Abstract
α-Amylases are essential biocatalysts representing a billion-dollar market with significant long-term global demand. They have varied applications ranging from detergent, textile, and food sectors such as bakery to, more recently, biofuel industries. Microbial α-amylases have distinct advantages over their plant and animal counterparts owing to generally good activities and better stability at temperature and pH extremes. With the scope of applications expanding, the need for new and improved α-amylases is ever-growing. However, scaling up microbial α-amylase technology from the laboratory to industry for practical applications is impeded by several issues, ranging from mass transfer limitations, low enzyme yields, and energy-intensive product recovery that adds to high production costs. This review highlights the major challenges and prospects for the production of microbial α-amylases, considering the various avenues of industrial bioprocessing such as culture-independent approaches, nutrient optimization, bioreactor operations with design improvements, and product down-streaming approaches towards developing efficient α-amylases with high activity and recyclability. Since the sequence and structure of the enzyme play a crucial role in modulating its functional properties, we have also tried to analyze the structural composition of microbial α-amylase as a guide to its thermodynamic properties to identify the areas that can be targeted for enhancing the catalytic activity and thermostability of the enzyme through varied immobilization or selective enzyme engineering approaches. Also, the utilization of inexpensive and renewable substrates for enzyme production to isolate α-amylases with non-conventional applications has been briefly discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
α-Amylases are the oldest carbohydrases that can replace chemical starch liquefaction in various sectors by the selective breaking of α-1,4 glycosidic bonds (Abraham et al. 2013; Aleem et al. 2018; Egbune et al. 2022). This class of amylases represents almost ~ 25% of the global market share of total enzymes with deployment into the textile, detergent, beverage, paper, pharma, bakery, and biofuel industries (Abd-Elhalem et al. 2015; Kaur et al. 2021; Hallol et al. 2022). The global market size was valued at USD 278.2 million in 2018 and is expected to increase to USD 353 million by 2026 (Niego et al. 2023). α-Amylases for bakery applications will witness the fastest geographical volume gains in the Asia Pacific at a CAGR of 5.9% (https://www.grandviewresearch.com), underpinned by end-user demand to improve the texture of the dough and the color, aroma, and softness of the final bakery products. Also, rising demand from the animal feed industry and discerning consumer needs for products with nutritional value, efficiency, and environmental impact considerations in the pulp and paper industry have helped ramp up global requirements of the enzyme with Novozymes (Denmark), Royal DSM N.V. (Netherlands), AB Enzymes (Germany), and DuPont (USA) being among the major market players (https://menafn.com; https://gminsights.com) (Ahuja and Malkani 2019).
In the realm of biological taxonomy, a multitude of organisms spanning the plant kingdom (including soybeans, sweet potatoes, barley, wheat, and maize) as well as the animal kingdom (encompassing Homo sapiens, canines, and avian species) exhibit the capacity to synthesize α-amylases (El-Gendi et al. (2022). However, when it comes to the production of enzymes, microorganisms are widely regarded as the most exemplary cellular factories due to their remarkable catalytic activity, stability, simplicity of production, and ability to optimize various parameters, as elucidated by Dakhmouche Djekrif et al. (2021).
α-Amylase, a canonical metalloenzyme, crucially relies on the presence of calcium ions (Ca2+) to attain its operational efficacy, stability, and overall structural integrity, as outlined by Nandi et al. (2022). This enzyme, through its interaction with starch, undertakes the conversion of the said polysaccharide into glucose, maltose, and dextrins, as discerned from the findings of Arnau et al. (2020). Notably, α-amylase (EC 3.2.1.1) functions as an endo-amylase, while β-amylase (EC 3.2.1.2) acts as an exo-amylase, and γ-amylase (EC 3.2.1.3) exists as another noteworthy class of amylases. These enzymes exhibit distinct mechanisms of action, executing diverse approaches in their cleavage of starch molecules. Particularly, α-amylase disrupts α-glycosidic linkages at random locations within the starch substrate, a characteristic distinguishing it from its counterparts resulting in maltose, dextrins, and oligosaccharides (depicted in Fig. 1). Conversely, β-amylase selectively targets the maltose-producing α-1,4 glycosidic linkage at the nonreducing end, thereby yielding maltose (Pan 2021). This enzyme is predominantly prevalent in plants and microorganisms (Lahiri et al. 2021). On the other hand, γ-amylase, known for its ability to break both α-1,4 and α-1,6 glycosidic linkages from the non-reducing end, exhibits a broader spectrum of cleavage activity directed towards glucose as the major product (Tong et al. 2021). Of the three classes above of amylases, α-amylases are favored in industrial applications due to their superior kinetic properties. However, their relatively lower selectivity than β and γ-amylases represents a potential trade-off.
This review provides an in-depth detail of the structural attributes of α-amylase, shedding light on various thermodynamic parameters that govern its functional properties and functional stability. It also provides a comprehensive understanding of the challenges associated with scale-up for different industrial applications of the enzyme with possible strategies to combat/mitigate the issues by considering the various factors governing the bioprocess, protein engineering, or enzyme immobilization, for which hardly any literature is available. To the best of our knowledge, this is the first review encompassing almost all the critical factors associated with α-amylase production for industrial application. The scope of α-amylase for possible application as a bioremediating agent as a non-conventional application has also been discussed.
α-Amylase: an insight into its structure
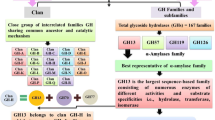
Genome sequencing projects have elucidated the sequence of several microbial amylases, enabling scientists to solve and model their 3D structures (Fig. 1). Conformationally, α-amylase generally has three domains. The catalytic or central domain, A, is the largest and consists of an eight-stranded α/β barrel referred to as the TIM barrel, first discovered in chicken muscle triphosphate isomerase—hence the name (MacGregor 1988). This region carries three active site residues (two Asp and one Glu residue) that are crucial in enabling substrate binding (Fig. 1) (Brayer et al. 1995). One Aspartate residue helps to maintain the integrity of the active site by interacting with the neighboring conserved Arginine through H-bonds. The other Aspartate molecule interacts directly with the substrate (such as starch), resulting in substrate distortion and elevating the pKa of the Glutamate, which acts as the proton donor to the glycosylated oxygen moiety and thereby enables hydrolysis through the formation of a beta-linked glycosyl-enzyme intermediate (Mehta and Satyanarayana 2016). Domain A is present in all α-amylases, whether obtained from bacteria, fungi, or animals.
On the other hand, domain B (a protrusion from domain A) has non-uniform β-sheets compared to the TIM barrel of domain A. It constitutes a significant portion of the substrate binding pocket of various α-amylases and differs significantly in conformation (Li et al. 2017a, b). It appears as an insertion between the C and A domains and remains attached to the latter through a disulfide linkage. Interestingly, the B domain is absent in liquefying α-amylases of B. subtilis, whereas the region remains slightly elongated and has distinct features in most thermostable bacterial amylases. It is assumed that the rigidity of the B domain enables substantial improvement in thermostability and plays a crucial part in imparting substrate specificity (Zeng et al. 2020). Domain C, the most variable domain, accounts for the C- terminal sequence assembled into a globular unit forming a Greek key motif. It remains loosely bound to the TIM barrel compared to the B domain and at its opposite end. The C terminal domain is structured with β sheets and connects to domain A by simple polypeptide linkage. This region shows significant irregularities in sequence and length between different α-amylases. Though its function is not entirely understood, some researchers have shown that this variable region can play an essential role in enzymatic activity and stability. Fort et al. (2021) indicated that domain C in Bacillus stearothermophilus plays a vital role in its enzymatic activity. Using C-terminal domain truncated mutants (CTDM) of Geobacillus thermoleovorans (Zeng et al. 2020), the researchers noted that although the wild type α-amylase could successfully bind the corn starch, the CTDM amylase could not attach to the substrate under identical conditions. Also, its kcat and half-life were reduced by 22.1% and by 1 h, respectively, which indicated that the CTD probably played a part in starch binding and enabled it to prolong the thermostability. The conserved calcium-binding groove is localized in central domain A towards the C terminal region. Mehta and Satyanarayana (2014) confirmed the function of domain C in starch binding by carrying out truncation analysis; α-amylase produced by G. thermoleovorans mutant could not bind the raw starch.
α-Amylases from Aspergillus awamori showed the presence of an additional domain (CBM), i.e., a carbohydrate-binding module. Also referred to as an ancillary module comprising 40–200 amino acid residues that facilitate the binding of various polysaccharides (Singh et al. 2021), an α-amylase starch binding domain (SBD) is such a CBM with specificity toward starch (Baroroh et al. 2019). SBD contains a β-sandwich fold and is categorized under family CBM20. A linker region facilitates the binding of CBM with the catalytic domain. It enhances the hydrolysis rate by increasing the concentration of raw starch at the site by adsorption (Cripwell et al. 2020). The amino acid residues W543, W590, W616, and W662 play crucial roles in the binding starch granule. The CAZY database contains ~ 2704 SBD entries (out of 292,679 hits) in amylases produced by Bacillus cereus, Bacillus circulans, and Aspergillus niger (http://www.cazy.org/). α-Amylase from Saccharomycopsis fibuligera R64 showed lower adsorption of raw starch due to a lack of a carbohydrate-binding domain (CBD) (Baroroh et al. 2019). α-Amylase from Bacillus aryabhattai showed the presence of CBM similar to amylase from soybean and other plants (Duan et al. 2021). SBD is present in various amylases, having the capability to degrade raw starch. These are additional domains apart from the specific domains found in different α-amylases (Mehta and Satyanarayana 2014).
Calcium: an inherent cofactor
Calcium plays a significant role in α-amylase activity. The number of bound metals typically varies from one to ten within the protein molecule (Navjot et al. 2022). On the other hand, Gopinath et al. (2017) reported seventeen binding sites for calcium in Bacillus amyloliquefaciens. Although the functions of α-amylases from various origins have changed due to evolution, catalytic residues, and calcium binding pockets are still conserved (Posoongnoen et al. 2021; Marengo et al. 2022). Calcium ions have proven to function critically in α-amylases, resulting in structure, activity, and stability improvement, especially in thermophilic ones (Yadav 2012). It has been noted that the potential residues binding to Calcium are conserved. The metal ions are necessary to maintain protein shape in their functional conformations (by stabilizing the interface of domains A and B) and resist enzymes' thermal inactivation (Liao et al. 2019). The authors reported that with loss of Calcium ions in thermophilic Anoxybacillus sp. GXS-BL, the denaturation temperature of the enzyme was substantially reduced by 10 °C, similar to those reported with Aspergillus oryzae. Calcium removal from the protein led to increased susceptibility to proteolytic degradation and loss of structural integrity. As an interesting fact, sequence analysis has revealed the presence of a metal triad at the interface of domains A and B, i.e., calcium–sodium–calcium (Ca–Na–Ca) (Machius et al. 1998; Lee et al. 2022) in certain Bacillus amylases, which probably account for their increased thermostabilities. The structured presence of sodium and calcium creates a unique arrangement surrounded by negatively charged residues. Ca and Na in the metal triad are responsible for the stability of thermophilic α-amylases (Yi et al. 2018). However, there are one or two reports that calcium ions also negatively impact the enzyme activity in some species. For example, α-amylase from A. oryzae is found to be inactivated in the presence of Calcium ions (Ng et al. 2021). The Calcium-binding site exhibits secondary interactions with Asp, Glu, and Asp catalytic residues, leading to the inactivation of the active site. Despite these exceptions, Calcium is an essential component of the enzyme that significantly enhances the catalytic efficiency of α-amylase through stable interactions with various amino acid residues within the protein molecule (Marengo et al. 2022).
Amino acid composition: a key determinant of stability
The amino acid composition within the α-amylases, apart from the catalytic activity, can also significantly influence the enzyme stability. It has been observed that elevated levels of non-polar amino acids, particularly those with hydrophobic properties, result in a reduction of charged amino acids, specifically Arginine and Glutamate residues. The charged amino acids, however, play a crucial role in ionic interactions, salt bridges, and H bondings within the proteins which promote stability. For example, an increase in aromatic amino acids, especially Tyrosine residues, enhances cation–pi interactions. George et al. (2020) performed an in-silico sequence analysis of several α-amylases and observed that certain charged amino acid residues such as Lysine, Arginine, Glutamate, and their corresponding dipeptides occur at a higher frequency in the thermostable versions of the enzyme compared to their mesophilic counterparts. Statistical data analysis has also shown that amino acid substitutions such as Glycine to Alanine and Lysine to Arginine in thermophilic α-amylases are favored. Whereas a decrease in Methionine residues promotes thermostability within a particular protein. It is assumed that thermodynamically, the distinctive compositions of amino acids are closely linked to specific characteristics, notably the shape and Gibbs free energy change of hydration for native enzymes. It is assumed that amino acid composition disparities can be a pivotal determinant of protein thermostability. However, to ascertain the intricate relationship between structure and function in α-amylases, further exploration of protein activity and stability needs to be carried out and modeled as a function of amino acid composition (across a larger extent in the database) using the advanced machine learning or AI-based) computational tools at the micro level.
Thermodynamics of α-amylase
The thermodynamic parameters for starch hydrolysis using α-amylases have been derived from the transition state theory by Eyring and Stearn.
where,
where h = Planck’s constant (6.63 × 10−34), R = gas constant (8.314 J/K mol), T = absolute temperature, ∆S* = change in entrophy, ∆H* = change in enthalpy, k = Boltzmann constant (1.38 × 10−23).
The change in enthalpy provides valuable information for the effectiveness of the transition state. In contrast, a change in entropy suggests the affinity of the substrate for the enzyme and the stability of the transition state. On the other hand, the change in Gibb's free energy (ΔG) is an indicator of the spontaneous reaction. The protein structure strictly regulates both parameters.
