Abstract
Purpose
Heavy metal accumulation produces significant physiological and biochemical responses in vascular plants. Plants growing on abandoned mine sites are of particular interest, since they are genetically tolerant to high metal concentrations. In this work, we examined the effect of heavy metals (HMs) on the morphology of T. officinale growing in pots with mine soils, with the following objectives: (1) to determine the evolution of HM concentration in leaves and roots over 3 years of cultivation; (2) to highlight possible damage at anatomical and cytological level.
Materials and methods
Wild specimens of Taraxacum officinale Web., with their soil clod, were gathered from three sites with different contamination levels by heavy metals (Cd, Cr, Cu, Fe, Pb, Zn) in the abandoned Imperina Valley mine (Northeast Italy). A control plant was also gathered from a non-contaminated site nearby. Plants were cultivated in pots at the botanical garden of the University of Florence (HBF), and appeared macroscopically not affected by toxic signals (reduced growth, leaf necrosis) possibly induced by soil HM concentration. Leaves and roots taken at the same growing season were observed by light microscopy and transmission electron microscopy.
Results and discussion
Light microscopy observations show a clear difference in the cellular organisation of non-contaminated and contaminated samples. The unpolluted samples present a well-organised palisade tissue and spongy photosynthetic parenchyma. Samples from contaminated sites, instead, present a palisade parenchyma less organised, and a reduction of leaf thickness proportional to HM concentration. The poor structural organisations, and the reduced foliar thickness of the contaminated plants, are related to soil contamination. Differences in root micromorphology concern the cortical parenchyma. Moreover, all the samples examined present mycorrhiza. Ultrastructure observations of the parenchyma cells show mitochondrial structure alteration, with lacking or reduced cristae of the internal membrane at increasing metal content. Instead, chloroplast organisation does not present significant differences, particularly in number and compartmentalization of thylakoids.
Conclusions
Although macromorphology does not present evidence of phytotoxicity, the recorded observations of the micromorphological characteristics of leaves and roots, show a suffering state of the plants, strictly related to HM content. Leaching reduced partly the HM content of the soil, therefore decreasing their phytotoxic effect. A gradual restoration of leaf organisation suggests that somewhat resilience occurred in plants. Moreover, the presence of stress-tolerant mycorrhizal fungi could contribute to reduce metal toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Trace elements are ordinarily present in rocks, sediments and soils, but locally may be concentrated in rocks as ore bodies and generally dispersed in the environment through pollution as a consequence of mining the ores (Davies 1987; Alloway 1995). Environmental threats arise when ores are mined, milled and smelted, and a certain amount of potentially harmful elements (PHEs) is released in the surrounding areas and to waterways. Depending on the nature of the waste rock and tailings, a wide dispersion of these PHEs both in solution and in particulate form is possible (Sivri et al. 2010).
Industrial extraction of metals from ore minerals usually results in large amounts of waste materials which often contain elevated concentrations of PHEs such as As, Cd, Cr, Cu, Pb, Zn (Helios-Rybicka 1996; Lee et al. 2001; Navarro et al. 2008). These elements can be transported, dispersed in the environment and accumulated in plants, and then may pass through the food chain to human people as the final consumer, causing serious health problems as intoxication, lead poisoning, mercurialism and also cancer (Bernard 1995; Steinnes 2009).
The metal-enriched areas, therefore, represent an ideal natural laboratory where to study the processes in order to provide descriptive models of the interactions between PHEs, the pedosphere, the biosphere and the hydrosphere. As stated by Preeti and Tripathi (2011), there is a direct relationship between chemical characteristics of soil, heavy metals concentration and morphological and biochemical responses of plants. Indeed, it is well known that PHEs may have toxic effects on living organisms (microbes, plants and animals, including humans): decline of soil fertility and yield depression (Zhao et al. 2011), decrease in microbial activity (Kucera et al. 2008), limited plant growth and root elongation (Kidd et al. 2009), reduction of the meristematic zone (Giuliani et al. 2008; Lösch 2004), damaged epidermal cells, plasmolysis, reduced chlorophyll and carotene production (Lopareva-Pohu et al. 2011).
In the last decades, the assessment of soil contamination has been extensively carried out through plant analysis (Ernst 1996; Zupan et al. 2003; Bini 2010 and references therein). There are plants that can survive in a metal-enriched environment, and other plants that can accumulate metals in their aerial parts; both are genetically tolerant to heavy metals. The first ones are diffused in abandoned mine sites or in naturally metal-enriched soils (e.g. serpentine soils); they are good (passive accumulative) bioindicators for large scale and local soil contamination (Baker 1981; Baker and Brooks 1989; Bargagli 1993; Zupan et al. 1995, 2003; Poschenrieder et al. 2001). The second ones are bioaccumulator plants, and have been proposed recently as tools to clean up contaminated soils by the environmental friendly technique of phytoremediation (Adriano et al. 1995; Baker et al. 2000; Bini 2009). A third group of plants do not tolerate metals, and are referred to as excluder (Baker 1981).
Although plants are metal-tolerant or bioaccumulators, heavy metals interfere with their metabolism (Lopareva-Pohu et al. 2011), and several morphological modifications were observed in their structure and ultrastructure (Mangabeira et al. 2001; Sarret et al. 2001; Maleci et al. 2001).
A large part of the phytotoxic effects of heavy metals have been assessed in laboratory studies on seeds germination or on plantlets (Mangabeira et al. 2001; Li et al. 2005; Preeti and Tripathi 2011). On the contrary, few contributions have been published on full-scale experiments and field observations carried out on wild plants (Madejon et al. 2002; Yoon et al. 2006; Unterbrunner et al. 2007; Llugany et al. 2009; Fontana et al. 2010; Bini et al. 2010; Wahsha et al. 2012b).
