Abstract
Previous works show the development of thicker leaves on tolerant plants growing under cadmium (Cd2+) contamination. The aim of this study was to evaluate the Cd2+ effects on the leaf meristems of the tolerant species Schinus molle. Plants were grown in nutrient solution containing 0, 10, and 50 μM of Cd2+. Anatomical analysis was performed on leaf primordia sampled at regular time intervals. Under the lowest Cd2+ level (10 μM), increased ground meristem thickness, diameter of the cells, cell elongation rate, and leaf dry mass were found. However, 50 μM of Cd2+ reduced all these variables. In addition, the ground meristem cells became larger when exposed to any Cd2+ level. The epidermis, palisade parenchyma, and vascular tissues developed earlier in Cd2+-exposed leaves. The modifications found on the ground meristem may be related to the development of thicker leaves on S. molle plants exposed to low Cd2+ levels. Furthermore, older leaves showed higher Cd2+ content when compared to the younger ones, preventing the Cd2+ toxicity to these leaves. Thus, low Cd2+ concentrations change the ground meristem structure and function reflecting on the development of thicker and enhanced leaves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants face the heavy metal contamination caused by both natural and anthropogenic sources. Likewise, the exposure to these pollutants causes several physiological and biochemical limitations for non-tolerant species (Singh et al. 2015). Cadmium (Cd2+) is a heavy metal which binds to sulfhydryl groups in proteins causing metabolism disruption and changes on the nutrient uptake, producing reactive oxygen species (Delmail et al. 2011).
Tolerant plants develop several adaptations to reduce the Cd toxicity (Singh et al. 2015). The Cd2+ perception and transport can be related to signal transduction cascades promoted by growth regulators (Asgher et al. 2015). Cadmium causes anatomical modifications particularly to roots and leaves. Leaves are the main site for photosynthesis, and its modification such as increased thickness and stomatal density are key traits for metal tolerance (Dickson and Isebrands 1991; Tsukaya 2006; Shi and Cai 2009; Pérez Chaca et al. 2014; Shi et al. 2014; Pereira et al. 2016). However, heavy metal effects have been investigated only for mature leaves in despite of the key role of meristems during leaf development.
Thus, the study of the meristematic activity during leaf ontogeny can give insight into the origin of cellular traits which are related to the structural changes found on mature leaves. Previously, it was shown that fully developed leaves from Schinus molle exposed to Cd2+ showed enhanced photosynthesis, thicker mesophyll, and higher stomatal density (Pereira et al. 2016). Therefore, the aim of this study was to evaluate the activity and structure of leaf meristems as related to the anatomical changes on mature leaves of S. molle exposed to Cd2+.
Material and methods
Plant growth conditions
S. molle (L.) plants were obtained from seeds collected from a cultivated population located at the southern region of Minas Gerais state, Brazil. These plants were grown in plastic bags of 0.35 L containing washed sand and nutrient solution in greenhouse at 25 ± 2 °C. The nutrient solution was composed of the salt concentrations described by Hoagland and Arnon (1950) using the following sources: NH4H2PO4, Ca(NO3)2, MgSO4·7H2O, KNO3, H2BO3, MnSO4·H2O, ZnSO4·7H2O, CuSO4·5H2O, H2MoO4·H2O, and FeSO4·7H2O. This solution was replaced at 15-day intervals and the water lost by evapotranspiration refilled daily. These plants remained under these conditions for five months.