Shukla and Singh (2015) performed deactivation studies varying from 50 to 100 °C to analyze the thermostable amylase from an actinobacteria Laceyella sacchari TSI-2. The change in entropy was estimated at around − 126.45 J/mol/K, which indicated that α-amylase binds with high affinity with its substrate, soluble starch. Also, the enzyme had a high energy of deactivation (21.16 kJ/mol), which corroborated its stability at elevated temperatures. Nwagu et al. (2020) discovered a high change entropy (~ -180 J/mol/K) by α-amylase from Paecilomyces variotii ATHUM 8891 while binding with starch. The enthalpy change was around 34.09 kJ/mol, which highlighted that higher free energies were necessary to inactivate the enzyme. In another report, Samanta et al. (2014) described an α-amylase derived from Bacillus licheniformis SKB4, which is highly thermostable with an optimum temperature of 90 °C. The free energy for substrate binding (∆G E–S) and transition state at 90 °C were found to be 5.53 and − 17.4 kJ/mol, respectively. Yandri et al. (2020) reported a thermostable enzyme from Bacillus subtilis ITBCCB148 with an optimum working temperature of 65 °C. Native α-amylase had a half-life (t1/2) of 1.89 h with a ΔG value of 107.3 kJ/mol.
Interestingly, immobilizing the α-amylase using chitin enhanced its half-life by ~ 12-fold and improved the ΔG value to 115.51 kJ/mol (Yandri et al. 2021). Table 1 highlights the thermodynamic properties of different α-amylases isolated from various bacterial and fungal species. The mesophilic α-amylase counterparts have significantly lower enthalpy and Gibb’s free energy values. Based on the thermodynamic parameters, the enzyme performance can be easily deduced.
Production methods
α-Amylase production has been attempted through different fermentation techniques. However, commercially only two production methods, (a) solid-state fermentation (SSF) and (b) submerged fermentation (SMF), have been successful (Elyasi et al. 2020) (Table 2). The conventional fermentation technique SMF employs a free-flowing liquid to grow the culture in an aerobic or anaerobic environment (Bakri et al. 2020). As a homogeneous medium, the substrate is utilized rapidly, and the α-amylase can be efficiently secreted in the liquid broth. This easily enables the recovery and purification of the enzyme for downstream applications. Besides, the physical parameters such as aeration, pH, and temperature can be smoothly regulated, which dictates the enzyme kinetics (Sharma et al. 2016).
SSF, on the other hand, employs a solid substrate such as bran, pulp, leave, peels, or raw biomass that is biodegradable for the growth of microbe at a solid–liquid interface (Cuadrado-Osorio et al. 2022). Unlike SMF, the substrates are utilized slowly but steadily in this method. The process does not require specialized equipment, resulting in a high concentration of products with minimal effluent generation (Ramachandran et al. 2010; Srivastava et al. 2019). However, yeast and fungi were considered most appropriate for SSF compared to bacterial cultures with their substantial water requirements (Prabhu et al. 2022). Also, bacterial cultures can be well maintained and exploited for SSF processes, as shown by recent research (Tsegaye and Gessesse 2014). A comparison of the two methods has shown that SSF is more appropriate for developing countries because of its cost-effectiveness with relatively little energy expenditure as compared to SMF.
Although the cost of α-amylase production is the most crucial factor for any process development with the enzyme, it is usually not taken into serious consideration. Very little information is available on α-amylase production costs, and the authors mostly provide an estimated price (US $3/kg to US $50/kg) without giving detailed information about the source or the models (Sóti et al. 2018). Among a few handfuls of reports available, Castro et al. (2010) estimated the cost of α-amylase production from A awamori using SSF of Babassu cake. The initial cost of production was estimated to be very high. With much optimization and strategic planning, the authors brought down the cost to US$ 10.4 kg of the enzyme (by selling the fermented cake as a co-product). In a more recent study, Balakrishnan et al. (2021) performed the production of α-amylase with scale-up (in a 600 L fermenter) from A oryzae using solid-state fermentation of edible cakes. Using statistical design and optimization tools, the production could be enhanced by ~ 12%. The economic analysis demonstrated that the partially purified enzyme had a tentative production cost of ~ US$ 7.59/L of the enzyme with very high activity (9868.12 U/g dry substrate). In an interesting study, reported by authors from Bangladesh, an in-house α-amylase could be produced through SSF at a production cost of US$ 0.54/L the enzyme which was ~ 10 times lower compared to the conventional α-amylase (Khalid-Bin-Ferdaus et al. 2018). Such a low-cost enzyme, if successfully scaled up, would be an instant hit for application in the textile and apparel industry.
α-Amylase: a host of endless opportunities
Starch conversion
The α-amylases are essential enzymes with vital applications in starch liquefaction (Paul 2016). The process of starch degradation involves three steps, namely a) Gelatinization, b) Liquefaction, and c) Saccharification (El-Fallal et al. 2012). During gelatinization, starch feedstock, corn, wheat, or cassava undergoes thorough cleansing and pulverization, yielding a fine powder. This meticulous step augments the surface area of the starch particles, ensuring enhanced accessibility for subsequent enzymatic actions. The solid starch slurry is next treated at high temperatures (> 100 °C) to ensure the removal of lipid-starch complexes. This step results in a dense suspension of starch dissolved in water, which enables the α-amylases to partially hydrolyze the polymer into short-chain dextrin, significantly reducing the solution viscosity. This is followed by the enzyme's breakdown of the oligomeric dextrin into reducing monomeric sugars D-glucose and D-fructose. The incubation period and the enzyme loading predominantly govern starch hydrolysis efficacy. Since the current industrial amylases cannot sustain such high temperatures, the gelatinization and liquefaction steps must be performed separately (Silano et al. 2018). The liquefaction process after gelatinization commences with a heat-intensive step, wherein the starch slurry, accompanied by water, is subjected to elevated temperatures. Simultaneously, thermostable α-amylase enzymes are introduced into the mixture. These enzymes catalyze the hydrolysis of α-1,4 glycosidic bonds, effectively deconstructing the starch molecules into shorter dextrin chains. Operating within the 95–105 °C temperature range, this phase facilitates optimal enzymatic activity. After the liquefaction stage, the temperature of the starch slurry is carefully decreased, accompanied by the introduction of additional enzymes. This crucial step encompasses the synergistic action of glucoamylase and α-amylase enzymes. Their concerted effort progressively disintegrates the dextrin chains into individual glucose molecules. The glucoamylase enzyme contributes to the hydrolysis of both α-1,4 and α-1,6 glycosidic bonds within the dextrins, liberating glucose. Saccharification predominantly transpires within a temperature range of 55–65 °C.
Once the desired glucose concentration is attained, meticulous control over the enzymatic activity is exercised by either elevating the temperature or adjusting the pH of the slurry. This serves the dual purpose of halting further enzymatic degradation of the starch and preserving the glucose for subsequent fermentation endeavors. However, there is a search for an enzyme that can sustain even higher temperatures and low pH so that the gelatinization and liquefaction steps may be performed simultaneously (which is still a great challenge in the industry to date).
Detergent industry
α-Amylases are the most used enzyme after proteases in the liquid detergent industry (Dakhmouche Djekrif et al. 2021). They are supplemented explicitly to the laundry and dishwashing detergents for the liquefaction of starch and breakdown of starchy stains from different food preparations and gravies. It principally breaks down the starch into tiny sugar molecules lifted from the clothes during the detergent wash. Additionally, the enzyme prevents swollen starch from adhering to the surface of laundry and glassware (Gürkök 2019). They are generally used in combination with proteases, which boost cleaning (in different industrial sectors) and restore the whiteness of clothes. The amylases that can function at low temperatures and in an alkaline pH range are significant in the detergent industry (Hamid et al. 2022). The Novozyme-developed Stainzyme Plus Evity 24T® and Amplify Prime 100 L® perform exceptionally well in cold washes and even in the presence of strong chelators (https://biosolutions.novozymes.com). One of the significant issues of α-amylase in the detergent industry is its oxidant sensitivity and calcium dependency (Samanta 2022). Genetic engineering has been tried to overcome the limiting issues with pH/temperature stability and calcium dependency. Yang et al. (2017) mutated α-amylase obtained from ciliated protozoan by introducing proline residues in the surface loop and changing valine to threonine near the catalytic site. It increased half-life (t1/2) by twofold at 50 °C compared to the wild type. Gai et al. (2018) created a double mutant α-amylase by deleting 179R and 180G from Bacillus stearothermophilus. Half-life was increased by 1.375 fold, and the mutant was stable at a lower pH than the wild type. Farooq et al. (2021) prepared an oxidant-resistant amylase by replacing methionine with leucine at 197 in (B. licheniformis α-amylase) BLA, resulting in better oxidative stability. Mutation of oxidation-prone M residues with I, A, and T resulted in improved catalytic activity and oxidative stability. Oxidative stability was increased by 5.4-fold due to mutant α-amylase (M145I-214A-229 T-247 T-47I) (Yang et al. 2013). Natalase® and Termamyl® are some of the amylases available commercially and widely used in the liquid detergent industry (Mehta and Satyanarayana 2016). B. licheniformis derived Termamyl®300L is a thermally stable α-amylase that hydrolyses starch at 95 °C (Guerrero-Navarro et al. 2019). Guerrero-Navarro et al. (2022) described an enzyme formulation having two commercial enzymes, Savinase® and Termamyl® Ultra 300 L, which could efficiently remove (~ 75%) fouling in a spray dryer and pilot-scale plate heat exchanger in a dairy industry, which was comparable to the chemical cleaning methods used conventionally in industries based on 1.2 mL/L protease and 1 ml/L Termamyl®300L. α-Amylase and protease are used to remove dirt from household streams and various industrial waste streams. Nowadays, oxidation resistant α-amylases are essential for application in the detergent industry as native α-amylases are sensitive to oxidation (https://www.creative-enzymes.com).
Bioethanol production
The rapid decline of fossil fuels (due to the increasing population) coupled with the issue of climate change has prompted the scientific fraternity to develop clean and renewable biofuels such as ethanol (Khan et al. 2021; Pham et al. 2022). Starch is the preferred carbon source for producing 1G ethanol due to its global availability. α-Amylases readily break down the starch (after liquefaction) into its constituent monomers, which are then rapidly converted by a fermenting yeast such as Saccharomyces cerevisiae (Singh et al. 2022) (Fig. 2). Kumar and Singh (2016) reported efficient ethanol production from corn-based starch using a novel vacuum-assisted fermentation method through SSF. Using commercial α-amylases with high enzyme activity (6400 µmol maltose/min mL) and a superior industrial fermenting yeast termed "ethanol red" (S. cerevisiae yeast, Lesaffre Advance Fermentation), 18% v/v ethanol titers were achieved. The vacuum-assisted SSF technique enabled rapid ethanol recovery within the system, allowing the yeast to ferment with high productivity without any toxic effects of the product alcohol at higher concentrations (Kumar and Singh 2016). Krajang et al. (2021) reported ethanol production in a single step by combining starch hydrolysis and fermentation plus scale-up studies (3000 L fermenter) from raw Cassava. Using a commercial α-amylase Stargen TM002®, 80.19% of raw Cassava starch was hydrolyzed by yielding ~ 176.4 g/L fermentable D-glucose. The “baker’s yeast” successfully fermented the broth with a high yield (8.97% v/v) and productivity (0.98 g/L/h). Since starch hydrolysis requires high-temperature hydrolysis followed by fermentation by mesophilic yeasts, a significant amount of cooling water is needed to lower the temperatures, which consumes energy, thereby increasing process costs. Yeasts with high-temperature ethanol fermenting capability, such as Kluyveromyces, would be a promising candidate for such conversions (Bilal et al. 2022). However, their ethanol fermenting capabilities need further improvement to be at par with the industrial Saccharomyces sp. Apart from the aforementioned points, bioethanol production faces several challenges: feedstock availability and cost, energy and water requirements, feedstock conversion efficiency, policy, and market factors. Ethanol production also needs to factor in environmental impact issues, such as increased water usage, emissions of greenhouse gases, and potential competition for land with food production. Mitigating these environmental impact elements and ensuring sustainable practices throughout the ethanol production lifecycle is essential for this sector.
Textile industry
α-Amylases are employed in the textile industry for designing and finishing fabric. Starch is applied to yarn before fabric generation as a strengthening agent (to avert splitting of a warp thread during the weaving procedure), typically ensuring a rapid and effective procedure. A wet treatment process later eliminates it from the fabric. The traditional processes of desizing involved using acid or alkali as the desizing material. This damages the cellulose fibers and lowers the cloth's tensile strength (Vaibav et al. 2022). The harsh chemical treatments involve colossal energy expenditure and cause environmental concerns in the textile industry, promoting the utilization of enzymatic desizing. The α-amylases penetrate the space between fibers and selectively remove the starch without adversely affecting the fabric (Al-bedak et al. 2022). The α-amylases from Bacillus sp. are nowadays widely used in the textile industries. Enzyme solutions such as Aquazyme 210L® and Termamyl® typically operate between 30 °C-60°C and efficiently remove starch with colossal water and energy savings within a pH range of 5.5–6.5 (https://biosolutions.novozymes.com). Another commercial enzyme, SuperLIQ™®, has been developed by the Bestzyme® Corporation to operate across a broad range of pH and temperatures, functioning on all starch-based sizes (https://www.bestzyme.com/).
Paper industry
α-Amylases are applied in the paper factory to modify the starch-layered paper. Starch is one of the cheapest and most crucial wet-end additives used in the paper industry. The coating or overlaying process makes the exterior of the paper polished, glossy, and robust (Paul 2016; Xu et al. 2018). Also, as a sizing agent, starch adds to the reusability and enhances paper quality. The cohesiveness of natural starch makes it unsuitable for direct application in the paper industry as a sizing agent. α-Amylases are employed for partially degrading starch-coating of paper to improve the viscosity and concentration to achieve the desired consistency (Gangadharan et al. 2020). Specific modifications in α-amylase can increase the viscosity, film-forming properties, and adhesion of the starch-based coating. Deinking is the process of removing ink from recycled paper fibers to produce high-quality recycled paper. Genetically modified amylases can be utilized to enhance the deinking process by breaking down the starch and carbohydrate-based components of ink and improving the efficiency of ink removal. Genetically modified amylases can also be used to modify the surface properties of paper fibers. By targeting the starch components present in the fibers, amylases can alter their structure, making them more receptive to various treatments such as sizing, coating, and dyeing. This modification improves the overall quality and performance of the paper. Bleaching is a crucial step in paper production to achieve the desired brightness and whiteness. Genetically modified amylases can assist in pulp bleaching by degrading starch residues that may interfere with the bleaching process. This ensures better penetration of bleaching agents, leading to improved color removal and brightness. α-Amylase G995® (Enzyme Biosystems, USA), BAN® (Novozymes), and Termamyl® are the few commercial amylases used in the paper and pulp industry (Mehta and Satyanarayana 2016). They also provide stiffness and strength.
