these components can be attributed to the local soil source.
Factor 3 represents the major contribution by anthropogenic activities such as vehicular emissions, biomass burning, fossil fuel burning and industrial emissions (a cluster of industries in Maharashtra Industrial Development Corporation (MIDC) in Mahad is about 60 km from the sampling site). This factor showed major contribution from SO42− and NO3− which is about 22% and 84%, respectively. In earlier studies, NOx emissions from the industries in the Western Ghats have been reported (Ghude et al. 2008). In addition, Factor 1 has good amount of contribution by SO42− indicating adequate role of fossil fuel burning (Roy et al. 2016). The presence of small amount of Cl− also indicates towards emissions from industrial sources, coal burning and incineration processes (McCulloch et al. 1999; EC Report 2007). Higher presence of SO42− and NO3− infers towards secondary inorganic aerosol formation (Yang et al. 2019a).
Whereas factor 4 signals towards combined presence of two separate sources; long range transported sea salt and dust which is characterised by 74%, 79%, 37% and 34% contribution respectively to Na+, Cl−, Mg2+ and Ca2+ components of the RW.
4 Conclusions
The study was conducted on RW chemistry at a hilly location, Mahabaleshwar in Peninsular India during summer monsoon of 2016 and 2017. RW was analysed in order to find its chemical composition, acidity of rains and prominent neutralizing ions along with the source apportionment for the measured ionic components. This has been the first comprehensive study on the RW composition from this less polluted hilly station in the Western Ghats mountainous region in south west India which falls under the global eco-sensitive zones. The major conclusions of the study can be summarised as:
-
The VWM pH value was found to be alkaline (5.95 ± 0.51) for the period 2016 and 2017. About 23% of wet precipitation episodes were recorded as acidic rain episodes (pH < 5.65). The acidic samples showed considerable concentrations of SO42− and NO3−.
-
VWM of ionic composition of RW showed dominance of sea salt and dust (Ca2+, Cl−, Na+ and Mg2+) forming about 76% of the total measured RW composition whereas anthropogenic components (SO42−, NH4+ and NO3−) together contributed only about 16%.
-
The neutralisation efficiency was maximum for Ca2+ followed by Mg2+ and NH4+. It was noticed that Mg2+ had about 50% higher NF value than NH4+.
-
Enrichment factor and non-sea salt fractions suggested that terrestrial sources of Ca2+, Mg2+, K+ and SO42− are predominant. In addition, the possible role of long-range transport from distant sources cannot be overlooked.
-
Source apportionment of ionic components of RW using the EPA PMF 5.0 model indicated four factors. Higher concentrations of NH4+ and K+ were related to agricultural activities, animal excretion and biomass burning activities. The main source of NO3− and SO42− was attributed to the vehicular and industrial emissions. Natural sources were split into two factors i.e. crustal (Ca2+ and Mg2+) and marine (Na+ and Cl−) together with long range transport from the Gulf region and Arabian Sea.
References
Akpo, A.B., Galy-Lacaux, C., Laouali, D., Delon, C., Liousse, C., Adon, M., Gardrat, E., Mariscal, A., Darakpa, C.: Precipitation chemistry and wet deposition in a remote wet savanna site in West Africa: Djougou (Benin). Atmos. Environ. 115, 110–123 (2015)
Al-Khashman, O.A.: Ionic composition of wet precipitation in the Petra region. Jordan. Atmos. Res. 78, 1–12 (2005a)
Al-Khashman, O.A.: Study of chemical composition in wet atmospheric precipitation in Eshidiya area, Jordan. Atmos. Environ. 39, 6175–6183 (2005b)
Al-Khashman, O.A.: Chemical characteristics of rainwater collected at a western site of Jordan. Atmos. Res. 91, 53–61 (2009)
