Abstract
Markedly increased withanolide content was found in transformed roots (TR) of Withania somnifera germplasm grown in low mineral minimal media and withanolides showed high antioxidant potential when analysed using acidic potassium permanganate chemiluminescence. Transformation frequency of explants infected with Agrobacterium rhizogenes strain A4 varied between the three germplasms tested with the highest observed as 75 ± 0.9. Transformed root production was explant specific with leaves being the most productive among the different explants used. Withanolides, namely withaferin A, withanolide A, withanolide B and 12-deoxywithastramonolide were detected in TR cultures and differences in their content were found between germplasms. The highest concentrations of secondary metabolites were found in 4-week-old cultures and concentrations declined by the 8th and 12th week of culture. In 4-week-old cultures, the biomass of TR cultures was 4.5 fold higher than their respective non-transformed roots (NTR). Withaferin A was found in TR at levels that were 28–34 times higher than that found in NTR. A rapid method for the determination of the antioxidant potential of W. somnifera TR extracts was developed using post-column acidic potassium permanganate chemiluminescence (APPC) detection. The APPC chromatographic peaks for extract constituents showed strong alignment with those found for ultraviolet absorbance detection. The methods developed in this study for TR culture establishment and the use of a fast and sensitive way for the qualitative and quantitative determination of the antioxidant activity of their metabolites provides a new platform that will have use for similar studies in other species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant derived secondary metabolites are an important component of various medicinal, industrial and agricultural formulations. Among the medicinal plants, Withania somnifera (Family Solanaceae) is a perennial that produces steroidal lactones, ‘withanolides’ (Khedgikar et al. 2013; Sivanandhan et al. 2012a), which have been shown to have pharmacological properties, for example, as anti-inflammatory, antimicrobial, antioxidant and anti-tumour agents (Kalra and Kaushik 2017; Vaishnavi et al. 2012). Extracts from field grown W. somnifera have been used in the preparation of medicines (Sangwan et al. 2007) and are commercially available as herbal health supplements that are GRAS (generally regarded as safe) products. Withania somnifera is considered the best source of withanolides, specifically withaferin-A (Johny et al. 2015) and is accepted in Ayurveda (traditional Indian medicine) as a plant with numerous medicinal properties.
Due to the increased demand for bioactive compounds (Sharada et al. 2007), such as those produced by W. somnifera, new ways of producing reliable quantities have been eagerly sought. The reliability of field grown plants as a source of secondary metabolites has been compromised by variable climatic conditions, lengthy plant growth cycles, plant disease, the requirement of significant manpower and time taken to harvest (Sivanandhan et al. 2012b). To overcome these impediments to reliable and efficient production of secondary metabolites approaches that have used tissue culture have been developed (Singh et al. 2017). Further, an additional and promising technique for increased production of metabolites is that of using Agrobacterium rhizogenes-transformed ‘hairy’ root cultures (Srivastava et al. 2016).
Hairy roots, or transformed roots more specifically, are formed during a proliferative root disease caused by infection with A. rhizogenes. Roots derived from such infection are fast growing and genetically stable (Dehdashti et al. 2017; Srivastava et al. 2016). Thus, medicinal and aromatic plants are now increasingly cultured for TR induction for the production of secondary metabolites (Danphitsanuparn et al. 2012; Kumar et al. 2014; Nagella et al. 2013). In addition, TR cultures are generally competent at producing secondary metabolites of similar profile to that found in roots of the native parent plant with no loss of the compound itself, a feature observed in callus or cell suspension cultures (Kim et al. 2002). The induction of TR morphology is recognised as a beneficial tool for biomass and secondary metabolite production (Sharma et al. 2013; Sheludko and Gerasymenko 2013).
Even though several other studies have reported on the presence of withanolides from TR cultures there has been no comprehensive analysis of withanolide production under low nutrient conditions or of the production of these metabolites across cultivars and ages. Also, the earlier studies were restricted to analysis of withaferin A (Vaishnavi et al. 2012), Withanolide A (Kuboyama et al. 2005) and D (Banerjee et al. 1994; Ray et al. 1996) and withanone (Sivanandhan et al. 2012b). No analysis has been performed of the production of withanolide B and 12-deoxywithastramonolide.
Secondary metabolites from plants are a major source of antioxidants that have important roles in human health (Galano et al. 2016). Transformed root cultures have become recognised as an equally important source of these antioxidants (Gai et al. 2015; Srivastava et al. 2016; Weremczuk-Jeżyna et al. 2013). Various antioxidant assays are used to test antioxidant activity in plant extracts (Cvetanović et al. 2015) and two very commonly used spectrometric methods are those based on 2,2-diphenyl-1-picrylhydrazyl (DPPH˙) and 2,2′-azinobis (3-ethylbenzothioazoline-6-sulfonic acid) ABTS˙ + (Nopo-Olazabal et al. 2013; Thiruvengadam et al. 2014). Many antioxidant assays have a number of shortcomings (Niederländer et al. 2008) which has paved the way for development of alternative techniques such as that based on chemiluminescence, that provide highly sensitive, fast response rates and low limits of detection (McDermott et al. 2011a). Moreover, chemiluminescence assays have been shown to be comparable with DPPH˙ and ABTS˙ + assays (Conlan et al. 2010; Francis et al. 2010) thus making them a reliable approach for analysing antioxidant potential activity.