Challenges and opportunities
α-Amylase holds great significance in various industrial sectors due to its versatile applications. However, the pursuit of advancements in this field is driven by the challenges posed by specific industrial demands and the opportunities to overcome them. Challenges include ensuring stability under harsh chemical conditions, optimizing substrate specificity, achieving cost-effective production, and addressing limitations in immobilization techniques. On the other hand, opportunities lie in enhancing reactivity and stability through genetic and protein engineering, tailoring enzymes for specific applications through advanced screening methods, exploring sustainable production methods, and leveraging immobilization for process optimization (Madhavan et al. 2021). By addressing these challenges and capitalizing on the opportunities, the field of α-amylase can evolve and offer improved performance, stability, and cost-effectiveness for a wide range of industrial applications. The following are the challenges in α-amylase:
Strain selection and culture-independent approaches
Selection of a suitable micro-organism with high α-amylase production capability is an essential yet challenging task (Elyasi et al. 2020). This is due to the significant variations that exist in enzyme production capabilities, yields, and productivities among different microbial species and strains. Screening a large pool of strains is often necessary to identify the most suitable one for efficient α-amylase production (Pranay et al. 2019) which makes it time, resource, and labor-intensive. Also, for scale-up and industrial applications, the desired strain should be fast-growing, easily culturable, and less prone to contamination. Recent research has emphasized the importance of using advanced screening methods, such as metagenomics and high-throughput screening techniques, to rapidly identify novel microbial strains with potential α-amylase production capabilities (Delavat et al. 2012; Motahar et al. 2021). For example, a cold-active α-amylase gene was found using the metagenomic approach, which showed a pH optimum of 8–9 and temperature optimum at 10–15 °C (Vester et al. 2015) which could be potentially used in the detergent industry. In another report, Motahar et al. (2020) screened sheep rumen metagee to find the thermostable and acidic amylolytic genes. In another interesting study, Nair et al. (2017) exploited marine sediments to discover potential microbes with substantial α-amylase activity with a view that the isolated enzyme may be able to withstand the typically harsh chemical conditions encountered in the industry. They created a metagenomic library in pUC19 from the marine sediments of the Arabian Sea obtained at a depth below 96 m, and the clone showed considerable amylolytic activity. The geothermal springs are believed to harbor an extensive array of undiscovered microorganisms. A thermostable amylase was isolated from the Odisha geothermal spring through metagenomics-based techniques, unveiling a gene comprising 1503 base pairs. This gene encodes a protein consisting of 469 amino acids, with a molecular weight of 53,895.05 Da and a pI of 7.78. Sequence analysis revealed a remarkable 98.95% identity with the α-amylase gene of B. licheniformis (Chauhan et al. 2023). The functional metagenomics approach in the Ethiopian soda lake led to the finding of various carbohydrate-degrading enzyme sequences (Jeilu et al. 2022). In cold environments, Proteobacteria and actinobacteria are widespread. Singh et al. (2022) in a similar study, reported careful examination of the metagenomic data from these environments to identify α-amylase enzymes with promising applications in the detergent industry.
One of the major challenges in the industry working under ambient operating conditions is that they are prone to contamination, resulting in product inconsistency and, at times, huge financial losses (Yassin et al. 2021). This is the primary reason that starch industries operate at high temperatures. But, at times, it leads to denaturation of the hydrolyzing enzymes. Thus, there is a need for a suitable strain/enzyme that can not only withstand severe operating conditions but also work effectively. To combat this issue, M/s Novozymes has developed an enzyme sold under the name of Termamyl Ultra from that can degrade cooked (gelatinized) starch with high efficiency at a temperature close to 90 °C with a working pH between 7 and 11 (https://www.ncbe.reading.ac.uk). Another challenge in strain selection for α-amylase production relates to regulatory and intellectual property considerations. Accessing and utilizing microbial strains may be restricted due to patent rights, biosafety regulations, or intellectual property conflicts. Recent discussions and developments in the field have emphasized the importance of open access to microbial resources, collaboration, and clear regulatory frameworks to overcome these challenges and promote innovation (O'Connor 2021).
Nutrient optimization
α-Amylase production relies on specific nutrients for bacterial growth and efficient enzyme synthesis. Identifying the optimal blend of carbon and nitrogen sources, trace elements, and other physical factors is not a simple task. Nutrient optimization studies are often required to maximize the yield of the enzyme (Kothakota, et al. 2021). Recent studies have investigated the effect of different carbon and nitrogen sources, on α-amylase production (Kamer et al. 2023) using statistical modeling and optimization strategies, such as Response Surface Methodology (RSM). By using the different techniques in RSM, such as the Box Behnken Design (BBD) and Central Composite Design (CCD), researchers have been able to study the intricate interactions between the different variables associated with α-amylase production and their influence on the response in the form of a non-linear regression model. By analyzing this model, researchers can identify optimal conditions that lead to the desired outcome, thereby improving efficiency, reducing costs, and enhancing product quality. A thermo-alkali stable α-amylase from Bacillus sp. was optimized using Box-Behnken design (BBD) by considering the physical factors such as pH, temperature, agitation speed, and incubation time. It was observed that the optimum conditions for α-amylase production were observed to be at more or less the central values of the variables. The variables pH, temperature, and incubation time showed both main effects as well as interaction effects with positive effects on the system response, enhancing the α-amylase activity by ~ 1.5 folds (Khusro et al. 2017). In a similar study, optimizing α-amylase production in Bacillus cereus using a rotatable central composite design enhanced the enzyme activity by 3.9 fold (Ojha et al. 2020). The authors reported that the temperature and pH of the production medium significantly influenced the enzyme production. An increase in temperature beyond 30 °C or a reducing pH below 6.3 (which were identified as optimal conditions) reduced the enzymatic activity by 20–40%. Both the factors displayed main as well as interaction effects within the system. In another study, Saad et al. (2021) optimized α-amylase production from starch using CCD from B. licheniformis sp. It was observed that the enzyme productivity was enhanced by ~ 90% compared to the traditional OVAT optimization technique. The 3-D surface plots showed that starch concentration, pH, and incubation period displayed positive effects on the response (α-amylase activity) up to their optimal levels (10.5 g/L, 6.0, and 45 h, respectively), beyond which the values resulted in α-amylase repression. These studieselucidated that the micronutrients and growth factors either individually or with interaction effects play vital roles as cofactors or regulators of the enzyme (Fatoki et al. 2023). An inducer and signal molecules such as influencers can affect α-amylase production by regulating gene expression and signal transduction pathways. However, identifying suitable inducers and understanding their optimal concentrations and timing pose challenges. Recent approaches such as transcriptome and proteome analysis have explored the effects of different inducers and signal peptides on α-amylase production and investigated their underlying mechanisms (Huang et al. 2022).
Simair et al. (2017) stated that the high cost of fermentation media for amylase production is a major challenge to looking for agro-waste as a cheap substitute for fermentation media to reduce the overall cost. Substrate cost is a significant challenge in α-amylase production, accounting for 30–40% of the process (Niyomukiza et al. 2022). Cassava starch is cheaper than soluble starch and showed 170 times better results than soluble starch, which can be used for reducing fermentation costs (Ayansina et al. 2017) for large-scale industrial applications. Addressing the challenges in nutrient optimization for α-amylase production requires a multidisciplinary approach, encompassing metabolic engineering, systems biology, and process optimization. Recent advancements in next-generation sequencing technologies, computational modeling, and high-throughput screening methods have offered opportunities to gain a deeper understanding of nutrient requirements and develop tailored strategies for enhanced α-amylase production.
Oxygen transfer
Oxygen availability in liquid broth is a crucial factor influencing α-amylase production. Ensuring appropriate aeration and agitation to meet the oxygen requirements of the microbe is a must (Zhou et al. 2018). Microbial cells consume oxygen for different parallel pathways functioning inside cells, thus reducing the available dissolved oxygen in the fermentation broth, which may negatively impact α-amylase production. Recent research has focused on understanding and controlling the oxygen uptake rate through process optimization and oxygen supply strategies (Wang et al. 2020). Strategies such as intermittent aeration, oxygen feedback control, and metabolic engineering approaches have been explored to regulate oxygen uptake and improve enzyme production. In addition, the role of oxygen carriers such as n-dodecane and biosurfactants have been investigated to enhance oxygen solubility within the system (Wang et al. 2023). Mass transfer limitations can sometimes arise due to inadequate oxygen transfer from the gas phase to the bulk liquid and limitations in oxygen diffusion within the culture broth. The issues can be mitigated by optimizing bioreactor design, impeller configurations, and aeration strategies (Puiman et al. 2022). The use of oxygen-enriched air or pure oxygen, along with advanced oxygen delivery methods, has been observed to improve oxygen transfer efficiency considerably during α-amylase production (Mostafa et al. 2021).
Foam formation in bioreactors can also hinder oxygen transfer by reducing the effective gas–liquid contact area. The foam can accumulate at the liquid surface, impeding oxygen transfer and causing reactor instability. Blaga et al. (2022) investigated foam control strategies, including using antifoam agents, foam level control systems, and improved bioreactor designs, to minimize the adverse effects of foam on oxygen transfer and enhance α-amylase production. It has been well observed that oxygen transfer issues become more pronounced during the scale-up of α-amylase production processes. As the fermentation volume increases, maintaining an efficient oxygen supply becomes challenging due to increased agitation power requirements, oxygen demand, and limitations in oxygen supply. de Souza Vandenberghe et al. (2022) crucially looked into these facts and reported addressing the scale-up challenges by optimizing bioreactor geometries, aeration strategies, and process control parameters. In addition, Computational modeling and advanced monitoring techniques have been employed to study the fluid dynamics and the gas holdup within the system to understand and optimize the oxygen transfer at larger scales. Overcoming the challenges in α-amylase oxygen transfer requires a comprehensive approach that combines engineering, bioprocess optimization, and microbial physiology. Recent advancements in bioreactor design, oxygen delivery systems, and process control strategies offer opportunities for improved oxygen transfer efficiency and enhanced α-amylase production.
Downstream processing
The purification of α-amylase from the fermentation broth can present certain challenges such as the presence of various cellular components and contaminants, low enzyme stability, and high viscosity of the fermentation broth which complicate and hinder the recovery process (Elyasi et al. 2020). For example, a high concentration of starch often leads to poor activity of α-amylase due to substrate inhibition (Božić et al. 2017). In such cases, recovery of the enzyme to achieve the desired purity in the industry becomes a demanding task (Wiltschi et al. 2020). Also, purification cost is an important consideration for the development of any α-amylase technology (Witazora et al. 2021). With the advent of technological advancements and the development of sophisticated purification methods, high-end purification of the enzyme may be possible but would also contribute significantly to the final production cost of the enzyme, increase the overall OPEX, and reduce the profit margin (Boodhoo et al. 2022).
Alternatively, to mitigate this issue, different purification techniques have been explored which include a combination of centrifugation, filtration, ultrafiltration, and chromatography to develop an efficient and cost-effective technology for enzyme recovery (Chen et al. 2022). In addition, chromatographic methods such as ion exchange chromatography, size exclusion chromatography, affinity chromatography, etc. have also been tried, to achieve high purity of α-amylase (Lim et al. 2020). In a more recent study, the use of novel materials, such as magnetic nanoparticles and molecularly imprinted polymers, has been explored for selective purification of α-amylase (Eivazzadeh-Keihan et al. 2021), with claims of 49.8% recovery.
Maintaining the stability of α-amylase during downstream processing is extremely crucial to preserve its activity and prolong its shelf life. However, α-amylase is susceptible to denaturation and degradation under certain processing conditions, such as high temperatures and extreme pH. Developing stabilization strategies, such as immobilization, encapsulation, formulation with stabilizing agents, and the use of additives and surfactants have been observed to considerably enhance enzyme stability and resistance to proteases (Li et al. 2022). In general, the recovery of α-amylase is cost-intensive due to the requirement for specialized equipment, consumables, and purification steps. The development of cost-effective downstream processing strategies is therefore extremely essential to make α-amylase production economically viable. Recent studies have explored process optimization, such as process intensification, process integration, and the use of novel separation techniques to reduce the overall cost of downstream processing (Meyer et al. 2021). Also, the utilization of renewable and low-cost raw materials for enzyme recovery and purification through innovative approaches needs to be investigated to obtain purity high-purity enzymes with substantial activities (Raina et al. 2022).
Scale-up considerations
Scaling up of α-amylase production presents significant challenges as it is extremely difficult to assess the most critical factors influencing the scale-up during fermentation due to a drastic change in environment (Crater and Lievense 2018). The transition from laboratory-scale to commercial-scale production requires careful consideration of several factors to ensure efficient and cost-effective processing for achieving the desired enzyme production levels (Deljou et al. 2018). Choosing the appropriate bioreactor design and mode of operation is of paramount importance. For example, impeller flooding is a condition that is often observed during α-amylase production, where the air stream along the stirrer shaft increases, which leads to poor mixing and low oxygen diffusion inside the medium (Mostafa et al. 2021). This typically occurs in industrial bioreactors where the pumping speed of the impellor is lower than the gas volume being introduced in the system. This issue can be mitigated by designing a broad blade impeller (within the bioreactor system) with a higher aeration number without compromising the aeration rates. This can efficiently prevent the microbial cells from settling down and ensure intimate contact between the air and the liquid medium with air dispersion and enhanced oxygen transfer rates. Besides oxygen transfer, other variables such as mixing efficiency and heat transfer effects must be carefully considered to ensure optimal growth and productivity of the microbial culture producing α-amylase. A few literature reports have even suggested the use of multiple bioreactors as a scale-up strategy for large-scale α-amylase production (Balakrishnan et al. 2021). Scaling up the downstream processing steps, such as harvesting, purification, and processing, is a complex task. Efficient separation and purification techniques need to be developed to handle larger volumes of the fermentation broth which requires both specialized facilities and skilled manpower. Additionally, optimizing the purification steps to achieve consistent enzyme quality is critical for successful scale-up. Robust quality control measures, including enzyme activity assays, purity analysis, and stability assessments, must be established and validated to ensure the product's quality meets the required specifications. Also, scaling up α-amylase production must consider cost optimization. Factors such as raw material sourcing, energy consumption, and process efficiency need to be carefully evaluated to achieve cost-effective production at a larger scale. Process modifications and optimization can help reduce overall production costs without compromising the quality and activity of the enzyme. Successful scale-up of α-amylase production requires a comprehensive understanding of the fermentation process, efficient bioreactor design, robust downstream processing techniques, rigorous quality control, and careful cost optimization. Collaborative efforts between scientists, engineers, and industry experts are essential to overcome these challenges and ensure a smooth transition from laboratory-scale to commercial-scale production (Crater and Lievense 2018).
