Abstract
Maize is an important crop, with genotype BRS-4154, 'Saracura', being tolerant to intermittent flooding. Cattail is an aquatic plant with known radial oxygen loss activity, being widely diffused around the world and highly invasive. We aimed to analyse the interaction of Maize and Cattail under waterlogging and field capacity. The experiment was conducted in pots containing red latosol and nutrient solution in a greenhouse, 10 days after germination, when maize plants reached the average height of 12.5 cm, the sources of variation were introduced, being the presence or absence of Cattail and waterlogged or at field capacity with the conditions being kept by refilling the water lost. The experiment was conducted in a factorial completely randomized design (2 × 2). Growth data were collected fortnightly, 5 times, with dry weight measured on the eightieth day. The analysis of gas exchange, chlorophyll content, and sampling for leaf anatomy was performed monthly, twice, using an infrared gas-exchange analyser and a SPAD unit meter. In the final 5 days of the experiment, the dissolved oxygen in waterlogged pots was measured consecutively and roots were sampled for usual microtechnique procedures. Waterlogging was detrimental however Cattail increased the dissolved oxygen content and benefitted maize showing no competition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cultivation in flooded areas can be damaged by the proliferation of invasive plants of rapid clonal propagation consuming resources. Crops, such as maize (Zea mays L.), Soybean (Glycine max (L.) Merr.), wheat (Triticum aestivum L.) and rice (Oryza sativa L.), are among the world's most-produced agricultural commodities, but, as Dai et al. (2014) shows, cultivation subjected to flooding promotes losses by competition with aquatic macrophytes.
Maize (Zea mays L.) is a crop of great economic importance, one of the five most-produced cereals worldwide, although most of its genotypes are susceptible to waterlogging damages, however, as seen in Pereira et al. (2009) the National Maize and Sorghum Research Center (EMBRAPA) developed a tolerant genotype called BRS-4154 ‘Saracura’.
Southern Cattail (Typha domingensis Pers.) is a cosmopolitan rooted aquatic macrophyte belonging to the family Typhaceae. Carvalho et al. (2014) describe it as having its underground parts consist of roots and rhizomes with its aerial parts being formed by leaves and inflorescences. Cervi et al. (2009) demonstrated aquatic macrophytes being used as shelter and food by animals showing the relevance for preservation and management of its environments which are experiencing constant changes and losses, as published by Sundar et al. (2015), due to rapid population growth, urbanization and agricultural needs which leads to the needs of a better understanding of those environments for sustainability.
Natural wetlands are susceptible to degradation and explosive proliferation of invasive species that use eutrophication favouring itself and decreasing biodiversity, which shows that natural and artificial wetlands are a great way to control eutrophication, such as agricultural runoff, serving as biological filters shown by Vymazal and Březinová (2015). Studies about the ecology and environment of aquatic macrophytes made by Cunha et al. (2012) show that interspecific competition between different macrophyte species is influenced by habitat structure, zonation of aquatic plants and spatial segregation promoted by water levels, probably because of the competitive factor and preferred habitat. Thus, more knowledge is needed on how plants cope and interact in these environments together.
Some plant species release oxygen in their substrate via their root system; this process is called radial oxygen loss (ROL) which was reported by Chabbi et al. (2000) for T. domingensis. This leaching of oxygen may promote a lower demand for gas storage and aerenchyma development, increasing water, and nutrient uptake. Interaction between crops and invasive weeds in flood susceptible areas can cause serious losses on productivity. The geographic region, latitude, proportion of plants to each other, and the genetic diversity are key factors in competitiveness and productivity decreases. Weed species are more competitive than crops, producing higher biomass, photosynthetic rate, and seed sets among other characteristics. (Dai et al. 2014).
There are few studies on the competition of aquatic macrophytes and crops, such as maize, with the conventional weed management practices being the removal of invasive plants, which can be expensive. The interaction between crops and aquatic macrophytes must be better understood to improve sustainable agriculture. Thus, a better understanding of the effects of the competition with T. domingensis can contribute to better sustainable use of aquatic ecosystems as well as lowland agriculture, reducing costs, increasing productivity and contributing to the preservation of these ecosystems and its diversity.