Here we describe the establishment of TR cultures of W. somnifera to examine the effect of age and germplasm on growth and withanolide production in low nutrient medium. Further, we have used a novel chemiluminescence detection assay for the determination, in W. somnifera TR root exatracts, of antioxidant potential.
Materials and methods
Plant material
Seeds of W. somnifera (Johny et al. 2015) were from The Energy and Resources Institute, Gurgaon, Haryana, India. Three germplasms (WS 1, 2 and 3) collected from Uttarakhand, Madhya Pradesh and Maharashtra were examined in this study. For surface sterilization, seeds were initially washed in running tap water followed by immersion in 0.2% v/v Tween 20 in water (Serva, Hyderabad, India) and then soaked for 10 min in 0.1% w/v mercuric chloride (Qualigens, Mumbai) in water. Seeds were then rinsed five times with sterilized distilled water to remove traces of mercuric chloride after which they were placed for germination on Murashige and Skoog (MS, Murashige and Skoog 1962) medium supplemented with 3% (w/v) sucrose (Qualigens, Mumbai, India) in Petri plates (90 mm in diameter) and incubated at 30 °C in the dark. After germination, plates were maintained at 25 ± 2 °C with a 47 µmol/m2/s photosynthetic photon flux density and then 30-day-old seedlings were used for transformation studies.
Agrobacterium rhizogenes strains
For transformation studies, four different strains of A. rhizogenes were used. Agrobacterium rhizogenes strains A4, 8196, 1600 were obtained from David Tepfer, INRA Versailles, France and A. rhizogenes strain 11325 was obtained from Late Prof. Gopi K Podila (University of Alabama, Huntsville). The Agrobacterium strains A4, 1600 and 8196 were maintained on Yeast Mannitol Agar medium (YMA, Himedia, Mumbai, India) and the strain 11325 was maintained on Luria Agar medium (LA, Himedia, Mumbai, India).
Agrobacterium rhizogenes mediated infection
Bacterial strains were streaked with a sterile loop onto YMA plates to result in single distinct colonies. The hypocotyl, internodes and young leaf explants were infected with the different strains of A. rhizogenes separately using a single colony by moderate wounding with a sterile needle (23 gauge, Dispovan, Haryana, India). Control leaves were wounded with a sterile needle without bacterial culture. All explants were then incubated on sterile filter paper discs (Whatman Grade 1) placed on MS plates supplemented with 3% (w/v) sucrose and 100 mM acetosyringone (Sigma, Bangalore, India) in the dark at 25 ± 2 °C.
Transformation frequency
Infected explants were observed for frequency of transformation by observing the root initiation response of an explant from the injured site and the transformation frequency (TF) calculated using the formula:
TF = (Number of explants showing root initiations from injured sites/total number of injured explants) × 100.
Selection of bacteria-free root cultures
Positive explants showing root initiation were washed with liquid MS medium supplemented with 3% (w/v) sucrose and 500 mg/L cefotaxime (Omnatax, Abbott Healthcare Ptv. Ltd., Mumbai, India) to remove excess A. rhizogenes bacteria. The explants were blot dried on filter paper and transferred to MS medium supplemented with 3% (w/v) sucrose and 250 mg/L cefotaxime for complete elimination of bacteria on the surface of the explant. The plates were incubated in the dark at 25 ± 2 °C. Root lines that had developed on the explants on the antibiotic-supplemented media were removed after 15 days incubation and transferred to MS medium without hormones. Root lines were then subcultured three times after a growth period of 25 days before they were transferred to Minimal White (MW) medium (Bécard and Fortin 1988). Transformed roots were maintained on MW for two subcultures at 25-day intervals, after which they were transferred to Minimal (M) medium (Bécard and Fortin 1988) and were thereafter maintained on M medium for further studies.