Previous studies of our research group (Bini et al. 2000, 2011; Fontana et al. 2010, 2011a, b; Bini 2012; Wahsha et al. 2012a, b) investigated the heavy metal concentration of soils developed from mine waste material. Attention was focused on the toxicity and influence of heavy metals on wild plants growing on those contaminated soils, and on the metal uptake by both known and unreported metal-tolerant plant species.
The plant selected for this study was dandelion (Taraxacum officinale Web.), a species well known for his tolerance to heavy metals (Królak 2003; Zupan et al. 2003; Rosselli et al. 2006). T. officinale is a very common plant, easy to identify and greatly adaptable to different geo-morpho-pedological and climatic conditions (Keane et al. 2001; Malawska and Wilkomirski 2001). Moreover, it is commonly collected to be eaten as a fresh salad or boiled vegetable; it is reported in European Pharmacopoeia (2006), and used in traditional medicine as hepatoprotector and diuretic. Therefore, when grown on heavily contaminated soils, it may be potentially harmful if introduced in dietary food or used as medicine.
Our preliminary results (Bini et al. 2012) confirmed that T. officinale is a species tolerant to high metal concentrations, and suggested to use it as a bioindicator plant. Metals accumulated preferentially in roots, but leaves also proved to be accumulator organs, showing only little morphological damage.
In this work, we report the results of the chemical content and its effect on the morphology of T. officinale plants growing on soils with different contamination levels from the mine area of Imperina Valley (North East Italy), with the following objectives:
-
To determine heavy metal (HM) concentration in leaves and roots
-
To highlight possible damage at anatomical and cytological level
-
To assess the possible plant resilience
2 Materials and methods
2.1 Site description
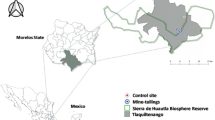
The Imperina Valley mining area is located in the Dolomites mountain district (NE Italy, Fig. 1), with an altitude ranging between 543 and 990 m above sea level. The geological substrate consists of rocks of the metamorphic basement (Pre-Permian), in tectonic contact with dolomite rocks (Dolomia Principale, Upper Triassic). The exploitation area is located along the tectonic contact; it consists of a deposit of mixed sulphides (Fe–Cu–Pb–Zn), composed primarily of cupriferous pyrite, pyrite and chalcopyrite, with minor amounts of other metallic minerals. Waste dump materials are dispersed over a large area in the territory, and contain relatively high amounts of toxic metals, with these average values: Cu = 1.3%, Pb = 0.2%, Zn = 1%, Cd = 8 mg kg−1, Cr = 75 mg kg−1, Ni = 62 mg kg−1. The recorded metal amounts confirm the waste composition to be determined by weathering products of primary minerals (cupriferous pyrite, sphalerite, galena), where Cr and Ni are present in traces. Mining activity in the investigated areas dates back at least to the Middle Ages and flourished in the nineteenth and twentieth centuries, until final closure in 1962.
Full information on the geological and environmental setting is available in Fontana et al. (2010) and references therein.
2.2 Soil sampling and laboratory analyses
Three contaminated areas, each with two sites (1–2, riverbed upstream; 3–4, roasting area; 5–6, permanent meadow downstream) and a non-contaminated site over dolomite (background control), were selected (Fig. 1) and sampled according to the procedures described by Hood and Jones (1997) and Margesin and Schinner (2005).
In the period between spring–summer 2011, soil pits were opened and described following Italian national guidelines (Costantini 2007). All locations were sampled for topsoil (0–30 cm). Afterwards, soil samples were recovered to the laboratory for routine and geochemical analyses. For the analysis of pseudo-total metal content, 0.2 g of soil samples was subjected to a complete digestion in the microwave (model 1600-Ethos, Milestone) in closed containers made of Teflon. The breakdown was performed in 5 mL of aqua regia (37% HCl + 65% HNO3, 1:3) and 1 mL of 48% HF, and then 1 mL of cold supersaturated H3BO3 was added. Two standard certified reference materials (Soil 5 from the International Atomic Energy Agency and MESS3 from National Research Council Canada) were analyzed as a part of the quality control.
Full information on field sampling and laboratory methods is available in Wahsha et al. (2012a, b).
2.3 Plant sampling
At four of the previously selected sites (sites 2, 4, 6, and control), during summer 2011, T. officinale specimens were sampled with their corresponding soil clod. Plants of the different sites, at gathering, presented normal appearance, and did not evidence particular diversities in shape and dimension of the leaves. The plants with their natural substrate were transferred and cultivated in pots (12 cm, one specimen for each pot, three replicates) at the Botanical Garden of the University of Florence, under the supervision of the authors.
2.3.1 Chemical analysis
Plant samples were rinsed gently with tap and distilled water to remove the adhering soil, then were divided into leaves and roots, dried in ventilated oven at 80 °C for 2 days. Dried tissues were grinded in an agate mill (<100 μm). Plant samples (0.5 g) were digested in an acid mixture of 5 mL 65% HNO3 and 3 mL 30% H2O2 in open vessels on the hot plate, followed by filtration with filter cellulose Whatman n. 42. After digestion, plant samples were analyzed for PHEs. The concentration of metals (Cd, Cr, Cu, Pb, Zn, Fe) was determined by inductively coupled plasma–optical emission spectroscopy (ICP–OES, Perkin Elmer model 5300DV) according to the method reported by Margesin and Schinner (2005).
Analyses were carried out at gathering (June, 2011), and repeated at the corresponding vegetative stage, during June, 2013.