Al-Momani, I.F.: Trace elements in atmospheric precipitation at northern Jordan measured by ICP-MS: acidity and possible sources. Atmos. Environ. 37, 4507–4515 (2003)
Alves, D.D., Backes, E., Rocha-Uriartt, L., Riegel, R.P., de Quevedo, D.M., Schmitt, J.L., da Costa, G.M., Osório, D.M.M.: Chemical composition of rainwater in the Sinos River basin. Southern Brazil: a source apportionment study. Environ. Sci. Pollut. Res. 25, 24150–24161 (2018)
Başak, B., Alagha, O.: The chemical composition of rainwater over Büyükçekmece Lake. Istanbul. Atmos. Res. 71, 275–288 (2004)
Bhaskar, V.V., Rao, P.S.P.: Annual and decadal variation in chemical composition of rain water at all the ten GAW stations in India. J. Atmos. Chem. 74, 23–53 (2017)
Bisht, D.S., Srivastava, A.K., Joshi, H., Ram, K., Singh, N., Naja, M., Srivastava, M.K., Tiwari, S.: Chemical characterization of rainwater at a high-altitude site “Nainital” in the Central Himalayas, India. Environ. Sci. Pollut. Res. 24, 3959–3969 (2017)
Budhavant, K.B.: Studies on Precipitation Chemistry at Different Locations over Pune Region, PhDThesis. Univ. Pune. (2011)
Budhavant, K.B., Rao, P.S.P., Safai, P.D., Ah, K.: Chemistry of monsoon and post-monsoon rains at a high altitude location, Sinhagad, India. Aerosol Air Qual. Res. 9, 65–79 (2009)
Budhavant, K.B., Rao, P.S.P., Safai, P.D., Granat, L., Rodhe, H.: Chemical composition of the inorganic fraction of cloud-water at a high altitude station in West India. Atmos. Environ. 88, 59–65 (2014)
Calvo, A.I., Olmo, F.J., Lyamani, H., Alados-Arboledas, L., Castro, A., Fernández-Raga, M., Fraile, R.: Chemical composition of wet precipitation at the background EMEP station in Víznar (Granada, Spain)(2002–2006). Atmos. Res. 96, 408–420 (2010)
Cao, Y.-Z., Wang, S., Zhang, G., Luo, J., Lu, S.: Chemical characteristics of wet precipitation at an urban site of Guangzhou. South China. Atmos. Res. 94, 462–469 (2009)
Chate, D.M., Murugavel, P., Ali, K., Tiwari, S., Beig, G.: Below-cloud rain scavenging of atmospheric aerosols for aerosol deposition models. Atmos. Res. 99, 528–536 (2011). https://doi.org/10.1016/j.atmosres.2010.12.010
Chester, R., Murphy, K.J.T., Lin, F.J., Berry, A.S., Bradshaw, G.A., Corcoran, P.A.: Factors controlling the solubilities of trace metals from non-remote aerosols deposited to the sea surface by the ‘dry’deposition mode. Mar. Chem. 42, 107–126 (1993)
Das, R., Das, S.N., Misra, V.N.: Chemical composition of rainwater and dustfall at Bhubaneswar in the east coast of India. Atmos. Environ. 39, 5908–5916 (2005)
EPA: Positive Matrix Factorization (PMF) 5.0-Fundamentals and User Guide. US Environmental Protection Agency, Washington DC. USA https://www.epa.gov/sites/production/files/2015-02/documents/pmf_5.0_user_guide.pdf. (2014)
Flues, M., Hama, P., Lemes, M.J.L., Dantas, E.S.K., Fornaro, A.: Evaluation of the rainwater acidity of a rural region due to a coal-fired power plant in Brazil. Atmos. Environ. 36, 2397–2404 (2002)
Fujita, S., Takahashi, A., Weng, J.-H., Huang, L.-F., Kim, H.-K., Li, C.-K., Huang, F.T.C., Jeng, F.-T.: Precipitation chemistry in East Asia. Atmos. Environ. 34, 525–537 (2000)
Ghude, S.D., Fadnavis, S., Beig, G., Polade, S.D., van der A, R.J.: Detection of surface emission hot spots, trends, and seasonal cycle from satellite-retrieved NO2 over India. J. Geophys. Res. Atmos. 113, 1–13 (2008). https://doi.org/10.1029/2007JD009615
Gobre, T., Salve, P.R., Krupadam, R.J., Bansiwal, A., Shastry, S., Wate, S.R.: Chemical composition of precipitation in the coastal environment of India. Bull. Environ. Contam. Toxicol. 85, 48–53 (2010)
Hegde, P.: Major ionic composition of aerosol, rainwater and its impact on surface and sub-surface waters, in and around Mangalore, West coast of India. Environ. Monit. Assess. 133, 119–125 (2007)