Confirmation of transformation
Ri-T DNA integration into the plant genome was detected using polymerase chain reaction (PCR). Root lines were harvested from the plates, rinsed with sterile distilled water and then, after freezing with liquid nitrogen, crushed to a powder. Genomic deoxyribonucleic acid (DNA) was extracted using a commercial kit (DNeasy Plant Mini Kit, Qiagen, USA) from the putatively transformed root lines and non-transformed root (NTR) lines taken as the negative control (Johny et al. 2015). Plasmid DNA from the A. rhizogenes strain, was used as a positive control and was extracted using a High–Speed Plasmid Mini Kit (Geneaid, Taiwan). Root genomic DNA and plasmid DNA were analysed using a rolA gene specific primer (forward 5′AGAATGGAATTAGCCGGACTA3′ and reverse 5′GTATTAATCCCGTAGGTTTGTTT3′ obtained from Sigma). To study the absence of bacterial contamination in the transformed root lines, virC gene-specific primers were used (forward 5′ATCATTTGTAGCGAC3′ and reverse 5′AGCTCAAACCTGCTTC3′, obtained from Sigma). The PCR reaction mixture (25 μl) contained, 1 × PCR buffer, 1.5 mM magnesium chloride (MgCl2), 0.2 μM of each primer, 50 μM nucleoside triphosphate (dNTPs), 1.0 U of taq polymerase and ~ 50 ng of the DNA as template. PCR was carried out in a programmable thermal cycler (BioRad, Mumbai, India) by amplifying at an initial denaturation temperature of 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for 1 min and then final extension for 10 min at 72 °C. PCR products were separated on a 1% agarose gel (Himedia, Mumbai, India) and visualised by staining with ethidium bromide.
Growth kinetic studies, measurement of root length, growth index analysis, and root biomass determination
Transformed root growth kinetics was examined at 4, 8 and 12 weeks after TR culture initiation by analysing root growth index, root length, biomass and secondary metabolite concentration. At each time point, roots were harvested and the media adhered to roots was deionized using 10 mM sodium citrate buffer (Doner and Becard 1991) by treating the root system with the buffer at 25 °C for 30 min in an incubator shaker (Kuhner Shaker, Basel, Switzerland) at 100 rotations per minute (rpm). Roots were collected using a sieve (52 British Standard Sieve, Industrial Wire Netting Co., New Delhi, India), and washed through with distilled water to eliminate traces of the media components.
Root length of the harvested roots was measured using image analysis software (WinRHIZO® version Pro2007, Quebec, Canada) and the root images were captured with a scanner (EPSON Perfection V 700, Delhi, India). Roots were blot dried on filter paper, and their fresh weights documented. Growth as an index of fresh weight (Verma et al. 2002) was measured in triplicate at each time point to determine the growth performance of each TR culture using the following formula:
After this, root samples were lyophilised (Labconco lyophilizer, USA) at − 94.3 °C and 141 kPa for 72 h. Dry weight of the lyophilized roots was recorded.
Extraction of withanolides and quantification using HPLC analysis
Sample preparation and extraction of metabolites from W. somnifera roots followed the method of Johny et al. (2015). Four withanolide standards (Fig. 1) purchased commercially were used in the analysis—withaferin A and withanolide A (Sigma, Bangaluru, Karnataka, India), and withanolide B and 12-deoxywithastramonolide (Natural Remedies, Bangaluru, Karnataka, India). Chromatographic separation and examination of plant extracts was carried out using a Shimadzu HPLC, CBM-20A, with a C18 Phenomenex column (Gemini® 250 × 4.6 mm, 5 µm). Individual withanolide separation was accomplished using a mobile phase of water (18.2 MΩ, filtered through a 0.2 µm filter was prepared in-house) containing 0.1% acetic acid (all solvents were HPLC Grade, Merck, Mumbai, India) as solvent A and solvent B consisting of methanol containing 0.1% acetic acid. A gradient program was used for the analysis (Johny et al. 2015) with modification. Here, the analysis was performed at 27 °C, firstly solvent B was run from 40 to 60% over 30 min, 60% was maintained for the next 2.0 min then a gradient to 75% over the next 13 min and to 95% over the next 5 min and finally to 100% by 55 min and maintained at 100% for a further 5 min. The flow rate was 1.0 mL/min and the wavelength scan range of the PDA detector was set to 190–350 nm with chromatograms recorded at 227 nm.
Determination of antioxidant potential: instrumentation
Flow injection analysis instrumentation was set up as previously reported (McDermott et al. 2011a). An Agilent Technologies 1200 Series liquid chromatography system (Agilent Technologies, VIC, Australia) using Agilent Chemstation software was used for system control and data acquisition for chromatographic examination. The chemiluminescence reagent was mobilized at 1.0 mL/min using a Gilson Minipuls 3 peristaltic pump (John Morris Scientific, NSW, Australia). A T-piece was used to mix analytes with the acidic potassium permanganate ahead of entering the coil-tubing detection flow-cell. The flow-cell was seated flush adjacent the window of a photomultiplier tube (Electron Tubes model 9828SB; ETP, NSW, Australia) wrapped in a light-tight housing and powered by a steady power supply at 950 V. Detectors for chemiluminescence were coupled to HPLC by replacing the carrier line with the output of the column. A Hewlett-Packard analogue-to-digital interface box (Agilent) was used to convert detector signals.