Strategy for α-amylase improvement
Although α-amylase has widespread applications occupying most of the world’s enzyme market, it is still far from the ideal enzyme. The extremely high temperatures in the starch conversion industry limit the application of wild-type α-amylase (Gangadharan et al. 2020). Also, the oxidative environment of the detergent industry denatures the natural enzyme (Lim and Oslan (2021; Samanta 2022). Likewise, wild type α-amylase does not function well in an acidic environment; hence, raw juice clarification in the beverage industry is challenging (Liu et al. 2017; Bamigboye et al. 2022). Conventionally, it requires increasing the pH of the juice for optimum functioning of wild α-amylases, which ultimately leads to a change in the physicochemical characteristics of natural raw juice.
Additionally, the essential requirement of calcium for the functioning of the α-amylase typically restricts its action in a Calcium-deficient environment (Marengo et al. 2022). To overcome these limitations, extensive research has been focused on modifying the existing amylases and improving their activity/stability for effective functioning per the industrial requirements (Elyasi et al. 2020). As an option, chemical methods for amylase improvement have been tried. Yandri et al. (2012) attempted a chemical modification of Bacillus subtilis derived α-amylase using citraconic anhydride, which enhanced the thermal stability in contrast to the native enzyme. Similarly, Abdella et al. (2020) reported immobilization on chitosan magnetic nano-particles through physical adsorption and covalent binding, which improved its activity by ~ 2.3-fold (Abdella et al. 2020). Although initial observations suggest that the chemical treatments enhance the enzyme properties, in the long run, they have not been effective. Also, in some instances, the chemicals applied have been reported to threaten the environment and are ill-advised for large-scale applications (Li et al. 2022). Hence, protein engineering is the most preferred choice to generate more active and stable mutants in an eco-friendly manner compared to the chemical process. Genetic transformation can be done in two significant ways, namely, a) Directed evolution and b) Site-directed mutagenesis (Rabbani et al. 2011).
Directed evolution
The directed evolution method involves productive protein-sequence substitutions through mutagenesis followed by natural selection for generating a library of enzyme variants (Wang et al. 2021). Ruan et al. (2022) created a random mutation library of maltogenic amylase from B. licheniformis R-53 using error-prone PCR and successfully selected mutations with enhanced activity and thermal stability. The mutant V296F/K418I showed 2.16 times higher specific activity compared to the native one with optimum temperature enhanced by 5 °C from 60 °C. The authors observed that apart from improvement in dough recrystallization, the mutant α-amylase demonstrated reduced hardness and improved elasticity during bread storage compared to the native one. In another report, the pH stability of α-amylase from B. licheniformis was amplified by directed evolution with the help of error-prone PCR, resulting in mutant G81R. It was selected by high throughput screening, retaining 10% of its initial activity at pH 4.5 after 40 min in contrast to the native one, which was inactive in similar conditions. This enhanced stability might be due to increased electrostatic interaction, helix propensity, and hydrophilicity (Huang et al. 2019). Wang et al. (2012) developed a focused mutant library using a modified method, i.e., coevolving site saturation mutagenesis (CCSM) in directed evolution to improve the thermostability of α-amylase from Bacillus subtilis CN7 (Amy7C). Co-evolution is the correlated variation of protein sites selected by various interactions like allosteric, hydrophobic, and synergistic effects. Correlated variation sites of protein were chosen as hotspots (H100, D144, T147, G89, D95, and N197) to prepare a focused mutant library, and a high throughput screening screened the hot spot residues through starch-iodine and DNS method. The novel mutation sites improved the thermal stability of wild-type amylase by 8 °C (Wang et al. 2012). The directed evolution method is in popular demand as it does not necessitate any requirement regarding the molecule's structure. Functional enzymes with desired properties can be easily attained by simply creating a mutant library of coding enzymes through random mutagenesis and selecting the desired mutant through high throughput screening, which can be extremely beneficial for industrial applications. For example, patent US10563186B2 (Andersen et al. 2017) describes random mutagenesis of a Termamyl-like α-amylase from M/s Novozymes. The mutation was targeted to enhance substrate specificity with enhanced thermal stability for application as a dishwashing detergent. The patent US10066222B2 (Callen et al. 2017) assigned to M/s BASF enzymes LLC, on the other hand, describes the involvement of random mutagenesis for the improvement of α-amylase to remove starch from oil during the steeping process of corn. Another US patent US8828460B2 (Cherry et al. 2013) from M/s Novozymes describes the preparation of maltogenic α-amylases through random mutagenesis. The variants had altered pH enhanced thermostability, with an increased ability to reduce bread staling in the baking industry.
Site-directed mutagenesis
The site-directed mutagenesis method involves creating specific and intentional changes in the gene's DNA sequence of interest by targeted mutation of particular amino acids that alter or modify the intramolecular bonding within the protein or intermolecular interactions with a substrate (Fig. 3) Zhang et al. 2021a, b). Torktaz et al. (2018) carried out a series of point mutations in Geobacillus stearothermophilus to enhance its thermostability for potential industrial application. With the help of a prediction algorithm, 13 different sites were selected for point mutations, and the folding free energy was calculated after each modification. Although these amino acids are not part of the catalytic site, their alteration significantly affected the active site cavity by changes in substrate binding area and, thus, in binding affinity. The authors reported that four-point mutations D137V, G243V, E244I, and E244Y contributed significantly to making the Geobacillus stearothermophilus α-amylase more thermotolerant with higher ligand binding avidity. Industrial amylases, generally produced by Bacillus sp., have been extensively modified to incorporate desired properties such as secretion and enzymatic stability. For example, methionine residues within the protein have been determined as the most oxidation-prone residue and mutated to get oxidatively stabilized amylases for the detergent industry. Methionine mutation also enhanced the thermostability of enzymes. Declerck et al. (2003) aimed to enhance enzyme thermostability by targeting residues of hydrophobic packing of surface indentation of B. licheniformis α-amylase. It was reported that the point mutations aimed towards increasing thermostability by targeting residues of hydrophobic packing of surface indentation of B. licheniformis α-amylase. The mutations A209V and H133V enhanced the ligand binding and hydrophobicity responsible for protecting the secondary structure. Also, it was observed that N190F is the most thermo-stabilizing single mutation that could be achieved in B. licheniformis. The double mutants N190F and N265Y extended complex aromatic interaction with hydrogen bonds around the Ca-Na-Ca. This interaction reduced the enzyme's flexibility and stopped the escape of metal ions. On the contrary, the point mutation N192A made α-amylase more thermolabile by disrupting the hydrogen bonds, leading to helix to coil transition (Fort et al. 2021).
Literature reports suggest that the catalytic cleft in B. licheniformis contains conserved residues D206, E230, and D297 between domains A and B. Like B. licheniformis, other species have similar conserved residue profiles. H122 and H296 have been reported to assist in substrate binding. Pijning et al. (2021) Reported that alteration in conserved residues aspartate 206 and 297, glutamate 230, and histidine 122 and 296 in BLA enhanced the thermostability of the α-amylase. Deletion of Gly179 and Lys180 resulted in improved thermal stability due to reduced flexibility. Li et al. (2017a, b) carried out SDM of selected amino acid residues and showed that the mutation N188T and N188S enhanced the non-bond interactions, such as hydrogen bonds and salt bridges with the substrate. Also, the mutations A269K/S187D and A269K/S187D/N188T resulted in 25% less Km and 30% more Kcat than the wild type α-amylase. The triple mutant showed a half-life of 270 min (at 95 °C) at pH 5.5 and played an active role in the starch industry. α-Amylase obtained by the fusion of Bacillus acidicola with Geobacillus thermoleovorans had a melting temperature higher than the native one (Parashar and Satyanarayana 2016). Changes in N75, S76, and H77 of Bacillus megaterium resulted in better thermostability. α-Amylase from B. licheniformis (BLA) was mutated to increase its stability. It was observed that the Asparagines and Glutamines are prone to deamidation, which is accountable for thermal instability. Seven Asparagine residues were mutated at positions 172, 192, 190, 264, 265, 204, and 237. The authors reported that the Asparginine mutations at the positions enhanced the enzyme's stability. They further observed that N190F is the best mutation, even with different combinations, compared to other stabilizing mutations.
Bacillus licheniformis and Bacillus amyloliquefaciens are strains of industrial interest. Zhang et al. (2021a, b) focused on improving autolysis issues during amylase production. They found genes responsible for peptidoglycan hydrolase by transcriptomic profile during log and stationary phases. lytd, lytE and sig D were detected and mutated, resulting in a sharp reduction in hydrolyzed cells and a 48.4% increase in amylase activity. Deng et al. (2014) stated that the best mutated AmyK (The alkaline α-amylase gene AmyK) had a 5.4 °C increase in Tm and more than sixfold thermostability compared to the native type when four residues were simultaneously mutated. The hydrophobic and electrostatic interactions improved specific activity and thermostability (Li et al. 2017a, b). For example, Silano et al. (2020) reported an industrial α-amylase produced through genetic modification of a B amyloliquefaciens strain by M/s DuPont Danisco. The enzyme could be extensively used as a food amylase for the production of glucose syrups from starch (up to a recommended level of 45 mg TOS/kg) without any adverse toxicological effects. In a similar report, M/s Novozymes produced a recombinant maltogenic α-amylase using submerged fed-batch fermentation. The enzyme demonstrated very high activity (19,000 IU/g) and could be used in baking, brewing, and other cereal-based. Various examples of directed evolution and site-directed mutagenesis are given in Table 3.
A few industrial reports have demonstrated that SDM of α-amylases has significantly enhanced the functional properties of the enzyme for varied applications. For example, SDM of α-amylase in B. licheniformis (at M15L, from M/s GENENCOR) considerably enhanced the pH stability, oxidation, and heat stability, which are considered the most critical factors for application in the suitable industry. Another study from M/s Novozymes illustrates that alteration of the amino acid residue methionine (in Bacillus sp. at either 197th or 200th position) with a Leu, Ile, Asn, Ser, Gln, Asp or Glu enhanced the oxidation stability of the enzyme by many folds which can be used as a detergent additive. Likewise, US11248193B2 (Andersen and Fevre 2019) describes a SDM strategy for improvement of α-amylase for potential detergent applications in the industry.
Immobilization: an effective recycling strategy
Immobilization of α-amylase is a worthwhile strategy for improving stability alongside its reusability (Beltagy et al. 2022). The method employs retaining or binding enzymes over an insoluble matrix (through entrapment, adsorption, or covalent and cross-linking), enabling easy separation from the reaction mixture for successive recycling (Pervez et al. 2019). This is particularly important in large-scale production systems where production and processing cost is of paramount importance. The different methods for enzyme immobilization and their advantages are highlighted in Table 4.
For successful immobilization, it is necessary to choose a carrier with reasonable cost, and availability, and that has a good affinity towards the enzyme. The physicochemical parameters of the carrier including particle size, type of functional groups placed on the surface, surface area, and pore structure are governing factors for the immobilization step. Also, the support material should be insoluble and rigid. This reduces the chance of product inhibition by reducing non-specific interactions. A host of matrices have been investigated for α-amylase immobilization which may be organic, inorganic, or composite (Sharma et al. 2021). However, recently, hybrid matrices have become more popular owing to the advantages of high overall surface area, excellent ion exchange capability, environmentally friendly, and chemically inert with ease of activation. Yandri et al. (2022a, b) immobilized an α-amylase produced by A. fumigatus over a chitin-bentonite (CB) hybrid matrix and tested it for up to six cycles. The hybrid matrix displayed superior thermal stability in contrast to the classical matrices. The thermal inactivation rate constant (ki) at 60 °C for the free and immobilized α-amylases were 0.0171 min−1 and 0.0045 min−1, respectively. Reduced ki indicated a decrease in denaturation due to higher flexibility in the water. The half-life (t1/2) of the immobilized α-amylases was evaluated as 154 min, ~ 4-fold more elevated than the free enzyme (40.53 min). The free energy change due to denaturation (\(\Delta {G}_{i}\)) was 104.47 kJ/mol for free enzyme, whereas 108.17 kJ/mol for the immobilized enzyme. The increased \(\Delta {G}_{i}\) was due to increased folding conformations in the tertiary structure. The free α-amylases wasted ~ 72% of their original activity, whereas immobilized ones showed less than 30% loss. Immobilized α-amylase on CB hybrid can be used for up to six cycles by retaining 38% residual activity. Zhouquan et al. (2022) immobilized α-amylase on cellulose-chitosan hybrid gel macro sphere (CCMs) prepared by a sol–gel method having a high specific surface area (235.4–325.3 m2/g), overcoming the limitation of the low specific surface area of cellulose. The Immobilized α-amylases displayed improved stability compared to free enzymes and reusability up to 10 cycles with 77.55% residual activity. The stability of α-amylase was improved by Karaca Açarı et al. (2022) due to the immobilization of carbon and graphene quantum dots (QDs). QD was prepared from Hypericum perforatum L flowers (QD-1) and Hypericum capitatum seeds (QD-2). Immobilization of α-amylase on QD-1 and QD-2 displayed activity efficiency of 71.15% and 81.51%. The difference in the activity efficiency was due to variation in the porosity of both Q.D.s. The free activation energy (Ea) was assessed to be 9.61 kJ/mol, 3.20 kJ/mol, and 4.81 kJ/mol for QD-1/α-amylase, QD-2/α-amylase, and free enzyme, respectively. Enhancement in the restriction of mobility of secondary structure resulted in more stability in the QD-1/amylase and QD-2/amylase in contrast to free α-amylase. Morais et al. (2013) immobilized α-amylase on Luffa operculate fibres. It was used in kitchen grease traps and showed 30% activity after 30 days. α-Amylase from R. solani AG-4 strain ZB-34 was immobilized on chitosan covalently. It retained 81% residual activity when used with Persil® detergent (for solid laundry). When tested for desizing ability, the immobilized enzyme demonstrated 31% desizing at 40 °C at pH 4.5 (Uzun and Akatin 2019).
