Abstract
The revegetation of areas degraded by iron ore mining is a difficult challenge mainly due to water availability and impoverished metal-rich substrates. We sought to understand the photosynthetic responses to drought of native tropical grasses Paspalum densum (Poir.) and Setaria parviflora (Poir.) grown in iron ore tailing. The grass P. densum presented better photosynthetic adjustments when grown in the iron ore tailing and S. paviflora in response to water stress. Both species accumulated iron above the phytotoxic threshold when grown in an iron ore tailing. The net photosynthesis, stomatal conductance, transpiration, and water use efficiency decreased followed by a reduction in leaf relative water content in response to water stress for both species. The photochemical efficiency of photosystem II only decreased at the point of maximum drought. At this point, the water-stressed grass grown in the iron ore tailing presented higher H2O2 concentrations, particularly S. parviflora. After rehydration, full recovery of photosynthetic variables was achieved with decreased malondialdehyde concentrations, increased catalase activity, and, consequently, decreased H2O2 concentrations in leaves for both species. The fast recovery of the native grasses P. densum and S. parviflora to drought in the iron ore tailing substrate is indicative of their resistance and potential use in the revegetation of impoverished mined areas with high iron content and seasonal water deficit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining activities are responsible for numerous environmental impacts, such as particulate emission, deforestation, pollution of water resources, and generation of byproducts (Chaturvedi et al. 2014). Mining generates two types of solid byproducts: sterile waste—waste rock and sediments discarded during ore extraction; and tailings —waste resulting from ore treatment/processing after extraction (Bigot et al. 2013). Mining industries produce more than 10 billion tons of waste annually worldwide (Adiansyah et al. 2015; Wang et al. 2017), which also represents risks due to possible accidents associated with their storage and management (Grangeia et al. 2011). An example of this was the environmental and social disasters that occurred in Brazil after the breach in tailing dams in the state of Minas Gerais (do Carmo et al. 2017; Silveira et al. 2019).
Considering the intensification of human activities, the restoration of degraded ecosystems is an urgent task and a difficult challenge (Wang et al. 2017). Revegetation of iron-ore mined areas with native plant species is a fundamental process as it should ensure the increase of biodiversity patterns, ecosystem functioning, and structure close to reference sites (Gastauer et al. 2019). Natural regeneration in areas degraded by iron mining is slow and often impossible (Malcová et al. 2001; Silva et al. 2006) due to the physicochemical characteristics of the substrate, such as high metal concentrations, low organic matter, and nutrient content and low water retention capacity (Liebenberg et al. 2013; López-Orenes et al. 2017; Wang et al. 2017). Vegetation in mined areas is therefore subjected not only to metal stress and nutrient deficiency but also to drought (López-Orenes et al. 2017), which change the water status in the substrate and consequently its metal availability (González-Alcaraz and van Gestel 2017), making it difficult to restore mined areas. The selected species for revegetation purposes must be able to thrive under these multi-stress scenarios often linked to marked fluctuations in environmental factors. However, little is known about the responses of native tropical plants when exposed to the interaction between water stress and iron mining residues.
Given the concomitant stresses in the areas impacted by iron mining, native species belonging to the Poaceae family have been indicated during the process of revegetation (Araújo et al. 2014; Rios et al. 2017; Siqueira-Silva et al. 2019). Grass species present greater tolerance to drought, rapid growth, covering the soil, thus decelerating the erosion process and improving local fertility through adding organic matter and promoting aeration capacity of the substrate, which improves the rooting of other plant species (Guittonny-Larchevêque et al. 2016).
In previous studies, the tropical native grasses P. densum and S. parviflora have proven to be potential candidates for revegetating iron-ore mined areas (Araújo et al. 2014, 2015; Rios et al. 2017). Those species, when exposed to potentially toxic iron concentrations in a nutrient solution, exhibit efficient uptake and accumulation of iron in the leaves above the toxicity threshold, without impairment of growth, foliar bronzing appearance or reduction of the photosynthetic rate, and chlorophyll degradation (Araújo et al. 2020; Rios et al. 2017; Siqueira-Silva et al. 2019).
The present study aimed to evaluate the photosynthetic responses to drought of the native tropical grasses P. densum and S. parviflora grown in iron ore tailing and reference soil. Considering the promising use for revegetating impacted sites with P. densum and S. parviflora, we hypothesize that those species will present efficient stomatal adjustments in photosynthetic responses to water stress and full recovery capacity, coupled with reactive oxygen species (ROS) scavenging mechanisms when grown in the iron ore tailing.
Materials and methods
Plant material and experimental conditions
The experiments were conducted in a greenhouse at the Federal University of Viçosa, Campus Florestal, Brazil (19° 53′ 20.23″ S and 44° 25′ 56.38″ W). The climate is classified as tropical according to the Köppen climate classification, with well-defined summers (rainy) and winters (dry) and a mean annual rainfall of 1500 mm. During the experiments, the mean temperature and relative humidity were 32.8 °C and 58%, respectively.
The native grasses Paspalum densum (Poir.) and Setaria parviflora (Poir.) Kerguélen (Poaceae) were chosen due to their recognized resistance to excess iron in the growth media (Araújo et al. 2014, 2015; Rios et al. 2017; Siqueira-Silva et al. 2019). P. densum is a tall (> 1 m) perennial grass with sharp leaf margins and a high potential for use in the paper industry due to its high cellulose content and yield (Barreto 1966). It is a C4 grass, belonging to the Quadrifaria group, with diversity centered in South America (Williams et al. 2011). S. parviflora is a perennial C4 grass that is native to the New World (Chuine et al. 2012; Darmency and Dekker 2011); it is preferentially allogamous (Pensiero et al. 2005) and has been used as a starch source in the human diet (Austin 2006).
Initially, seeds of both species were collected from plants near an iron ore pelletization facility located in Anchieta, state of Espírito Santo, Brazil (20° 46′ 49.52″ S and 40° 35′ 08.69″ W). These seeds were germinated in the sand, and after germination, the seedlings were transplanted to the soil in field conditions near the greenhouse.
For the experiments, plants of P. densum and S. parviflora were obtained from those matrices, then were pruned and transferred to half-strength Hoagland nutrient solution (Hoagland and Arnon 1950), at pH 5. The plants remained in nutrient solution for 53 (P. densum) and 120 (S. parviflora) days for acclimatization. The plants were then transferred to plastic pots (5 kg) containing two different substrates: a reference soil (non-mining substrate), originating from areas adjacent to an iron mine; and an iron ore tailing (mining substrate) originating from the Mina de Fábrica, belonging to VALE S.A. (Ouro Preto, MG, 20° 25′ 12″ S and 43° 52′ 32″ W). In two independent essays, P. densum and S. parviflora seedlings were grown in these two substrates for 47 days and 23 days, respectively, under constant irrigation. When the seedling presented satisfactory acclimation responses, evaluated by chlorophyll content and chlorophyll a fluorescence measurements every 2 days, the water stress treatments were applied.