Online chemiluminescence detection: secondary metabolites analysis
Sample preparation and the secondary metabolite extraction methods of Johny et al. (2015) were followed. Chromatographic examination of the filtered extract was carried out on an Agilent Technologies 1200 Series liquid chromatography system (Agilent Technologies, VIC, Australia) with a C18 Altima™ column (250 × 4.6 mm, 5 µm). For withanolides, the HPLC gradient program described above was used but the solvent flow rate was 0.6 mL/min until 55 min and then maintained at 1.0 mL/min for the following 5 min. All the gradient segments were linear and the chromatograms were recorded at a wavelength of 280 nm.
To analyse the antioxidant potential of extracted metabolites, an acidic potassium permanganate (Chem Supply, Australia) assay was used (Fig. 2) following the method of McDermott et al. (2011b). The potassium permanganate reagent standard was prepared freshly before use by dissolving sodium polyphosphate (10 g/L) (Sigma-Aldrich, Australia) in water followed by addition of potassium permanganate (1.0 mM). The pH of the solution was adjusted to 2.5 using analytical grade sulphuric acid (Merck, Australia).
Establishment of W. somnifera TR cultures and instrument set up for HPLC with post column acidic potassium permanganate chemiluminescence detection. (a) Seed germination. (b) Root initiation from infected sites. (c) Root elongation and branching from single root tip. (d) Developed TR culture in Petri plate. The chromatogram generated via digital interface gives both UV-absorbance and CL signals providing both qualitative and quantitative data for the analysed sample
Statistical analysis
All experiments were performed in triplicate. One-way analysis of variance was used to detect significant difference between means. Variability around the mean was represented as the standard error. Statistical significance was determined at the p < 0.05 level using the Tukey post-hoc test to determine variation in growth parameters and secondary metabolite concentration in different germplasms. All data were analysed using a commercial software package (SPSS Statistics 22, IBM).
Results
Establishment, transformation frequency and morphology of transformed root cultures
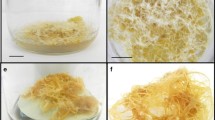
Explants were inoculated with four different A. rhizogenes strains and those that were infected with A. rhizogenes strain 8196 showed no response. The performance of the other three strains was in order of A4 > 11325 > 1600 (Fig. 3). Transformation frequency among germplasms of infected explants with A. rhizogenes strain A4 was in the order of WS3 TR > WS2 TR > WS1 TR. Transformed roots were thus successfully induced in W. somnifera. Initially, swelling of wounded areas was observed within 7–8 days of inoculation and the start of TR commenced within 17–20 days (Fig. 4). Among the different explants tested for transformation only leaves and specifically leaf mid rib showed root initiation (Fig. 4a–c). There were single and multiple root initiations from the infected sites. Hypocotyl and internodal explants showed no response to bacterial infection and became necrotic within 30 days. The presence of bacteria was eliminated after washing and growing the TRs on media supplemented with cefotaxime (Fig. 4d–f). The TRs (Fig. 4g–i) were subsequently subcultured onto MS, MW and M media and grew rapidly on hormone free solid medium. After continuous culture, extensive formation of root hairs was observed and two distinct morphologies were noted in the three different germplasms: WS1 TR, were thinner and showed slow growth with less branching whereas WS2 TR and WS3 TR were fast growing with extensive branching.
Representative figures showing establishment of the three germplasms of W. somnifera TR. (a–c) Root initiation from mid rib region of positive explants. (d–f) Single TR lines growing on hormone free media. (g–i) TR culture growth and spread on the whole media plate. (a, d, g) represent WS1 TR, (b, e, h) represent WS2 TR (c, g, i) represent WS3 TR
PCR based confirmation of transformed root cultures
The Ri plasmid T-DNA integration into the plant genome was confirmed using PCR to detect the presence of rolA genes (Fig. 5). A 308 bp (bp) fragment of rolA was amplified showing rolA integration into the plant genome. The virC gene was not amplified showing no Agrobacterium contamination (data not shown). Similarly, there was no amplification products obtained from NTR lines.