Keene, W.C., Pszenny, A.A.P., Galloway, J.N., Hawley, M.E.: Seasalt corrections and interpretation of constituent ratios in marine precipitation. J. Geophys. Res. Atmos. 91, 6647–6658 (1986)
Keresztesi, A., Nita, I.-A., Boga, R., Birsan, M.-V., Bodor, Z., Szep, R.: Spatial and long-term analysis of rainwater chemistry over the conterminous United States. Environ. Res. 188, 109872 (2020)
Khare, P., Goel, A., Patel, D., Behari, J.: Chemical characterization of rainwater at a developing urban habitat of northern India. Atmos. Res. 69, 135–145 (2004)
Khemani, L.T., Momin, G.A., Naik, M.S., Rao, P.S.P., Kumar, R., Murty, B.V.R.: Impact of alkaline particulates on pH of rain water in India. Water Air Soil Pollut. 25, 365–376 (1985)
Khemani, L.T., Momin, G.A., Naik, M.S., Rao, P.S.P., Safai, P.D., Murty, A.S.R.: Influence of alkaline particulates on pH of cloud and rain water in India. Atmos. Environ. 21, 1137–1145 (1987)
Khemani, L.T., Momin, G.A., Rao, P.S.P., Safai, P.D., Singh, G., Kapoor, R.K.: Spread of acid rain over India. Atmos. Environ. 23, 757–762 (1989)
Kulshrestha, M.J., Kulshrestha, U.C., Parashar, D.C., Vairamani, M.: Estimation of SO4 contribution by dry deposition of SO2 onto the dust particles in India. Atmos. Environ. 37, 3057–3063 (2003)
Kulshrestha, U.C., Sarkar, A.K., Srivastava, S.S., Parashar, D.C.: Investigation into atmospheric deposition through precipitation studies at New Delhi (India). Atmos. Environ. 30, 4149–4154 (1996)
Lelieveld, J., Klingmuller, K., Pozzer, A., Burnett, T., Haines, A., Ramanathan, V.: Effects of fossil fuel and total anthropogenic emission removal on public health and climate. PNAS. 116(15), 7192–7197 (2019)
Li, C., Kang, S., Zhang, Q., Kaspari, S.: Major ionic composition of precipitation in the Nam co region, Central Tibetan Plateau. Atmos. Res. 85, 351–360 (2007)
Losno, R., Bergametti, G., Carlier, P., Mouvier, G.: Major ions in marine rainwater with attention to sources of alkaline and acidic species. Atmos. Environ. Part A. Gen. Top. 25, 763–770 (1991)
Mahajan, A.S., De Smedt, I., Biswas, M.S., Ghude, S., Fadnavis, S., Roy, C., van Roozendael, M.: Inter-annual variations in satellite observations of nitrogen dioxide and formaldehyde over India. Atmos. Environ. 116, 194–201 (2015). https://doi.org/10.1016/j.atmosenv.2015.06.004
McCulloch, A., Aucott, M.L., Benkovitz, C.M., Graedel, T.E., Kleiman, G., Midgley, P.M., Li, Y.: Global emissions of hydrogen chloride and chloromethane from coal combustion, incineration and industrial activities: reactive chlorine emissions inventory. J. Geophys. Res. Atmos. 104, 8391–8403 (1999)
Migliavacca, D., Teixeira, E.C., Wiegand, F., Machado, A.C.M., Sanchez, J.: Atmospheric precipitation and chemical composition of an urban site, Guaiba hydrographic basin. Brazil. Atmos. Environ. 39, 1829–1844 (2005)
Migliavacca, D.M., Teixeira, E.C., Rodriguez, M.T.R., Wiegand, F., Pereira, F.N.: Analysis of the sulfate aerosol scavenging processes in the metropolitan area of Porto Alegre (MAPA), RS. Brazil. Atmos. Pollut. Res. 1, 82–93 (2010)
Momin, G.A.: The Role of Alkaline Particulates on pH of Rain Water in India. M. Sc. Thesis, University of Pune, Pune. (1990)
Mouli, P.C., Mohan, S.V., Reddy, S.J.: Rainwater chemistry at a regional representative urban site: influence of terrestrial sources on ionic composition. Atmos. Environ. 39, 999–1008 (2005)
Mphepya, J.N., Pienaar, J.J., Galy-Lacaux, C., Held, G., Turner, C.R.: Precipitation chemistry in semi-arid areas of southern Africa: a case study of a rural and an industrial site. J. Atmos. Chem. 47, 1–24 (2004)
Mukherjee, S., Singla, V., Pandithurai, G., Safai, P.D., Meena, G.S., Dani, K.K., Kumar, V.A.: Seasonal variability in chemical composition and source apportionment of sub-micron aerosol over a high altitude site in Western Ghats, India. Atmos. Environ. 180, 79–92 (2018)