A randomized block experimental design was used for all essays, with a factorial combination (2 × 2), with the factors being the two substrates (reference soil and iron ore tailing) and the two water regimes (with and without water stress), with five replicates. The treatments without water stress received daily irrigation during the entire experiment. The treatments with water stress received no irrigation until the point of maximum stress was reached, i.e., when photosynthetic rate (A) was close to zero (day 9 for P. densum in both substrates; days 12 and 19 for S. parviflora grown in the iron ore tailing and reference soil, respectively). After reaching the point of maximum stress, irrigation was resumed until photosynthesis was fully recovered (16 days after the beginning of the drought treatment for P. densum in both substrates; 19 and 26 days for S. parviflora grown in the mining and non-mining substrates, respectively).
Substrate water content and leaf relative water content measurements
The water content of soil and iron ore tailing samples were determined on a gravimetric basis, calculated as the difference between wet weight (W) and dry weight (DW) using the following equation:
For S. parviflora, the evaluations were made in 1, 8, 19, 21, and 26 days after treatments (DAT) and for P. densum in DATs 1, 3, 7, 9, 13, and 16. Leaf relative water content (RWC) was measured at approximately noon on the first fully expanded leaf. Three disks with a 1-cm diameter were removed from each leaf sample, and the fresh weight (FW) was immediately measured. The turgid weight (TW) was determined after rehydration of the leaf disks immersed in distilled water, and kept at 4 °C for 24 h. The dry weight (DW) was determined by placing the leaf discs in an oven at 65 °C to reach a constant weight. The RWC was calculated as the difference between the different leaf weights, using the following equation according to Barrs and Weatherley (1962):
Evaluation of photosynthetic responses of grasses to drought and iron ore tailing exposition
Gas exchange measurements were performed daily between 07:00 and 11:00, on the third fully expanded leaf of each plant, using a LI-6400xt infrared gas analyzer (Li-Cor Inc., Lincoln, NE, USA). Light (1500 μmol m−2 s−1) was provided by the LED light source in the leaf chamber fluorometer (6400-40, Li-Cor Inc.), which had an area of 2 cm2. The measurements were performed using a CO2 control system (6400-01, Li-Cor Inc.), at 400 μmol mol−1 CO2, with a mean leaf temperature and air humidity of 30 °C and 45%, respectively. The following gas exchange variables were evaluated: net photosynthetic rate (A, μmol m−2 s−1), stomatal conductance (gs, mol m−2 s−1), transpiration (E, mmol m−2 s−1), the ratio of internal to external CO2 concentration (Ci/Ca), and the instantaneous water use efficiency (Wt = A/E, μmol CO2 mmol H2O m−2 s−1).
The chlorophyll content index was measured daily using the portable ClorofiLOG meter (CFL1030, Falker, Porto Alegre, Rio Grande do Sul, Brazil). Besides the total chlorophyll content index, the indexes of chlorophyll a, chlorophyll b, and chlorophyll a/b ratio were evaluated. The measurements were performed three times in the middle part of the third fully expanded leaf, and the mean of the three measurements was calculated as a replicate.
Chlorophyll a fluorescence was measured daily on the same leaves used for the gas exchange measurements, using a Mini-PAM fluorometer (Heinz Walz, Effeltrich, Germany). The initial fluorescence (F0) and the maximum quantum yield of photosystem II (PSII) (Fv/Fm) was determined in dark adapted leaves for at least 30 min (Genty et al. 1989). Then, the leaves were exposed to 1000 μmol m−2 s−1 photosynthetic photon flux density (PPFD) for 60 s, followed by a saturating light pulse, to determine the following parameters: effective quantum yield of PSII (ϕII) (Genty et al. 1989), non-photochemical quenching (NPQ) (Bilger and Björkman 1990). The apparent electron transport rate (ETR) was calculated as ETR = 0.5 × lA × ϕΙΙ × PPFD, where 0.5 is the assumed proportion of absorbed quanta used by PSII reaction centers (Melis et al. 1987) and lA is the leaf absorbance.
Determination of oxidative damage, hydrogen peroxide (H2O2) concentration, and antioxidant enzyme activities
The extension of lipid peroxidation was measured after malondialdehyde (MDA) concentrations determined throughout the experiments in fully expanded leaves, according to Hodges et al. (1999). A portion from the same samples was used to determine H2O2 concentration according to Velikova et al. (2000). The activities of superoxide dismutase (SOD; EC 1.15.1.1) (Beauchamp and Fridovich 1971), catalase (CAT; EC 1.11.1.6) (Havir and McHale 1987), and ascorbate peroxidase (APX; EC 1.11.1.11) (Nakano and Asada 1981) were also evaluated. Total protein was determined in crude extracts, according to Bradford (1976), using bovine serum albumin (BSA) as the standard. These analyses were performed in S. parviflora on DATs 1, 8, 12, 14, and 19 when exposed to iron ore tailing and on DATs 1, 8, 19, 21, and 26 when exposed to reference soil. For P. densum, the evaluations were performed on the DATs 1, 3, 7, 9, 13, and 16 for both substrates.
Evaluation of plant nutrient concentration and substrate properties
At the end of the experiments, the plants were separated into roots and shoots, washed and dried in an oven at 75 °C for 72 h. The nitrogen (N), phosphorus (P), and potassium (K) concentrations were determined using the Kjeldahl method, the molybdenum blue colorimetric method, and emission flame photometry, respectively, and quantification of iron (Fe), manganese (Mn), and zinc (Zn) was performed using an atomic absorption spectrometer (Malavolta 1997).
Soil and iron ore tailing chemical compositions were determined at the beginning of the essays using five replicates of composite samples from each treatment.pH in water and KCl (Jackson 1970); P, K, Ca, Mg, and Al concentrations (Braga and Defelipo 1974); potential acidity (de Castro et al. 1972); residual P (Alvarez et al. 2000); N concentration (Kjeldahl 1883); and Fe, Mn, and Zn concentrations (Jackson 1970) were evaluated. A portion from the same samples used for chemical traits was used for substrate physical characterization. Soil texture/grain size was determined according to de Almeida et al. (2012).
Statistical analysis
The data were subjected to a two-way repeated measures analysis of variance (ANOVA), and means were compared using the Tukey test (p < 0.05) with the SAEG 9.2 software (Fundação Arthur Bernardes, UFV, Viçosa, Brazil). All data were evaluated for homogeneity of variance and normality prior to the ANOVA (Cochran’s Q and Lilliefors tests, respectively).
