Abstract
The objective of this work was to test the effect of water limitation on Copaifera langsdorffii Desf. cultivated in mining tailings from the dam rupture in Mariana City, Brazil. Plants were grown in the mining tailing and under two conditions: field capacity (FC) and 50% FC for 60 days. The effects of water restriction on growth, gas exchange, water potential, and leaf anatomy of C. langsdorffii were evaluated. The experimental design was completely randomized with two treatments and 15 replicates, and data was submitted to one-way ANOVA to p < 0.05. Mining tailings showed adequate nutrient levels and the presence of Al, Cd, Pb, and Cr as well as very small particles of 1.19 μm in diameter. The reduction in water availability promoted no changes in the shoot fresh weight, however, increased this parameter for the roots. In addition, water limitation increased plant investment in the root system while reduced biomass allocation to shoots. Lower water levels also increased the root length, number of leaves, and leaf area. However, both water potential and content were not changed by reduced water availability. Lower water levels also increased gas exchange parameters and chlorophyll content. In addition, 50% FC increased the stomatal length/width ratio and their size though no effect in stomatal density was found. Thus, Copaifera langsdorffii grows and thrives in mining tailings even under reduced water availability up to 50% FC showing potential for reforestation systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Several anthropic activities increase environmental pollution on both its biotic or abiotic aspects (Reis et al. 2017). Mining industry is one of the main anthropic sources of pollution and its environmental impact may be greater than those promoted by other production chains (Lacaz et al. 2017). Dam failures are the most impactful disasters related to mining industry and several cases have been reported worldwide (Rico et al. 2008). These disasters cause soil and water pollution in addition to deforestation and soil acidification (Foulds et al. 2014; Karaca et al. 2018; Silva Jr et al. 2018). Unfortunately, dam failure episodes have been more frequent in Brazil (Lacaz et al. 2017) and elsewhere. The biggest and more recent failure of mining tailings dam occurred in November 2015 at Mariana City, State of Minas Gerais, Brazil, at the “Fundão” dam (Lacaz et al. 2017; Carmo et al. 2017) which received global attention. The impact of this disaster spread over hundreds of kilometers and it had destroyed 1200 ha of the original vegetation (Silva Junior et al. 2018). Mining tailing backlog also may cause silting in river basins, particularly during the rainy seasons (Foulds et al. 2014). Thus, recovering these areas by reforestation is an urgent matter since it provides ecological services and it creates conditions for natural regeneration.
Reforestation systems face difficulties resulting from the lack of information from species with potential to be used (Barbosa 2000). Some methods, such as the soil correction, are efficient for reforestation systems as well as for agriculture (Andrade et al. 2018). However, physical-chemical methods are unviable for large-scale application because they are expensive and time-demanding (Karaca et al. 2018). Therefore, reforestation systems using tolerant plant species can be a viable solution at lower costs for affected communities.
Physical-chemical properties of mining tailings are important for the success of reforestation systems, for instance, the size of the particles, the organic matter content, and the concentration of chemical elements (Segura et al. 2016; Andrade et al. 2018). Secondary environmental factors can also inflict difficulties for the success of reforestation. For instance, the region affected by the Fundão dam failure shows a dry season that last from 6-7 months (Alvares et al. 2013) and recent climate change scenario may increase the frequency of drought events (Marengo 2008). Thus, it is important to test how secondary environmental factors affect plant growth and survival in mining tailings. Copaifera langsdorffii Desf. (Fabaceae) is a widely distributed tree that is used for restoration of impacted environments (Nunes et al. 2015; Lima et al. 2016; Carvalho et al. 2019). This species is native to Brazil that can reach up to 30 m in height and shows a high seed yield (Pedroni et al. 2002), providing food and shelter for the fauna among other ecosystem services. In addition, this is a deciduous plant (Pedroni et al. 2002), periodically providing organic matter to soils.
Anatomical and ecophysiological responses are key features when studing species with potential for reforestation programs. Mining tailings from the Mariana’s dam failure reduced the shoot length, chlorophyll content, and leaf area of some tree species (Cruz et al. 2020). Mining tailings from South Africa also reduced growth parameters in Chloris gayana such as plant and organ biomasses and shoot height which were reverted by the application of additional nutrition (Lukashe et al. 2020). Mining tailings from Guangdong, China reduced the biomass production, leaf water potential, chlorophyll content, and photosynthetic rate in Vetiveria zizanioides plants (Pang et al. 2003). The morpho-physiological parameters were used in these previous works to distinguish potential tolerant species to be used in reforestation systems as well as necessary amendments to these soils that favor plant growth. It is clear that mining tailings reduce plant growth due to detrimental effects on its physiological parameters; however, information on anatomical responses promoted by mining tailings is not available. Plant anatomy can be important to understand plant tolerance to environmental stresses such as heavy metals (Pereira et al. 2014; Oliveira et al. 2018), drought (Melo et al. 2007; Cruz et al. 2020), and nutritional deficiency or excess (Santos et al. 2015). However, the effects of mining tailings in anatomical aspects of plants with potential for reforestation were neglected over the years as evidenced by the lack of works in this subject.