Confirmation of putatively transformed roots of W. somnifera transformed with A. rhizogenes strain A4 using PCR amplification using rolA region of T DNA. Lane 1: 100 bp DNA ladder, Lane 2: plasmid DNA Ri A4 (positive control), Lane 3: Genomic DNA NTR (negative control), Lane 4, 5, 6 Genomic DNA of W. somnifera TR cultures (WS1 TR, WS2 TR, WS3 TR)
Growth parameters of transformed root cultures
Transformed roots of WS were harvested at three time intervals (4, 8 and 12 weeks) and their fresh weight (FW), dry weight (DW), root length and GI were determined (Fig. 6). Biomass accumulation among the germplasms at 12 weeks was in the order of WS2 TR > WS3 TR > WS1 TR. For WS1 TR at 12 weeks, biomass was not significantly different from its respective 4 and 8-week-old cultures. The WS2 TR germplasm showed maximum fresh weight (1.18 ± 0.06 g), DW (0.14 ± 0.02 g), root length (430.75 ± 3.71 cm) and growth index of 57.79 ± 0.32. This was followed with WS3 TR germplasm fresh weight (0.92 ± 0.07 g), DW (0.12 ± 0.03 g), root length (386.568 ± 22.87 cm) with growth index of 45.14 ± 3.63. Root length of WS2 TR was highest in the 12th week followed by WS3 TR. WS1 TR germplasm showed the least biomass (FW = 0.07 ± 0.03 g and DW = 0.008 ± 0.01 g), root length (272.59 ± 12.41 cm) and GI (2.29 ± 0.50). Among the three germplasms, WS2 TR of 12 weeks was statistically significant from WS1 TR and WS3 TR. At 12 weeks of age, all growth parameters were significantly higher than for those recorded at the earlier time points.
Growth parameters of W. somnifera TR culture germplasms at different time points. a and b Biomass accumulation. c Time course growth on M media. d Root length. Different letters indicate significant differences (p ≤ 0.05) according to Tukey post-hoc test. Concentrations with the same letters are not statistically different. Data reported are mean ± SE for three replicates
HPLC analysis of withanolides
All extracts prepared were analysed using HPLC and three replicates for each germplasm were examined. Calibration curves for four withanolide standards, withaferin A, withanolide A, withanolide B and 12-deoxywithastramonolide were prepared. The calibration curve for withaferin A showed a coefficient of determination of r2 = 0.9992 for the regression equation of y = 23964x − 11676, for withanolide A a coefficient of determination of r2 = 0.997for the linear regression equation of y = 141394x − 39802, for withanolide B a coefficient of determination, r2 = 0.9997 and linear regression equation of y = 91936x − 24683 and for 12-deoxywithastramonolide a coefficient of determination of r2 = 0.9994 and linear regression equation of y = 210343x − 752930.
Withanolide production in transformed roots of Withania somnifera
Transformed root cultures harvested at the three time points (4, 8 and 12 weeks) were analysed for withanolides (Fig. 7) using HPLC. The retention times determined for the withanolide standards were used to identify relevant peaks derived from the root extracts. Withanolide production in WS TR germplasms at different time points showed that for WS1 TR there was no withanolide production except for a very low concentration of withaferin A (0.02 ± 0.01 mg/g DW) at 12 weeks of age (Fig. 8). WS2 TR germplasm exhibited maximum withanolide production at 4 weeks of age. For WS2 TR and WS3 TR germplasms at 4 weeks of age (Fig. 8a), there was no statistically significant difference between the concentrations between the two germplasms in terms of withaferin A production (0.93 ± 0.03 mg/g DW compared with 0.95 ± 0.04 mg/g DW respectively). WS3 TR produced the most withanolide A (0.05 ± 0.003 mg/g DW) whereas WS2 TR produced the most withanolide B (0.05 ± 0.001 mg/g DW) and 12-deoxywithastramonolide (0.18 ± 0.03 mg/g DW) (Fig. 8b–e). By the 12th week the concentration of all withanolides decreased compared with the 4th and 8th week samples (withaferin A: 0.46 ± 0.02 to 0.56 ± 0.08 mg/g DW, withanolide B: 0.013 ± 0.004 to 0.016 ± 0.002 mg/g DW and 12-deoxywithastramonolide: 0.078 ± 0.01 to 0.080 ± 0.01 mg/g DW and withanolide A: 0.029 ± 0.03 mg/g DW). Among all withanolides produced in WS2 TR and WS3 TR, the order of their concentration produced from highest to lowest was withaferin A > 12-deoxywithastramonolide > withanolide B > withanolide A.
Withanolide production in germplasms of W. somnifera TR cultures at three different time points of their growth on M media. a Withaferin A. b Withanolide A. c Withanolide B. d 12-Deoxywithastramonolide. Different letters indicate significant differences (p ≤ 0.05) according to Tukey post-hoc test. Bars with the same letters are not statistically different. Data reported as mean ± SE for three replicates
Comparison of growth parameters and secondary metabolite production between TR and NTR cultures
For all growth parameters tested, the TR lines outperformed those examined in the NTR cultures with the exception of WS1 TR, which showed no difference to the NTR cultures. 4-week-old cultures of TR and NTR were used for comparative studies (Fig. 9). The biomass of NTR in all the germplasms was less than that in the TR cultures. Culture WS2 TR was 6.3 fold higher in biomass (DW) than WS2 NTR and WS3 TR culture was 7.7 fold higher than WS3 NTR. Root lengths in WS2 TR (103.43 ± 0.91 cm) and WS3 TR (121.28 ± 7.83 cm) germplasms were significantly higher than that for the NTR (WS2: 76.25 ± 5.90 and WS3: 68.20 ± 7.83 cm). On comparing withanolide production (Table 1) TR clearly outperformed NTR, for example, withaferin A was produced to levels that were 28–34 times higher in TR compared with that in the NTR for both germplasms. Withanolide A and withanolide B in TR were 1.8–2.1 and 5.0-5.2 times higher than NTR respectively in both the germplasms. 12-deoxywithastramonolide was not detected in NTR of both germplasms.