Results
Photosynthetic responses of grass species to changes in substrate and leaf water content
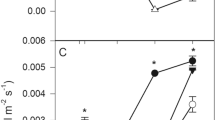
The depletion of substrate water content (SWC) after the suppression of irrigation was more pronounced for the iron ore tailing, which presented significantly (p < 0.05) lower water content than the reference soil (Fig. 1). After the resuming of irrigation, it was observed a rapid recovering of substrate moisture. Despite the differences in SWC, no significant differences (p > 0.05) were found for leaf relative water content (RWC) in both S. parviflora and P. densum grown in well-watered distinct substrates (Fig. 1). The RWC for both grass species decreased until the point of maximum water stress was reached. For S. parviflora grown in reference soil, the reduction of RWC started after 8 days of water deprivation. The RWC from both species recovered with rehydration to values found before the beginning of the water deficit treatments (Fig. 1).
Substrate water content (SWC) (%) (a and b) and leaf relative water content (RWC) (%) (c and d) for S. parviflora (a and c) and P. densum (b and d) grown in the iron ore tailing (IT) or the reference soil (RS), well-watered (WW), or under water stress (WS). Dashed lines indicate the moment when irrigation was resumed. For S. parviflora, the first line represents the treatments with the iron ore tailing, and the second line represents the treatments with the reference soil. Asterisks indicate a significant difference between treatments by the Tukey test (p < 0.05). Values are the means ± standard error (n = 5)
The point of maximum water stress (i.e., when net photosynthesis approached 0 μmol m−2 s−1) occurred at 12 and 19 days after the beginning of the water deficit treatment for S. parviflora grown in the iron ore tailing and reference soil, respectively, and 9 days after the beginning of the water deficit treatment for P. densum grown in both substrates (Fig. 2). In general, only S. parviflora presented significantly (p < 0.05) lower photosynthetic rate and stomatal conductance when grown in the iron ore tailing than in the reference soil. Significant (p < 0.05) decrease at 8 days after the suspension of irrigation occurred in photosynthesis, stomatal conductance, and transpiration of S. parviflora, with lower values observed for water-stressed plants grown on iron ore tailing (Fig. 2 and Fig. S1). On the other hand, P. densum presented reduction (p < 0.05) in those gas exchange variables 5 days after the suspension of irrigation (Fig. 2 and Fig. S1) without significant differences between both substrates. The water use efficiency decreased in the days preceding irrigation for both species growing in the reference soil and the iron ore tailing, with recovering after the irrigation (Fig. 2). The ratio of internal to external CO2 (Ci/Ca) increased under water deficit for both grass, regardless of the substrate (Fig. S1). Overall, after 3 to 4 days of rehydration, these photosynthetic variables recovered in water-stressed plants to equal or higher values than the well-watered ones.
Photosynthesis (A) (a and b), stomatal conductance (gs) (c and d) and the instantaneous water use efficiency (Wt) (e and f) for S. parviflora (a, c, and e) and P. densum (b, d, and f) grown in the iron ore tailing (IT) or the reference soil (RS), well-watered (WW), or under water stress (WS). Dashed lines indicate the moment when irrigation was resumed. For S. parviflora, the first line represents the treatments with the iron ore tailing, and the second line represents the treatments with the reference soil. Asterisks indicate a significant difference between treatments by the Tukey test (p < 0.05). Values are the means ± standard error (n = 5)
Both grass species presented a higher chlorophyll content index when grown in reference soil (Fig. 3). They had decreased total chlorophyll content when subjected to water stress, regardless of the substrate (Fig. 3). These same responses were observed for the content index of chlorophyll a and chlorophyll b (Fig. S2). However, the recovering in chlorophyll synthesis after the onset of water stress was slowly in P. densum, despite presenting higher chlorophyll content than S. parviflora. The chlorophyll a/b ratio increased gradually with the onset of water stress in both grass species (Fig. S2).
Chlorophyll content index (a and b), initial fluorescence (F0) (c and d) and maximum quantum yield of PSII (Fv/Fm) (e and f) for S. parviflora (a, c, and e) and P. densum (b, d, and f) grown in the iron ore tailing (IT) or the reference soil (RS), well-watered (WW), or under water stress (WS). Dashed lines indicate the moment when irrigation was resumed. For S. parviflora, the first line represents the treatments with the iron ore tailing, and the second line represents the treatments with the reference soil. Asterisks indicate a significant difference between treatments by the Tukey test (p < 0.05). Values are the means ± standard error (n = 5)
Lower F0 values (p < 0.05) were observed in both grass species grown in the iron ore tailing substrate, regardless of the water deficit treatment (Fig. 3). However, only S. parviflora presented a significant (p < 0.05) increase in F0 under water stress when grown in the reference substrate (Fig. 3). In reference soil as well as in iron ore tailing, significant (p < 0.05) reduction in Fv/Fm (Fig. 3), ϕII, and ETR (Fig. S3) values was observed in both grasses following the suspension of irrigation. For S. parviflora, full recovery in the Fv/Fm values occurred 1 day after the resuming of irrigation, while in P. densum, it took 2 days (Fig. 3). The reductions in ϕII and ETR for S. parviflora occurred after 8 and 12 days from the beginning of water withholding in plants grown in the iron ore tailing and reference soil, respectively (Fig. S3). In P. densum, ϕII and ETR were decreased after 7 days of exposure to water stress in both substrates (Fig. S3). After rehydration, fast recovery of ϕII and ETR in water-stressed plants reached similar levels than plants without water stress in both grasses (Fig. S3).
Both grass species in reference soil or iron ore tailing exhibited significant (p < 0.05) increase in NPQ under water deficit (Fig. S3). However, in S. parviflora grown in the reference soil, a consistent increase in NPQ dissipation occurred after the resumption of irrigation at 19 days of water stress (Fig. S3).
H2O2 concentration, lipid peroxidation, and antioxidant enzyme activity upon water stress in mining substrate
Only water stress promoted a significant increase (p < 0.05) in H2O2 and MDA concentration for both grasses (Fig. 4). After irrigation resumption, the values of H2O2 reached similar levels to the respective treatments without water stress. The same was observed for the MDA concentration in S. parviflora in both substrates, but for P. densum grown in the iron ore tailing, MDA values began to decrease before plant rehydration (Fig. 4).