This study was aimed to identify the effects in maize from T. domingensis association and how it is influenced by the water level, with the ‘Saracura’ genotype tolerating intermittent periods of flooding, akin to the seasonality of how wetlands work, it is necessary to know how the association behaves in both conditions.
Experimental
The maize seeds were acquired from EMBRAPA – Maize and Sorghum located at Sete Lagoas, MG. Typha domingensis plants were collected from natural populations in wetlands located at the Federal University of Lavras, Lavras, State of Minas Gerais, Brazil (21°14′43″ S, 45°59′59″ W).
Maize seeds were germinated in a greenhouse in pots of 15 L capacity containing 11.65 L of soil (red latosol) which was collected and sieved through a 4 mm sieve and irrigated at its field capacity with 2.90 L of Hoagland and Arnon (1950) nutrient solution at 100% of its ionic power. The soil field capacity was calculated to be 24.87% by subtracting the water-saturated weight of the sample by its dry weight and dividing it by the water-saturated weight and multiplying by 100 (Loss et al. 2014).
After maize plants achieved average 12.5 cm height and two completely expanded leaves, one cattail plant was introduced per pot (cattail plants were 15 cm tall average) and the waterlogging introduced in the designated treatments, with the conditions being maintained throughout the whole experiment by weighing the pots daily. The pots in field capacity had the water lost by evapotranspiration refilled by weighing and refilling it to the weight it had when the nutrient solution was first applied, the waterlogged plots were kept by measuring the height of the water above soil level and refilling it to that same height.
The red latosol was evaluated for its nutrient concentration via soil analysis (Table q supplementary file), the soil analysis chart demonstrated adequate pH, and soil fertility with the nutrient solution added providing the necessary nutrients for plant growth as described by Souza et al. (2016). The Soil chemical analysis was conducted with a sample of soil extracted from each pot and was carried out at the Soil Science Department (DCS) at the Federal University of Lavras.
The experiment was conducted in a 2 × 2 factorial design with two water levels, being field capacity (24.87% of the soil weight) and (layer of water 2 cm above soil level). The total number of plots was 40 with four treatments and ten replications being each replication constituted of one plant.
The maize plants were fortnightly evaluated for its height, the number of fully expanded leaves, dead leaves from the day the sources of variation were introduced until the end of the experiment, 80 days after the introduction of sources of variation. Twenty maize plants (n = 20) were collected at the end of the experiment and oven-dried at 60 °C for 48 h and the shoots, roots and total dry weight were measured using an analytical scale (AY220, Shimadzu, Japan).
The allocation of biomass was calculated by dividing the organ dry mass, root or shoot, by the total dry mass. The total plant height elongation rate was calculated by subtracting the final plant height by the initial plant height, dividing it by the final height and multiplying by 100. The plant height elongation rate by day was calculated by dividing the final height by the number of days passed since the first measurement.
Gas exchange analysis was performed twice using an infrared gas exchange analyzer (IRGA) model LI-6400XT (Li-COR Biosciences, Lincoln, Nebraska, USA) equipped with a 6 cm2 cuvette for the measurements. The readings were made on the second fully expanded leaf that could fit the cuvette per plant, eliminating the outliers and considering the means of the two replications in time, making the number of evaluations equals 32 (n = 32). The evaluations occurred past 30 and 60 days after the beginning of the experiment and started at 08:00 a.m. and did not go beyond 10:00 a.m. The density of photosynthetically active photons was fixed at 1000 μmol m−2 s−1 in the chamber. The following characteristics were evaluated: stomatal conductance (gs), transpiratory rate (E) and photosynthetic rate (Pn). The chlorophyll content in leaves was undirected estimated with the portable chlorophyll meter SPAD-502 (Konica Minolta, Tokyo, Japan). The second fully expanded leaf per plant in three positions per leaf (base, medium, and apex) was evaluated two times with the data evaluated excluding the outliers averaged to one plant (n = 32).