The hypothesis of this work is that C. langsdorffii can grow in mining tailings from Fundão dam failure and will tolerate lower water availability, showing ecophysiological and anatomical modifications. Therefore, the objective of this work was to evaluate the effect of water limitation in the mining tailings from the Fundão dam failure on growth, development, gas exchange, and leaf anatomy of Copaifera langsdorffii, a tree species with potential for restoration programs.
2 Material and Methods
2.1 Plant Material and Experimental Conditions
Seeds were collected from individuals of Copaifera langsdorffii found at the Universidade Federal de Lavras, Lavras City, State of Minas Gerais, Brazil (21° 13′ 42.1″ S 44° 59′ 15.9″ W), and stored in paper bags at room temperature until the start of experiments. Seeds were sampled in the first two weeks of September 2018.
Experiments were conducted in a growth chamber located at the Universidade Federal de Alfenas, Alfenas City, State of Minas Gerais, Brazil (21° 25′ 44″ S 45° 56′ 49″ W). Growth conditions for experiments were: 26 °C, 12 h photoperiod, and 40 μmol m−2 s−1 of photosynthetically active radiation. Seeds were sowed in mining tailings that were sampled 4 km away from the Fundão dam location. Mining tailings formed a layer of 1.0 m in depth at sampling sites. Mining tailings from Fundão dam failure covered large areas, reaching several meters in depth and preventing plants to have direct contact with original soils (Carmo et al. 2017; Silva Junior et al. 2018). Therefore, we decided to use only mining tailings as a substrate, providing a situation similar to that found at impacted areas, with no soil available.
Mining tailings were sieved and then 400 ml (650 g) of this substrate was placed in 500-ml plastic pots. Field capacity was used in this experiment to define the maximum water content held by the mining tailings and was determined as reported by Souza et al. (2000). Water loss by evapotranspiration was replaced daily by weighing the pots and then replacing the water to match the weigh at the previous day; all measurements were performed in an analytical scale (Quimis, Diadema, Brazil). Seeds were singly sown in each pot and kept under field capacity until seedling emergence and then two water availabilities were applied: field capacity (FC) and 50% of the field capacity (50% FC). Experimental design was completely randomized with two treatments and 15 replicates (n = 30). The experimental plot was considered as one plant for each variable analyzed; thus, each replicate represents one plant. Plants were kept under experimental conditions by 60 days.
2.2 Mining Tailings Analysis
The concentrations of macronutrients (P, Ca, K, and Mg), micronutrients (Mn, Fe, Zn, Cu, and Na), and toxic metals (Al, Cr, Cd, and Pb) were measured. Mining tailings samples (500 mg) were oven dried at 40 °C for 72 h and then acid digested. Element concentration was determined by atomic absorption spectrometry with a Perkin-Elmer Elemental Analyzer 2400 (CHNS/O).
For the granulometry analysis, samples of the mining tailings were sieved and then oven dried at 60 °C for 48 h. Further, the material was spread over a microscope slide containing glycerol 50% (v v−1) then covered with coverslips. Slides were photographed in a Zeiss Axio Scope.A1 microscope (Zeiss, Oberkochen, Germany) and the diameter of particles was measured in the ImageJ software. Ten slides, three fields, and ten particles per field (n = 300) were measured.
2.3 Plant Biometry
Shoot height was measured weekly from the substrate level until the shoot apex using a ruler. At the end of the experiment, plants were sampled and separated in roots, stem, and leaves. Furthermore, the size of the longest root was measured and leaves were photographed for the leaf area measurement which was performed using the ImageJ software. Fresh mass of roots, shoot, and leaves were measured in an analytical scale AY220 (Marte Científica, Santa Rita do Sapucaí, Brazil). Furthermore, each plant part was oven dried at 60 °C until constant mass and then weighed in an analytical scale.
2.4 Gas Exchange Analyses
Gas exchange analyses were performed at the end of the experiment using a LI-6400XT infrared gas analyzer (Li-Cor, Lincoln, USA) coupled to a 6.0 cm2 cuvette with light source (LI-6400-02B, Li-Cor, Lincoln, USA). Measurements were taken in the morning from 8 to 10 a.m. in the first fully expanded leaf at shoot apex and on three leaflets per leaf. Previously to gas exchange analysis, a light response curve was used to determine the light saturation point (400 μmol m−2 s−1) that was fixed in the cuvette. Net photosynthesis (A), transpiration rate (E), and stomatal conductance were measured. The chlorophyll content was estimated with a SPAD 502-Plus (Konica Minolta, Osaka, Japan). Measurements were taken at the basis, middle, and apex of leaflets from the first fully developed leaf from the shoot apex.
2.5 Leaf Water Potential and Plant Water Content
Leaf water potential (Ψw) was measured at the end of the experiment with a pressure pump model 3115 (Soilmoisture Equipment Corp., Santa Barbara, USA). Measurements were taken starting at 6 a.m. in one leaf per plant. The water content was calculated as follows: PWC = [(FM−DM)/FM]*100 where PWC is the plant water content, FM is the fresh mass, and DM is the dry mass.