Leaf hydrogen peroxide (H2O2) (a and b) and malondialdehyde (MDA) (c and d) concentrations for S. parviflora (a and c) and P. densum (b and d) grown in the iron ore tailing (IT) or the reference soil (RS), well-watered (WW), or under water stress (WS). Dashed lines indicate the moment when irrigation was resumed. For S. parviflora, the first line represents the treatments with the iron ore tailing, and the second line represents the treatments with the reference soil. Asterisks indicate a significant difference between treatments by the Tukey test (p < 0.05). Values are the means ± standard error (n = 5)
Antioxidant enzyme activities in both S. parviflora and P. densum showed different responses between substrates, irrespective of water stress treatments (Fig. 5). Overall, APX activity was lower in S. parviflora plants grown in the iron ore tailing, regardless of the water deficit treatment. However, those water-stressed S. parviflora plants from iron ore tailing presented significantly (p < 0.05) lower APX activity during the onset of water stress and increased APX activity after the resumption of irrigation in comparison with well-watered plants (Fig. 5). For P. densum, although variations in APX activity were also observed through time, no major significant changes occurred, except for the higher APX activity in plants under water stress grown in the reference soil 3 days after the irrigation withheld (Fig. 5). CAT activity significantly (p < 0.05) increased for both water-stressed grass species after the resumption of irrigation, but only in plants grown in the reference soil (Fig. 5). No changes in SOD activity were observed for both S. parviflora and P. densum due to water stress (Fig. 5) or for P. densum in response to the different substrates (Fig. 5). Punctual higher SOD activity was observed in S. parviflora plants growing in reference soil after 19 days in comparison with plants grown in the iron ore tailing, regardless of the water stress (Fig. 5).
Leaf ascorbate peroxidase (APX) (a and b), catalase (CAT) (c and d) and superoxide dismutase (SOD) (e and f) activity for S. parviflora (a, c, and e) and P. densum (b, d, and f) grown in the iron ore tailing (IT) or the reference soil (RS), well-watered (WW), or under water stress (WS). Dashed lines indicate the moment when irrigation was resumed. For S. parviflora, the first line represents the treatments with the iron ore tailing, and the second line represents the treatments with the reference soil. Asterisks indicate a significant difference between treatments by the Tukey test (p < 0.05). Values are the means ± standard error (n = 5)
Substrate physicochemical properties and grasses’ nutrient concentrations after water stress
Significant (p < 0.05) lower concentrations of N, Cu, Pb, and Al, and higher concentrations of P, Ca, Mn, S, Ni, and As were found in the iron ore tailing substrate in comparison with the reference soil (Table 1). No significant differences were observed between substrates for K, Mg, Fe, Zn, Cd, Cr, or residual P (res-P) values (Table 1). Cation exchange capacity at pH 7, aluminum saturation index, and organic matter were lower (p < 0.05) in the iron ore tailing, while the base saturation index and effective cation exchange capacity and substrate pH were higher (p < 0.05) when compared to the reference soil (Table 1). Regarding grain size, the iron ore tailing presented significantly higher (p < 0.05) coarse sand, fine sand, and silt concentrations and was classified as loam, whereas the reference soil presented a higher (p < 0.05) clay concentration and was classified as clayey (Table 1).
Root Mn concentration was 4 times higher in S. parviflora plants grown in the iron ore tailing when compared with those grown in the reference soil (Fig. 6). In P. densum, Mn concentration was significantly (p < 0.05) higher in roots and lower in shoots of plants grown on the iron ore tailing (Fig. 6). Water stress did not affect significantly (p > 0.05) shoot and root Mn concentrations for the two grasses, regardless of the substrate (Fig. 6).
Concentration of micronutrients Mn (a and b), Fe (c and d), and Zn (e and f) in leaves and roots of S. parviflora (a, c, and e) and P. densum (b, d, and f) grown in the iron ore tailing (IT) or the reference soil (RS), well-watered (WW), or under water stress (WS). Values are the means ± standard error (n = 5). Means followed by the same letter were not significantly different according to the Tukey test (p ≤ 0.05). Uppercase letters compare the substrates for the same plant organ, and lowercase letters compare the treatments with or without WS
Both grasses accumulate higher Fe concentrations in roots than shoots (Fig. 6). Roots and shoots of S. parviflora grown in the iron ore tailing presented significantly (p < 0.05) higher Fe concentration than plants grown in the reference soil. The same was observed in the shoots of P. densum grown in the iron ore tailing, regardless of the water stress treatment. However, water stress resulted in decreased (p < 0.05) root Fe concentration in P. densum grown in the reference soil (Fig. 6). Water stress significantly (p < 0.05) decreased root Zn concentration in S. parviflora, regardless of the substrate (Fig. 6). On the other hand, the increase in shoot Zn concentration under water stress was observed for P. densum (Fig. 6). The Cu concentration was lower in both species when exposed to iron ore tailing (Table S1).
Nitrogen concentration was lower (p < 0.05) in the shoots of S. parviflora and P. densum grown in the iron ore tailing, regardless of the water deficit treatment (Fig. S4). In both substrates, water-stressed P. densum plants showed significant (p < 0.05) higher N concentration in shoots than non-stressed ones (Fig. S4). Water stress decreased (p < 0.05) shoot P concentration in S. parviflora grown in the reference soil (Fig. S4). In addition, P. densum grown in iron ore tailing significantly (p < 0.05) increased shoot and root P concentrations (Fig. S4). S. parviflora presented lower root K concentration under water stress, regardless of the substrate (Fig. S4). On the contrary, the suspension of irrigation resulted in increased (p < 0.05) shoot K concentration in P. densum (Fig. S4). Calcium concentrations were significantly (p < 0.05) higher in leaves and roots of both species when grown in iron ore tailing (Table S1). The S concentration in the roots of P. densum was higher when exposed to iron ore tailing. In contrast, S. parviflora grown in reference soil under well-watered conditions showed a higher leaf S concentration when compared to plants from the iron ore tailing. The concentration of Mg was higher in roots of P. densum plants grown in reference soil as well as in leaves of plants under water stress. The roots of S. parviflora exposed to iron ore tailing presented higher concentrations of Mg when compared to plants grown in the reference soil (Table S1).
Discussion
The photosynthetic adjustments and the antioxidant metabolism of P. densum and S. parviflora under drought growing in the iron ore tailing, and their capacity for physiological recovery after rehydration, confirm the hypothesis that those grasses present satisfactory resistance to these abiotic stresses and are potential candidates for the revegetation of mining-impacted environments. Both grass species when grown in the tailing were able to cope with shoot Fe concentrations above values considered phytotoxic (> 500 mg Fe kg−1 leaf DW) for most plants (Broadley et al. 2012; Pugh et al. 2002). The absence of visual symptoms of Fe toxicity, despite the potentially phytotoxic Fe levels in plant tissues, indicates tolerance strategies of the studied native grasses (Araújo et al. 2014, 2015, 2020; Rios et al. 2017). However, higher Fe concentrations in the roots than in shoots, as observed for P. densum and S. parviflora in the present study, can be considered an additional avoidance strategy (Araújo et al. 2014; Rios et al. 2017; Siqueira-Silva et al. 2019).