Dissolved oxygen was evaluated 80 days after the beginning of the experiment for five consecutive days in the morning from 8:00 a.m to 10:00 a.m. using the portable dissolved oxygen meter Oakton DO600 (Cole Parmer, Illinois, USA). These measurements were performed by introducing the probe 2 cm away from maize plants on waterlogged plots (with and without T. domingensis). A total of 100 measurements were made with the data submitted to analysis of variance excluding outliers (n = 86). The substrates from field capacity treatments were not evaluated due to its natural oxygenated condition provided by the soil pores. The device was calibrated before every evaluation according to the manufacturer instructions, with the mean substrate temperature of the five consecutive days measured by the device being 27.3, 22.5, 27.2, 21.7 and 23.1 °C, respectively, from the first day to the fiftieth day of evaluation.
Leaves were collected twice for the anatomical analysis, at 30 and 60 days of the experiment beginning, with the data from the two samples being averaged as one, and were fixed in FAA70% solution (formaldehyde, acetic acid and ethanol 70% in a ratio of 1: 1: 18), for 48 h and then transferred to a solution of ethanol 70% (Johansen, 1940). The sections were performed in the median region of the leaves.
For the analysis of adaxial and abaxial epidermis, paradermic impressions were made on the surface of the leaves with cyanoacrylate ester resin (Superbonder®, Loctite, Dusseldorf, Germany). After the resin’s drying, four prints per replication (n = 160) were removed and mounted in slides using 50% glycerol (m v−1).
Five cross sections per replication (n = 200) were obtained by hand with the use of steel blades and clarified with 50% sodium hypochlorite and washed in distilled water for 10 min and then stained with safrablau solution (safranin 1% and Astra blue 0.1% in a ratio of 7:3) and mounted in slides with 50% glycerol (m v−1) (Bukatsch 1972).
Root samples were collected at the end of the experiment at 80 days after the introduction of sources of variation. Cross sections were obtained approximately 2 cm away from the root tip by hand using steel blades and stained with 1% aqueous safranin (m v−1). Two roots per replication (n = 40) with one section per root were mounted in slides with 50% glycerol (m v−1). The sections were clarified with 50% sodium hypochlorite and washed in distilled water for 10 min, stained and mounted on slides with 50% glycerol (Johansen 1940).
The slides were photographed using an Olympus microscope model CX31 (Olympus, Tokyo, Japan). These images were analysed using the ImageJ software, calibrated with microscopic rulers photographed in the same configuration of the images obtained from the material where the quantitative parameters of the tissues of leaves and roots were evaluated according to the methodologies described by Pereira et al. (2009).
Data were submitted to ANOVA and the means compared by the Scott-Knott test, at 5% probability with the aid of the statistical software SISVAR, version 5.0 (Ferreira 2011).
Results
Under waterlogged conditions, the presence of T. domingensis, significantly increased the dissolved oxygen concentration in the substrate (Fig. 1).
Oxygen concentration in waterlogged soils containing maize plants in a contrasting condition of T. domingensis. Bars = Standard Error. Different letters in the columns show significantly different means according to the Scott-Knott test to p < 0.05 (p = 0.0326). M + T = Maize + T. domingensis association; M maize plants only (no Association)
Growth parameters showed no significant interaction between factors (p > 0.05). Association with T. domingensis increased the number of fully expanded leaves of maize under waterlogging (Table 1). However, waterlogging increased the number of maize dead leaves and association with T. domingensis did not affect it (Table 1). Waterlogging decreased maize height and elongation rate whereas the association with T. domingensis increased these parameters (Table 1).
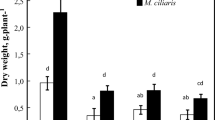
Waterlogging decreased the dry weight of maize while T. domingensis association did not affect it (Table 1). However, biomass allocation showed significant interaction between sources of variation (p < 0.0422) and the association with T. domingensis increased the biomass allocation to shoots in maize plants under waterlogging (Fig. 2).
Biomass allocation of maize plants grown under contrasting water levels and T. domingensis association. Means followed by the lowercase letter in the same colour columns in each plant part and by uppercase letter in the same soil condition in each plant part does not differ by the Scott-Knott test for p < 0.05. Bars = Standard Error. Interaction effect p-value of 0.0422. M + T = Maize + T. domingensis association, M maize plants only (no Association)
Gas exchange parameters showed no interaction between sources of variation (p > 0.09). Waterlogging reduced maize’s net photosynthesis, transpiration rate and stomatal conductance (Table 2), whereas these parameters were increased by the association with T. domingensis (Table 2). However, waterlogging decreased the maize chlorophyll content measured in SPAD units and no significant effect was found under association with T. domingensis (Table 2).