Experimental design
Five-month-old plants were exposed to 0, 10, and 50 μM of Cd2+ using the same Hoagland and Arnon solution described for plant growth plus Cd(NO3)2 as the Cd2+ source according to Pereira et al. (2016). The Cd2+ concentrations applied in our work were based in the control (0 μM), the Cd2+ level that caused leaf thickening (10 μM), and toxicity (50 μM) for S. molle plants according to Pereira et al. (2016). The Cd(NO3)2 was selected as the Cd source because NO3 − causes no secondary toxicity and the N levels were balanced by the NH4H2PO4 levels of the nutrient solution. Developing leaves were sampled at regular time intervals, and each leaf primordium received a label to ensure the correct determination of age. The experimental design was completely randomized in a factorial scheme, considering three concentrations of Cd2+ and four leaf ages for the analysis of the ground meristem (2, 6, 8, and 10 days factorial 3 × 4) or six ages for the analysis of leaf elongation (6, 8, 10, 14, 20, and 24 days factorial 3 × 6). The sampling of the leaf primordia for meristem analysis stopped at 10 days because older leaves were already differentiated. These plants were maintained in these conditions for 30 days. Five replicates were used per treatment both for the analysis of meristematic tissues (n = 60) or the analysis of leaf elongation (n = 90). Each replicate was constituted of one different plant.
Leaf development analysis
Leaves were sampled and fixed in Karnovsky’s solution (4% paraformaldehyde and 2.5% glutaraldehyde in sodium cacodylate buffer 0.1 M pH 7.2) for 72 h. Further, the samples were dried with increasing ethanol concentrations (70, 90, and 100%) at 2-h intervals and embedded in historesin according to the manufacturer’s instructions (Leica Microsystems, Wetzlar, Germany). Transversal and longitudinal sections were obtained using a semi-automated rotary microtome Yidi YD-335 (Jinhua Yidi Medical Appliance CO., LTD, Zhejiang, China). The sections were stained with toluidine blue 1% (w v−1) and mounted on slides with Entellan (Merck, Darmstadt, Germany). The slides were photographed using a microscope attached to an image capture system (CX31, Olympus, Tokyo, Japan), and quantitative anatomical analysis was performed using ImageJ software. The quantitative analysis of meristem traits was performed only for the ground meristem. The reason for this method was because this meristem is the precursor of the parenchyma cells that fill most of the mesophyll area and determine leaf thickness. In addition, the protodermis is one-layered and the procambium is early changed to xylem and phloem making it hard to evaluate cell division. S. molle shows compound leaves with 13 to 15 leaflets when mature. We evaluate the development of one leaflet per leaf considering the Cd2+ effects to whole leaf.
Quantitative meristematic traits were evaluated according to equations proposed by Ivanov and Dubrovsky (1997), and the variables evaluated were the cell elongation rate, the number of meristematic cells undergoing mitosis, the cell production, and division rates as well as the cell cycle time. In addition, we evaluated the thickness, the cell diameter, and the number of cell layers in the ground meristem.
The leaf growth was evaluated by the following parameters: leaf area, length, width, dry mass, and marginal growth. Leaf area was measured scanning the leaves and measuring the area in the ImageJ software. The dry mass was measured in a precision scale (AY 220, Shimadzu, Japan) from oven-dried leaves for 72 h at 60 °C. The marginal growth was measured by tracing the distance between the midrib and the leaflet margin at each age. The leaf length and width were measured with a digital pachymeter. The following parameters were calculated: the specific leaf area (leaf area/dry mass) and leaf elongation rate (leaf length at the end of experiment − the length at the first day sampled).
Cadmium measurements
At the end of the experiment, the Cd2+ contents in young (6 days old) and old (30 days old) leaves were measured. Leaves were dried at 45 °C for 48 h. Dried mass (500 mg) was triturated in small parts and then digested in 10 mL of HNO3 for 30 min at 150 °C in a block digestion system. Further, 1.0 mL of HClO4 was added, and the temperature was elevated to 210 °C for 20 min. The digested material was diluted to 25 mL with distilled water, and the Cd2+ content was determined with an atomic absorption spectrometer.
Statistical analysis
Statistical analyses were performed using the SISVAR 5.0 software (Ferreira 2011). Prior to parametric analysis, data were tested for a normal distribution using the Shapiro–Wilk test. Further, data were subjected to analysis of variance and means compared by the Scott–Knott test at 5% probability.