2.6 Anatomical Analysis
At the end of the experiment, one leaf per plant was sampled and fixed in an FAA 70% solution (formaldehyde, acetic acid, and 70% ethanol at a 0.5:0.5:9 ratio) for 48 h and then stored in 70% ethanol until further analysis. For stomatal analysis, paradermal imprints from the abaxial surface of the leaves were taken with cyanoacrylate resin (Castilloa and Ferrarotto 1998). One slide and four fields were evaluated for each leaflet. Slides were photographed in an Axio Scope.A1 microscope (Zeiss, Oberkochen, Germany) coupled to a Powershot G10 digital camera (Canon, Tokyo, Japan). Images were evaluated using the ImageJ software. The stomatal length (STL), width (STW), STL/STW ratio, and stomatal density were measured.
Transversal sections were taken at the middle part of the leaflet using steel blades. Sections were clarified with sodium hypochlorite 50% (v v−1) and then washed twice in distilled water for 10 min. Furthermore, sections were stained with a safrablau solution (safranine 1.0% and astra blau 0.1% at 3:7 rate), mounted in slides with glycerol 50% (v v−1), and then covered with coverslips. Slides were photographed in an Axio Scope.A1 microscope (Zeiss, Oberkochen, Germany) coupled to a Powershot G10 digital camera (Canon, Tokyo, Japan). For each plant, one slide and four fields were evaluated with the ImageJ software. In adaxial and abaxial epidermis, palisade, and spongy parenchyma thicknesses, in addition, the areas of vascular bundles, xylem, and phloem as well as the diameter of xylem vessels were measured.
2.7 Statistical Analysis
Data were averaged to one plant per replicate in the cases where multiple sections, fields, and leaves were measured. Data was submitted to one-way ANOVA and means compared by F test to p < 0.05 using the Sisvar software (Ferreira 2011).
3 Results
Mining tailings characteristics are shown in Table 1 that evidenced the presence of macro and micronutrients as well as Al, Cr, Cd, and Pb. In addition, mining tailings particles were very small, with an average diameter of 1.187 μm.
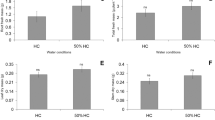
Lower water availability does not changed the fresh mass of stem and leaves (p > 0.05) as plants showed average 0.46 ± 0.17 g for stem and 0.51 ± 0.17 g for leaves. However, 50% FC increased the total (Fig. 1a) and root (Fig. 1b) fresh masses. Reduction of the water availability also increased the leaf (Fig. 1c), root (Fig. 1d), and total (Fig. 1e) dry masses, but promoted no effect on stem dry mass (p > 0.05; average 0.19 ± 0.08 g). The 50% FC treatment increased the biomass allocation to roots (Fig. 1h) while reduced its allocation to stem (Fig. 1f) and leaves (Fig. 1g).
Growth parameters of Copaifera langsdorffii exposed to mining tailings from “Fundão” dam failure at Mariana, Brazil, 2015 and at two water conditions. FC = field capacity of the substrate (mining tailing); 50% FC = water availability reduced to 50% of the FC. Bars = standard error. Asterisks (*) indicate significant modifications according to F test (P ≤ 0.05)
The 50% FC treatment increased the shoot elongation (Fig. 2a) and the root length (Fig. 2b). The number of leaves (Fig. 2c) and leaf area (Fig. 2c) were increased at lower water levels. Lower water availability promoted no effect in water potential (Fig. 2e) and water content (Fig. 2f). The reduction of the water availability increased the net photosynthesis, stomatal conductance, transpiration rate, and chlorophyll content of Copaifera langsdorffii (Table 2).
Shoot elongation, leaf development, and water status of Copaifera langsdorffii exposed to mining tailing from “Fundão” dam failure at Mariana, Brazil, 2015 and at two water conditions. FC = field capacity of the substrate (mining tailing); 50% FC = water availability reduced to 50% of the FC. Bars = standard error. Asterisks (*) and “ns” respectively indicate significant and not significant modifications according to F test (P ≤ 0.05)
The anatomical structure from the interveinal region of the Copaifera langsdorffii leaflets shows one-layered epidermis on both adaxial and abaxial leaf sides, comprising of table-shaped cells that are usually larger in the adaxial side (Fig. 3a and 3b). Leaves are hypostomatous and show dorsiventral mesophyll (Fig. 3a and 3b). Palisade parenchyma is found at the adaxial side and shows elongated cells with few intercellular spaces (Fig. 3a and 3b); at the abaxial side, there is the spongy parenchyma with cells of several morphologies from round to horizontally elongated and large to narrow intercellular spaces (Fig. 3a and 3b). The midrib region shows one-layered epidermis with smaller cells compared with the epidermis of the interveinal region but with thicker cuticle (Fig. 3c and 3d). In addition, the epidermis of the midrib region shows non-glandular trichomes which are absent in the interveinal region (Fig. 3c and 3d). Internally, two or three layers of ground parenchyma are found at the adaxial side while four to six layers of this tissue are found at the abaxial side (Fig. 3c and 3d). The abaxial ground parenchyma shows larger cells and more intercellular spaces compared with this tissue from the adaxial side; in addition, abaxial ground parenchyma shows secretory cavities (Fig. 3c and 3d). At the central part of the midrib, a single and large collateral vascular bundle is found (Fig. 3c and 3c). The vascular bundle contains two to five layers of sclerenchyma fibers with thick cell walls forming rings circling the transport tissues (Fig. 3c and 3d). Vascular tissues are found in several layers with xylem located at the adaxial side and phloem at the abaxial side (Fig. 3c and 3d).