Stomatal size and density had significant interaction between sources of variation (p < 0.0046). Waterlogging increased stomatal size on both adaxial and abaxial epidermal surfaces of maize in association with T. domingensis with no significant effects occurring in maize plants alone (Table 3). Besides, association with T. domingensis under waterlogging promoted lower adaxial stomata size (Table 3) and increased the stomatal density while these structures became smaller (Table 3).
Only the leaf vascular bundle diameter and abaxial epidermis thickness showed a significant interaction between the sources of variation (p < 0.0001; p < 0.0004). However, the maize leaf vascular bundle diameter showed no significant modification in both soil water conditions in the absence of association but it was decreased by waterlogging in association when compared to the field capacity and association and the waterlogging with no association treatments (Fig. 3 and Table 4). Soil water condition had no significant effect in the adaxial epidermis thickness of maize plants grown alone but waterlogging reduced this parameter under association with T. domingensis. However, the association with T. domingensis had a polar effect in the different water soil conditions with it decreasing under waterlogging while increasing it under field capacity (Fig. 3 and Table 4).
Transversal sections of maize leaves grown under under contrasting water levels and T. domingensis association. a, c field capacity, b, d Waterlogged, a, b = no association, c, d association with T. domingensis. Ade adaxial epidermis, abe abaxial epidermis, bfc bulliform cells, st stomata, xl xylem, phl phloem, bs bundle sheath, pa chlorophyll parenchyma. Bars = 100 µm
Waterlogging modified the maize leaf anatomy as all tissues became thinner compared to plants under field capacity (Fig. 3 and Table 5). Also, waterlogging reduced the proportion of the mesophyll and the vascular bundles in maize leaves whereas no significant effect was found in the proportions of the bundle sheath (Fig. 3 and Table 5). Association with T. domingensis promoted no significant changes for most of the leaf anatomical traits, except for the adaxial epidermis thickness and mesophyll proportion which were reduced (Fig. 3 and Table 5).
Waterlogging reduced the xylem vessel area, vascular cylinder area, vascular cylinder proportion and it increased the cortex proportion as well in maize roots (Fig. 4 and Table 6). Also, association with T. domingensis promoted no significant changes for most of the maize root’s tissues whereas the exodermis and endodermis thicknesses were reduced (Fig. 4 and Table 6).
Transversal sections of maize roots grown under under contrasting water levels and T. domingensis association. a, c field capacity, b, d waterlogged, a, b no association, c, d in association with T. domingensis. ed endodermis, ct cortex, ae aerenchyma, xl xylem vessel, phl phloem, pp pith parenchyma. Bars = 50 µm
Discussion
An important aspect from the results found in this work is that Typha domingensis causes no significant competition and reduction in maize’s growth parameters both under field capacity and waterlogged conditions. This is relevant since maize is considered sensitive to competition reducing its yield (Maddonni and Otegui 2006). Besides, T. domingensis can turn into an invasive species causing problems to plants on these sites (Surratt, Shinde and Aumen 2012). Moreover, we used the recommended soil nutritional conditions for maize and the absence of competition between these two species is, in fact, relevant to reduce the crop management costs and to open the possibility of associated cultivation. Intercropping of maize and bean can be more advantageous than isolated cultivation under unfavorable environmental conditions, such as the Brazilian semi-arid region (Morgado and Willey 2008). However, this aspect was never previously explored for waterlogged conditions and this work can open the possibility of its investigation.
Further discussion in this matter raises the necessity of information on the main problem for waterlogged soils, which is the low O2 levels, triggering root O2 deprivation that causes metabolic alterations with consequences to the whole plant (Drew 1997). Hypoxia condition by water saturation reduces growth (Else et al. 1995), and Conaty et al. (2008) state that it increases the reactive oxygen species (ROS) production and decreases oxygen levels, leading to lower effectiveness of the mitochondrial electron transport chain. These stresses promoted by environments under hypoxia lead plants to develop a higher rate of dead leaves as well as lower height and biomass (Bailey-Serres; Lee and Brinton 2012; Herzog et al. 2016). Thus, oxygen limitation under drought is the main cause of environmental stress under drought.