Alternatively, immobilization of α-amylases on magnetic nanoparticles has been shown to improve the stability and activity of the enzyme under different operating conditions. In addition, these support materials have proven to offer extensive reusability by enabling simple recovery with negligible enzyme losses. Salem et al. (2021) immobilized α-amylase from Bacillus subtilis on Iron oxide magnetic nanoparticles (IO-MNP) by electrostatic bonds. The authors reported that the immobilized enzyme could work for ~ 15 cycles, retaining ~ 82% of the initial activity. In a similar study, Abouelkheir et al. (2023) created IOMNPs using Bacillus subtilis SE05, which showed very high α-amylase activity (592.92 U/mg) during starch hydrolysis for bio-ethanol production. The enzyme could be recycled up to 9 batches for starch hydrolysis with ~ 50% activity retained. Desai et al. (2021) alternatively immobilized α-amylases on graphene oxide-magnetite nanoparticles through covalent bonding. The immobilization enhanced the enzyme's half-life to ~ 20 h (from 13 h) at 50 °C with a marked increase in alkali tolerance. The immobilized enzyme could be used for up to 11 cycles and showed potential application in the production of high maltose containing syrup. Chitosan-coated Fe3O4 magnetic nanoparticles were used for α-amylase immobilization by Dhavaler et al. (2018). The immobilized enzyme showed no loss in activity up to 20 cycles and retained 66% activity after 3 weeks as compared to 18% activity of the free enzyme. α-Amylase immobilized on Magnesium ferrite nanoparticles functionalized with silane displayed catalytic activity even after 12 cycles of reaction (Rana et al. 2022). A more recent report by Hallol et al. (2022) produced chitosan-loaded barium ferrite nano-particles (CLBFNPs). α-Amylase derived from soil isolates was immobilized on them. The specific activity of nano-particle bound α-amylases was increased to 246.85 U/mg in contrast to the free enzyme (177.12 U/mg). The immobilized enzyme retained more than 90% activity even after five recycles. The authors concluded that the magnetic nano-particles were suitable enzyme carriers owing to their high surface area (SA) to volume (V) ratio, super magnetic characteristics, and biocompatibility. The outcome of immobilization has pointed out that the activity and the stability of the α-amylase could be significantly enhanced through the different immobilization methods and demonstrate excellent advantages from an industrial viewpoint.
Summary and future recommendations
α-Amylases are pivotal enzymes and have prevailed in the chemical industry for many decades with applications in the food, detergent, textile, and paper industries. The ever-expanding global market with diverse application areas has prompted both the industry and academia in this field to isolate and develop new and improved versions of the enzyme. However, there exist a few technological and economic challenges in the successful scale-up of these laboratory-based processes to industrial/commercial production scales. Designing a suitable bioreactor by considering the fermentation broth's hydrodynamics, mixing effects, and heat and mass transfer effects can enable maximum enzyme yield by lowering process energy requirements. Similarly, the utilization of inexpensive and renewable substrates for enzyme production can lead to significant cost reduction. For example, Litchee seeds, which are primarily considered as waste (after the consumption of pulp) and generally thrown away, contain ~ 40% starch and pose an excellent renewable feedstock for the cultivation of α-amylases (Aqilah et al. 2023). Recently, we have been able to isolate 5 thermophilic novel bacterial strains from the soil below the Litchi tree with considerable α-amylase activity (data unpublished). Likewise, bakery wastes such as stale bread, bread rolls, and cookies, and vegetable and fruit wastes from local mandis/markets may also serve as a low-cost feedstock for the production of these enzymes.
One interesting application of the α-amylase could be in the field of bioremediation, particularly for the breakdown of petroleum-derived compounds (PDC). A few recent studies have shown that these enzymes (like bacterial P450s) are capable of degrading hydrocarbons such as n-alkanes (ranging from C10 to C14 carbon atoms) and in some cases low-density polyethylene (LDPE) (Karimi and Biria 2019). The addition of starch to the medium has been observed to accelerate the breakdown of PDCs by enabling some kind of surfactant activity, which reduces the issue of hydrocarbon mass transfer thereby enabling better uptake. This could have immense applications in the areas where oil spillage occurs or in the case of petroleum refineries where petroleum sludge is an issue with millions of dollars spent on its disposal. Site-specific mutagenesis may further enable the synthesis of tailored α-amylases with enhanced reactivity and durability which may be capable of degradation of polyaromatic hydrocarbons from PDCs. With the advent of new immobilization techniques using different carriers, the recyclability of the enzyme may be further improved for a stretch of 6–10 cycles without affecting its performance and stability. A perfect combination of the approaches would enable addressing the existing challenges for successfully deploying large-scale α-amylase technologies.
Data availability
The manuscript is a review article and does not contain any research data.
References
Abd-Elhalem BT, El-Sawy M, Gamal RF, Abou-Taleb KA (2015) Production of amylases from Bacillus amyloliquefaciens under submerged fermentation using some agro-industrial by-products. Ann Agric Scie 60(2):193–202. https://doi.org/10.1016/j.aoas.2015.06.001
Abdel-Hameed SA, Ahmed SA, Mostafa FA, Almasarawi ON, Wahab WAA (2022) Preparation and characterization of sugilite glass from basalt for α-amylase immobilization, statistical optimization of the immobilization process and description of free and immobilized enzyme. Heliyon 8(7):e09960. https://doi.org/10.1016/j.heliyon.2022.e09960
Abdella MA, El-Sherbiny GM, El-Shamy AR, Atalla SM, Ahmed SA (2020) Statistical optimization of chemical modification of chitosan-magnetic nano-particles beads to promote Bacillus subtilis MK1 α-amylase immobilization and its application. Bull Natl Res Centre 44(1):1–13. https://doi.org/10.1186/s42269-020-00301-3
Abouelkheir SS, Ibrahim HA, Beltagy EA (2023) Functionalized maghemite superparamagnetic iron oxide nanoparticles (γ-Fe2O3-SPIONs)-amylase enzyme hybrid in biofuel production. Sci Rep 13(1):11117
Abraham A, Narayanan SP, Philip S, Nair DG, Kochupurackal J (2013) Molecular modelling and docking studies of an α-1, 4-amylase from endophytic Bacillus amyloliquefaciens. Front Life Sci 7(3–4):140–147. https://doi.org/10.1080/21553769.2013.852993
Ahuja K, Malkani T (2019) Enzymes market size, share and industry analysis report by product (proteases, lipases, carbohydrases [amylases, xylanases, cellulases, pectinases, lactases], polymerases and nucleases, phytases, catalyses) and application (food and beverage, processed food, diary, bakery, confectionary), regional outlook, growth potential, competitive market share and forecast, 2018–2024. Global Market Insights Inc. https://www.gminsights.com/industry-analysis/enzymes-market. Accessed 22 Jan 2023
Al-Bedak OA, Sakr RS, Al-Kolaibe AMG (2022) The microbial amylases: an overview with practical consequences and applications. J Microbiol Exp 10(4):130–134
Aleem B, Rashid MH, Zeb N, Saqib A, Ihsan A, Iqbal M, Ali H (2018) Random mutagenesis of super Koji (Aspergillus oryzae): improvement in production and thermal stability of α-amylases for maltose syrup production. BMC Microbiol 18(1):1–13. https://doi.org/10.1186/s12866-018-1345-y
Almanaa TN, Vijayaraghavan P, Alharbi NS, Kadaikunnan S, Khaled JM, Alyahya SA (2020) Solid state fermentation of amylase production from Bacillus subtilis D19 using agro-residues. J King Saud Univ Sci 32(2):1555–1561
Al-Najada AR, Almulaiky YQ, Aldhahri M et al (2019) Immobilization of α-amylase on activated amidrazone acrylic fabric: a new approach for the enhancement of enzyme stability and reusability. Sci Rep 9:12672. https://doi.org/10.1038/s41598-019-49206-w
Alkaline Amylase—Creative Enzymes (2019). Ariaeenejad/similar/alkaline-amylase_51.html. Accessed 23 Jan 2023
Anand U, Vaishnav A, Sharma SK, Sahu J, Ahmad S, Sunita K et al (2022) Current advances and research prospects for agricultural and industrial uses of microbial strains available in world collections. Sci Total Environ 842:156641. https://doi.org/10.1016/j.scitotenv.2022.156641
Andersen C, Fevre A (2019) Stabilized alpha-amylase variants and use of the same. US Patent 11248193B2
Andersen C, Oestdal H, Skagerlind JP (2017) Alpha-amylase variants with altered properties. US Patent 10563186B2
Apostolidi ME, Kalantzi S, Hatzinikolaou DG, Kekos D, Mamma D (2020) Catalytic and thermodynamic properties of an acidic α-amylase produced by the fungus Paecilomyces variotii ATHUM 8891. 3Biotech 10(7):311. https://doi.org/10.1007/s13205-020-02305-2
Aqilah R et al (2023) A review on the compressive strength and workability of concrete with agricultural waste ash as cement replacement material. Earth Environ Sci. https://doi.org/10.1088/1755-1315/1135/1/012058
Aquazym®|Novozymes (2023) https://www.novozymes.com/en/products/textile/desizing/aquazym. Accessed 23 Jan 2023
Arnau J, Yaver D, Hjort CM (2020) Strategies and challenges for the development of industrial enzymes using fungal cell factories. Grand Chall Fungal Biotechnol. https://doi.org/10.1007/978-3-030-29541-7_7
Augustine M, Madhusudhanan DT, Velayudhan MP (2023) Thermal deactivation studies of alpha-amylase immobilized onto core-shell structured aniline formaldehyde crosslinked polyaniline magnetic nanocomposite. Biotechnol Biotechnol Equip 37(1):273–285. https://doi.org/10.1080/13102818.2023.2182150
Ayansina A, Adelaja A, Mohammed S (2017) Characterization of amylase from some Aspergillus and Bacillus species associated with cassava waste peels. Adv Microbiol 7(4):280–292. https://doi.org/10.4236/aim.2017.74023
Baking enzyme market business opportunity and future growth analysis report 2028: reports and data. https://menafn.com/1103923735/Alpha-Amylase-Baking-Enzyme-Market-Business-Opportunity-And-Future-Growth-Analysis-Report-2028-Reports-and-Data. Accessed 23 Jan 2023
Baking enzyme market size and share report, 2024. https://www.grandviewresearch.com/industry-analysis/alpha-amylase-baking-enzyme-market. Accessed 23 Jan 2023
Bakri Y, Elkhouri S, Harba M, Akeed Y (2020) Amylase production by Bacillus subtilis SY134D strain under submerged fermentation: production of amylase. Biol Sci (PJSIR) 63(2):113–118. https://doi.org/10.52763/PJSIR.BIOL.SCI.63.2.2020.113.118
Balakrishnan M, Jeevarathinam G, Kumar S, Muniraj I, Uthandi S (2021) Optimization and scale-up of α-amylase production by Aspergillus oryzae using solid-state fermentation of edible oil cakes. BMC Biotechnol. https://doi.org/10.1186/s12896-021-00686-7
Bamigboye CO, Okonji RE, Oluremi IO, James V (2022) Stain removing, juice-clarifying, and starch-liquefying potentials of amylase from Pleurotus tuberregium in submerged fermentation system. J Genet Eng Biotechnol. https://doi.org/10.1186/s43141-022-00298-4
Baroroh U, Yusuf M, Rachman SD, Ishmayana S, Hasan K, Subroto T (2019) Molecular dynamics study to improve the substrate adsorption of Saccharomycopsis fibuligera R64 α-amylase by designing a new surface binding site. Adv Appl Bioinform Chem. https://doi.org/10.2147/AABC.S198110
Beltagy EA, Abouelwafa A, Barakat KM (2022) Bioethanol production from immobilized amylase produced by marine Aspergillus flavus AUMC10636. Egypt J Aquat Res. https://doi.org/10.1016/j.ejar.2022.02.003
Berlina YY, Petrovskaya LE, Kryukova EA, Shingarova LN, Gapizov SS, Kryukova MV et al (2021) Engineering of thermal stability in a cold-active oligo-1, 6-glucosidase from Exiguobacterium sibiricum with unusual amino acid content. Biomolecules 11(8):1229. https://doi.org/10.3390/biom11081229
Bilal M, Ji L, Xu Y, Xu S, Lin Y, Iqbal HM, Cheng H (2022) Bioprospecting Kluyveromyces marxianus as a robust host for industrial biotechnology. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2022.851768
Blaga AC, Caşcaval D, Galaction AI (2022) Improved production of α-amylase by Aspergillus terreus in presence of oxygen-vector. Fermentation 8(6):271. https://doi.org/10.3390/fermentation8060271
Boodhoo KVK, Flickinger MC, Woodley JM, Emanuelsson EAC (2022) Bioprocess intensification: a route to efficient and sustainable biocatalytic transformations for the future. Chem Eng Process Process Intensif. https://doi.org/10.1016/j.cep.2022.108793M