Results
The Cd2+ promoted no toxic effects on the shoot apical meristem of S. molle plants (Fig. 1). Plants from all treatments showed the same overall structure with the shoot apical meristem located at the center and three parallel leaf primordia (Fig. 1). The leaf primordia showed one-layered protodermis and procambium bundles surrounded by several layers of ground meristem (Fig. 1). Increased number and size of the leaf primordia were found for the Cd2+-exposed plants as compared to the control (Fig. 1).
Shoot apex of Schinus molle plants exposed to cadmium. Increased leaf primordia size and number of leaflets are shown for the cadmium-exposed plants (10 and 50 μM) as compared to control (0 μM). Leaf primordia were labeled from the youngest to oldest as follows: p1 youngest leaf primordium, p2 intermediary leaf primordium, p3 oldest leaf primordium, gm ground meristem. a 0 μM. b 10 μM. c 50 μM
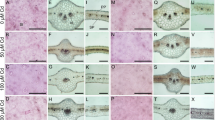
The 2-day-old leaflets of control plants are comprised only by the midrib with one-layered prododermis, procambium bundles, and several ground meristem layers. The outmost layer of the ground meristem showed partial differentiation to the hypodermis, which is found in mature leaves (Fig. 2a, d). The inner parts of the ground meristem are composed of lighter cells with round shapes, and the outer layers show smaller cells which are found undergoing cell division (Fig. 2a, d). Secretory ducts are found amidst the ground meristem and show large intercellular spaces surrounded by the one-layered epithelium (Fig. 2a). The innermost part of the midrib of the leaflet contains ground parenchyma showing larger and lighter cells. This overall structure was found for all 2-day-old leaflets; however, leaflets exposed to Cd2+ were larger and showed xylem vessels, larger secretory ducts, and hypodermis (Fig. 2b, e). The 2-day-old leaflet morphology is heart-shaped in transversal section; however, Cd2+ strongly increased the marginal growth causing the expansion of the adaxial side borders (Fig. 2b, e), which remarkably changed the shape of the leaflet, promoting earlier mesophyll development.
Leaflet development and tissue differentiation of Schinus molle plants (showed on a daily basis for leaf age in rows) exposed to cadmium (10 and 50 μM of Cd2+) and control treatments (0 μM of Cd2+). Leaflets under cadmium treatments developed quicker, achieving the final number of cell layers earlier than those of control treatment. Asterisks in e, f are indicating xylem vessels and arrows are indicating stomata (o). pr protodermis, hp hypodermis, gm ground meristem, sc secretory duct, vb vascular bundle, pp palisade parenchyma, ep epidermis, control = 0 μM
The leaflets of control plants started the marginal growth at the sixth day, and a few xylem vessel elements can be found at the central part of the structure (Fig. 2g). However, leaflets exposed to Cd2+ showed longer marginal growth and developed protoxylem and protophloem, which can be found associated to the secretory ducts (Fig. 2h, i). The 8-day-old leaves of control plants showed longer marginal growth, and the first xylem and phloem elements were found in the midrib and mesophyll (Fig. 2j, m). Cadmium promoted enhanced leaflet development at the eighth day where the mesophyll was found to be well developed with several vascular bundles and epidermis showing its first stomata; the palisade parenchyma at both leaf sides started to show palisade cells (Fig. 2n, o). Further, at the tenth day of development, leaves from all treatments showed full-developed midrib and mesophyll regions, but those exposed to Cd2+ were thicker (Fig. 2p–r). Leaflets older than 10 days were already differentiated.