The decrease in water content in the soil due to drought affects the uptake of micronutrients by roots, as observed for Fe concentration in P. densum grown under water stress in the reference soil, since the supply of micronutrients by plants occurs mainly from the absorption of water via mass flow driven by transpiration rate (Silva et al. 2018). The lower water drainage of the clayey reference soil leads to lower mass flow and lower Fe absorption by plants. The higher organic matter of the reference soil also immobilizes the nutrients in the form of organic compounds and reduces their availability to the plants due to the formation of chelates even at pH values ideal for absorption (Benites et al. 2007). Compacted soils with a high concentration of metals present less mobility of nutrients which limits its absorption by plants (Silva et al. 2018). Despite the high Mn concentrations of iron ore tailing (approximately 13 times higher than for the reference soil), the mean shoot Mn concentration of grasses was above the adequate for satisfactory plant growth, 50 mg kg−1 DW (Kirkby 2012); a higher uptake of Mn and Fe was not reached due to the slightly basic pH of mining substrate, which may have resulted in lower nutrient bioavailability.
Both grass species in all treatments presented P concentrations below levels considered satisfactory for most plants (Broadley et al. 2012). P deficiency compromises photosynthesis since it decreases ATP and NADPH consumption and production, interferes in the carboxylation/regeneration of ribulose-1,5-bisphosphate (Prodhan et al. 2019), and downregulates the expression of genes related to photosynthesis (Flügge et al. 2003). However, P. densum and S. parviflora may have not been P-deficient since no visual symptoms were observed and the photosynthetic rate in the plants that were irrigated daily was similar to plants grown in nutrient solution as reported by Rios et al. (2017).
Leaf Zn concentrations for both grass species were below the critical toxicity threshold (300 mg Zn kg−1 DW) (Broadley et al. 2012). This may have been due to the low Zn values (~ 3–5 mg dm−3) in both substrates. However, leaf Zn concentrations were higher for P. densum than for S. parviflora. Zn plays a role in the detoxification of superoxide radicals since CuZn superoxide dismutase (CuZnSOD) is the most abundant SOD in plant cells (Abreu and Cabelli 2010). This explains the observed higher SOD activity for P. densum than for S. parviflora.
Except for S. parviflora grown in the reference soil, the shoot N concentrations in both grasses were below the nutrient requirements reported for plant satisfactory growth (15 g kg−1 DW) (Kirkby 2012). Nitrogen is an essential element for plants; however, in the iron ore tailing, N availability is a limiting factor, which hampers plant growth. Therefore, to obtain greater success in the process of revegetation, it is necessary to reduce N losses in the soil, as well as to improve the uptake and metabolization of N within the plant (Cione et al. 2002; Cross et al. 2019). P. densum stood out responding positively with greater absorption of N under water stress. During the rehydration period after drought, the increase in transpiratory flow may have led to an increase in N absorption trough nitrate reductase activity (Ferrario-Méry et al. 1998), which resulted in the higher N concentration at the end of the experiment.
Plants under water stress have higher K demands (Zahoor et al. 2017), which was observed in the present study for P. densum. A high leaf K content allows plants to maintain high photosynthetic capacity, minimizing the drought effects. Leaf K concentrations for both grasses were within the nutrient requirements for plant satisfactory growth (20–50 g kg−1) (Hawkesford et al. 2012). On the other hand, the decrease of soil water content directly affected the uptake of K by the roots of S. parviflora, since potassium moves on the ground via diffusive flux (Silva et al. 2018).
Overall, water stress results in decreased stomatal conductance, photosynthesis, and transpiration (Najafabadi and Ehsanzadeh 2017; Vandegeer et al. 2020). Although water stress caused limitations to leaf gas exchange in P. densum and S. parviflora, the recovering of photosynthetic traits after plant rehydration highlights physiological adjustments that avoided severe damage. The capacity for photosynthetic rate recovery with the resumption of irrigation following a period of drought depends on plant efficiency and will determine plant resistance to drought (Chaves et al. 2009). The lower water retention capacity of the iron ore tailing, due to its sandy texture, may have contributed to S. parviflora reaching the point of maximum water stress earlier in this soil than in the reference soil. More clayey soils, such as the reference soil, have a higher water retention capacity and are better able to able to aid plant recovery from stress than sand soil (de Andrade Bonetti et al. 2017). The decrease in stomatal conductance and transpiration observed for S. parviflora grown in the iron ore tailing explains why the point of maximum stress was reached earlier for plants grown in this substrate than for those in the reference soil.
The results of gas exchange indicate a better performance of P. densum in the iron ore tailing, but S. parviflora had a higher resistance to water stress when grown in this substrate. The C4 photosynthetic metabolism of P. densum and S. parviflora (Beloni et al. 2017; Chuine et al. 2012) is directly related to the fast recovery from water stress. The C4 photosynthetic metabolism involves a series of biochemical and anatomical changes in which the enzyme phosphoenolpyruvate carboxylase (PEPCase) concentrates CO2 around ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) (Furbank 2016). C4 photosynthesis enables plants to dominate warm, dry, and often salinized habitats, and to colonize areas that are too stressful for most plant groups, allowing carbon fixation even when the stomata are partially closed due to water stress (Sage and Stata 2015).
As observed for both tested species in the present study, water stress may cause decreases in chloroplastidic pigment contents (Frosi et al. 2017; Najafabadi and Ehsanzadeh 2017). This decrease in the chlorophyll content indexes was intensified by the low concentration of nitrogen in the substrates, outstanding in the iron ore tailing, which resulted in the significant differences in comparison with the plants exposed to the reference soil. In addition, even Fe being considered an essential mineral element for the synthesis of chlorophyll (Broadley et al. 2012; Jeong and Guerinot 2009), high concentrations of Fe as observed in the shoot of grasses exposed to the tailing, may cause degradation of chloroplastic pigments due to oxidative stress (Pereira et al. 2013; Silva et al. 2015). The decreased chlorophyll content indexes observed for plants subjected to water deficit and grown in the iron ore tailing resulted in changes to chlorophyll a fluorescence variables.
The increase in initial fluorescence (F0) observed for S. parviflora subjected to water stress and grown in the reference soil may have been caused by the decreased capacity of energy transfer from the light-harvesting complex II (LHCII) to the reaction center. The decrease in Fv/Fm observed for both grasses under water stress indicates that the light energy use efficiency was reduced. This is an important variable to evaluate the integrity of the photosynthetic mechanism and subsequent detection of plants tolerant to water stress. However, for P. densum and S. parviflora, there was no permanent damage to the photosynthetic complex since the fast recovery of the photosynthetic complex and other physiological variables after rehydration were observed. Despite some studies with other grass species showed a reduction in Fv/Fm with the onset of water stress (Jagtap et al. 1998; Pour-Aboughadareh et al. 2017), the photochemical efficiency commonly presents higher resistance to drought effects on grasses (Carmo-Silva et al. 2007; Ghannoum et al. 2003; Sánchez et al. 2018).