Mukhopadhyay, B., Datar, S.V., Srivastava, H.N.: Precipitation chemistry over the Indian region. Mausam. 43, 249–258 (1992)
Naik, M.S.: Study of Ionic Constituents in Rain Water in Polluted and Non-polluted Regions of India. PhDThesis. Univ. Pune, Pune. (2001)
Paatero, P.: Least squares formulation of robust non-negative factor analysis. Chemom. Intell. Lab. Syst. 37, 23–35 (1997). https://doi.org/10.1016/S0169-7439(96)00044-5
Paatero, P., Eberly, S., Brown, S.G., Norris, G.A.: Methods for estimating uncertainty in factor analytic solutions. Atmos. Meas. Tech. 7, 781–797 (2014)
Pachon, J.E., Weber, R.J., Zhang, X., Mulholland, J.A., Russell, A.G.: Revising the use of potassium (K) in the source apportionment of PM2. 5. Atmos. Pollut. Res. 4, 14–21 (2013)
Pillai, A.G., Naik, M.S., Momin, G.A., Rao, P.S.P., Safai, P.D., Ali, K., Rodhe, H., Granat, L.: Studies of wet deposition and dustfall at Pune, India. Water. Air. Soil Pollut. 130, 475–480 (2001)
Polissar, A.V., Hopke, P.K., Paatero, P., Malm, W.C., Sisler, J.F.: Atmospheric aerosol over Alaska: 2. Elemental composition and sources. J. Geophys. Res. Atmos. 103, 19045–19057 (1998)
Possanzini, M., Buttini, P., Di Palo, V.: Characterization of a rural area in terms of dry and wet deposition. Sci. Total Environ. 74, 111–120 (1988)
Prasad, B.C.N., Pasha, G.S.M., Shivashankara, G.P.: Precipitation chemistry at an urban area of Karnataka, India (2005–2007). Pollution Research. 27, 747–750 (2008)
Prathibha, P., Kothai, P., Saradhi, I.V., Pandit, G.G., Puranik, V.D.: Chemical characterization of precipitation at a coastal site in Trombay, Mumbai, India. Environ. Monit. Assess. 168, 45–53 (2010)
Praveen, P.S.: Aerosol Properties in Different Environments. PhDThesis. Univ. Pune, Pune. (2008)
Ramaswamy, V., Muraleedharan, P.M., Babu, C.P.: Mid-troposphere transport of middle-east dust over the Arabian Sea and its effect on rainwater composition and sensitive ecosystems over India. Sci. Rep. 7, 1–8 (2017)
Rao, P.S.P.: Some Studies on the Deposition of Atmospheric Pollutants in Different Environments in India. PhDThesis. Univ. Pune, Pune. (1997)
Rao, P.S.P., Tiwari, S., Matwale, J.L., Pervez, S., Tunved, P., Safai, P.D., Srivastava, A.K., Bisht, D.S., Singh, S., Hopke, P.K.: Sources of chemical species in rainwater during monsoon and non-monsoonal periods over two mega cities in India and dominant source region of secondary aerosols. Atmos. Environ. 146, 90–99 (2016). https://doi.org/10.1016/j.atmosenv.2016.06.069
Rastogi, N., Sarin, M.M.: Chemical characteristics of individual rain events from a semi-arid region in India: three-year study. Atmos. Environ. 39, 3313–3323 (2005)
Reff, A., Eberly, S.I., Bhave, P. V.: Receptor modeling of ambient particulate matter data using positive matrix factorization: review of existing methods. J. Air Waste Manage. Assoc. 57, 146–154 (2007)
Roy, A., Chatterjee, A., Tiwari, S., Sarkar, C., Das, S.K., Ghosh, S.K., Raha, S.: Precipitation chemistry over urban, rural and high altitude Himalayan stations in eastern India. Atmos. Res. 181, 44–53 (2016)
Safai, P.D.: Study of the Air Pollutants in the Environment of the Nilgiri Biosphere Reserve, South India. PhDThesis. Univ. Pune, Pune. (2000)
Safai, P.D., Rao, P.S.P., Momin, G.A., Ali, K., Chate, D.M., Praveen, P.S.: Chemical composition of precipitation during 1984–2002 at Pune, India. Atmos. Environ. 38, 1705–1714 (2004)
Sakihama, H., Ishiki, M., Tokuyama, A.: Chemical characteristics of precipitation in Okinawa Island, Japan. Atmos. Environ. 42, 2320–2335 (2008)
Samara, C., Tsitouridou, R., Balafoutis, C.: Chemical composition of rain in Thessaloniki, Greece, in relation to meteorological conditions. Atmos. Environ. Part B. Urban Atmos. 26, 359–367 (1992)