2.3.2 Microscope observations: Optical Microscopy and Transmission Electron Microscopy
Microscope observations were carried out in autumn, 2011, and summer, 2012 and 2013. Small pieces of leaves (taken in the middle part of the leaf length), and thin roots, with prominent absorbing function, were withdrawn from plants of each contaminated site (Adriano et al. 1995; Ashraf et al. 2011; Baker and Brooks 1989) and from the control. Fresh material was pre-fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer at a pH 6.8, post-fixed in 2% OsO4 in the same buffer, then dehydrated and embedded in Spurr’s epoxy resin. Semi-thin sections of embedded material were stained with Toluidine Blu, and observed with a Leitz Light Microscope for a general overview of the leaf and root morphology. Ultrathin sections were stained with uranyl acetate and subsequently with lead citrate to observe leaf ultrastructure. Observations were performed with a Philips EM 300 transmission electron microscope.
2.4 Data analysis
Statistical analysis of metal content in soils and plants was based on ANOVA and is presented as means ± S.D. The statistical significance was declared when p value was equal to or less than 0.05. Statistical analyses were performed using Sigma Stat statistical software version 3.5.
3 Results
3.1 Soil characters and heavy metals accumulation in soils
The soils of the study area, except control soil, are classified as Spolic Technosols (Wahsha et al. 2012b). Soils are shallow, sandy loam in texture and typically unsaturated with respect to water; they have low cation exchange capacity and relatively high hydraulic conductivity. Organic carbon content is highly variable, ranging between 4 and 12 g kg−1 (33 g kg−1 at the control site, Mollisol over dolomite), with the lowest values at the most contaminated sites. The pH value is 7.5 at site 2, 5.3 at site 4, and 7.6 at site 6, while the control pH is 7.8, the range depending on the lithology of parent material. Full description of soil data is reported in Fontana et al. (2010).
The average concentrations of Cd, Cr, Cu, Pb, Zn, Fe in the soils examined are reported in Table 1. All the studied sites, out of the non-contaminated one, are strongly enriched in metals. The total concentrations of most of the investigated metals (Cd, Cu, Pb, Zn and Fe) in the soil samples were significantly higher than those of the non-contaminated site, and almost all above the toxicity threshold according to the Italian Legislative Decree (D.L. 152/2006). Chromium concentration, instead, is below the regulatory limits, and considerably higher in non-contaminated soil than at contaminated sites. Site 4 is the most contaminated, presenting very high metal concentrations (Cd up to 4.35 mg kg−1, Cu up to 4,100 mg kg−1, Pb up to 14,150 mg kg−1, Zn up to 2,700 mg kg−1), with Fe up to 58% in the roasting area. Sites 2 and 6 too are highly contaminated by Cu, Pb, Zn, Fe, while Cd concentration is slightly above the non-contaminated site, and Cr is well below the control value and under the detection limit at site 6.
Univariate statistics show that there is a positive linear correlation, significant at p < 0.05, between Pb, Cu, Zn and Fe (Cu/Pb 0.867; Pb/Zn 0.616; Cu/Zn 0.688; Cu/Fe 0.933). This is consistent with the composition of ore deposits found in the Imperina Valley, as chalcopyrite (CuFeS2), sphalerite (ZnS) and galena (PbS). Instead, Cr is negatively correlated with Cu (−0.847), Pb (−0.816), Zn (−0.604) and Fe (−0.754). Iron presents a significant positive correlation with Pb (Fe/Pb 0.734). Furthermore, Fe it is not significantly correlated with Cd, as expected considering the counteracting geochemical behaviour of the two elements.
3.2 Heavy metals accumulation in T. officinale plants
The concentrations of heavy metals in roots and leaves of dandelion plants collected in Imperina Valley are presented in Table 2. Data show that at contaminated sites this plant is able to accumulate metals, out of Cd, at much higher concentrations than at the unpolluted site, and above the toxicity threshold indicated by Kabata-Pendias (2011); metal accumulation in shoots was proportional to soil metal content; therefore, the greatest metal amount was found at the most contaminated site (sample 4).
Cadmium concentrations in both shoots and roots of plants from sites 2, 4, 6, are below the control value (up to 1.46 mg kg−1), and below the phytotoxicity threshold, with site 2 presenting the highest value (1.05 mg kg−1).
Chromium concentrations in plants from contaminated sites are slightly higher than the detection limit (1.00 mg kg−1), and very low in comparison to the phytotoxicity threshold reported by Kabata-Pendias (2011), consistently with concentration levels recorded in soil (see Table 1).
Copper and lead concentrations in shoots present much higher levels (Cu up to 64 mg kg−1; Pb up to 193 mg kg−1) than at the non-contaminated site. However, Cu is nearly equally accumulated in shoots and roots, while Pb is slightly more abundant in shoots than in roots.
Zinc levels in T. officinale shoots from sites 2 and 6 fall within the normal range (27–150 mg kg−1) given by Kabata-Pendias (2011), while plants from site 4 present Zn concentrations slightly above the normal values (189 mg kg−1).
Iron concentrations in leaves show a wide range of variation, with the highest level up to 890 mg kg−1 at site 4. However, this metal is not considered toxic unless at very high concentration: above 1,000 mg kg−1 according to Kabata-Pendias (2011).
Heavy metals in roots of T. officinale present lower concentrations with respect to leaves, with the exception of Cu at site 4. This suggests the ability of this plant to translocate most metals from roots to shoots. This is particularly evident for Fe and Zn (two- to threefold), which are essential micronutrients, while Pb and especially Cu are less prone (0.20 and 0.0 fold, respectively) to translocate from roots.