The decrease in the effective quantum yield of PSII (ϕII) during water stress was accompanied by an increase in non-photochemical quenching (NPQ). This photoprotective mechanism is mediated by the xanthophyll-regulated non-photochemical energy dissipation (Demmig-Adams and Adams 2006) and contributed to the fast recovery of the photosynthetic rate and ϕII of S. parviflora and P. densum after rehydration. Mainly under drought, the excess energy is dissipated as heat, acting as an important protection mechanism of the photosynthetic complex of both grasses against overexcitation and subsequent oxidative damage of the PSII reaction center (Baker 2008; Pereira et al. 2013).
High Fe concentrations in the substrate and, consequently, higher uptake and accumulation in plant tissues, might result in ROS overproduction by the Fenton reaction. ROS-induced by excess Fe may cause changes in cellular redox balance resulting in a diversity of morphological, biochemical, and physiological symptoms (Hell and Stephan 2003). MDA has been considered an important indicator of oxidative stress in plants (Oliveira Jucoski et al. 2013; Pinto et al. 2016) and damage to the cell membrane (Boda et al. 2017). The reduction in MDA concentrations observed for P. densum and S. parviflora after resuming irrigation indicates a mechanism of ROS control. This was confirmed by the increase in CAT activity observed on the day following the resuming of irrigation for S. parviflora grown in the reference soil and the consequent decrease in H2O2 concentration due to the CAT reduction of H2O2 into H2O and O2 (Yanik and Donaldson 2005).
Excessive Fe uptake and accumulation may increase the formation of ROS (Neves et al. 2009; Rout et al. 2015), as observed for P. densum and S. parviflora. Water stress also increases the probability of transferring excitation energy to O2, producing O2− and 1O2 and leading to lipid peroxidation (Smirnoff 1993). However, both species presented efficient enzymatic antioxidant mechanisms capable of preventing oxidative damage. The activities of antioxidant enzymes such as SOD, CAT, and APX are key mechanisms for ROS detoxification in cells, and they confer stress tolerance (Gill and Tuteja 2010). In addition, antioxidant enzymes, the phenolic compounds (Michalak 2006), reduced glutathione, tocopherols and tocotrienols, ascorbic acid, and carotenoids (Ashraf et al. 2019; Muñoz and Munné-Bosch 2019), which act as antioxidant molecules and neutralize ROS, might also have contributed to ROS control in the native grass species and will be considered in future studies.
Conclusions
Both grasses presented photosynthetic and antioxidant adjustments, that prove their capacity to be grown in the iron ore tailing, and accumulated iron above the phytotoxic threshold. P. densum presented better responses when grown in the iron ore tailing, whereas S. parviflora was more resistant to water stress. Although water stress resulted in decreased net photosynthetic rates and chlorophyll content indexes, those drought effects could be reversed quickly after plant rehydration. Both species possess efficient enzymatic mechanisms of ROS elimination to avoid severe damage to plants, as indicated by the H2O2 and MDA concentrations.
The fast recovery of the native grasses P. densum and S. parviflora confirms their resistance to the water stress and their potential use for the revegetation of mined soils with high iron contents, seasonal constraints, and nutrient-impoverished substrates.
References
Abreu IA, Cabelli DE (2010) Superoxide dismutases—a review of the metal-associated mechanistic variations. Biochim Biophys Acta, Proteins Proteomics 1804:263–274
Adiansyah JS, Rosano M, Vink S, Keir G (2015) A framework for a sustainable approach to mine tailings management: disposal strategies. J Clean Prod 108:1050–1062. https://doi.org/10.1016/j.jclepro.2015.07.139
Alvarez V, Novais R, Dias L, Oliveira J (2000) Determinação e uso do fósforo remanescente: boletim informativo Revista Brasileira de Ciência do Solo. Viçosa 25:121–127
Araújo TO, de Freitas-Silva L, Santana BVN, Kuki KN, Pereira EG, Azevedo AA, da Silva LC (2014) Tolerance to iron accumulation and its effects on mineral composition and growth of two grass species. Environ Sci Pollut Res 21:2777–2784. https://doi.org/10.1007/s11356-013-2201-0
Araújo TO, Freitas-Silva L, Santana BVN, Kuki KN, Pereira EG, Azevedo AA, Silva LC (2015) Morphoanatomical responses induced by excess iron in roots of two tolerant grass species. Environ Sci Pollut Res 22:2187–2195
Araújo TO, de Freitas-Silva L, Silva FMO, Ribeiro C, Kuki KNK, Pereira EG, Nunes-Nesi A, da Silva LC (2020) Understanding photosynthetic and metabolic adjustments in iron hyperaccumulator grass. Theor Exp Plant Physiol 32:147–162. https://doi.org/10.1007/s40626-020-00176-9
Ashraf MA, Riaz M, Arif MS, Rasheed R, Iqbal M, Hussain I, Salman M (2019) The role of non-enzymatic antioxidants in improving abiotic stress tolerance in plants. Plant Tolerance to Environmental Stress: Role of Phytoprotectants, CRC Press, pp 129–144
Austin DF (2006) Fox-tail millets (Setaria: Poaceae) - abandoned food in two hemispheres. Econ Bot 60:143–158. https://doi.org/10.1663/0013-0001(2006)60[143:fmspfi]2.0.co;2
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Barreto IL (1966) Las especies afines a Paspalum quadrifarium (Gramineae) en la América del Sur de clima sub-tropical y templado. Darwiniana 14:130–155
Barrs H, Weatherley P (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428. https://doi.org/10.1071/BI9620413
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beloni T, Pezzopane CG, Rovadoscki G, Favero A, Dias-Filho M, Santos P (2017) Morphological and physiological responses and the recovery ability of Paspalum accessions to water deficit and waterlogging. Grass Forage Sci 72:840–850
Benites VM, Schaefer CEG, Simas FN, Santos HG (2007) Soils associated with rock outcrops in the Brazilian mountain ranges Mantiqueira and Espinhaço. Braz J Bot 30:569–577
Bigot M et al (2013) Metal-binding hydrogel particles alleviate soil toxicity and facilitate healthy plant establishment of the native metallophyte grass Astrebla lappacea in mine waste rock and tailings. J Hazard Mater 248:424–434. https://doi.org/10.1016/j.jhazmat.2013.01.025
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Boda RK, Majeti NVP, Suthari S (2017) Ricinus communis L. (castor bean) as a potential candidate for revegetating industrial waste contaminated sites in peri-urban Greater Hyderabad: remarks on seed oil. Environ Sci Pollut Res 24:19955–19964. https://doi.org/10.1007/s11356-017-9654-5