Santos, P.S.M., Otero, M., Santos, E.B.H., Duarte, A.C.: Chemical composition of rainwater at a coastal town on the southwest of Europe: what changes in 20 years? Sci. Total Environ. 409, 3548–3553 (2011)
Saxena, A., Kulshrestha, U.C., Kumar, N., Kumari, K.M., Srivastava, S.S.: Characterization of precipitation at Agra. Atmos. Environ. 30, 3405–3412 (1996)
Singh, A.K., Mondal, G.C., Kumar, S., Singh, K.K., Kamal, K.P., Sinha, A.: Precipitation chemistry and occurrence of acid rain over Dhanbad, coal city of India. Environ. Monit. Assess. 125, 99–110 (2007)
Singla, V., Mukherjee, S., Kashikar, A.S., Safai, P.D., Pandithurai, G.: Black carbon: source apportionment and its implications on CCN activity over a rural region in Western Ghats, India. Environ. Sci. Pollut. Res. 26, 7071–7081 (2019)
Tiwari, S., Chate, D.M., Bisht, D.S., Srivastava, M.K., Padmanabhamurty, B.: Rainwater chemistry in the North Western Himalayan region, India. Atmos. Res. 104, 128–138 (2012)
Tiwari, S., Hopke, P.K., Thimmaiah, D., Dumka, U.C., Srivastava, A.K., Bisht, D.S., Rao, P.S.P., Chate, D.M., Srivastava, M.K., Tripathi, S.N.: Nature and sources of ionic species in precipitation across the indo-Gangetic Plains, India. Aerosol Air Qual. Res. 16, 943–957 (2015)
Tiwari, S., Srivastava, M.K., Bisht, D.S.: Chemical composition of rainwater in Panipat, an industrial city in Haryana Indian. J. Radio Space Phys. 37, 443–449 (2008)
Varma, G.S.: Impact of soil-derived aerosols on precipitation acidity, in India. Atmos. Environ. 23, 2723–2728 (1989)
Vet, R., Artz, R.S., Carou, S., Shaw, M., Ro, C.-U., Aas, W., Baker, A., Bowersox, V.C., Dentener, F., Galy-Lacaux, C.: A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos. Environ. 93, 3–100 (2014)
Wu, Y., Xu, Z., Liu, W., Zhao, T., Zhang, X., Jiang, H., Yu, C., Zhou, L., Zhou, X.: Chemical compositions of precipitation at three non-urban sites of Hebei Province. North China: influence of terrestrial sources on ionic composition. Atmos. Res. 181, 115–123 (2016)
Xiao, J.: Chemical composition and source identification of rainwater constituents at an urban site in Xi’an. Environ. Earth Sci. 75, 209 (2016)
Yang, L., Mukherjee, S., Pandithurai, G., Waghmare, V., Safai, P.D.: Influence of dust and sea-salt sandwich effect on precipitation chemistry over the Western Ghats during summer monsoon. Sci. Rep. 9, 1–13 (2019a)
Yang, L., Pandithurai, G., Chate, D.M., Rao, P.S.P., Waghmare, V., Iyer, U.: Evidence of precedent wind role on controlling PM1 wet scavenging of aerosols during monsoon rain events. Atmos. Environ. 201, 265–277 (2019b)
Yu, S., Gao, C., Cheng, Z., Cheng, X., Cheng, S., Xiao, J., Ye, W.: An analysis of chemical composition of different rain types inMinnan Golden Triangle’region in the southeastern coast of China. Atmos. Res. 47, 245–269 (1998)
Acknowledgments
Authors are grateful to the Director, IITM Pune for providing required technical and financial support to conduct this analysis. All the samples were collected at the High Altitude Cloud Physics Laboratory (HACPL), Mahabaleshwar which is fully funded by the Ministry of Earth Sciences, Government of India. We express our gratitude to the people who have helped in sample collection during the study period at Mahabaleshwar.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the paper entitled “Inorganic Ionic Composition of Rainfall at a High Altitude Station over the Western Ghats in Peninsular India”.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 47 kb)
Rights and permissions
About this article
Cite this article
Waghmare, V.V., Aslam, M.Y., Yang, L. et al. Inorganic Ionic Composition of Rainwater at a High Altitude Station over the Western Ghats in Peninsular India. J Atmos Chem 78, 59–76 (2021). https://doi.org/10.1007/s10874-021-09416-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-021-09416-x