Results showed that of association maize with Typha domingensis can be beneficial for its growth under waterlogged conditions. Moreover, no significant competition was found between maize and T. domingensis and its association under waterlogging conditions may be viable. The main service provided by T. domingensis was the increase in dissolved oxygen levels which alleviate the hypoxia caused by waterlogging. This is particularly important since increased dissolved oxygen in soils is shown to minimize ROS effects (Blokhina et al. 2003). Also, low level of oxygen in soil decreases photosynthesis in different plant species (Bai et al. 2013) and waterlogging reduces photosynthesis on maize plants (Ren et al. 2016). Therefore, reduced growth verified in maize under waterlogging is related to low oxygen levels under waterlogging conditions and this stress was alleviated by the presence of T. domingensis which increased oxygen concentration in the soil.
Increased photosynthetic-related traits of maize plants when associated with Typha domingensis promoted higher growth and this response is related to the stress alleviation by the radial oxygen loss. Association with T. domingensis increased several characteristics that are related to photosynthesis, such as higher production of leaves, increased shoot elongation and height, higher net photosynthesis, stomatal conductance, and transpiration. Waterlogging and hypoxia severely damage the gas exchanges and mainly the photosynthesis (Bai et al. 2013; Ren et al. 2016), thus limiting plant growth and biomass production (Bailey-Serres; Lee and Brinton 2012; Herzog et al. 2016). The reasons for photosynthesis reductions in maize under waterlogging may be caused by damage to chloroplast structure hindering its quantity (Ren et al. 2016) and lowering chlorophyll content (Tian et al. 2019) and stomatal closure for pea plants (Zhang and Zhang 1994). In the present study, the chlorophyll content remained unaffected, and no significant modifications were found for the mesophyll and bundle sheath proportions of maize plants by the association with T. domingensis. As the maize photosynthesis depends on both mesophyll (chlorophyll parenchyma) and bundle sheath cells, as well as the most of chlorophyll content, is found in mesophyll cells, these results seem reasonable and indicate that the increased photosynthesis found in maize may be related to stomatal parameters instead.
Waterlogging reduces the stomatal conductance in maize and wheat at different plant stages or pre-treatments to stress (Ren et al. 2016; Wang et al. 2016). In fact, in the present study, we found reduced gs in maize plants under waterlogging. Interestingly, the association with T. domingensis increased the stomatal conductance and this may be related to higher photosynthesis found in maize. Stomatal closure reduces its conductivity and is found under waterlogged plants by the influence of abscisic acid (Zhang and Zhang 1994) or by the excessive reactive oxygen species which action can be alleviated by ethylene (Wang et al. 2016). Moreover, this higher stomatal conductance promoted by the association with T. domingensis may be due to anatomical modifications in maize plants leaves. The main factor that triggers stomatal closure and decreases stomatal conductance is drought (Xu et al. 2010; Cruz et al. 2019). However, when subjected to water limitation, stomatal density is often increased in drought-tolerant species (Zhang et al. 2006) and this higher stomatal density turns its behaviour more efficient because stomata stays open for shorter time intervals (Berry et al. 2010; Cruz et al. 2019). This is the typical behaviour to preserve water and lower plant transpiration under water-limited environments. Additionally, Pereira et al. (2016) reports that higher stomatal density leads to increased CO2 uptake consequently increasing Pn. Thus, association with T. domingensis increased the stomatal density of maize and this may have favoured stomatal conductance and photosynthesis as well. This effect or T. domingensis is probably related to increased oxygen levels in soil that was released by this plant and this may have reduced the ROS production of maize and stomatal closure caused by these compounds.