Transversal sections of leaflets from C. langsdorffii grown at mining tailing from “Fundão” dam failure at Mariana, Brazil, 2015 and at two water conditions. a and c Plants under field capacity (FC). b and d 50% FC treatment. a and b Interveinal region. c and d Midrib region. ade = adaxial epidermis; abe = abaxial epidermis; pp = palisade parenchyma; sp = spongy parenchyma; fb = sclerenchymal fibers; xl = xylem; phl = phloem; sc= secretory cavity; tt = trichome; gp = ground parenchyma; ct = cuticle. Bars = 25 μm (a and b); 50 μm (c and d)
Lower water availability reduced both stomatal length and width while increased its length/width ratio (Table 3). However, 50% FC treatment had no effect in stomatal density (Table 3). Reduced water availability did not change the percentage of xylem (61.09 ± 4.96%) or phloem (35.46 ± 4.41%) in vascular bundles of C. langsdorffii. The lower water level significantly increased the xylem vessel diameter (Fig. 4a) and the thickness of the adaxial epidermis (Fig. 4b). In addition, the palisade parenchyma (Fig. 4c) and whole leaf (Fig. 4d) thicknesses were increased by lower water levels. However, the 50% FC treatment did not change the thicknesses of the adaxial epidermis (14.7 ± 1.54 μm) and spongy parenchyma (61.22 ± 7.15 μm) or the palisade/spongy parenchyma ratio (0.68 ± 0.1).
Leaf anatomical traits of Copaifera langsdorffii exposed to mining tailing from “Fundão” dam failure at Mariana, Brazil, 2015 and at two water conditions. FC = field capacity of the substrate (mining tailing); 50% FC = water availability reduced to 50% of the FC. Bars = standard error. Asterisks (*) indicate significant modifications according to F test (P ≤ 0.05)
4 Discussion
Mining tailing elemental analysis revealed the presence of all macro and micronutrients tested and this substrate may provide adequate nutrition for plant growth. However, potentially toxic metals were also found, despite our samples showed concentrations below the prevention limits of the Brazilian National Environmental Council (CONAMA 2009). High concentrations of Fe and Al found in mining tailings samples from Fundão dam may be related to iron mining activity in that region. Al concentrations are often high in Brazilian soils, and according to Andrade et al. (2012), Fe and Al levels are elevated in soils from the region affected by Fundão dam failure. Segura et al. (2016) analyzed both the soils from the Mariana region and the mining tailings from the Fundão dam failure and reported relative low heavy metal concentrations. It is important to note that variation of rain regime may change the availability of metals in soils (Reis et al. 2017). The presence of heavy metals (Cr, Cd, and Pb) in the mining tailings may be of concern because these elements may cause problems for plant establishment. For instance, mining soils containing Cd, Cr, and Pb promote the formation of less organized palisade and spongy parenchyma as well as reduced leaf thickness in Taraxacum officinale plants (Maleci et al. 2014). Heavy metals such as Cd can reduce the activity of specific meristems and alter the plant investment in leaf primary tissues (Pereira et al. 2017). Tolerant plant species may overcome heavy metal effects and keep its leaf anatomical structure unchanged (Oliveira et al. 2018) or may improve its leaf anatomy increasing the stomatal density and leaf thickness to favor its photosynthetic rate (Pereira et al. 2016). Results showed (Fig. 3) that no deformations were present in the leaf anatomy of C. langsdorffii grown in the mining tailings showing no evident toxicity of these metals. However, the mining tailings tested are not homogeneous and may show variation on its composition along the large area impacted.
Mining tailings did not cause lethal damage to C. langsdorffii since all plants survived. Another interesting trait of mining tailings from Fundão dam is the very small granulometry (1.19 μm) which is similar to that of clay (≤ 2 μm); this feature gives particular features to this substrate. Clay soils often show higher capacity to retain water due to its smaller particles (Beutler et al. 2002). This trait is interesting, since C. langsdorffii plants performed poorly under field capacity as compared with 50% FC. This result may be related to excessive water retained in mining tailings under field capacity, which reduced the availability of O2. Interestingly, 50% FC treatment caused not a stress by lower water condition, but results evidenced a hypoxia condition under field capacity. Several plant species grown under water limitation or drought show lower growth and development (Martinez et al. 2013; Rahmati et al. 2015; Padilha et al. 2016). However, providing 50% of field capacity in mining tailings increased growth parameters and ecophysiological responses of C. langsdorffii. These results suggest that, as a consequence of the small particles of mining tailings, this substrate may enter a state of waterlogging very quickly creating a hypoxia condition at field capacity.