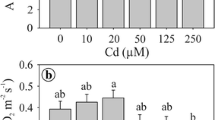
Cadmium changed the ground meristem activity and structure of S. molle leaves (Fig. 3). The leaf age and Cd2+ concentration showed significant interaction, and combined effect was found for the ground meristem anatomy and activity (P < 0.01). Leaves exposed to 10 μM Cd2+ showed thicker ground meristem as compared to control; however, at 50 μM Cd2+, reduced means were found (Fig. 3a). In addition, leaves showed increased ground meristem thickness with aging (Fig. 3a). The ground meristem cells had larger diameter when exposed to 10 μM Cd2+ as compared to both control and 50 μM Cd2+ treatments (Fig. 3b). Furthermore, the diameter of the cells from the ground meristem increased over time under all Cd2+ levels (Fig. 3b). Interestingly, the number of cell layers of the ground meristem responded to Cd2+ application only on younger leaves (Fig. 3c). Leaves older than 8 days showed no differences for the number of cell layers. However, leaves under Cd2+ effect developed quicker, achieving the final number of cell layers earlier (Fig. 3c).
Thickness (a), cell diameter (b), and number of cell layers (c) of the ground meristem of Schinus molle leaf primordia (showed on a daily basis for leaf age) exposed to cadmium (10 and 50 μM of Cd2+) and control treatments (0 μM of Cd2+). The lowercase letters compare the cadmium concentration at the same leaf age, and the uppercase letters compare the leaf age amongst the same cadmium concentration. Means followed by the same letter do not differ by the Scott–Knott test at 5% significance level
The cell elongation rate of the ground meristem from leaves exposed to 10 μM Cd2+ was always higher as compared to control and 50-μM Cd2+ treatments (Fig. 4a). In addition, control plants showed higher cell elongation rate on the older leaves (Fig. 4a). However, increased cell elongation rate was found on the leaves exposed to 10 μM Cd2+ at early developmental stages (Fig. 4a). Furthermore, under 50 μM Cd2+, the cell elongation rate was similar for all leaf ages (Fig. 4a).
Cell elongation rate (a), number of cells undergoing mitosis (b), cell production rate (c), and time to accomplish the cell cycle (d) of the ground meristem in Schinus molle leaf primordia exposed to cadmium. The lowercase letters compare the cadmium concentration at the same leaf age, and the uppercase letters compare the leaf age amongst the same cadmium concentration. Means followed by the same letter do not differ by the Scott–Knott test at 5% significance level
The number cell divisions (number of cells undergoing mitosis) was higher at the intermediary leaf ages (6- and 8-day-old leaves) for all treatments (Fig. 4b). However, leaves showed the highest means for this trait at 6 days under Cd2+ treatments although the control plants achieved this peak only passed 8 days (Fig. 4b). The cell production rate was lower in plants exposed to 50 μM of Cd2+ for all leaf ages (Fig. 4c). Although 10-day-old leaves showed reduced cell production rate for the plants of control and 50 μM treatments, 10 μM Cd2+ showed no modification of this parameter for any leaf age (Fig. 4c). The cell cycle time (time of accomplish the cell cycle) was longer on 8-day-old leaves from the control plants; however, on Cd2+-treated plants, this trait showed higher means for the 6-day-old leaves (Fig. 4d).
The exposure to Cd2+ also promoted significant morphological changes on the development of S. molle leaves (Fig. 5). Leaves exposed to 10 μM Cd2+ developed quicker as compared to control and 50-μM Cd2+ treatments (Fig. 5); the 6-day-old leaves developed under 10 μM Cd2+ already showed leaflets while other treatments had only leaflet primordia (Fig. 5).
The leaf area increased until the eighth day of development, and older leaf ages showed no significant differences (Fig. 6a). The Cd2+ promoted no effect on the leaf area for any age assessed (Fig. 6a). However, the leaf dry mass was higher for leaves developed under 10 μM Cd2+ at all leaf ages (Fig. 6b). In addition, the leaf dry mass was reduced in older leaves developed under 50 μM Cd2+ (Fig. 6b). Likewise, leaves from control plants showed increasing dry mass during 14 days, although this trait increased only by 8 days for Cd2+-exposed plants (Fig. 6b). Leaves developed under 50 μM Cd2+ showed higher specific leaf area compared to other treatments (Fig. 6c). The lowest means for specific leaf area were found for plants exposed to 10 μM Cd2+ (Fig. 6c). The specific leaf area increased from 6 to 8 days of development with no further changes (Fig. 6c). The marginal growth increased with time for all treatments achieving the highest mean at 20 days of development (Fig. 6d). However, 10 and 14-day-old leaves showed higher marginal growth when exposed to 10 μM Cd2+ (Fig. 6d). In addition, the diameter of mature cells of the mesophyll was higher on plants exposed to 10 μM Cd2+ (Fig. 6e) and the leaf elongation rate was reduced only under 50 μM Cd2+ (Fig. 6f).