Waterlogging significantly reduced the transpiration of maize, however, association with T. domingensis increased this parameter. The increased stomatal density permits a higher control of plant transpiration avoiding excessive water loss under drought (Cruz et al. 2019). Maize plants developed higher stomatal density when grown in association with T. domingensis and this may have increased the stomatal conductance and the transpiration. However, waterlogging may trigger stomatal closure (Zhang and Zhang 1994) limiting transpiration. It is important to note that a proper amount of water must be absorbed and transported to reach the leaves, preventing the abscisic acid or ROS production and stomatal closure. The vascular bundles of maize remain unaffected by waterlogging or the association with T. domingensis. This is an important result since these structures were kept functional under these conditions and proper water transport was found in maize. Likewise, anatomical modifications in the roots of maize may be useful to understand the water uptake of maize in these conditions. Waterlogging increased cortex thickness and reduced the vascular cylinder proportion of maize roots, both modifications negatively affecting the root water conductance. Cheng et al. (2012a, b) attribute a decrease in gas-exchange parameters to increased cortex length and apoplastic barriers under hypoxia. Thicker root cortex reduces the water conductance because of the longer distances to reach the vascular cylinder which contains the xylem. Additionally, with a lower proportion of the vascular cylinder roots may show reduced xylem tissue, reducing water transport. Association with T. domingensis prevents these two modifications preserving the water conductance capacity of maize roots. Also, apoplastic barriers of maize roots (exodermis and endodermis) were reduced by the association with T. domingensis and this modification may improve water uptake. Thicker apoplastic barriers minimize damages by ROS at the cost of decreasing hydraulic conductance and transpiration rate (Cheng et al. 2012a, b). Therefore, increased transpiration found in maize grown in association with T. domingensis was sustained by more efficient roots and preserved transport tissues. Besides, as maize plants were under waterlogged conditions excessive water loss is not a relevant problem.
New form of food production systems, such as aquaculture, is a viable form of sustainable agriculture but it still lacks understanding about the management of its high oxygen demand (Fang et al. 2017). The use of expensive tools, such as compressed oxygen, is applied; however, Goto et al. (1996) emphasize the need for cheaper and innovative alternatives. In addition, reports suggest that both maize and T. domingensis are viable as bioenergy crops showing high biomass production (Nkemka et al. 2015). In this work, we found that increased dissolved oxygen promoted by T. domingensis enhanced maize growth, anatomy, and physiology and further experiments may establish viable and environmentally sustainable intercropping systems.
Conclusion
Typha domingensis increased dissolved oxygen in the waterlogged soil without developing competitive traits with maize, instead, favouring its growth by increasing its net photosynthesis, which was promoted by a higher stomatal density and decreased root apoplastic barriers.
Author contributions statement
Each author contributed significantly to the final version of the work. Rodrigo Barbosa Kloss conduced most of the experiment, data sampling, analysis and writing of the first draft of the work, it was par of its dissertation for master degree in Applied Botany in the Universidade Federal de Lavras. Evaristo Mauro de Castro contributed to anatomical analysis and critical review. Paulo César Magalhães contributed with critical review and manuscript revision. Vinícius Politi Duarte contributed to data sampling. Felipe Fogaroli Corrêa contributed to data sampling, cattail propagation and experiment handling. Fabricio José Pereira was the adviser of the first author, conduced the experimental design, data sampling and analysis and the writing of the final version of the work.
References