Božić N, Lončar N, Slavić MŠ, Vujčić Z (2017) Raw starch degrading α-amylases: an unsolved riddle. Amylase 1(1):12–25. https://doi.org/10.1515/amylase-2017-0002
Brayer GD, Luo Y, Withers SG (1995) The structure of human pancreatic alpha-amylase at 1.8 A resolution and comparisons with related enzymes. Protein Sciences 4(9):1730–42. https://doi.org/10.1002/pro.5560040908
Callen W, Richardson T, Frey G, Miller CA, Kazaoka M, Mathur EJ, Short JM (2017) Amylases, nucleic acids encoding them and method of producing an oil. US Patent 10066222B2
Castro AMD, Carvalho DF, Freire DMG, Castilho LDR (2010) Economic analysis of the production of amylases and other hydrolases by Aspergillus awamori in solid-state fermentation of babassu cake. Enzyme Res. https://doi.org/10.4061/2010/576872
CAZy (2023) http://www.cazy.org/Citing-CAZy. Accessed 22 Jan 2023
Cerda A, El-Bakry M, Gea T, Sánchez A (2016) Long term enhanced solid-state fermentation: inoculation strategies for amylase production from soy and bread wastes by Thermomyces sp. in a sequential batch operation. J Environ Chem Eng 4(2):2394–2401. https://doi.org/10.1016/j.jece2016.04.022
Chauhan G, Kumar V, Arya M et al (2023) Mining of thermostable alpha-amylase gene from geothermal springs using a metagenomics approach. J Pure Appl Microbiol 17(1):362–370. https://doi.org/10.22207/JPAM.17.1.26
Chen S, Tan Y, Zhu Y, Sun L, Lin J, Zhang H (2022) Application of ultrafiltration and ion exchange separation technology for lysozyme separation and extraction. Fermentation 8(7):297. https://doi.org/10.3390/fermentation8070297
Cherry J, Syendsen A, Andersen C, Beier L, Frandsen TP (2013) Method for preparing maltogenic alpha-amylase variants. US Patent 8828460B2
Crater JS, Lievense JC (2018) Scale-up of industrial microbial processes. FEMS Microbiol Lett 1:365
Cripwell RA, Favaro L, Viljoen-Bloom M, Vanzyl WH (2020) Consolidated bioprocessing of raw starch to ethanol by Saccharomyces cerevisiae: achievements and challenges. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2020.107579
Cuadrado-Osorio PD, Ramírez-Mejía JM, Mejía-Avellaneda LF, Garriga LM, Bautista EJ (2022) Agro-industrial residues for microbial bioproducts: a key booster for bioeconomy. Bioresour Technol Rep. https://doi.org/10.1016/j.biteb.2022.101232
Cui X, Yuan X, Li S, Hu X, Zhao J, Zhang G (2022) Simultaneously improving the specific activity and thermostability of α-amylase BLA by rational design. Bioprocess Biosyst Eng 45(11):1839–1848. https://doi.org/10.1007/s00449-022-02790-0
Dahiya P, Rathi B (2015) Characterization and application of alkaline α-amylase from Bacillus licheniformis MTCC1483 as a detergent additive. Int Food Res J 22(3):1293–1297
Dakhmouche Djekrif S, Bennamoun L, Labbani FZK, AitKaki A, Nouadri T, Pauss A et al (2021) An alkalothermophilic amylopullulanase from the yeast Clavispora lusitaniae ABS7: purification, characterization and potential application in laundry detergent. Catalysts 11(12):1438. https://doi.org/10.3390/catal11121438
de Souza-Vandenberghe, Porto L, Herrmann LW, de Oliveira-Penha R, de Mello AFM, Martínez-Burgos WJ, Magalhães-Junior AI, de Souza-Kirnev PC, de Carvalho JC, Soccol CR (2022) Engineering aspects for scale-up of bioreactors. Curr Dev Biotechnol Bioeng. https://doi.org/10.1016/B978-0-323-91167-2.00002-2
Declerck N, Machius M, Joyet P, Wiegand G, Huber R, Gaillardin C (2003) Hyperthermostabilization of Bacillus licheniformis alpha-amylase and modulation of its stability over a 50 degrees C temperature range. Protein Eng 16(4):287–293. https://doi.org/10.1093/proeng/gzg032
Delavat F, Phalip V, Forster A, Plewniak F, Lett MC, Lievremont D (2012) Amylases without known homologues discovered in an acid mine drainage: significance and impact. Sci Rep 2(1):1–6. https://doi.org/10.1038/srep00354
Deljou A, Arezi I, Khanahmadi M (2018) Scale-up thermostable α-amylase production in lab-scale fermenter using rice husk as an elicitor by Bacillus licheniformis-AZ2 isolated from Qinarje Hot Spring (Ardebil Prov. of Iran). Period Biol 120(1):11–21. https://doi.org/10.18054/pb.v120i1.6775
Deng Z, Yang H, Shin HD, Li J, Liu L (2014) Structure-based rational design and introduction of arginines on the surface of an alkaline α-amylase from Alkalimonas amylolytica for improved thermostability. Appl Microbiol Biotechnol 98(21):8937–8945. https://doi.org/10.1007/s00253-014-5790-8
Desai RP, Dave D, Suthar SA, Shah S, Ruparelia N, Kikani BA (2021) Immobilization of α-amylase on GO-magnetite nanoparticles for the production of high maltose containing syrup. Int J Biol Macromol 169:228–238
Dhavaler RP, Parit SB, Sahoo SC, Kollu P, Patil PS, Patil PB, Chougale AD (2018) α-Amylase immobilized on magnetic nanoparticles: reusable robust nano-biocatalyst for starch hydrolysis. Mater Res Express 5(7):075403
Duan X, Zhu Q, Zhang X, Shen Z, Huang Y (2021) Expression, biochemical and structural characterization of high-specific-activity β-amylase from Bacillus aryabhattai GEL-09 for application in starch hydrolysis. Microb Cell Fact 20(1):1–14. https://doi.org/10.1186/s12934-021-01649-5
EFSA Panel, Silano V, Barat Baviera JM, Bolognesi C, Cocconcelli PS et al (2020) Safety evaluation of the food enzyme α-amylase from the genetically modified Bacillus amyloliquefaciens strains DP-Czb53. EFSA J 18(10):e06185. https://doi.org/10.2903/j.efsa.2020.6185
Egbune EO, Avwioroko OJ, Anigboro AA, Aganbi E, Amata AI, Tonukari NJ (2022) Characterization of a surfactant-stable α-amylase produced by solid-state fermentation of Cassava (Manihot esculenta Crantz) tubers using Rhizopus oligosporus: Kinetics, thermal inactivation thermodynamics and potential application in laundry industries. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2022.102290
Eivazzadeh-Keihan R, Dogari H, Ahmadpour F et al (2021) Design and synthesis of a novel nanocomposite based on magnetic dopamine nanoparticles for purification of α-amylase from the bovine milk. Sci Rep 11:13428. https://doi.org/10.1038/s41598-021-92919-0
El-fallal A, Abou-Dobara M, El-Sayed A, Omar N (2012) starch and microbial α-amylases: from concepts to biotechnological applications. IntechOpen, London. https://doi.org/10.5772/51571
El-Gendi H, Saleh AK, Badierah R, Redwan EM, El-Maradny YA, El-Fakharany EM (2022) A comprehensive insight into fungal enzymes: structure, classification, and their role in mankind’s challenges. J Fungi 8(1):23. https://doi.org/10.3390/jof8010023
Elyasi F, Ahmadi Y, Khosroshahi AY, Dilmaghani A (2020) Microbial α-amylase production: progress, challenges and perspectives. Adv Pharm Bull 10(3):350. https://doi.org/10.34172/apb.2020.043
Farooq MA, Ali S, Hassan A, Tahir HM, Mumtaz S, Mumtaz S (2021) Biosynthesis and industrial applications of α-amylase: A review. Arch Microbiol 203(4):1281–1292. https://doi.org/10.1007/s00203-020-02128-y
Fatoki OA, Onilude AA, Ekanola YA, Akanbi CT (2023) Optimisation of alpha-amylase inhibitor production in solid state fermentation. Front Pharmacol. https://doi.org/10.3389/fphar.2023.1073754
Fort J, Nicolàs-Aragó A, Palacín M (2021) The ectodomains of rBAT and 4F2hc are fake or orphan α-glucosidases. Molecules. https://doi.org/10.3390/molecules26206231
Gai Y, Chen J, Zhang S, Zhu B, Zhang D (2018) Property improvement of α-amylase from Bacillus stearothermophilus by deleting amino acid residues arginine 179 and glycine 180. Food Technol Biotechnol 56(1):58–64. https://doi.org/10.17113/ftb.56.01.18.5448
Gangadharan D, Jose A, Nampoothiri KM (2020) Recapitulation of stability diversity of microbial α-amylases. Amylase 4(1):11–23. https://doi.org/10.1515/amylase-2020-0002
George R, Georrge JJ, Singh SP, Vaidya A (2020) Comparative analysis of thermophilic alpha-amylase using in silico approach. In: Proceedings of the national conference on innovations in biological sciences (NCIBS). https://doi.org/10.2139/ssrn.3573327
Gopinath SC, Anbu P, Arshad MM, Lakshmipriya T, Voon CH, Hashim U, Chinni SV (2017) Biotechnological processes in microbial amylase production. Biomed Res Int. https://doi.org/10.1155/2017/1272193
Guerrero-Navarro AE, Ríos-Castillo AG, Avila CR, Hascoët AS, Felipe X, Jerez JR (2019) Development of a dairy fouling model to assess the efficacy of cleaning procedures using alkaline and enzymatic products. LWT 106:44–49. https://doi.org/10.1016/j.lwt.2019.02.057
Guerrero-Navarro AE, Ríos-Castillo AG, Ripolles-Avila C, Zamora A, Hascoët A-S, Felipe X, Castillo M, Rodríguez-Jerez JJ (2022) Effectiveness of enzymatic treatment for reducing dairy fouling at pilot-plant scale under real cleaning conditions. LWT 154:112634. https://doi.org/10.1016/j.lwt.2021.112634
Gürkök S (2019) Microbial enzymes in detergents: a review. Int J Sci Eng Res 10(9):75–81
Hallol M, Helmy O, Shawky AE, El-Batal A, Ramadan M (2022) Optimization of α-amylase production by a local Bacillus paramycoides isolate and immobilization on chitosan-loaded barium ferrite nanoparticles. Fermentation. https://doi.org/10.3390/fermentation8050241
Hameed U, Ul-Haq I (2020) Kinetics and thermodynamics of catalysis and thermal inactivation of a novel α-amylase (Tp-AmyS) from Thermotoga petrophila. Biocatal Biotransform 38(3):227–233. https://doi.org/10.1080/10242422.2020.1736573
Hamid B, Bashir Z, Yatoo AM, Mohiddin F, Majeed N, Bansal M et al (2022) Cold-active enzymes and their potential industrial applications—a review. Molecules. https://doi.org/10.3390/molecules27185885
Huang L, Shan M, Ma J, Li Y, Xu Z, Shao S et al (2019) Directed evolution of α-amylase from Bacillus licheniformis to enhance its acid-stable performance. Biologia 74(10):1363–1372. https://doi.org/10.2478/s11756-019-00262-7
Huang Q, Liu H, Zhang J, Wang S, Liu F, Li C, Wang G (2022) Production of extracellular amylase contributes to the colonization of Bacillus cereus 0–9 in wheat roots. BMC Microbiol 22(1):205
Jeilu O, Simachew A, Alexandersson E, Johansson E, Gessesse A (2022) Discovery of novel carbohydrate degrading enzymes from soda lakes through functional metagenomics. Front Microbiol. https://doi.org/10.3389/fmicb.2022.1059061
Kamer A, Abd-El-salam M et al (2023) A promising microbial α-amylase production, and purification from Bacillus cereus and its assessment as antibiofilm agent against Pseudomonas aeruginosa pathogen. Microb Cell Fact. https://doi.org/10.1186/s12934-023-02139-6
Karaca Açarı İ, Dik G, Bakar B, Ulu A, Önal Y, Ateş B (2022) Immobilization of α-amylase onto quantum dots prepared from Hypericum perforatum L. flowers and Hypericum capitatum seeds: its physicochemical and biochemical characterization. Top Catal. https://doi.org/10.1007/s11244-022-01699-y
Karam EA, Wahab WAA, Saleh SA, Hassan ME, Kansoh AL, Esawy MA (2017) Production, immobilization and thermodynamic studies of free and immobilized Aspergillus awamori amylase. Int J Biol Macromol 102:694–703. https://doi.org/10.1016/j.ijbiomac.2017.04.033
Karimi M, Biria D (2019) The promiscuous activity of alpha-amylase in biodegradation of low-density polyethylene in a polymer-starch blend. Sci Rep 9(1):1–10. https://doi.org/10.1038/s41598-019-39366-0
Kaur N, Kumar V, Nayak SK, Wadhwa P, Kaur P, Sahu SK (2021) α-Amylase as molecular target for treatment of diabetes mellitus: A comprehensive review. Chem Biol Drug Des 98(4):539–560. https://doi.org/10.1111/cbdd.13909
Khalid-Bin-Ferdaus KM, Hossain MF, Mansur SA, Sajib SA, Miah MM, Hoque KMF, Reza MA (2018) Commercial production of α amylase enzyme for potential use in the textile industries in Bangladesh. Int J Biosci 13(4):149–157. https://doi.org/10.12692/ijb/13.4.149-157
Khan N, Sudhakar K, Mamat R (2021) Role of biofuels in energy transition, green economy and carbon neutrality. Sustainability. https://doi.org/10.3390/su132212374
Khusro A, Barathikannan K, Aarti C, Agastian P (2017) Optimization of thermo-alkali stable amylase production and biomass yield from Bacillus sp. Under Submerged Cultivation Ferment 3(1):7. https://doi.org/10.3390/fermentation3010007
Kikani BA, Kourien S, Rathod U (2019) Stability and thermodynamic attributes of starch hydrolyzing α-amylase of Anoxybacillus rupiensis TS-4. Starch (Stärke) 72(1–2):1900105. https://doi.org/10.1002/star.201900105
Kothakota A, Pandiselvam R, Siliveru K, Pandey JP, Sagarika N, Srinivas CHS, Kumar A, Singh A, Prakash SD (2021) Modeling and optimization of process parameters for nutritional enhancement in enzymatic milled rice by multiple linear regression (MLR) and artificial neural network (ANN). Foods 10(12):2975. https://doi.org/10.3390/foods10122975