Comparison between growth parameters in TR and NTR of three germplasms of W. somnifera: a Fresh weight, b dry weight, c root length. Different letters indicate significant differences (p ≤ 0.05) according to Tukey post-hoc test. Concentrations with same letters are not statistically different. Data reported as mean ± SE for three replicates
Determination of antioxidant potential
Based on withanolide content, WS2 TR was selected for post-column chemiluminescence detection. For extracts taken from WS2 TR, there were 13 significant chemiluminescence (CL) signals detected (Table 2). Similarly, under UV–Vis detection 13 peaks were visible with retention times corresponding with those observed following CL detection. Peaks at retention times 40.01, 43.32, 44.29, 52.80 min in CL chromatograms represented withaferin A, 12-deoxywithastramonolide, withanolide A and withanolide B respectively. The presence of a CL signal at the aforementioned retention times confirms the presence of antioxidant activity in the extract. All other CL signals showed the presence of molecules with antioxidant potential in the TR extract. Individual targeted withanolide contribution to the total antioxidant content was determined by comparing their peak area with the peak area of total antioxidants. Withanolides antioxidant activity contributed 6.7% (withaferin A), 0.8% (12-deoxywithastramonolide), 5.2% (withanolide A) and 1.8% (withanolide B) of the total antioxidant activity in WS2 TR extracts.
Comparative analysis of chemiluminescence detection between TR and NTR cultures
Based on the chromatograms derived from extracts of TR and NTR of WS2, there were 13 significant chemiluminescence signals (CL) observed via post-column chemiluminescence detection (Table 3). The presence of a CL signal in WS extracts of TR and NTR showed the presence of molecules with antioxidant potential and indicated that, qualitatively, the antioxidant potential from both extracts was based on the same chemical compounds. Peak 8 in TR CL and UV and peak 13 in NTR CL and UV were the most significant peaks observed in the detection assay. In addition to the specific withanolides targeted a broad range of antioxidant signals were observed in the sample matrix (Fig. 10).
Chromatograms for W. somnifera 2 TR and NTR. Potassium permanganate chemiluminescence detection is presented as CL and UV-absorbance detection is presented as UV. Peak numbers (1–13) are key signals which correspond to peak assignments indicated in the chromatograms. The most prominent peaks in TR and NTR are circled in red. Letters a–d indicate the withanolides- withaferin A, 12-deoxywithastramonolide, withanolide A and withanolide B respectively. (Color figure online)
Peak numbers 9 and 10 in TR and NTR respectively, corresponding to withaferin A and withanolide A, showed prominent antioxidant activity (Fig. 10a–c). The peak at retention time 34.3 min also showed high antioxidant potential in TR CL but was low in NTR CL. Similarly, peak 13 was observed to show high antioxidant potential in NTR CL but was low in TR CL. Peaks a, b, c, d in TR UV absorbance trace (Fig. 10 b) are withaferin A, 12-deoxywithastramonolide, withanolide A and withanolide B respectively. A similar pattern was observed in NTR CL with the exception of 12-deoxywithastramonolide which was not detected in the extract (Fig. 10d). The total peak area corresponding to antioxidant activity of the TR samples was found to be greater than that for the NTR samples.
Discussion
We have transformed root cultures of W. somnifera by infection with several strains of A. rhizogenes to produce elite root lines for which we have subsequently examined growth parameters and secondary metabolite production. We have found that these cultures produce withanolides in a cultivar- and age-specific manner and that withanolides are produced under low nutrient conditions. We have also shown that withanolide B and 12-deoxywithastramonolide have now been found in transformed hairy root cultures. Additionally, we also analysed the antioxidant potential of root extracts using a novel APPC assay and found that the antioxidant potential of the TR root lines was much greater than their respective NTR lines.