Leaves of dandelion taken directly from the assisted pots at University of Florence (HBF) during June, 2013 were analyzed for heavy metals following the previously described procedure. Roots could not be sampled, to avoid death of plants. The results obtained are shown in Table 3.
A comparison of HM concentrations in leaves between the two sampling periods indicates that metal uptake and transfer to leaves decreased significantly (at p < 0.05). Not all the metals, however, presented the same decreasing trend: Cd, Cr, Cu and Fe contents decreased by one- to fivefolds, while Pb content decreased up to 100-folds at sites 2 and 6, and up to tenfolds at site 4; Zn content decreased by tenfolds at all contaminated sites. It is likely that leaching with rain and tap water decreased the metal content in soils (data not shown), and consequently in plants.
3.3 LM observations
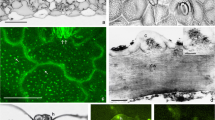
Light microscope observations were primarily carried out in October 2011, few months after plants were gathered (i.e. the leaves are those grown during spring and summer in the different sites). As clearly evidenced in Fig. 2, the thickness and especially the organisation of the leaf blade is different in the samples observed. Indeed, control samples present a well-developed photosynthetic parenchyma organised in an evident palisade and spongy parenchyma (Fig. 2a). In plants taken from polluted soils, the photosynthetic parenchyma is less developed (i.e. the leaf is less thick), it is constituted of a lower cell number and scarcely organised (Fig. 2c, e, g). Yet, plants from site 4, the most polluted one, show a photosynthetic parenchyma with small roundish cells and large intercellular spaces (Fig. 2e). Moreover, chloroplasts appear less numerous and smaller in comparison to the control. Plants from sites 6 and 2 show the absence of an actual palisade and spongy parenchyma, but the cellular organisation (shape, distribution and intercellular spaces) tends towards an arrangement similar to that of the control plant (Fig. 2c, g), particularly in plants from site 2, the less polluted.
A new series of observations were set up on leaves of the early vegetative phase, in June, 2012 and 2013. Small differences in the leaves micromorphology in the 2 years were observed; therefore, a unique observation set is reported (Fig. 2b, d, f, h). It is noteworthy to point out that both these observations were carried out on new leaves born in spring, after winter dormancy, on plants irrigated with tap water or/and by rain. As clearly evidenced in Fig. 2, the lamina thickness of the different samples is more or less the same in all the plants observed and similar to the control. The parenchyma cells present a better organisation in comparison to that of 2011. Indeed, the cells present intercellular spaces thinner on the adaxial side of the leaf and larger on the abaxial side (Fig. 2b, d, f, h). However, these cells have still a roundish shape and present smaller and less numerous chloroplasts in comparison to the control (Fig. 3a, b).
a, b Transverse section (LM) of the leaf lamina in 2013 at greater magnification (bar 50 μm). a Control plant; b plant from site 4 (ch chloroplasts). c, d transverse section of the mibrid in 2013 (bar 500 μm). c Control plant; d plant from site 4. e, f, g, h Transverse section of the roots (LM); arrows indicate the mycorrhizal sheath, asterisk the intercellular spaces (bar 100 μm). e Control plant f plant from site 2; g plant from site 4; h plant from site 6
The mibrid of the leaf appears well developed in the control (Fig. 3c) with numerous vascular bundles of different dimensions. The central part of the mibrid is constituted of a large wide space. In sample 4 (Fig. 3d) the shape appears different, but the dimensions are almost similar. The differences concern the vascular bundles, which are reduced in number (one large and two small bundles), and the absence of the empty central part, although several large intercellular spaces are evident. In samples from less contaminated soil (samples 2, 6) intermediate development between the most contaminated plant (sample 4) and control is recorded.
Primary roots, with a moderately developed cortical reserve parenchyma, were examined. All the samples observed present externally a well-developed mycorrhizal fungi sheath (Fig. 3e, f, g, h). Differences between control and polluted samples concern, in particular, the cortical parenchyma that in the control is compact with few and small intercellular spaces. The polluted samples present parenchyma cells with larger dimensions in comparison to the control and numerous large intercellular spaces, particularly developed in samples 4 and 6, the more polluted ones.
3.4 TEM observations
Transmission electron microscopy (TEM) observations were performed on leaves, primarily in October 2011, coincident with light microscopy (LM) observations. In all the samples observed, the photosynthetic parenchyma presents normal cells bearing numerous chloroplasts with thylakoids intergrana and grana, similar to the control (Fig. 4a). Instead, the mitochondria appear strongly damaged in samples gathered at the most contaminated sites, cristae are few in sample 6 (Fig. 4d) or lacking at all in sample 4 (Fig. 4c). In sample 2, grown on less polluted soil, mitochondria appear normal (Fig. 4e).
Observations carried out in June, 2012 and 2013 show somewhat recalcitrant ultrastructure in all samples examined. The damage observed in the mitochondria disappeared; indeed, these organelles present normal cristae in all the examined samples (Fig. 5a, b, c, d). No particular differences were observed in the root ultrastructure of the control and those of all the contaminated sites.