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Braga J, Defelipo B (1974) Determinacao espectrofotometrica de fosforo em extratos de solo e material vegetal. Revista Ceres 21:73–85
Broadley M, Brown P, Çakmak İ, Rengel Z, Zhao F (2012) Function of nutrients: micronutrients. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, San Diego, pp 191–248. https://doi.org/10.1016/B978-0-12-384905-2.00007-8
Carmo-Silva AE, Soares AS, da Silva JM, da Silva AB, Keys AJ, Arrabaça MC (2007) Photosynthetic responses of three C4 grasses of different metabolic subtypes to water deficit. Funct Plant Biol 34:204–213
Chaturvedi N, Ahmed MJ, Dhal NK (2014) Effects of iron ore tailings on growth and physiological activities of Tagetes patula L. J Soils Sediments 14:721–730
Chaves M, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Chuine I et al (2012) Climate change might increase the invasion potential of the alien C4 grass Setaria parviflora (Poaceae) in the Mediterranean Basin. Divers Distrib 18:661–672. https://doi.org/10.1111/j.1472-4642.2011.00880.x
Cione NK, Padgett PE, Allen EB (2002) Restoration of a native shrubland impacted by exotic grasses, frequent fire, and nitrogen deposition in southern California. Restor Ecol 10:376–384
Cross AT, Ivanov D, Stevens JC, Sadler R, Zhong H, Lambers H, Dixon KW (2019) Nitrogen limitation and calcifuge plant strategies constrain the establishment of native vegetation on magnetite mine tailings. Plant Soil https://doi.org/10.1007/s11104-019-04021-0
Darmency H, Dekker J (2011) Setaria. In: Kole C (ed) Wild crop relatives: genomic and breeding resources: millets and grasses. Springer, Berlin Heidelberg, pp 275–296. https://doi.org/10.1007/978-3-642-14255-0_15
de Almeida BG, Donagemma GK, Ruiz HA, Braida J A, Viana JHM, Reichert JMM, Oliveira LB, Ceddia MB, Wadt PS, Fernandes RBA, Passos RR, Dechen SCF, Klein VA, Teixeira WG (2012) Padronização de métodos para análise granulométrica no Brasil. Embrapa Solos-Comunicado Técnico (INFOTECA-E)
de Andrade Bonetti J, Anghinoni I, de Moraes MT, Fink JR (2017) Resilience of soils with different texture, mineralogy and organic matter under long-term conservation systems. Soil Tillage Res 174:104–112
de Castro AF, de Oliveira Barreto W, Anastácio MLA (1972) Correlação entre pH e saturação de bases de alguns solos brasileiros. Pesq Agrop Brasileira 7:9–17
Demmig-Adams B, Adams WW (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol 172:11–21
do Carmo FF, Kamino LHY, Junior RT, de Campos IC, do Carmo FF, Silvino G, de Castro KJSX, Mauro ML, Rodrigues NUA, Miranda MPS, Pinto CEF (2017). Fundão tailings dam failures: the environment tragedy of the largest technological disaster of Brazilian mining in global context. Perspect Ecol Conserv 15(3):145–151
Ferrario-Méry S, Valadier M-H, Foyer CH (1998) Overexpression of nitrate reductase in tobacco delays drought-induced decreases in nitrate reductase activity and mRNA. Plant Physiol 117:293–302
Flügge UI, Häusler RE, Ludewig F, Fischer K (2003) Functional genomics of phosphate antiport systems of plastids. Physiol Plant 118:475–482
Frosi G, Harand W, Oliveira MT, Pereira S, Cabral SP, Montenegro AAA, Santos MG (2017) Different physiological responses under drought stress result in different recovery abilities of two tropical woody evergreen species. Acta Bot Bras 31:153–160
Furbank RT (2016) Walking the C4 pathway: past, present, and future. J Exp Bot 67:4057–4066
Gastauer M, Souza Filho PWM, Ramos SJ, Caldeira CF, Silva JR, Siqueira JO, Neto AEF (2019) Mine land rehabilitation in Brazil: goals and techniques in the context of legal requirements. Ambio 48:74–88
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Ghannoum O, Conroy JP, Driscoll SP, Paul MJ, Foyer CH, Lawlor DW (2003) Nonstomatal limitations are responsible for drought-induced photosynthetic inhibition in four C4 grasses. New Phytol 159:599–608
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
González-Alcaraz MN, van Gestel CAM (2017) Changes in soluble metal concentrations induced by variable water table levels as response to liming and Phragmites australis growth in metal-polluted wetland soils: management effectiveness. Geoderma 289:20–28. https://doi.org/10.1016/j.geoderma.2016.11.019
Grangeia C, Ávila P, Matias M, da Silva EF (2011) Mine tailings integrated investigations: the case of Rio tailings (Panasqueira Mine, Central Portugal). Eng Geol 123:359–372. https://doi.org/10.1016/j.enggeo.2011.10.001
Guittonny-Larchevêque M, Meddeb Y, Barrette D (2016) Can graminoids used for mine tailings revegetation improve substrate structure? Botany 94:1053–1061
Havir EA, McHale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450–455
Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerring J, Møller IS, White P (2012) Functions of macronutrients. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, San Diego, pp 135–189. https://doi.org/10.1016/b978-0-12-384905-2.00006-6
Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216:541–551
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. California Agricultural Experiment Station, Berkeley
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Jackson ML (1970) Analisis quimico de suelos. Omega, Barcelona, 662p
Jagtap V, Bhargava S, Streb P, Feierabend J (1998) Comparative effect of water, heat and light stresses on photosynthetic reactions in Sorghum bicolor (L.) Moench. J Exp Bot 49:1715–1721
Jeong J, Guerinot ML (2009) Homing in on iron homeostasis in plants. Trends Plant Sci 14:280–285
Kirkby E (2012) Introduction, definition and classification of nutrients. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, San Diego, pp 3–5
Kjeldahl J (1883) Neue methode zur bestimmung des stickstoffs in organischen körpern. Fresenius J Anal Chem 22:366–382
Liebenberg D, Claassens S, Van Rensburg L (2013) Insights and lessons learned from the long-term rehabilitation of an iron ore mine. Int J Environ Res 7:633–644
López-Orenes A, Bueso MC, Conesa HM, Calderón AA, Ferrer MA (2017) Seasonal changes in antioxidative/oxidative profile of mining and non-mining populations of Syrian beancaper as determined by soil conditions. Sci Total Environ 575:437–447. https://doi.org/10.1016/j.scitotenv.2016.10.030
Malavolta E (1997) Avaliação do estado nutricional das plantas: princípios e aplicações/Eurípedes Malavolta, Godofredo Cesar Vitti, Sebastião Alberto de Oliveira.—2. ed., ver. e atual Piracicaba: Potafos
Malcová R, Albrechtová J, Vosátka M (2001) The role of the extraradical mycelium network of arbuscular mycorrhizal fungi on the establishment and growth of Calamagrostis epigejos in industrial waste substrates. Appl Soil Ecol 18:129–142