Morphological traits of Schinus molle leaves from the sixth to the 24th day exposed to three cadmium concentrations (a–d). Diameter of mature cells in ground tissues (e) and leaf elongation rate evaluated in the ground tissues (f). Control = 0 μM. The lowercase letters compare the cadmium concentration at the same leaf age, and the uppercase letters compare the leaf age amongst the same cadmium concentration. Means followed by the same letter do not differ by the Scott–Knott test at 5% significance level
The Cd2+ content increased on both old and young leaves of S. molle with increasing Cd2+ concentration (Fig. 7). The control plants showed no difference in Cd2+ content for either old and young leaves. However, Cd2+-treated plants showed higher allocation of the metal in old leaves as compared to younger ones (Fig. 7).
Cadmium content of Schinus molle leaves at different ages (young leaves are considered as 6-day-old ones and old leaves 30-day-old ones). Control = 0 μM. The lowercase letters compare the cadmium content at different nutrient solutions, and the uppercase letters compare the leaf age amongst the same cadmium concentration. Means followed by the same letter do not differ by the Scott–Knott test at 5% significance level
Discussion
Previous works reported increased chlorenchyma thickness on leaves exposed to heavy metals (Shi and Cai 2009; Souza et al. 2011; Pereira et al. 2014). Likewise, Pereira et al. (2016) showed that low Cd2+ concentrations promote thicker chlorenchyma development and enhance the photosynthesis in S. molle leaves. However, in despite of the known effects for mature leaves, the Cd2+ effects in leaf meristems have been little investigated.
The ground meristem is the precursor of several parenchyma types, including the chlorenchyma (Evert 2006). Therefore, a given modification on mature tissue is likely a meristem-driven response. Likewise, the ground meristem of S. molle leaves showed several modifications when exposed to Cd2+. These modifications may be related to the thicker mature leaves grown under Cd2+ contamination.
The Cd2+ effects on meristems are mostly reported as toxic, causing several problems such as mitosis inhibition, abnormal microtubule organization during division, and aberrant chromosomes (Shi et al. 2016). These toxic effects of Cd2+ are remarkable for roots, as the reduction of the meristem size and number of cells have been reported (Yuan and Huang 2016). Therefore, the effects of Cd2+ on meristems are mainly reported for the root apex. The mitotic index as well as the number of cells in the root apical meristem decreases depending on the concentration of Cd2+ (Liu et al. 2003; Fusconi et al. 2006). All these Cd2+ effects may explain the lower results found for meristem traits at 50 μM Cd2+. However, the limited meristem activity found to S. molle leaf meristems at 50 μM Cd2+ cannot be considered toxic once the mean values are statistically similar to those of control plants. Therefore, this corroborates the Cd2+ tolerance of S. molle plants as reported by Pereira et al. (2016).
Interestingly, S. molle plants exposed to 10 μM Cd2+ showed improved leaf ground meristem parameters. However, the anatomical modifications and positive effects on plant growth promoted by low Cd2+ concentrations have been little discussed in literature (Arduini et al. 2004; Pereira et al. 2016). According to Kennedy and Gonsalves (1987), low Cd2+ levels can hyperpolarize the cell membranes, increasing the trans-membrane potential and cation uptake. In addition, low metal concentrations can stimulate enzymatic activity (Sawidis 2008). According to Asgher et al. (2015), the perception and transport of Cd2+ may activate signal transduction cascades in plants. It has been reported that Cd2+ stress signaling is tightly bound to the levels of both endogenous and exogenous plant growth regulators. Plant growth regulators play significant roles during plant development, which may be related to structural changes in important organs such as leaves.