Bai T, Li C, Li C, Liang D, Ma F (2013) Contrasting hypoxia tolerance and adaptation in Malus species is linked to differences in stomatal behaviour and photosynthesis. Physiol Plant 147(4):514–523. https://doi.org/10.1111/j.1399-3054.2012.01683.x
Bailey-Serres J, Lee SC, Brinton E (2012) Waterproofing crops: effective flooding survival strategies. Plant Physiol 160(4):1698–1709. https://doi.org/10.1104/pp.112.208173
Berry JA, Beerling DJ, Franks PJ (2010) Stomata: key players in the earth system, past and present. Curr Opin Plant Biol 13:232–239. https://doi.org/10.1016/j.pbi.2010.04.013
Blokhina O, Virolainen E, Fagerstedt EV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91(2):179–194. https://doi.org/10.1093/aob/mcf118
Bukatsch F (1972) Bemerkungen zur Doppelfärbung: Astrablau-Safranin. Mikrokosmos 61:225–255
Cervi AC, Bona C, Moço MCC, von Linsingen L (2009) Macrófitas aquáticas do Município de General Carneiro, Paraná. Brasil Biota Neotrop 9(3):215–222. https://doi.org/10.1590/S1676-06032009000300022
Chabbi A, Mckee KL, Mendelssohn IA (2000) Fate of oxygen losses from Typha domingensis (Typhaceae) and Cladium jamaicense (Cyperaceae) and consequences for root metabolism. Am J Bot 87(8):1081–1090. https://doi.org/10.2307/2656644
Cheng H, Chen DT, Tam NFY, Chen GZ, Li SY, Ye ZH (2012a) Interactions among Fe2+, S2-, and Zn2+ tolerance, root anatomy, and radial oxygen loss in mangrove plants. J Exp Bot 63(7):https://doi.org/10.1093/jxb/err440
Cheng H, Tam NFY, Wang YS, Li SY, Chen GZ, Ye ZH (2012b) Effects of copper on growth, radial oxygen loss and root permeability of seedlings of the mangroves Bruguiera gymnorrhiza and Rhizophora stylosa. Plant Soil 359(1–2):255–266. https://doi.org/10.1007/s11104-012-1171-1
Conaty WC, Tan DK, Constable GA, Sutton BG, Field DJ, Mamum EA (2008) Genetic variation for W tolerance in cotton. J Cotton Sci 12:53–61
Cruz YC, Scarpa ALM, Pereira MP, Castro EM, Pereira FJ (2019) Growth of Typha domingensis as related to leaf physiological and anatomical modifications under drought conditions. Acta Physiol Plant 41:2–9. https://doi.org/10.1007/s11738-019-2858-1
Cunha NL, Delatorre M, Rodrigues RB, Vidotto C, Gonçalves E, Scremin-Dias G, Damasceno-Junior G, Pott VJ, Pott A (2012) Structure of aquatic vegetation of a large lake, western border of the Brazilian Pantanal. Braz J Biol 72(3):519–531. https://doi.org/10.1590/S1519-69842012000300015
Dai L, Dai W, Song X, Lu B, Qiang S (2014) A comparative study of competitiveness between different genotypes of weedy rice (Oryza sativa) and cultivated rice. Pest Manag Sci 70:113–122. https://doi.org/10.1002/ps.3534
Carvalho MLS de, Lima CT de, Oliveira RP de, Giulietti AM (2014) Flora of Bahia: Typhaceae. SITIENTIBUS Sér Ciênc Biol https://doi.org/10.13102/scb420
Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol 48:223–250. https://doi.org/10.1146/annurev.arplant.48.1.223
Else MA, Davies WJ, Malone M, Jackson MB (1995) A negative hydraulic message from oxygen-deficient roots of tomato plants? (Influence of soil flooding on leaf water potential, leaf expansion, and synchrony between stomatal conductance and root hydraulic conductivity). Plant Physiol 109:1017–1024. https://doi.org/10.1104/pp.109.3.1017
Fang Y, Hu Z, Zou Y, Fan J, Wang Q, Zhu Z (2017) Increasing economic and environmental benefits of media-based aquaponics through optimizing aeration pattern. J Clean Prod 162:1111–1117. https://doi.org/10.1016/j.jclepro.2017.06.158
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Ciência Agrotec 35(6):1039–1042. https://doi.org/10.1590/S1413-70542011000600001
Goto E, Both AJ, Albright LD, Langhans RW, Leed AR (1996) Effect of dissolved oxygen concentration on lettuce growth in floating hydroponics. Acta Hortic 440:205–210. https://doi.org/10.17660/ActaHortic.1996.440.36
Herzog M, Striker GG, Colmer TD, Pedersen O (2016) Mechanisms of waterlogging tolerance in wheat–a review of root and shoot physiology. Plant Cell Environ 39(5):1068–1086. https://doi.org/10.1111/pce.12676
Hoagland DR, Arnon D (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn 347:139
Loss A, Costa EM, Pereira MG, Beutler SJ (2014) Agregação, matéria orgânica leve e carbono mineralizável em agregados do solo. Rev Fac Agron Univ Nac la Plata 113(1):1–8