Ultrastructure of parenchyma cells (TEM) showing the restored internal membrane of mitochondria. Same samples as in Fig. 4. a bar 1 μm; b, c, d bar 0.5 μm
4 Discussion
4.1 HM in soils and plants
In this study, we have examined the toxic effect of selected HM on T. officinale, and its detoxification and resilience. Consistently with previous reports regarding the effects of heavy metals on plants (Simon et al. 1996; Malawska and Wilkomirski 2001; Zupan et al. 2003; Li et al. 2005; Savinov et al. 2007; Bini et al. 2012), our results show that HM concentration in soils affects their concentration in T. officinale tissues, in a proportional way with respect to the contamination level of different sites, the most contaminated being site 4. It is worthy to note that the extremely high content of iron is at site 4 (up to 58%), to which corresponds the highest Fe level in leaves and roots. Site 4 is a proper roasting area, forming a true iron pan, quite difficult to penetrate by plants, and has an acidic reaction (pH = 5.3). The acidic environment enhances metal solubility, and therefore their transfer from soil to plants, although there was no apparent visual phytotoxicity, as reported also by Giordani et al. (2012) in plants grown with iron nanoparticles.
Metals accumulate in leaves more than in roots, which qualifies dandelion as an indicator plant, as proposed by Baker (1981). The metal translocation ability, expressed by the ratio of metal concentration in shoots and roots (Malik et al. 2010), in almost all the investigated samples, presents positive translocation factors (TF > 1) (data not shown), indicating that T. officinale does not present a barrier effect against metals. Conversely, other studies (Mangabeira et al. 2001; Kabata-Pendias and Mukherjee 2007; Bini et al. 2008) report some root barrier for non-essential metals (e.g. Cd, Cr, Pb) uptake, suggesting some exclusion strategy by plants. Yet, as reported by Memon et al. (2001), plant species that have no exclusion mechanism in the roots absorb and translocate metals and accumulate them in their shoots, especially in leaves, without showing any toxicity symptoms, via a sort of internal resistance or accumulation mechanism.
Concerning cadmium, it is interesting to note that its concentration in both leaves and roots from the unpolluted site is higher than that from contaminated sites. The different soil parent material is likely to regulate the uptake of Cd as Ca-substituted element in dolomite at the control site, as reported by Dubois et al. (1998) in soils from Switzerland.
The differential HM content decrease in leaves collected in 2013 in comparison to 2011 (up to 100-folds for Pb) suggests that metals are leached with different pathways, according to their available fraction in the soil. Metals with the highest concentrations in 2011 leaves (Pb, Zn) were decreased to a major extent in comparison to the less concentrated ones. Lead was the easiest to be removed, as indicated by its strong decrease in 2013 leaves. Despite their less abundant content, Cd and Cr presented a minor decrease, which suggests some exclusion strategy for these non-essential elements. Copper and iron concentrations were relatively high (Cu up to 15 mg kg−1, Fe up to 636 mg kg−1) in 2013 leaves, with a less than fivefold decrease in comparison to 2011, in agreement with their essential/critical role as micronutrients, and do not determine any visual symptoms of phytotoxicity, suggesting a Cu–Fe tolerance (Abreu et al. 2008).
4.2 Plant morphology
In this study, any evident variation of the macromorphology was recorded in T. officinale by HM excess; indeed, all the plants examined (control and samples 2, 4, 6), even considering the range of species variability, present similar morphology and dimensions. Consistently, any apparent toxicity symptoms were visible in numerous accumulator plants, as reported by Memon et al. (2001). However, we observed a little difference in leaves colour (a less intense green colour in specimens of contaminated sites in comparison to control). These statements are consistent with findings by Kupper et al. (1998), who noted that in water plants transition metals such as Cd, Cu, Ni, Pb, Zn may substitute for Mg in the chlorophyll molecule, thus reducing the photosynthetic function, which results in colour change (i.e. chloroplasts number decrease, and consequently the chlorophyll production). It is likely that analogous process would occur in our studied plants.
A modified growth of leaves and roots, instead, was recently observed by Ashraf et al. (2011) in several plants grown on mine tailings. Although the leaf morphology of T. officinale does not present particular differences among the specimens of various sites, at microscopic level morphological and histological changes were observed. The actual thickness measured by microscopy (and which is not appreciable with the visual observation), is different in the observed samples (Fig. 2), and decreases with increasing HM content of plants. The different leaf thickness is consistent with the reduced, or even lacking, photosynthetic parenchyma normally structured as palisade on the adaxial leaf surface, and spongy parenchyma on the abaxial surface, as observed in control plants.
In the mibrid, the reduced number of vascular bundles (proportional to the contamination level), in comparison to control, have, as a consequence, less water availability for leaves, which certainly influences the plant metabolic activity.
Vacuoles constitute the cell compartment where HM, taken up by active transport systems, are deposited; in vacuoles, plants, according to a metal-adaptive strategy, isolate HM, thus inhibiting any interference with the plant metabolic reactions ( Memon et al. 2001; Schutzendubel and Polle 2002).
At relatively low metal concentrations, metals isolated in cell walls and vacuoles, are confined in roots, and therefore it is unlike to relate HM contents in soil with those in leaves, as reported by Rosselli et al. (2006). It is possible that plants grown at site 2 (the less contaminated) deposited all HM in cell walls and in vacuoles, since no macroscopic neither cellular deformations were observed. With higher soil metal contents, plants are able to translocate metals in the aerial parts, particularly in leaves (Keunen et al. 2011). In our study, plants from the most contaminated sites (samples 4, 6) presented HM contents in leaves related to those of the corresponding soils (with the exception of Cu which probably has a less effective transporter). The metal amount transported to leaves provokes cell morphology modification, as shown in figures above. Yet, as observed in samples 4 and 6, the cell metal content is responsible for serious deformations of mitochondria, the organelles performing aerobic respiration. Keunen et al. (2011) reported that PHEs provoke an increase in reactive oxygen species (ROS) production, and a reduction of mitochondria respiration functions. Similarly, Karuppanapandian et al. (2011) found that ROS generated under stress conditions provoke cell damage in various cellular compartments, including chloroplasts, mitochondria, endoplasmic reticulum and plasma membranes. Our morphological study ascertained mitochondria cristae reduction in sample 6, and even lack in sample 4, confirming recent physiological observations by Karuppanapandian et al. (2011), Keunen et al. (2011) and Lopareva-Pohu et al. (2011).