Here, we have also shown that transformation efficiency and frequency depends upon host source and Agrobacterium strain used. Different plant ecotypes vary in their response (Srivastava et al. 2016). The type of explants used also has a major role in determining the incidence of infection and among the various explants used in our experiments young leaves exhibited a higher response to infection with respect to root initiation compared with other explant types. Similar observations were made for Artemisia vulgaris (Sujatha et al. 2013), Gymnema sylvestre (Nagella et al. 2013), and Ocimum tenuiflorum (Vyas and Mukhopadhyay 2014). This difference between explant types may be because the cells of young leaves are continuously dividing and their viability is high even after inoculation injury to their surface. In the current study, only the A4 strain of A. rhizogenes successfully infected the W. somnifera explants which aligns with earlier reports (Bansal et al. 2014; Ooi et al. 2013). The response of W. somnifera explants to A4 strain infection but not the other A. rhizogenes strains tested is likely to be associated with the difference in virulence of the bacterial strains (Nourozi et al. 2014). Such differences in frequencies of root initiation has also been observed for A. vulgaris (Sujatha et al. 2013) and W. somnifera (Murthy et al. 2008).
Among the different transformed root lines obtained in each germplasm, putative transformed root lines were selected based on active growth and formation of lateral roots. There were phenotypic differences in the root lines observed in the different germplasms of the same species. In the present study, two different phenotypic traits were observed similar to those previously reported (Bandyopadhyay et al. 2007; Bansal et al. 2014; Thimmaraju et al. 2008) and may be due to the difference in copy number of the gene inserted into the plant genome. Our results showed that germplasms WS2 TR and WS3 TR had faster growth rate, higher biomass and higher secondary metabolite accumulation compared with WS1 TR. Putative transformed root lines were selected for study based on active growth and formation of lateral roots. Variation in growth rate and secondary metabolite production has been reported in several hairy root systems (Srivastava et al. 2016; Triplett et al. 2008) and the location of the insertion site of introduced T-DNA and genomic methylation patterns may be the factors that account for such differences. Some previous studies have documented the faster growth of transformed root cultures and enhanced secondary metabolite production when compared to NTR (Bandyopadhyay et al. 2007; Murthy et al. 2008; Sivanandhan et al. 2013). Similar results were observed in the present study wherein the biomass in WS NTR in all the germplasms was less than that in the TR. Biomass of WS TR cultures was more than six fold higher than their respective NTR. Withanolides production was also higher in TR as compared to NTR in both germplasms.
The results presented here for withaferin A production in WS TR 3 four-week-old cultures are in close accordance with several other studies (Sil et al. 2015; Sivanandhan et al. 2013) but not with others where higher withanolide production has been reported (Mukundan and Hjortso 1990; Murthy et al. 2008; Praveen and Murthy 2012, 2013). The lower withanolide concentration may be attributed to the low sucrose and macroelement concentration in the minimal medium used for root growth. Earlier reports found that the growth of transformed roots was influenced by sucrose concentration (Praveen and Murthy 2012; Wang and Weathers 2007) and by altering the concentration of macroelements (Praveen and Murthy 2013). Our study opens up a platform to analyse the response of elicitors, specifically biotic elicitors, such as mycorrhiza, which require low nutrient medium as a pre-requisite for association with roots to be explored for their influence on secondary metabolite production during symbiosis. In the current study, we found a decrease in the withanolide concentration with an increase in root age. This correlation with root age may be different in different species and different in transformed roots of the same species (Srivastava et al. 2016).
The withaferin A concentration found in our study was higher than that reported by Sivanandhan et al. (2012a) for adventitious roots of W. somnifera. Additionally, withanolide A and withaferin A concentration in in vitro and green house grown plants of W. somnifera (Dewir et al. 2010) was much less than that found in the transformed root cultures developed in the current study. Thus, the fast growth of transformed root cultures and the stability of secondary metabolite production found in our study provides significant advantages over conventional systems for efficient production of secondary metabolites.
Determination of antioxidants in a plant extract has been conventionally based on various off-line batch techniques (Nopo-Olazabal et al. 2013; Thiruvengadam et al. 2014). However, due to the complex nature of crude extracts these methodologies were not efficient or accurate for the determination of individual antioxidants (Karadag et al. 2009; Magalhaes et al. 2008; Moon and Shibamoto 2009). Recently, on-line assays have been developed where detection of antioxidants was coupled with HPLC, these assays include DPPH˙, ABTS˙ + and acidic potassium permanganate chemiluminescence detection assays (Niederländer et al. 2008; Shi et al. 2009; Srivastava et al. 2016). Within the on-line group of assays the acidic potassium permanganate chemiluminescence detection method is much more simplistic, robust and rapid to perform than the DPPH˙ and ABTS˙ + assays and total analysis time is significantly improved (Niederländer et al. 2008). The instrument set up and preparation of reagents in both DPPH˙ and ABTS˙ + assays are both more complex and detailed than the simple preparation required for acidic potassium permanganate. For example, the preparation of the ABTS˙ + reagent requires an incubation of 16–17 h followed by degassing before use. Similarly, in on-line DPPH˙ degassing of the reagent and all chromatography solvents is required. However, the acidic potassium permanganate solution can be used immediately. Secondly, both the assays require additional HPLC pumps and UV–Vis absorbance detectors whereas with acidic potassium permanganate chemiluminescence detection only a peristaltic pump and flow through luminometer are required. Further to this, the acidic potassium permanganate working solution can be used continuously and even stored for prolonged periods whereas in online DPPH˙ and ABTS˙ +, working solutions need to be prepared freshly on a daily basis (McDermott et al. 2011a). This experimental set up enabled a simple, rapid and antioxidant selective determination of key withanolides in our W. somnifera extracts. The utility offered by this system will afford operators a system that is less prone to analytical error due to the stability of the reagents enabling longer HPLC sequences to be performed for screening of samples of interest.