Maddonni GA, Otegui M (2006) Intra-specific competition in Maize: Contribution of extreme plant hierarchies to grain yield, grain yield components and kernel composition. Field Crops Res 97(2):155–166. https://doi.org/10.1016/j.fcr.2005.09.013
Morgado LB, Willey RW (2008) Optimum plant population for maize-bean intercropping system in the Brazilian semi-arid region. Sci agric 65(5):474–480. https://doi.org/10.1590/S0103-90162008000500005
Nkemka VN, Gilroyed B, Yanke J, Gruninger R, Vedres D, McAllister T, Hao X (2015) Bioaugmentation with an anaerobic fungus in a two-stage process for biohydrogen and biogas production using corn silage and cattail. Bioresour Technol 185:79–88. https://doi.org/10.1016/j.biortech.2015.02.100
Pereira JP, de Castro EM, de Souza TC, Magalhães PC (2009) Evolução da anatomia radicular do milho “Saracura” em ciclos de seleção sucessivos. Pesq Agropec Bras 43(12):1649–1656. https://doi.org/10.1590/S0100-204X2008001200002
Pereira MP, Rodrigues LCA, Corrêa FF, de Castro EM, Ribeiro VE, Pereira FJ (2016) Cadmium tolerance in Schinus molle trees is modulated by enhanced leaf anatomy and photosynthesis. Trees 30(3):807–814. https://doi.org/10.1007/s00468-015-1322-0
Ren B, Zhang J, Dong S, Liu P, Zhao B (2016) Effects of waterlogging on leaf mesophyll cell ultrastructure and photosynthetic characteristics of summer maize. PLoS ONE 11(9):e0161424. https://doi.org/10.1371/journal.pone.0161424
Souza MF, Neto MDC, Marinho MI, Saraiva DT, Faria AT, Silva AA, Silva DV (2016) Persistence of imidazolinones in soils under a clearfield system of rice cultivation. Planta Daninha 34(3):589–596. https://doi.org/10.1590/s0100-83582016340300020
Sundar KG, Chauhan AS, Kittur S, Babu S (2015) Wetland loss and waterbird use of wetlands in Palwal district, Haryana, India: the role of agriculture, urbanization and conversion to fishponds. Wetlands 35(1):115–125. https://doi.org/10.1007/s13157-014-0600-8
Surratt D, Shinde D, Aumen N (2012) Recent cattail expansion and possible relationships to water management: changes in upper Taylor Slough (Everglades National Park, Florida, USA). Environ Manage 49(3):720–733. https://doi.org/10.1007/s00267-011-9798-x
Tian L, Bi W, Liu X, Sun L, Li J (2019) Effects of waterlogging stress on the physiological response and grain-filling characteristics of spring maize (Zea mays L.) under field conditions. Acta Physiol Plant 41:63. https://doi.org/10.1007/s11738-019-2859-0
Vymazal J, Březinová T (2015) The use of constructed wetlands for removal of pesticides from agricultural runoff and drainage: a review. Environ Int 75:11–20. https://doi.org/10.1016/j.envint.2014.10.026
Wang X, Huang M, Zhou Q, Cai J, Dai T, Cao W, Jiang D (2016) Physiological and proteomic mechanisms of waterlogging priming improves tolerance to waterlogging stress in wheat (Triticum aestivum L.). Environ Exp Bot 132:175–182. https://doi.org/10.1016/j.envexpbot.2016.09.003
Xu Z, Zhou G, Shimizu H (2010) Plant responses to drought and rewatering. Plant Signal Behav 5:649–654. https://doi.org/10.4161/psb.5.6.11398
Zhang J, Zhang X (1994) Can early wilting of old leaves account for much of the ABA accumulation in flooded pea plants? J Exp Bot 45:1335–1342. https://doi.org/10.1093/jxb/45.9.1335
Zhang YP, Wang ZM, Wu YC, Zhang X (2006) Stomatal characteristics of different green organs in wheat under different irrigation regimes. Acta Agron Sin 32:70–75
Acknowledgements
The authors thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico [National Counsel of Technological and Scientific Development]), this study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, and FAPEMIG (Fundação de Amparo à Pesquisa do estado de Minas Gerais [Minas Gerais State Research Foundation]) for funding and research grants awarded to complete the present study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Montanaro.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kloss, R.B., de Castro, E.M., Magalhães, P.C. et al. Anatomical and physiological traits of maize under contrasting water levels and cattail occurrence. Acta Physiol Plant 43, 16 (2021). https://doi.org/10.1007/s11738-020-03192-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03192-z