Micromorphological studies on plant cells subjected to HM stress are nearly lacking. In a recent paper, Zhao et al. (2011) report the effects on the morphology and ultrastructure of tomato plants subjected to lead-induced stress. Besides a reduction in size of different parts (fruits, leaves, stems and roots), thinner cell walls, swollen and deformed chloroplasts are recorded at ultrastructural level. Reduction in thickness and dimensions of leaf blade has been recorded also in soybean by Weryszko-Chmielewska and Chwil (2005). More recently, Giordani et al. (2012), in an experimental work on metal nanoparticles (NP) application to tomato seedlings, found clear effects at both morphological and genetic level (e.g. root hair formation, epidermal cells outgrowth). In our study, no deformation was observed in chloroplasts; however, in sample 6, and in sample 4 in particular, chloroplasts are smaller and numerically less than in the control (Fig. 3a, b).
Considering the HM–plants relationships, and their adaption to contaminated environment, it is important to remind that in geophytes like Taraxacum complete detoxification occurs at the end of the vegetative season, when plants loose leaves, which accumulate on the ground, and after winter dormancy, during the subsequent spring season, a new leaf generation may start again with metal deposition in vacuoles.
It is worthy to note also that all the investigated samples, including the control plants, resulted to being mycorrhized. Several studies have dealt with a possible alleviation of metal toxicity by mycorrhization, but only few presented evidence of such effects (Peterson et al. 2004 and references therein). In our samples, the PHEs effect is certainly attenuated, since there is a hindered access of metals to the root surface, which suggests the metal-induced stress response to be significantly reduced, as stated also by Schutzendubel and Polle 2002.
5 Conclusions
-
Soil analysis shows that PHEs are released from mine tailing because of low pH and low available water capacity; part of their bioavailable fraction is taken up by Taraxacum and transferred from roots to shoots, as it is common in indicator plants.
-
The study shows that there is a relationship between high metal content in Taraxacum plants and their modified morphology. PHEs do not determine an evident modification of the macromorphology. However, the leaf thickness decreases, and the absence of a regularly structured cellular organisation is likely related to the soil contamination degree.
-
It is likely that a 2-year leaching reduced partly the HM content of the soil, therefore decreasing their phytotoxic effect. A gradual restoration of leaf organisation suggests that, somewhat, resilience occurred in plants.
-
Since all samples, including control, present mycorrhized roots, it is suggested that stress-tolerant mycorrhizal fungi could contribute to reduce metal sorption. A comparison with non-mycorrhized plants could clarify the effective role of mycorrhizal fungi.
-
Data which is generated through this study could be helpful in detecting the lethal levels of heavy metals for particular plant species, their tolerance and remediation capacity.
-
The presence of heavy metals in dandelion has two consequences: a beneficial consequence, because the land affected by anthropogenic pollution may be restored by natural way; a fatal consequence, because both humans and animals feed dandelion, and, if plants are contaminated, through this way toxic metals enter the food chain.
References
Abreu MM, Tavares MT, Batista MJ (2008) Potential use of Erica avandulensis and Erica australis in phytoremediation of sulphide mine environments: Sao Domingos, Portugal. J Geochem Explor 96:210–222
Adriano DC, Chlopecka A, Kapland DI, Clijsters H, Vangrosvelt J (1995) Soil contamination and remediation philosophy, science and technology. In: Prost R (ed) Contaminated soils. INRA, Paris, pp 466–504
Alloway BJ (1995) Heavy metals in soils. Blackie, London, p 368
Ashraf M-L, Maah MJ, Yusoff I (2011) Heavy metals accumulation in plants growing in ex tin mining catchment. Int J Environ Sci Tech 8(2):401–416
Baker AJM (1981) Accumulators and excluders strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654
Baker AMJ, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Baker A, Mc Grath S, Reeves R, Smith J (2000) Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soils. Lewis, London, pp 85–107
Bargagli R (1993) Plant leaves and lichens as biomonitors of naturals or anthropogenic emissions of mercury. In Markert B (ed) Plants as Biomonitors. Weinheim WCH, pp 468-484
Bernard AM (1995) Effects of heavy metals in the environment on the human health. In: Prost R (ed) Contaminated soils. INRA, Paris, pp 21–34
Bini C (2009) Soil restoration: remediation and valorisation of contaminated soils. In: Manual of Methods for Soil and Land Evaluation (E.A.C. Costantini edit), Science, Enfield, 137–160. (ISBN 978-1-57808-571-2)
Bini C (2010) From soil contamination to land restoration. In: Contaminated soils: environmental impact, disposal and treatment (R.V. Steinberg edit.), Nova, New York, 97-137 (ISBN 978-1-60741-791-0)