Copaifera langsdorffii showed ecophysiological responses that compensate lower water levels in mining tailings. One of these responses was the higher investment in the root system as evidenced by a higher root length and biomass allocation. Larger root systems increase both water and nutrient uptakes being important under lower water levels. The unchanged water potential and content of C. langsdorffii individuals exposed to 50% FC may be related to this investment in the root system. In fact, 50% FC can be a severe drought condition that usually lowers plant water potential (Cruz et al. 2019). However, tree species, such as Schinus molle L., may show higher water potentials along the dry season and authors attributed this to soil waterlogging at the rainy season (Ewe and Sternberg 2002). Waterlogging of mining tailing is likely to happen in regions with pronounced rainy seasons. The viability of revegetation of these areas depends on the use of trees that can grow in both dry and rainy seasons.
The development of smaller stomata may reduce water loss at environments showing lower water availabilities (Melo et al. 2007). Copaifera langsdorffii grown under 50% FC showed smaller stomata, but no significant modification in stomatal density was found. In addition, heavy metals can increase the stomatal density in different plant species as a mechanism to increase the stomatal conductance and photosynthesis (Pereira et al. 2014; Oliveira et al. 2018). The stomatal modifications may have balanced gas exchange of C. langsdorffii and permitted the observed increases in stomatal conductance, photosynthesis, and transpiration under 50% FC in mining tailings. Increased leaf area and number of leaves also evidenced that 50% FC increased the photosynthetic potential of these plants growing in mining tailings. The higher photosynthetic rate together with a larger leaf area provided conditions to increase C. langsdorffii growth under 50% FC. These results may be related to a better aeration in mining tailings under 50% FC, preventing hypoxia stress. This may be true for other mining tailings samples with similar granulometry, making these results important for the planning and management of restoration programs in affected areas.
Reduced photosynthesis and growth of plants under field capacity may be related to a lower stomatal conductance, which diminished CO2 uptake. According to Rodríguez-Gamir et al. (2011), stomatal closure reduces its conductance. This effect was reported for different species under waterlogging (Blanke and Cooke 2004; Sibbernsen and Mott 2010). In addition, C. langsdorffii grown under field capacity showed high activity of antioxidant enzymes (Rosa et al. 2017) suggesting that field capacity causes oxidative stress to this species. Limited leaf area also contributed to lower photosynthesis and growth of C. langsdorffii. In fact, reduced leaf area is a common stress response in plants under waterlogging (Luquez et al. 2012; Martinez et al. 2011). Therefore, mining tailings under field capacity may cause similar effects to those of waterlogging condition reducing stomatal conductance, photosynthesis, and growth of potential of C. langsdorffii.
Leaf tissues at transversal sections showed just minor changes of a few micrometers in some parameters and not all anatomical changes will necessarily promote modification in physiological responses. Stomatal changes of Copaifera langsdorffii may explain more reasonably the photosynthesis and growth results. However, transversal sections showed that leaves remained almost unchanged suggesting the absence of significant damage. Minor changes were also found on vascular tissues and the most significant may be the increased xylem vessel diameter under 50% FC. Waterlogging can reduce vessel diameter (Medri et al. 2011) and the size of the metaxylem vessels (Kloss et al. 2021), and this corroborates the possibility that mining tailings at field capacity generate hypoxia stress. In addition, heavy metals can reduce the vessel diameter of different plant species to reduce its transport to shoots and protect the photosynthetic tissues (Pereira et al. 2014; Ribeiro et al. 2015). However, reduced xylem vessels may cause limitation to water transport, reducing transpiration and stomatal conductance and these responses were also verified for C. langsdorffii under field capacity.
5 Conclusion
Copaifera langsdorffii can grow in mining tailings and shows potential for restoration programs. Mining tailings promoted no toxicity to C. langsdorffii despite the presence of heavy metals. Reduced water availability favors growth and ecophysiological traits of C. langsdorffii growing in mining tailings. The small size of the mining tailing grains provides conditions for waterlogging under field capacity. The leaf anatomy of C. langsdorffii preserved its structure in plants growing in mining tailings under both field capacity and reduced water availability but showed improved stomatal parameters and thicker tissues under lower water levels.
References
Alvares, C. A., Stape, J. L., Sentelhas, P. C., Gonçalves, J. L. M., & Sparovek, G. (2013). Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift, 22(6), 711–728. https://doi.org/10.1127/0941-2948/2013/0507.
Andrade, L. N., Leite, M. G. P., & Bacellar, L. A. P. (2012). Composição mineralógica e geoquímica dos solos do parque estadual do Itacolomi - Ouro Preto/MG. Quaternary and Environmental Geosciences, 3, 1–8. https://doi.org/10.5380/abequa.v3i1-2.16838.
Andrade, G. F., Paniz, F. P., Martins Jr., A. C., Rocha, B. A., Lobato, A. K. S., Rodrigues, J. L., et al. (2018). Agricultural use of Samarco’s spilled mud assessed by rice cultivation: A promising residue use? Chemosphere, 193, 892–902. https://doi.org/10.1016/j.chemosphere.2017.11.099.