The largest cell elongation rate in S. molle leaves exposed to 10 μM Cd2+ may be related to cell membrane modifications. Cell walls are very plastic, and modifications of its composition and structure are related to cell growth and differentiation (Parrotta et al. 2015). High Cd2+ concentration reduces the cell wall plastic extensibility causing morphological and structural alterations in pollen grains (Sawidis 2008). However, previous works have shown that low heavy metal levels may stimulate pollen tube growth (Searcy and Mulcahy 1985; Sawidis and Reiss 1995). Increased cell wall extensibility may be related to the higher cell elongation, diameter, and ground meristem thickness found on plants grown at 10 μM Cd2+. Likewise, the larger ground meristem cells may be related to thicker mature leaves under Cd2+ pollution. This effect was reported for fully developed leaves of different plant species (Shi and Cai 2009; Souza et al. 2011; Pereira et al. 2016). Therefore, the thicker mature leaves found on tolerant plants under Cd2+ contamination are related to the enlargement of ground meristem cells.
Positive effects found on tolerant plants under Cd2+ contamination also include the increased growth (Jia et al. 2015), enhanced plastid differentiation on the shoot apical meristem (Stoyanova and Tchakalova 1999), upregulation of the AtMRP6 gene transporter (Gaillard et al. 2008), and increased photosynthesis (Jia et al. 2015; Pereira et al. 2016). All these positive modifications and particularly those related to growth and photosynthesis depend on the leaf chlorenchyma developed after the ground meristem. Therefore, the Cd2+ effects are found early in leaf primordia in tolerant plants such as S. molle.
The typical effect of Cd2+ in meristems of non-tolerant plants is the inhibition of mitosis (Fusconi et al. 2006; Siddiqui et al. 2009; Yuan and Huang 2016). The toxicity of Cd2+ to meristems may be related to abnormal mitosis caused by the disorganization of microtubule, cytoskeleton, and tubulin structures (Shi et al. 2016); the repression of auxin production and signaling (Yuan and Huang 2016); the formation of mitotic aberrations (Fusconi et al. 2006); and the inhibition of biosynthesis of preribosomal RNA precursors (Marcano et al. 2002). However, on tolerant species, positive Cd2+ effects on meristems can be found (Stoyanova and Tchakalova 1999). The cell division parameters evaluated in this work were positively affected by 10 μM Cd2+ in S. molle leaves. These traits are related to the higher ground meristem activity and associated with the production of larger cells, which may explain the thicker mature leaves under Cd2+ pollution.
The toxicity of Cd2+ includes the reduced area, biomass, and elongation on leaves of non-tolerant species (Lunáčová et al. 2003; Anjum et al. 2016; Jinadasa et al. 2016). All these responses are very common for non-tolerant species under Cd2+ contamination and may be related to the lower meristem activity found in this work. However, this effect was found only for S. molle plants exposed to 50 μM Cd2+.
The Cd2+ accumulation in tolerant plants occurs mainly in older leaves. This mechanism of Cd2+ allocation prevents the toxicity for younger leaves which are physiologically active (Delmail et al. 2011; De Maria et al. 2013; Xin et al. 2013). In this work, we found that younger leaves responded differently to Cd2+ compared to older leaves. Thus, this suggests that the Cd2+ tolerance depends on differential metal allocation in young and old leaves.
In conclusion, the Cd2+ at low concentrations causes cell enlargement and improves mitosis in the ground meristem of S. molle leaves. Higher cell enlargement under Cd2+ contamination results in thicker ground meristem related to thicker and more functional mature leaves in S. molle. The development of thicker leaves in Cd-tolerant species depends on the primary effects on the ground meristem. Older leaves uptake more Cd2+ to protect the younger ones.