Krajang M, Malairuang K, Sukna J, Rattanapradit K, Chamsart S (2021) Single-step ethanol production from raw cassava starch using a combination of raw starch hydrolysis and fermentation, scale-up from 5-L laboratory and 200-L pilot plant to 3000-L industrial fermenters. Biotechnol Biofuels 14(1):1–15. https://doi.org/10.1186/s13068-021-01903-3
Kumar D, Singh V (2016) Dry-grind processing using amylase corn and superior yeast to reduce the exogenous enzyme requirements in bioethanol production. Biotechnol Biofuels 9(1):1–12. https://doi.org/10.1186/s13068-016-0648-1
Lahiri D, Nag M, Banerjee R, Mukherjee D, Garai S, Sarkar T (2021) Amylases: biofilm inducer or biofilm inhibitor? Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2021.660048
Lee Y, Wiriyasermkul P, Kongpracha P et al (2022) Ca2+-mediated higher-order assembly of heterodimers in amino acid transport system b0,+ biogenesis and cystinuria. Nat Commun. https://doi.org/10.1038/s41467-022-30293-9
Li Z, Duan X, Wu J (2016) Improving the thermostability and enhancing the Ca2+ binding of the maltohexaose-forming α-amylase from Bacillus stearothermophilus. J Biotechnol 222:65–72. https://doi.org/10.1016/j.jbiotec.2016.02.013
Li Y, Wei L, Zhu Z, Li S, Wang JW, Xin Q et al (2017a) Rational design to change product specificities and thermostability of cyclodextrin glycosyltransferase from Paenibacillus sp. RSC Adv 7(23):13726–13732. https://doi.org/10.1039/C7RA00245A
Li Z, Duan X, Chen S (2017b) Improving the reversibility of thermal denaturation and catalytic efficiency of Bacillus licheniformis α-amylase through stabilizing a long loop in domain B. PLoS ONE. https://doi.org/10.1371/journal.pone.0173187
Li B, Sun Y, Yao J, Wu H, Shen Y, Zhi C, Li J (2022) An environment-friendly chemical modification method for thiol groups on polypeptide macromolecules to improve the performance of regenerated keratin materials. Mater Des. https://doi.org/10.1016/j.matdes.2022.110611
Liao SM, Liang GE, Zhu J, Lu BO, Peng LX, Wang QY (2019) Influence of calcium ions on the thermal characteristics of α-amylase from thermophilic Anoxybacillus sp. GXS-BL. Protein Pept Lett 26(2):148–157. https://doi.org/10.2174/0929866526666190116162958
Lim SJ, Oslan SN (2021) Native to designed: microbial α-amylases for industrial applications. PeerJ. https://doi.org/10.7717/peerj.11315
Lim SJ, Oslan SNH, Oslan SN (2020) Purification and characterisation of thermostable α-amylases from microbial sources. BioResources 15(1):2005–2029. https://doi.org/10.15376/biores.15.1.2005-2029
Liu Y, Huang L, Jia L, Gui S, Fu Y, Zheng D et al (2017) Improvement of the acid stability of Bacillus licheniformis α-amylase by site-directed mutagenesis. Process Biochem 58:174–180. https://doi.org/10.1111/j.1365-2672.2012.05359.x
MacGregor EA (1988) Alpha-amylase structure and activity. J Protein Chem 7(4):399–415. https://doi.org/10.1007/BF01024888
Machius M, Declerck N, Huber R, Wiegand G (1998) Activation of Bacillus licheniformis alpha-amylase through a disorder–>order transition of the substrate-binding site mediated by a calcium-sodium-calcium metal triad. Structure 6(3):281–292. https://doi.org/10.1016/s0969-2126(98)00032-x
Madhavan A, Arun KB, Binod P, Sirohi R, Tarafdar A, Reshmy R (2021) Design of novel enzyme biocatalysts for industrial bioprocess: harnessing the power of protein engineering, high throughput screening and synthetic biology. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.124617
Marengo M, Pezzilli D, Gianquinto E, Fissore A, Oliaro-Bosso S, Sgorbini B (2022) Evaluation of porcine and Aspergillus oryzae α-amylases as possible model for the human enzyme. Processes 10(4):780. https://doi.org/10.3390/pr10040780
Mehta D, Satyanarayana T (2014) Domain C of thermostable α-amylase of Geobacillus thermoleovorans mediates raw starch adsorption. Appl Microbiol Biotechnol 98(10):4503–4519. https://doi.org/10.1007/s00253-013-5459-8
Mehta D, Satyanarayana T (2015) Structural elements of thermostability in the maltogenic amylase of Geobacillus thermoleovorans. Int J Biol Macromol 79:570–576. https://doi.org/10.1016/j.ijbiomac.2015.04.011
Mehta D, Satyanarayana T (2016) Bacterial and archaeal α-amylases: diversity and amelioration of the desirable characteristics for industrial applications. Front Microbiol. https://doi.org/10.3389/fmicb.2016.01129
Meyer F, Johannsen J, Liese A, Fieg G, Bubenheim P, Waluga T (2021) Evaluation of process integration for the intensification of a biotechnological process. Chem Eng Process Process Intensif 167:108506. https://doi.org/10.1016/j.cep.2021.108506
Morais R, Aline M, Samantha S, Flavio M, Kátia F (2013) Immobilization of α-amylase onto Luffa operculata fibers. Enzyme Res. https://doi.org/10.1155/2013/803415
Mostafa E, Abo Elsoud M, Salem MS, El-Tabakh E, Sidkey NM (2021) Optimization of aeration and agitation for α-amylase production by Aspergillus flavus from water hyacinth. Int J Ind Sustain Dev 2(1):25–35. https://doi.org/10.21608/IJISD.2021.145562
Motahar SFS, Khatibi A, Salami M, Ariaeenejad S, Emam-Djomeh Z, Nedaei H (2020) A novel metagenome-derived thermostable and poultry feed compatible α-amylase with enhanced biodegradation properties. Int J Biol Macromol 164:2124–2133. https://doi.org/10.1016/j.ijbiomac.2020.08.064
Motahar SFS, Ariaeenejad S, Salami M, Emam-Djomeh Z, Mamaghani ASA (2021) Improving the quality of gluten-free bread by a novel acidic thermostable α-amylase from metagenomics data. Food Chem. https://doi.org/10.1016/j.foodchem.2021.129307
Msarah MJ, Ibrahim I, Hamid AA, Aqma WS (2020) Optimisation and production of alpha amylase from thermophilic Bacillus spp. and its application in food waste biodegradation. Heliyon. https://doi.org/10.1016/j.heliyon.2020.e04183
Nair HP, Vincent H, Puthusseri RM, Bhat SG (2017) Molecular cloning and characterization of a halotolerant α-amylase from marine metagenomic library derived from Arabian Sea sediments. 3 Biotech 7(1):1–9. https://doi.org/10.1007/s13205-017-0674-0
Nandi S, Bose T, Mahato S, Chatterjee S, Chatterjee A (2022) Isolation and partial characterization of amylase produced by fungal isolates from the agro-industrial waste source. J Appl Biol Biotechnol 10(4):45–49. https://doi.org/10.7324/JABB.2022.100406
Narula A (2023) Kinetics of immobilized alpha amylase impregnated with silver nanoparticles in Egg Shell Membrane for enhanced starch hydrolysis. Egypt J Chem 66(2):1–12. https://doi.org/10.21608/EJCHEM.2022.110860.5050
Navjot K, Baljit S, Savita S, Ramesh K (2022) Study of relationships between independent extrusion variables and dependent product properties during quality protein maize extrusion. Appl Food Res 2(1):100048. https://doi.org/10.1016/j.afres.2022.100048
Ng YJ, Tham PE, Khoo KS, Cheng CK, Chew KW, Show PL (2021) A comprehensive review on the techniques for coconut oil extraction and its application. Bioprocess Biosyst Eng 44(9):1807–1818. https://doi.org/10.1007/s00449-021-02577-9
Niego AGT, Lambert C, Mortimer P, Thongklang N, Rapior S, Grosse M (2023) The contribution of fungi to the global economy. Fungal Divers 121(1):95–137. https://doi.org/10.1007/s13225-023-00520-9
Ningsih DR, Kartika D, Kurniasih M, Nofiani R, Fatoni A (2020) Improved reuse and affinity of enzyme using immobilized amylase on alginate matrix. J Phys 1494(1):012028. https://doi.org/10.1088/1742-6596/1494/1/012028
Niyomukiza S, Owino W, Maina JM, Maina N, Issifu M (2022) Concomitant production of α-amylase and protease by Bacillus aerius strain FPWSHA isolated from food wastes. Biointerface Res Appl Chem 13:1–15. https://doi.org/10.33263/BRIAC134.310
Nwagu TN, Aoyagi H, Okolo B, Moneke A, Yoshida S (2020) Citraconylation and maleylation on the catalytic and thermodynamic properties of raw starch saccharifying amylase from Aspergillus carbonarius. Heliyon 6(7):e04351. https://doi.org/10.1016/j.heliyon.2020.e04351
O’Connor KE (2021) Microbiology challenges and opportunities in the circular economy. Microbiology (Reading) 167(1):001026. https://doi.org/10.1099/mic.0.001026
Ojha SK, Singh PK, Mishra S, Pattnaik R, Dixit S, Verma SK (2020) Response surface methodology based optimization and scale-up production of amylase from a novel bacterial strain, Bacillus aryabhattai KIIT BE-1. Biotechnol Rep 27:e00506. https://doi.org/10.1016/j.btre.2020.e00506
Oshoma E, Akor O, Ikhajiagbe B, Ikenebomeh J (2022) Mutation of Aspergillus sp. using ultraviolet light and nitrous acid for amylase production from banana peels. Makara J Sci 26(3):7. https://doi.org/10.7454/mss.v26i3.1357
Pan I (2021) Exploration for thermostable β-amylase of a Bacillus sp. isolated from compost soil to degrade bacterial biofilm. Microbiol Spectrum 9(2):e00647-21. https://doi.org/10.1128/Spectrum.00647-21
Parashar D, Satyanarayana T (2016) A chimeric α-amylase engineered from Bacillus acidicola and Geobacillus thermoleovorans with improved thermostability and catalytic efficiency. J Ind Microbiol Biotechnol 43(4):473–484. https://doi.org/10.1007/s10295-015-1721-7
Paul D (2016) Microorganisms and α-amylase: a concise review. Innovare J Sci 4:1–5
Pervez S, Nawaz MA, Jamal M, Jan T, Maqbool F, Shah I et al (2019) (2019) Improvement of catalytic properties of starch hydrolyzing fungal amyloglucosidase: utilization of agar-agar as an organic matrix for immobilization. Carbohydr Res 486:107860. https://doi.org/10.1016/j.carres.2019.107860
Pham V, Kim J, Shim J, Chang S, Chung W (2022) Purification and characterization of strong simultaneous enzyme production of protease and α-amylase from an extremophile-Bacillus sp. FW2 and its possibility in food waste degradation. Fermentation 8(1):12. https://doi.org/10.3390/fermentation8010012
Pijning T, Gangoiti J, Te Poele EM, Börner T, Dijkhuizen L (2021) Insights into broad-specificity starch modification from the crystal structure of Limosilactobacillus Reuteri NCC 2613 4, 6-α-glucanotransferase GtfB. J Agric Food Chem 69(44):13235–13245. https://doi.org/10.1021/acs.jafc.1c05657
Posoongnoen S, Ubonbal R, Klaynongsruang S, Daduang J, Roytrakul S, Daduang S (2021) Characterization and molecular cloning of secreted α-amylase with dominant activity from mon thong durian (Durio zibethinus Murr. Cv. mon thong). Rev Bras Frutic 43(3):e-231. https://doi.org/10.1590/0100-29452021231
Prabhu G, Bhat D, Bhat RM, Selvaraj S (2022) A critical look at bioproducts co-cultured under solid state fermentation and their challenges and industrial applications. Waste Biomass Valoriz 13:3095–3111. https://doi.org/10.1007/s12649-022-01721-0
Pranay K, Padmadeo SR, Prasad B (2019) Production of amylase from Bacillus subtilis sp strain KR1 under solid state fermentation on different agrowastes. Biocatal Agric Biotechnol 21(4):101300. https://doi.org/10.1016/j.bcab.2019.101300
Puiman L, Abrahamson B, van der Lans RGJM, Haringa C, Noorman HJ, Picioreanu C (2022) Alleviating mass transfer limitations in industrial external-loop syngas-to-ethanol fermentation. Chem Eng Sci 259:117770. https://doi.org/10.1016/j.ces.2022.117770
Rabbani M, Mohammad M, Sadeghi H, Moazen F, Rahimi M, Salehi G (2011) Cloning and expression of randomly mutated Bacillus subtilis α-amylase genes in HB101. Biotechnol Res Int. https://doi.org/10.4061/2011/305956
Raina D, Kumar V, Saran S (2022) A critical review on exploitation of agro-industrial biomass as substrates for the therapeutic microbial enzymes production and implemented protein purification techniques. Chemosphere 294:133712. https://doi.org/10.1016/j.chemosphere.2022.133712
Ramachandran P, Manoharan S, Hemalatha D, Gunasekaran P (2010) Repeated random mutagenesis of α-amylase from Bacillus licheniformis for improved pH performance. J Microbiol Biotechnol 20(12):1696–1701. https://doi.org/10.4014/jmb.1008.08013
Rana S, Sharma A, Batoo KM, Kanwar SS, Singh M (2022) Fabrication and characteristic studies of doped metal oxide-silane magnetic nanocomposite for enhancement of stability of α-amylase. Appl Phys A 128(8):729. https://doi.org/10.1007/s00339-022-05882-6
Raplong H, Odeleye O, Hammuel C, Idoko O, Asanato I, Odeke H (2014) Production of alpha amylase by Bacillus cereus in submerged fermentation. Aceh Int J Sci Technol 3(3):124–130. https://doi.org/10.13170/aijst.3.3.1592
Remove Starch Stains with Amylase Enzymes|Novozymes. https://biosolutions.novozymes.com/en/laundry/starch-stains. Accessed 22 Jan 2022
Ruaida A (2021) Karim KMR (2020) Effect of chemical and physical mutagens on amylase producing potentiality of Aspergillus flavus NSH9. J Adv Biotechnol Exp Ther 4(1):53–59. https://doi.org/10.5455/jabet.2021.d106