Barbosa, L. M. (2000). Considerações Gerais e Modelos de Recuperação de Formações Ciliares. In R. R. Rodrigues & H. F. Leitão (Eds.), Matas Ciliares: Conservação e Recuperação (pp. 289–312). São Paulo: EDUSP, FAPESP.
Beutler, A. N., Centurion, J. F., Souza, Z. M., Andrioli, I., & Roque, C. G. (2002). Retenção de água em dois tipos de Latossolo sob diferentes usos. Revista Brasileira de Ciências do Solo, 26, 829–834. https://doi.org/10.1590/S0100-06832002000300029.
Blanke, M. M., & Cooke, D. T. (2004). Effects of flooding and drought on stomatal activity, transpiration, photosynthesis, water potential and water channel activity in strawberry stolons and leaves. Plant Growth Regulation, 42(2), 153–160. https://doi.org/10.1023/B:GROW.0000017489.21970.d4.
Carmo, F. F., Kamino, L. H. Y., Junior, R. T., Campos, I. C., Carmo, F. F., Silvino, G., et al. (2017). Fundão tailings dam failures: The environment tragedy of the largest technological disaster of Brazilian mining in global context. Perspectives in Ecology and Conservation, 15(3), 145–151. https://doi.org/10.1016/j.pecon.2017.06.002.
Carvalho, T. F., Pereira, I. M., Botelho, S. A., Titon, M., & José, A. C. (2019). Restoration strategies in an area invaded by Pteridium aquilinum (L.) Kuhn. Floresta Ambiente, 26(2), 2019. https://doi.org/10.1590/2179-8087.114617.
Castilloa, J. J., & Ferrarotto, M. (1998). Evaluation of cyanoacrylate glues for making attached living-leaves epidermis replicas and its scanning electron microscopy observations. Scanning, 20(8), 557–563. https://doi.org/10.1002/sca.4950200804.
Conselho Nacional do Meio Ambiente (CONAMA). Resolução n° 420, de 28 de dezembro de 2009. Dispõe sobre critérios e valores orientadores de qualidade do solo quanto à presença de substâncias químicas e estabelece diretrizes para o gerenciamento ambiental de áreas contaminadas por essas substâncias em decorrência de atividades antrópicas. Diário Oficial da União, Brasília, DF, n° 249, 2009, pp. 81–84.
Cruz, Y. C., Scarpa, A. L. M., Pereira, M. P., Castro, E. M., & Pereira, F. J. (2019). Growth of Typha domingensis as related to leaf physiological and anatomical modifications under drought conditions. Acta Physiologiae Plantarum, 41(64), 1–9. https://doi.org/10.1007/s11738-019-2858-1.
Cruz, F. V. S., Gomes, M. P., Bicalho, E. M., Torre, F. D., & Garcia, Q. S. (2020). Does Samarco’s spilled mud impair the growth of native trees of the Atlantic Rainforest? Ecotoxicology and Environmental Safety, 189, 110021. https://doi.org/10.1016/j.ecoenv.2019.110021.
Ewe, S. M. L., & Sternberg, L. S. L. (2002). Seasonal water-use by the invasive exotic, Schinus terebinthifolius, in native and disturbed communities. Oecologia, 133, 441–448. https://doi.org/10.1007/s00442-002-1047-9.
Ferreira, D. F. (2011). Sisvar: A computer statistical analysis system. Ciência e Agrotecnologia, 35, 1039–1042. https://doi.org/10.1590/S1413-70542011000600001.
Foulds, S. A., Brewer, P. A., Macklin, M. G., Haresign, W., Betson, R. E., & Rassner, S. M. E. (2014). Flood-related contamination in catchments affected by historical metal mining: An unexpected and emerging hazard of climate change. Science of The Total Environment, 476–477, 165–180. https://doi.org/10.1016/j.scitotenv.2013.12.079.
Karaca, O., Cameselle, C., & Reddy, K. R. (2018). Mine tailing disposal sites: Contamination problems, remedial options and phytocaps for sustainable remediation. Reviews in Environmental Science and Bio/Technology, 17(1), 205–228. https://doi.org/10.1007/s11157-017-9453-y.
Kloss, R. B., Castro, E. M., Magalhães, P. C., Duarte, V. P., Corrêa, F. F., & Pereira, F. J. (2021). Anatomical and physiological traits of maize under contrasting water levels and cattail occurrence. Acta physiologiae Plantarum, 43, 16. https://doi.org/10.1007/s11738-020-03192-z.
Lacaz, F. A. C., Porto, M. F. S., & Pinheiro, T. M. M. (2017). Tragédias brasileiras contemporâneas: o caso do rompimento da barragem de rejeitos de Fundão/Samarco. Revista Brasileira de Saúde Ocupacional, 42(9). https://doi.org/10.1590/2317-6369000016016.
Lima, P. A. F., Gatto, A., Albuquerque, L. B., Malaquias, J. V., & Aquino, F. G. (2016). Crescimento de mudas de espécies nativas na restauração ecológica de matas ripárias. Neotropical Biology and Conservation, 11, 72–79.