Bini C (2012) Environmental impact of abandoned mine waste: a review. Nova, New York, p 92
Bini C, Casaril S, Pavoni B (2000) Fertility gain and heavy metal accumulation in plants and soils. Toxicol Environ Chem 77:131–142
Bini C, Maleci L, Romanin A (2008) The chromium issue in soils of the leather tannery district in Italy. J Geochem Explor 96(2–3):194–202
Bini C, Fontana S, Wahsha M (2010) Land contamination by mine dumps, plant toxicity and restoration perspectives by phytoremediation. Int J Environ Qual EQA 4:173–180, Tipografia Fanti, Imola. ISBN 10: 88-901261-7-5
Bini C, Fontana S, Wahsha M (2011) Environmental impact of PTEs (Cu, Fe, Pb, Zn) from mixed sulphides mines in Italy. Proc. XI ICOBTE, I, 505-506. Florence, July, 3–7, 2011
Bini C, Wahsha M, Fontana S, Maleci L (2012) Effect of heavy metals on morphological characteristics of Taraxacum officinale Web growing on mine soils in NE Italy. J Geochem Explor 123:101–108
Costantini EAC (2007) Soil survey methods and information of soil data. S.E.L.C.A, Firenze (in Italian)
D.L.—Legislation Act no 152/2006. Official Gazette n.88, 14/04/2006—Supplement no 96 (in Italian)
Davies BE (1987) Consequences of environmental contamination by lead mining in Wales. Hydrobiologia 149:213–220
Dubois JP, Okopnik F, Benitez N, Vedy JC (1998) Origin and spatial variability of cadmium in some soils of the Swiss Jura. Proc. 16th IUSS Congress, Montpellier. Symposia 25:1–8
Ernst WHO (1996) Bioavailability of heavy metals and decontamination of soils by plants. Appl Geochem 11:163–167
European Pharmacopoeia (2006)—VI ed., Council of Europe, Strasburg
Fontana S, Wahsha M, Bini C (2010) Preliminary observations on heavy metal contamination in soils and plants of an abandoned mine in Imperina Valley (Italy). Agrochimica 4:218–231, LIV
Fontana S, Bini C, Wahsha M, Bullo M (2011a) Heavy metals contamination in soils and their transfer to common wheat (Triticum aestivum L.): a case study. Geophys Res Abstr 13:1751
Fontana S, Bini C, Wahsha M, Bullo M (2011b) PTEs in agroecosystems and implications for the food chain. Proc. XI ICOBTE, I, 507–508. Florence, July, 3–7, 2011
Giordani T, Fabrizi A, Guidi L, Natali L, Giunti G, Ravasi F, Cavallini A, Pardossi A (2012) Response of tomato plants exposed to treatment with nano particles. Int J Environ Qual 8:27–38
Giuliani C, Pellegrino F, Tirillini B, Maleci L (2008) Micromorphological and chemical characterization of Stachys recta subsp serpentini (Fiori) Arrigoni in comparison to S. recta subsp. recta (Lamiaceae). Flora 203:376–385
Helios-Rybicka E (1996) Impact of mining and metallurgical industries on the environment in Poland. Appl Geochem 11(1–2):3–11
Hood TM, Jones BJ Jr (1997) Soil and plant analysis in sustainable agriculture and environment. Marcel Dekker, New York, p 877
Kabata-Pendias A (2011) Trace elements in soils and plants, 3rd edn. CRC, Boca Raton, p 365
Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human. Springer, Berlin, p 550
Karuppanapandian T, Moon J, Kim C, Manoharan K, Kim W (2011) Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust J Crop Sci 5(6):709–725
Keane B, Collier MH, Shann JR, Rogstad SH (2001) Metal content of dandelion (Taraxacum officinale) leaves in relation to soil contamination and airborne particulate matter. Sci Total Environ 281:63–78
Keunen E, Remans T, Bohler S, Vangronsveld J, Cuypers A (2011) Metal-induced oxidative stress and plant mitochondria. Int J Mol Sci 12:6894–6918
Kidd P, Barcelo J, Bernal MP, Navari Izzo F, Poschenrieder C, Shilev S, Clemente R, Monterroso C (2009) Trace element behaviour at the root-soil interface: implications in phytoremediation. Environ Exp Bot 67:243–259
Królak E (2003) Accumulation of Zn, Cu, Pb and Cd by dandelion (Taraxacum officinale Web.) in environments with various degrees of metallic contamination. Pol J Environ Stud 12(6):713–721
Kucera T, Horáková H, Šonská A (2008) Toxic metal ions in photoautotrophic organisms. Photosynthetica 46:481–489
Kupper H, Kupper F, Spiller M (1998) In situ detection of heavy metal substituted chlorophylls in water plants. Photosynth Res 58(2):123–133
Lee CG, Chon H, Jung MC (2001) Heavy metal contamination in the vicinity of the Daduk Au–Ag–Pb–Zn mine in Korea. Appl Geochem 16:1377–1386
Li WJ, Khan MA, Yamaguchi S, Kamiya Y (2005) Effects of heavy metals on seed germination and early seedling growth of Arabidopsis thaliana. Plant Growth Regul 46:45–50
Llugany M, Lombini A, Dinelli E, Poschenrieder C, Barcelo J (2009) Transfer of selected mineral nutrients and trace elements in the host–hemiparasite association, Cistus–Odontides lutea, growing on and off metal-polluted sites. Plant Biol 11:170–178
Lopareva-Pohu A, Verdin A, Garçon G, Sahraoui AL, Pourrut B, Debiane D, Waterlot C, Laruelle F, Bidar G, Douay F, Shirali P (2011) Influence of fly ash aided phytostabilisation of Pb, Cd and Zn highly contaminated soils on Lolium perenne and Trifolium repens metal transfer and physiological stress. Environ Pollut 159:1721–1729
Lösch R (2004) Plant mitochondrial respiration under the influence of heavy metals. In: Prasad (ed) Heavy metal stress in plants. From biomolecules to ecosystems, 2nd edn. Springer, Berlin, Germany, pp 182–200