References
Anjum SA, Tanveer M, Hussain S, Ullah E, Wang L, Khan I, Samad RA, Tung SA, Anam M, Shahzad B (2016) Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. Clean (Weinh) 44:29–36
Arduini I, Masoni A, Mariotti M, Ercoli L (2004) Low cadmium application increase miscanthus growth and cadmium translocation. Environ Exp Bot 52:89–100
Asgher M, Khan MIR, Anjum NA, Khan NA (2015) Minimising toxicity of cadmium in plants—role of plant growth regulators. Protoplasma 252:399–413
De Maria S, Puschenreiter M, Rivelli AR (2013) Cadmium accumulation and physiological response of sunflower plants to Cd during the vegetative growing cycle. Plant Soil Environ 59:254–261
Delmail D, Labrousse P, Hourdin P, Larcher L, Moesch C, Botineau M (2011) Physiological, anatomical and phenotypical effects of a cadmium stress in different-aged chlorophyllian organs of Myriophyllum alterniflorum DC (Haloragaceae). Environ Exp Bot 72:174–181
Dickson RE, Isebrands JG (1991) Leaves as regulators of stress response. In: Mooney HA, Winner WE, Pell EJ (eds) Response of plants to multiple stresses. Academic Press, San Diego, pp 3–34
Evert RF (2006) Esau's plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development. Wiley, New Jersey, p 601
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Ciênc Agrotec 35:1039–1042
Fusconi A, Repetto O, Bona E, Massa N, Gallo C, Dumas-Gaudot E, Berta G (2006) Effects of cadmium on meristem activity and nucleus ploidy in roots of Pisum sativum L. cv. Frisson seedlings. Environ Exp Bot 58:253–260
Gaillard S, Jacquet H, Vavasseur A, Leonhardt N, Forestier C (2008) AtMRP6/AtABCC6, an ATP-binding cassette transporter gene expressed during early steps of seedling development and up-regulated by cadmium in Arabidopsis thaliana. BMC Plant Biol 22:1–11
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular. Calif Agr Expt Sta 347:1–39
Ivanov VB, Dubrovsky JG (1997) Estimation of the cell-cycle duration in the root apical meristem: a model of linkage between cell-cycle duration, rate of cell production, and rate of root growth. Int J Plant Sci 158:757–763
Jia L, Liu Z, Chen W, Ye Y, Yu S, He X (2015) Hormesis effects induced by cadmium on growth and photosynthetic performance in a hyperaccumulator, Lonicera japonica Thunb. J Plant Growth Regul 34:13–21
Jinadasa N, Collins D, Holford P, Milham PJ, Conroy JP (2016) Reactions to cadmium stress in a cadmium-tolerant variety of cabbage (Brassica oleracea L.): is cadmium tolerance necessarily desirable in food crops? Environ Sci Pollut R 23:5296–5306
Kennedy CD, Gonsalves FAN (1987) The action of divalent zinc, cadmium, mercury, copper and lead on the trans-root potential and H+ efflux of excised roots. J Exp Bot 38:800–817
Liu D, Jiang W, Gao X (2003) Effects of cadmium on root growth, cell division and nucleoli in root tip cells of garlic. Biol Plant 47:79–83
Lunáčová L, Šottníková A, Masarovičová E, Lux A, Streŝko V (2003) Comparison of cadmium effect on willow and poplar in response to different cultivation conditions. Biol Plant 47:403–411
Marcano L, Carruyo I, Del Campo A, Montiel X (2002) Effect of cadmium on the nucleoli of meristematic cells of onion Allium cepa L: an ultrastructural study. Environ Res 88:30–35
Parrotta L, Guerriero G, Sergeant K, Cai G, Hausman JF (2015) Target or barrier? The cell wall of early-and later-diverging plants vs cadmium toxicity: differences in the response mechanisms. Front Plant Sci 6:1–16