Lukashe, N. S., Mnkeni, P. N. S., & Mupambwa, H. A. (2020). Growth and elemental uptake of Rhodes grass (Chloris gayana) grown in a mine waste-contaminated soil amended with fly ash-enriched vermicompost. Environmental Science and Pollution Research, 27, 19461–19472. https://doi.org/10.1007/s11356-020-08354-7.
Luquez, V. M. C., Achinelli, F. G., & Cortizo, S. (2012). Evaluation of flooding tolerance in cuttings of Populus clones used for forestation at the Paraná River Delta, Argentina. Southern Forests, 74, 61–70. https://doi.org/10.2989/20702620.2012.686214.
Maleci, L., Buffa, G., Wahsha, M., & Bini, C. (2014). Morphological changes induced by heavy metals in dandelion (Taraxacum officinale Web.) growing on mine soils. Journal of Soils and Sediments, 14, 731–743. https://doi.org/10.1007/s11368-013-0823-y.
Marengo, J. A. (2008). Water and climate change. Estudos avançados, 22(63), 83–96. https://doi.org/10.1590/S0103-40142008000200006.
Martinez, G. B., Mourão, M., & Brienza Junior, S. (2011). Respostas morfofisiológicas de plantas de açacu (Hura crepitans L.) provenientes de várzeas do rio Amazonas: efeito da anoxia do solo. Revista Árvore, 35, 1155–1164. https://doi.org/10.1590/S0100-67622011000700001.
Martinez, C. F., Cavagnaro, J. B., Roing Juñent, F. A., & Catón, M. A. (2013). Response to water deficit on tree growth from urban forestry of Mendoza city: Comparative analysis in sapling trees. Revista de la Facultad de Ciencias Agrarias, Universidad Nacional de Cuyo, 45(2), 47–64.
Medri, C., Medri, M. E., Ruas, E. A., Souza, L. A., Medri, P. A., Sayhun, S., et al. (2011). Morfoanatomia de órgãos vegetativos de plantas juvenis de Aegiphila sellowiana Cham. (Lamiaceae) submetidas ao alagamento do substrato. Acta Botanica Brasilica, 25(2), 445–454. https://doi.org/10.1590/S0102-33062011000200020.
Melo, H. C., Castro, E. M., Soares, A. M., Melo, L. A., & Alvez, J. D. (2007). Alterações anatômicas e fisiológicas em Setaria anceps Stapf ex Massey e Paspalum paniculatum L. sob condições de déficit hídrico. Hoehnea, 34, 145–153. https://doi.org/10.1590/S2236-89062007000200003.
Nunes, Y. R. F., Fagundes, N. C. A., Veloso, M. D. M., Gonzaga, A. P. D., Domingues, E. B. S., Almeida, H. S., et al. (2015). Sobrevivência e crescimento de sete espécies arbóreas nativas em uma área degradada de floresta estacional decidual, norte de minas gerais. Revista Árvore, 39(5), 801–810. https://doi.org/10.1590/0100-67622015000500003.
Oliveira, J. P. V., Pereira, M. P., Duarte, V. P., Corrêa, F. F., Castro, E. M., & Pereira, F. J. (2018). Cadmium tolerance of Typha domingensis Pers. (Typhaceae) as related to growth and leaf morphophysiology. Brazilian Journal of Biology, 78(3), 509–516. https://doi.org/10.1590/1519-6984.171961.
Padilha, N. S., Silva, C. J., Pereira, S. B., Silva, J. A. N., Heid, D. M., Bottega, S. P., & Scalon, S. P. Q. (2016). Crescimento inicial do pinhão-manso submetido a diferentes regimes hídricos em latossolo vermelho distrófico. Ciência Florestal, 26(2), 513–521. https://doi.org/10.5902/1980509822752.
Pang, J., Chan, G. S. Y., Zhang, J., Liang, J., & Wong, M. H. (2003). Physiological aspects of vetiver grass for rehabilitation in abandoned metalliferous mine wastes. Chemosphere, 52, 1559–1570. https://doi.org/10.1016/S0045-6535(03)00496-X.
Pedroni, F., Sanchez, M., & Santos, F. A. M. (2002). Fenologia da copaíba (Copaifera langsdorffii Desf. – Leguminosae, Caesalpinoideae) em uma floresta semidecídua no sudeste do Brasil. Revista Brasileira de Botânica, 25, 183–194. https://doi.org/10.1590/S0100-84042002000200007.
Pereira, F. J., Castro, E. M., Oliveira, C., Pires, M. F., Pereira, M. P., Ramos, S. J., & Faquin, V. (2014). Lead tolerance of water hyacinth (Eichhornia crassipes Mart. - Pontederiaceae) as defined by anatomical and physiological traits. Anais da Academia Brasileira de Ciências, 86(3), 1423–1433. https://doi.org/10.1590/0001-3765201420140079.