Melis A, Spangfort M, Andersson B (1987) Light-absorption and electron-transport balance between photosystem II and photosystem I in spinach chloroplasts. Photochem Photobiol 45:129–136
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15:523–530
Muñoz P, Munné-Bosch S (2019) Vitamin E in plants: biosynthesis, transport, and function. Trends Plant Sci 24(11):1040–1051
Najafabadi MY, Ehsanzadeh P (2017) Photosynthetic and antioxidative upregulation in drought-stressed sesame (Sesamum indicum L.) subjected to foliar-applied salicylic acid. Photosynthetica 55:611–622. https://doi.org/10.1007/s11099-017-0673-8
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Neves NR, Oliva MA, da Cruz Centeno D, Costa AC, Ribas RF, Pereira EG (2009) Photosynthesis and oxidative stress in the restinga plant species Eugenia uniflora L. exposed to simulated acid rain and iron ore dust deposition: potential use in environmental risk assessment. Sci Total Environ 407:3740–3745
Oliveira Jucoski G, Cambraia J, Ribeiro C, de Oliveira JA, de Paula SO, Oliva MA (2013) Impact of iron toxicity on oxidative metabolism in young Eugenia uniflora L. plants. Acta Physiol Plant 35:1645–1657
Pensiero JF, Gutiérrez HF, Exner E (2005) Pollination system and its effect on seed production and weight in nine South American species of Setaria. Interciencia 30:495–500+447
Pereira EG, Oliva MA, Rosado-Souza L, Mendes GC, Colares DS, Stopato CH, Almeida AM (2013) Iron excess affects rice photosynthesis through stomatal and non-stomatal limitations. Plant Sci 201:81–92
Pinto SS, Souza AE, Oliva MA, Pereira EG (2016) Oxidative damage and photosynthetic impairment in tropical rice cultivars upon exposure to excess iron. Sci Agric 73:217–226
Pour-Aboughadareh A, Ahmadi J, Mehrabi AA, Etminan A, Moghaddam M, Siddique KHM (2017) Physiological responses to drought stress in wild relatives of wheat: implications for wheat improvement. Acta Physiol Plant 39:106. https://doi.org/10.1007/s11738-017-2403-z
Prodhan MA, Finnegan PM, Lambers H (2019) How does evolution in phosphorus-impoverished landscapes impact plant nitrogen and sulfur assimilation? Trends Plant Sci 24:69–82
Pugh RE, Dick DG, Fredeen AL (2002) Heavy metal (Pb, Zn, Cd, Fe, and Cu) contents of plant foliage near the Anvil Range lead/zinc mine, Faro, Yukon Territory. Ecotoxicol Environ Saf 52:273–279
Rios CO, Souza BC, Siqueira-Silva AI, Pereira EG (2017) Assessment of iron toxicity in tropical grasses with potential for revegetation of mined areas. Pol J Environ Stud 26:1643–1649
Rout JR, Behera S, Keshari N, Ram SS, Bhar S, Chakraborty A, Sudarshan M, Sahoo SL (2015) Effect of iron stress on Withania somnifera L.: antioxidant enzyme response and nutrient elemental uptake of in vitro grown plants. Ecotoxicology 24:401–413
Sage RF, Stata M (2015) Photosynthetic diversity meets biodiversity: the C4 plant example. J Plant Physiol 172:104–119
Sánchez E, Lino G, Arias C, Serrat X, Nogués S (2018) Photosynthesis, resource acquisition and growth responses of two biomass crops subjected to water stress. J Plant Sci 6:68
Silva GP, Fontes MPF, da Costa LM, Venegas VHA (2006) Potencialidade de plantas para revegetação de estéreis e rejeito da mineração de ferro da Mina de Alegria, Mariana-MG. Pesqui Agropecu Trop 36:165–172
Silva LC, Araújo TO, Martinez CA, Almeida Lobo F, Azevedo AA, Oliva MA (2015) Differential responses of C3 and CAM native Brazilian plant species to a SO2-and SPMFe-contaminated Restinga. Environ Sci Pollut Res 22:14007–14017
Silva SR, Barros NFd, Silva IRd, Comerford NB (2018) Diffusive fluxes of phosphorus, potassium and metallic microelements as affected by soil compaction. Commun Soil Science Plant Anal 49(19):2369–2378
Silveira FA, Gama EM, Dixon KW, Cross AT (2019) Avoiding tailings dam collapses requires governance, partnership and responsibility. Biodivers Conserv 28:1933–1934
Siqueira-Silva AI, Rios CO, Pereira EG (2019) Iron toxicity resistance strategies in tropical grasses: the role of apoplastic radicular barriers. J Environ Sci 78:257–266
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Vandegeer RK, Tissue DT, Hartley SE, Glauser G, Johnson SN (2020) Physiological acclimation of a grass species occurs during sustained but not repeated drought events. Environ Exp Bot 171:103954
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Wang L, Ji B, Hu Y, Liu R, Sun W (2017) A review on in situ phytoremediation of mine tailings. Chemosphere 184:594–600. https://doi.org/10.1016/j.chemosphere.2017.06.025
Williams WM, Williamson ML, Real D (2011) Paspalum. In: Kole C (ed) Wild crop relatives: genomic and breeding resources: millets and grasses. Springer, Berlin Heidelberg, pp 197–216. https://doi.org/10.1007/978-3-642-14255-0_12
Yanik T, Donaldson RP (2005) A protective association between catalase and isocitrate lyase in peroxisomes. Arch Biochem Biophys 435:243–252
Zahoor R, Zhao W, Dong H, Snider JL, Abid M, Iqbal B, Zhou Z (2017) Potassium improves photosynthetic tolerance to and recovery from episodic drought stress in functional leaves of cotton (Gossypium hirsutum L). Plant Physiol Biochem 119:21–32. https://doi.org/10.1016/j.plaphy.2017.08.011
Funding
The authors received financial support from FAPEMIG (Minas Gerais State Foundation for Research Development, grant FORTIS-TCT-10254/2014) and Vale S.A. (ACA 5500023606/5900022781) and also for the scholarship granted to Rios CO. EG Pereira is a recipient of a research productivity grant from the National Council for Scientific and Technological Development (CNPq) (311532/2017-9).
Author information
Authors and Affiliations
Contributions
EGP: Funding acquisition and resources, project administration, supervision, conceptualization, formal analysis, writing-reviewing, and editing. COR: conceptualization, investigation, formal analysis, writing-original draft. AIS: conceptualization, investigation, formal analysis, writing-reviewing, and editing. All authors approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable
Consent to participate
Not applicable
Consent to publish
Not applicable
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 278 kb)
Rights and permissions
About this article
Cite this article
Rios, C.O., Siqueira-Silva, A.I. & Pereira, E.G. How does drought affect native grasses’ photosynthesis on the revegetation of iron ore tailings?. Environ Sci Pollut Res 28, 14797–14811 (2021). https://doi.org/10.1007/s11356-020-11599-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11599-x