Madejon P, Murillo JM, Marañon T, Cabrera F, Lopez R (2002) Bioaccumulation of As, Cd, Cu, Fe and Pb in wild grasses affected by the Aznalcollar mine spill (SW Spain). Sci Total Environ 290:105–120
Malawska M, Wilkomirski B (2001) An analysis of soil and plant (Taraxacum officinale) contamination with heavy metals and polycyclic aromatic hydrocarbons (PAHs) in the area of the railway junction Ilawa Glòwna, Poland. Water Air Soil Pollut 127:339–349
Maleci L, Bini C, Paolillo A (2001) Chromium (III) uptake by Calendula arvensis L. and related phytotoxicity. Proc. VI ICOBTE, Guelph, On., 384 (abstract)
Malik RN, Husain SZ, Nazir I (2010) Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. J Bot 42(1):291–301
Mangabeira P, Almeida AA, Mielke M, Gomes FP, Mushrifah I, Escaig F, Laffray D, Severo MI, Oliveira AH, Galle P (2001) Ultrastructural investigations and electron probe X-ray microanalysis of chromium-treated plants. Proc. VI ICOBTE, Guelph, 555 (abstract)
Margesin R, Schinner F (2005) Manual for soil analysis—monitoring and assessing soil bioremediation, 1st edn. Springer, Berlin, Germany, p 359
Memon AR, Aktoprakligul D, Zdemur A, Vertii A (2001) Heavy metal accumulation and detoxification mechanisms in plants. Turk J Bot 25:111–121
Navarro MC, Pérez-Sirvent C, Martínez-Sánchez MJ, Vidal J, Tovar PJ, Bech J (2008) Abandoned mine sites as a source of contamination by heavy metals. A case study in a semi-arid zone. J Geochem Explor 96:183–193
Peterson RL, Massicotte HB, Melville LH (2004) Mycorrhizas: anatomy and cell biology. NRC Research, Ottawa
Poschenrieder C, Bech J, Llugany M, Pace A, Fenes E, Barcelo J (2001) Copper in plant species in a copper gradient in Catalonia (North East Spain) and their potential for phytoremediation. Plant Soil 230:247–256
Preeti P, Tripathi AK (2011) Effect of heavy metals on morphological and biochemical characteristics of Albizia procera (Roxb.) Benth. seedlings. Int J Environ Sci 1(5):1009
Rosselli W, Rossi M, Sasu I (2006) Cd, Cu and Zn contents in the leaves of Taraxacum officinale. Snow Landsc Res 80(3):361–366
Sarret G, Vangronsveld J, Roux M, Coves J, Manceau A (2001) Bioaccumulation of metal in plants and microorganisms studied by electron microscopy and EXAFS spectroscopy. Proc. VI ICOBTE, Guelph, 131 (abstract)
Savinov AB, Kurganova LN, Shekunov YI (2007) Lipid peroxidation rates in Taraxacum officinale Wigg. and Vicia cracca L. from biotopes with different levels of soil pollution with heavy metals. Russ J Ecol 38(3):174–180
Schutzendubel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53(372):1351–1365
Simon L, Martin HW, Adriano DC (1996) Chicory (Cichorium intybus L.) and dandelion (Taraxacum officinale Web.) as phytoindicators of cadmium contamination. Water Air Soil Pollut 91(3–4):351–362
Sivri Y, Munoz M, Sappin-Didier V, Riotte J, Denaix L, de Parceval P, Destrigneville C, Dupré B (2010) Multimetallic contamination from Zn-ore smelter: solid speciation and potential mobility in riverine floodbank soils of the upper Lot River (SW France). Eur J Mineral 22:679–691
Steinnes E (2009) Soils and geomedicine. Environ Geochem Health 31:523–535
Unterbrunner R, Puschenreiter M, Sommer P, Wieshammer G, Tlustos P, Zupan M, Wenzel WW (2007) Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environ Pollut 148:107–114
Wahsha M, Bini C, Fontana S, Zilioli D, Wahsha A (2012a) Toxicity assessment of contaminated soils from a mining area in northeast Italy by using lipid peroxidation assay. J Geochem Explor 113:112–117
Wahsha M, Bini C, Argese E, Minello F, Fontana S, Wahsheh H (2012b) Heavy metals accumulation in willows growing on Spolic Technosols from the abandoned Imperina Valley mine in Italy. J Geochem Explor 123:19–24
Weryszko-Chmielewska E, Chwil M (2005) Lead-induced histological and ultrastructural changes in the leaves of soybean (Glycine max (L.) Merr.). Soil Sci Plant Nutr 51(2):203–212
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368:456–464
Zhao S, Ye X, Zheng J (2011) Lead-induced changes in plant morphology, cell ultrastructure, growth and yields of tomato. Afr J Biotechnol 10(50):10116–10124
Zupan M, Hudnik V, Lobnik F, Kadunc V (1995) Accumulation of Pb, Cd, Zn from contaminated soil to various plants and evaluation of soil remediation with indicator plant (Plantago lanceolata). In: Prost R (ed) Contaminated soils. INRA, Paris, pp 325–335
Zupan M, Kralj T, Grcman H, Hudnik V, Lobnik F (2003) The accumulation of Cd, Zn, Pb in Taraxacum officinale and Plantago lanceolata from contaminated soils. Proc VII ICOBTE, Uppsala Sv.
Acknowledgments
The authors wish to thank Corrado Tani, Pietro Di Falco and Flavia Visin for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jaume Bech
Rights and permissions
About this article
Cite this article
Maleci, L., Buffa, G., Wahsha, M. et al. Morphological changes induced by heavy metals in dandelion (Taraxacum officinale Web.) growing on mine soils. J Soils Sediments 14, 731–743 (2014). https://doi.org/10.1007/s11368-013-0823-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-013-0823-y