Pereira FJ, Castro EM, Oliveira C, Pires MF, Pereira MP, Ramos SJ, Faquin V (2014) Lead tolerance of water hyacinth (Eichhornia crassipes Mart.-Pontederiaceae) as defined by anatomical and physiological traits. An Acad Bras Ciênc 86:1423–1433
Pereira MP, Rodrigues LCA, Corrêa FF, Castro EM, Ribeiro VE, Pereira FJ (2016) Cadmium tolerance in Schinus molle trees is modulated by enhanced leaf anatomy and photosynthesis. Trees 30:807–814
Pérez Chaca MV, Vigliocco A, Reinoso H, Molina A, Abdala G, Zirulnik F, Pedranzani H (2014) Effects of cadmium stress on growth, anatomy and hormone contents in Glycine max (L.) Merr. Acta Physiol Plant 36:2815–2826
Sawidis T (2008) Effect of cadmium on pollen germination and tube growth in Lilium longiflorum and Nicotiana tabacum. Protoplasma 23:95–106
Sawidis T, Reiss HR (1995) Effects of heavy metals on pollen tube growth and ultrastructure. Protoplasma 185:113–122
Searcy KB, Mulcahy DL (1985) The parallel expression of metal tolerance in pollen and sporophytes of Silene dioica (L.) Clairv., S. alba (Mill.) and Mimulus guttatus DC. Theor Appl Genet 69:597–602
Shi G, Cai Q (2009) Leaf plasticity in peanut (Arachis hypogaea L.) in response to heavy metal stress. Environ Exp Bot 67:112–117
Shi G, Sun L, Wang X, Liu C (2014) Leaf responses to iron nutrition and low cadmium in peanut: anatomical properties in relation to gas exchange. Plant Soil 375:99–111
Shi Q, Wang J, Zou J, Jiang Z, Wu H, Wang J, Jiang W, Liu D (2016) Cadmium localization and its toxic effects on root tips of barley. Zemdirbyste 103:151–158
Siddiqui S, Meghvansi MK, Wani MA, Jabee F (2009) Evaluating cadmium toxicity in the root meristem of Pisum sativum L. Acta Physiol Plant 31:531–536
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2015) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics and ionomics. Front Plant Sci 6:1–36
Souza VL, Almeida AAF, Lima SG, Cascardo JCDM, Silva DDC, Mangabeira PA, Gomes FP (2011) Morphophysiological responses and programmed cell death induced by cadmium in Genipa americana L.(Rubiaceae). Biometals 24:59–71
Stoyanova D, Tchakalova E (1999) Cadmium induces ultrastructural changes in shoot apical meristem of Elodea canadensis Rich. Photosynthetica 37:47–52
Tsukaya H (2006) Mechanism of leaf-shape determination. Annu Rev Plant Biol 57:477–496
Xin J, Huang B, Yang Z, Yuan J, Zhang Y (2013) Comparison of cadmium subcellular distribution in different organs of two water spinach (Ipomoea aquatica Forsk.) cultivars. Plant Soil 372:431–444
Yuan HM, Huang X (2016) Inhibition of root meristem growth by cadmium involves nitric oxide-mediated repression of auxin accumulation and signaling in Arabidopsis. Plant Cell Environ 39:120–135
Acknowledgements
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq [National Counsel of Technological and Scientific Development]), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES [Coordination for the Improvement of Higher Education Personnel]), and Fundação de Amparo à Pesquisa do estado de Minas Gerais (FAPEMIG [Minas Gerais State Research Foundation]) for funding and research grants awarded to complete the present study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bhumi Nath Tripathi
Rights and permissions
About this article
Cite this article
Pereira, M.P., Corrêa, F.F., de Castro, E.M. et al. Leaf ontogeny of Schinus molle L. plants under cadmium contamination: the meristematic origin of leaf structural changes. Protoplasma 254, 2117–2126 (2017). https://doi.org/10.1007/s00709-017-1103-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1103-2