Ruan Y, Zhang R, Xu Y (2022) Directed evolution of maltogenic amylase from Bacillus licheniformis R-53: Enhancing activity and thermostability improves bread quality and extends shelf life. Food Chem. https://doi.org/10.1016/j.foodchem.2022.132222
Saad WF, Othman AM, Abdel-Fattah M, Ahmad MS (2021) Response surface methodology as an approach for optimization of α-amylase production by the new isolated thermotolerant Bacillus licheniformis WF67 strain in submerged fermentation. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2021.101944
Saha K, Maity S, Roy S, Pahan K, Pathak R, Majumdar S, Gupta S (2014) Optimization of amylase production from B. amyloliquefaciens (MTCC 1270) using solid-state fermentation. Int J Microbiol. https://doi.org/10.1155/2014/764046
Salem K, Jabalera Y, Puentes-Pardo JD, Vilchez-Garcia J, Sayari A, Hmida-Sayari A et al (2021) Enzyme storage and recycling: nanoassemblies of α-amylase and xylanase immobilized on biomimetic magnetic nanoparticles. ACS Sustain Chem Eng 9(11):4054–4063. https://doi.org/10.1021/acssuschemeng.0c08300
Samanta S (2022) Structural and catalytical features of different amylases and their potential applications. Jordan J Biol Sci 15(2):311–337. https://doi.org/10.54319/jjbs/150220
Samanta S, Das A, Haider SK, Jana A, Mohapatra PKD, Pad BR, Mondal KC (2014) Thermodynamic and kinetic characteristics of an a-amylase from Bacillus licheniformis SKB4. Acta Biol Szegediensis 58(2):147–156
Sharma R, Oberoi HS, Dhillon GS (2016) Fruit and vegetable processing waste: renewable feed stocks for enzyme production. In: Agro-industrial wastes as feedstock for enzyme production. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-12-802392-1.00002-2
Sharma S, Kumar S, Kaur R, Kaur R (2021) Multipotential alkaline protease from a novel Pyxidicoccus sp. 252: ecofriendly replacement to various chemical processes. Front Microbiol. https://doi.org/10.3389/fmicb.2021.722719
Shukla RJ, Singh SP (2015) Characteristics and thermodynamics of α-amylase from thermophilic actinobacterium, Laceyella sacchari TSI-2. Process Biochem 50(12):2128–2136. https://doi.org/10.1016/j.procbio.2015.10.013
Silano V, Bolognesi C, Castle L, Chipman K, Cravedi JP (2018) Safety evaluation of the food enzyme α-amylase from a genetically modified Aspergillus niger (strain NZYM-SB). EFSA J Eur Food Saf Authority 16(7):e05320. https://doi.org/10.2903/j.efsa.2018.5320
Silva FS, Pio FS, Ribeiro EJ, Resende MM (2023) Immobilization of α-amylase (Termamyl® 2X) in Duolite® A-568 resin. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2023.102661
Simair AA, Khushk I, Qureshi AS, Bhutto MA, Chaudhry HA, Ansari KA, Lu C (2017) Amylase production from thermophilic Bacillus sp. BCC 021–50 isolated from a marine environment. Fermentation. https://doi.org/10.3390/fermentation3020025
Singh A, Bajar S, Devi A, Pant D (2021) An overview on the recent developments in fungal cellulase production and their industrial applications. Bioresour Technol Rep. https://doi.org/10.1016/j.biteb.2021.100652
Singh R, Langyan S, Rohtagi B, Darjee S, Khandelwal A, Shrivastava M et al (2022) Production of biofuels options by contribution of effective and suitable enzymes: technological developments and challenges. Mater Sci Energy Technol 5:294–310. https://doi.org/10.1016/j.mset.2022.05.001
Sóti V, Lenaerts S, Cornet I (2018) Of enzyme use in cost-effective high solid simultaneous saccharification and fermentation processes. J Biotechnol 270:70–76. https://doi.org/10.1016/j.jbiotec.2018.01.020
Srivastava N, Srivastava M, Ramteke PW, Mishra PK (2019) Solid-state fermentation strategy for microbial metabolites production: an overview. New Future Dev Microb Biotechnol Bioeng. https://doi.org/10.1016/B978-0-444-63504-4.00023-2
SuperAA Tex Desizing Amylase (2023) https://www.bestzyme.com/products/superaa-tex-desizing-amylase.html. Accessed 23 Jan 2023
T 1849/09 (Alpha-amylase/GENENCOR) 08-12-2010. https://www.epo.org/en/boards-of-appeal/decisions/t091849eu1. Accessed 24 Oct 2023
Tabassum R, Khaliq S, Rajoka MI, Agblevor F (2014) Solid state fermentation of a raw starch digesting alkaline alpha-amylase from Bacillus licheniformis RT7PE1 and its characteristics. Biotechnol Res Int. https://doi.org/10.1155/2014/495384
Termamyl Ultra – NCBE. https://www.ncbe.reading.ac.uk/termamyl-ultra/. Accessed 22 Jan 2023
Tong L, Zheng J, Wang X, Wang X, Huang H, Yang H (2021) Improvement of thermostability and catalytic efficiency of glucoamylase from Talaromyces leycettanus JCM12802 via site-directed mutagenesis to enhance industrial saccharification applications. Biotechnol Biofuels 14(1):1–9. https://doi.org/10.1186/s13068-021-02052-3
Torktaz I, Hemmat J, Karkhane AA, Rigi G, Rostami A, Khezri J, Behroozi R (2018) Molecular engineering of the Geobacillus stearothermophilus α-amylase and Cel5E from Clostridium thermocellim in silico approach. Iran J Biotechnol. https://doi.org/10.15171/ijb.1284
Tsegaye KN, Gessesse A (2014) Amylase production under solid state fermentation by a bacterial isolate W74. Afr J Biotechnol 13(21):2145–2153. https://doi.org/10.5897/AJB2013.13317
Uzun U, Akatin MY (2019) Immobilization and some application of α-amylase purified from Rhizoctonia solani AG-4 strain ZB-34. Turk J Biochem 44(3):397–407. https://doi.org/10.1515/tjb-2018-0240
Vaibav S, Halima S, Meena C, Rehman N, Devi S (2022) Enzymes in textile chemical. Int J Adv Res Innov Ideas Educ 8(2):604–612
Verma NK, Raghav N (2022) Cellulose tosylate as support for α-amylase immobilization. Int J Biol Macromol 222:413–420. https://doi.org/10.1016/j.ijbiomac.2022.10.032
Vester JK, Glaring MA, Stougaard P (2015) An exceptionally cold-adapted α-amylase from a metagenomic library of a cold and alkaline environment. Appl Microbiol Biotechnol 99(2):717–727. https://doi.org/10.1007/s00253-014-5931-0
Wang C, Huang R, He B, Du Q (2012) Improving the thermostability of α-amylase by combinatorial coevolving-site saturation mutagenesis. BMC Bioinform. https://doi.org/10.1186/1471-2105-13-263
Wang CH, Lu LH, Huang C, He BF, Huang RB (2020) Simultaneously improved thermostability and hydrolytic pattern of α-amylase by engineering central beta strands of TIM barrel. Appl Biochem Biotechnol 192(1):57–70. https://doi.org/10.1007/s12010-020-03308-8
Wang Y, Xue P, Cao M, Yu T, Lane ST, Zhao H (2021) Directed evolution: methodologies and applications. Chem Rev 121(20):12384–12444. https://doi.org/10.1021/acs.chemrev.1c00260
Wang J, Wang S, Zhao S, Sun P, Zhang Z, Xu Q (2023) Productivity enhancement in l-lysine fermentation using oxygen-enhanced bioreactor and oxygen vector. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2023.1181963
Wiltschi B, Cernava T, Dennig A, Casas MG, Geier M, Gruber S, Haberbauer M et al (2020) Enzymes revolutionize the bioproduction of value-added compounds: from enzyme discovery to special applications. Biotechnol Adv 40:107520. https://doi.org/10.1016/j.biotechadv.2020.107520
Witazora Y, Suhartati T, Satria H, Hadi S (2021) Production, purification and characterization of the α-amylase from local bacteria isolate Bacillus subtilis ITBCCB148. J Phys 1751(1):012096. https://doi.org/10.1088/1742-6596/1751/1/012096
Xu Y, Wang YH, Liu TQ, Zhang H, Zhang H, Li J (2018) The GlaA signal peptide substantially increases the expression and secretion of α-galactosidase in Aspergillus niger. Biotechnol Lett 40(6):949–955. https://doi.org/10.1007/s10529-018-2540-5
Yadav JK (2012) A differential behavior of α-amylase, in terms of catalytic activity and thermal stability, in response to higher concentration CaCl2. Int J Biol Macromol 51(1–2):146–152. https://doi.org/10.1016/j.ijbiomac.2012.04.013
Yandri SS, Suhartati T, Hadi S (2012) The chemical modification of α-amylase from locale bacteria of Bacillus subtilis ITBCCB148 using citraconic anhydride. Orient J Chem 28(4):1613–1618
Yandri Y, Suhartati T, Satria H, Widyasmara A, Hadi S (2020) Increasing stability of α-amylase obtained from Bacillus subtilis ITBCCB148 by immobilization with chitosan. Mediterr J Chem 10(2):155–161. https://doi.org/10.13171/mjc10202002131126ysh
Yandri Y, Suhartati T, Satria H, Karlinasari S, Yuwono SD, Hadi S (2021) Stability enhancement of Bacillus subtilis ITBCCB148 originating α-amylase by immobilization using chitin. J Adv Pharm Educ Res 11(3):63–69. https://doi.org/10.51847/FzQcrN5wmD
Yandri Y, Tiarsa ER, Suhartati T, Irawan B, Hadi S (2022a) Immobilization and stabilization of Aspergillus fumigatus α-amylase by adsorption on a chitin. Emerg Sci J 7(1):77–89. https://doi.org/10.28991/ESJ-2023-07-01-06
Yandri Y, Tiarsa ER, Suhartati T, Satria H, Irawan B, Hadi S (2022b) The stability improvement of α-amylase enzyme from Aspergillus fumigatus by immobilization on a bentonite matrix. Biochem Res Int. https://doi.org/10.1155/2022/3797629
Yandri Y, Ropingi H, Suhartati T, Irawan B, Hadi S (2023) Immobilization of α-amylase from Aspergillus fumigatus using adsorption method onto zeolite. Phys Sci Rev. https://doi.org/10.1515/psr-2022-0258
Yang H, Liu L, Shin HD, Li J, Du G, Chen J (2013) Structure-guided systems-level engineering of oxidation-prone methionine residues in catalytic domain of an alkaline α-amylase from Alkalimonas amylolytica for significant improvement of both oxidative stability and catalytic efficiency. PLoS ONE 8(3):e57403. https://doi.org/10.1371/journal.pone.0057403
Yang G, Yao H, Mozzicafreddo M, Ballarini P, Pucciarelli S, Miceli C (2017) Rational engineering of a cold-adapted α-amylase from the Antarctic ciliate Euplotes focardii for simultaneous improvement of thermostability and catalytic activity. Appl Environ Microbiol 83(13):e00449-17
Yassin SN, Jiru TM, Indracanti M (2021) Screening and characterization of thermostable amylase-producing bacteria isolated from soil samples of afdera, Afar region, and molecular detection of amylase-coding gene. Int J Microbiol. https://doi.org/10.1155/2021/5592885
Yi Z, Fang Y, He K, Liu D, Luo H, Zhao D (2018) Directly mining a fungal thermostable α-amylase from Chinese Nong-flavor liquor starter. Microb Cell Fact 17(1):1–14. https://doi.org/10.1186/s12934-018-0878-y
Yihan L, Huang L, Jia L, Gui S, Fu Y, Zheng D, Guo W, Lu F (2017) Improvement of the acid stability of Bacillus licheniformis alpha amylase by site-directed mutagenesis. Process Biochem 58:174–180. https://doi.org/10.1016/j.procbio.2017.04.040
Zeng J, Guo J, Tu Y, Yuan L (2020) Functional study of C-terminal domain of the thermoacidophilic raw starch-hydrolyzing α-amylase Gt-amy. Food Sci Biotechnol 29(3):409–418. https://doi.org/10.1007/s10068-019-00673-x
Zhang H, Hua S, Zhang L (2020) Effect of graphene oxide prepared under different conditions on immobilized α-amylase. Catal Lett 150:1244–1255. https://doi.org/10.1007/s10562-019-03055-4
Zhang J, Xu X, Li X, Chen X, Zhou C, Liu Y et al (2021a) Reducing the cell lysis to enhance yield of acid-stable α-amylase by deletion of multiple peptidoglycan hydrolase-related genes in Bacillus amyloliquefaciens. Int J Biol Macromol 167:777–786. https://doi.org/10.1016/j.ijbiomac.2020.11.193
Zhang K, Yin X, Shi K, Zhang S, Wang J, Zhao S et al (2021b) A high-efficiency method for site-directed mutagenesis of large plasmids based on large DNA fragment amplification and recombinational ligation. Sci Rep 11(1):1–16. https://doi.org/10.1038/s41598-021-89884-z
Zhou Y, Han LR, He HW, Sang B, Yu DL, Feng JT, Zhang X (2018) Effects of agitation, aeration and temperature on production of a novel glycoprotein GP-1 by Streptomyces kanasenisi ZX01 and scale-up based on volumetric oxygen transfer coefficient. Molecules 23(1):125. https://doi.org/10.3390/molecules23010125
Zhouquan S, Su H, Zhong Y, Xu H, Wang B, Zhang L et al (2022) Preparation of 3D porous cellulose-chitosan hybrid gel macrospheres by alkaline urea system for enzyme immobilization. Polym Adv Technol 33(2):546–555. https://doi.org/10.1002/pat.5536
Acknowledgements
Authors thankfully acknowledge Dr. Thallada Bhaskar, Head of, the Material Resource Efficiency Division for their constant motivation and support of this study. Ms. Pratima would also like to thank the Department of Biotechnology (DBT) for providing a fellowship.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
PPA: Investigation, writing—original draft; DD: writing—review and editing, manuscript representation, supervision, and guidance; AR: Supervision and guidance; SKS: manuscript representation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ashok, P.P., Dasgupta, D., Ray, A. et al. Challenges and prospects of microbial α-amylases for industrial application: a review. World J Microbiol Biotechnol 40, 44 (2024). https://doi.org/10.1007/s11274-023-03821-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03821-y