Pereira, M. P., Rodrigues, L. C. A., Corrêa, F. F., Castro, E. M., Ribeiro, V. E., & Pereira, F. J. (2016). Cadmium tolerance in Schinus molle trees is modulated by enhanced leaf anatomy and photosynthesis. Trees, 30, 807–814. https://doi.org/10.1007/s00468-015-1322-0.
Pereira, M. P., Corrêa, F. F., Castro, E. M., Oliveira, J. P. V., & Pereira, F. J. (2017). Leaf ontogeny of Schinus molle L. plants under cadmium contamination: The meristematic origin of leaf structural changes. Protoplasma, 254, 2117–2126. https://doi.org/10.1007/s00709-017-1103-2.
Rahmati, M., Davarynejad, G. H., Génard, M., Bannayan, M., Azizi, M., & Vercambre, G. (2015). Peach water relations, gas exchange, growth and shoot mortality under water deficit in semi-arid weather conditions. PLoS ONE, 10(4), 1–19. https://doi.org/10.1371/journal.pone.0120246.
Reis, D. A., Santiago, A. F., Nascimento, L. P., & Roeser, H. M. P. (2017). Influence of environmental and anthropogenic factors at the bottom sediments in a Doce River tributary in Brazil. Environmental Science and Pollution Research, 7456–7467. https://doi.org/10.1007/s11356-017-8443-5.
Ribeiro, E. S., Pereira, M. P., Castro, E. M., Baroni, G. R., Corrêa, F. F., & Pereira, F. J. (2015). Relações da anatomia radicular na absorção, no acúmulo e na tolerância ao chumbo em Echinodorus grandiflorus. Revista Brasileira de Engenharia Agrícola e Ambiental, 19(6), 605–612. https://doi.org/10.1590/1807-1929/agriambi.v19n6p605-612.
Rico, M., Benito, G., Salgueiro, A. R., Díez-Herrero, A., & Pereira, H. G. (2008). Reported tailings dam failures: A review of the European incidents in the worldwide context. Journal of Hazardous Materials, 152(2), 846–852. https://doi.org/10.1016/j.jhazmat.2007.07.050.
Rodríguez-Gamir, J., Ancillo, G., Gonzáles-Mas, M. C., Primo-Millo, E., Iglesias, D. J., & Forner-Giner, A. (2011). Root signalling and modulation of stomatal closure in flooded citrus seedlings. Plant Physiology and Biochemistry, 49, 636–645. https://doi.org/10.1016/j.plaphy.2011.03.003.
Rosa, D. B. C. J., Scalon, S. P. Q., Cremon, T., Ceccon, F., & Dresch, D. M. (2017). Gas exchange and antioxidant activity in seedlings of Copaifera langsdorffii Desf. under different water conditions. Anais da Academia Brasileira de Ciências, 89(4), 3039–3050. https://doi.org/10.1590/0001-3765201720170499.
Santos, K. R., Pereira, M. P., Ferreira, A. C. G., Rodrigues, L. C. A., Castro, E. M., Corrêa, F. F., & Pereira, F. J. (2015). Typha domingensis Pers. growth responses to leaf anatomy and photosynthesis as influenced by phosphorus. Aquatic Botany, 122, 47–53. https://doi.org/10.1016/j.aquabot.2015.01.007.
Segura, F. R., Nunes, E. A., Paniz, F. P., Paulelli, A. C. C., Rodrigues, G. B., Braga, G. Ú. L., et al. (2016). Potential risks of the residue from Samarco’s mine dam burst (Bento Rodrigues, Brazil). Environmental Pollution, 218, 813–825. https://doi.org/10.1016/j.envpol.2016.08.005.
Sibbernsen, E., & Mott, K. A. (2010). Stomatal responses to flooding of the intercellular air spaces suggest a vapor-phase signal between the mesophyll and the guard cells. Plant Physiology, 153, 1435–1442. https://doi.org/10.1104/pp.110.157685.
Silva Jr., C. A., Oliveira-Júnior, J. F., Teodoro, P. E., Lima, M., Shakir, M., et al. (2018). Analysis of the impact on vegetation caused by abrupt deforestation via orbital sensor in the environmental disaster of Mariana, Brazil. Land Use Policy, 76, 10–20. https://doi.org/10.1016/j.landusepol.2018.04.019.
Souza, C. C., Oliveira, F. A., Silva, I. F., & Amorim Neto, M. S. (2000). Avaliação de métodos de determinação de água disponível e manejo da irrigação em terra roxa sob cultivo de algodoeiro herbáceo. Revista Brasileira de Engenharia Agrícola e Ambiental, 4(3), 338–342. https://doi.org/10.1590/S1415-43662000000300006.
Funding
The authors received funding and research grants from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Counsel of Technological and Scientific Development)), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001, and FAPEMIG (Fundação de Amparo à Pesquisa do estado de Minas Gerais (Minas Gerais State Research Foundation)) to complete the present study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Pádua, M.P., Caetano, A.L., Polo, M. et al. Ecophysiological Responses of Copaifera langsdorffii Grown in Mining Tailings Under Lower Water Availability. Water Air Soil Pollut 232, 57 (2021). https://doi.org/10.1007/s11270-021-05037-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05037-y