The secondary metabolites examined in the present study that were derived from a Withania species have specific roles, for example, in plant defense but are also of value as bioactives of pharmaceutical importance. The National Medicinal Plant Board of India has recognized W. somnifera as a plant that is in high demand in both Indian and international markets (Prajapati et al. 2007; Ramawat and Goyal 2008; Sharada et al. 2007). The major bioactives, withanolides, are produced via the isoprenoid pathway, probably via both the mevalonate and non-mevalonate pathways (Sabir et al. 2013). The first branching point in the pathway is at the intermediate, 24-methylene cholesterol, which is the precursor of different withanolides that are synthesized through an array of steps (Chaurasia et al. 2012; Madina et al. 2007). Withaferin A is one such metabolite that is produced and other withanolides formed are a combination of different alkyl groups associated with the primary skeleton of withaferin A (Patel et al. 2015).
Various studies in W. somnifera, have identified the importance of root extracts as an antioxidant source (Pandey et al. 2014). Lab scale assessment of antioxidant activity for W. somnifera extracts has been carried out using a number of different assays for example superoxide dismutase, 2,2-diphenyl, 1-picryl hydrazyl radical (DPPH˙) scavenging activity, hydroxyl group reducing activity (Alam et al. 2012). However, a rapid and efficient way of determination of antioxidant potential of withanolides and along with it, a complete profile of root extract of the plant has been undertaken in the present study using a chemiluminescence detection assay. To date, our study represents the first online determination of antioxidant-based molecules in W. somnifera (TR and NTR). The benefits of using the acidic potassium permanganate chemiluminescence detection technique in W. somnifera extracts were related to the complex nature of the crude extract (Chatterjee et al. 2010) and chromatographic separation was required. The online detection technique presented here gives both qualitative and quantitative information and has the potential to quantify the antioxidant activity of individual compounds within a complex matrix (Srivastava et al. 2016). Greater sensitivity and rapid analysis times are an added advantage over the many conventional assays (McDermott et al. 2011a) as has been reported by our group in comparative studies between chemiluminescence assays and conventional antioxidant assays (Conlan et al. 2010; Francis et al. 2010).
The number of prominent peaks in the chromatograms of W. somnifera indicated the presence of antioxidant activity in NTR and TR extracts, not only for the targeted withanolides, but also other molecules that were potentially withanolides and that may have antioxidant properties. Several unidentified peaks in the APPC chromatograms derived from NTR and TR extracts were identified as potentially important compounds due to their significant signal intensity. The chromatograms and data generated for NTR and TR extracts with the aid of CL and UV–Vis detection showed a complex sample matrix containing at least 13 key components. The targeted withanolides eluted quite late in the methylene-selective separation process indicating that the molecules were less water-soluble than the earlier eluting compounds. Importantly this offers the potential for plants grown in TR culture to be used in a selective bioprocessing system that can target the production and subsequent extraction of withaferin A, a medicinally important metabolite found in W. somnifera.
Conclusion
This study has shown that transformed root cultures are a very useful, amenable system for biomass and secondary metabolite production. The transformed root cultures developed showed high growth rates and enhanced metabolite production compared with non-transformed roots. Further, this study has found that transformed root cultures offer significant potential for the generation of targeted antioxidants in plant matrices and that the APPC assay should be the assay of choice for their determination. Finally, we suggest that this plant-based system may be exploited for mass production of medicinally important withanolides.
Abbreviations
- ABTS˙ + :
-
2,2-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) radical cation
- APPC:
-
Acidic potassium permanganate chemiluminescence
- CL:
-
Chemiluminescence
- DPPH˙ :
-
2,2-diphenyl-1-picrylhydrazyl
- DW:
-
Dry weight
- HPLC:
-
High performance liquid chromatography
- M:
-
Minimal medium
- MS:
-
Murashige and Skoog medium
- MW:
-
Modified White’s medium
- NTR:
-
Non-transformed roots
- PCR:
-
Polymerase chain reaction
- PDA:
-
Photodiode array
- Ri:
-
Root inducing
- T-DNA:
-
Transfer DNA
- TR:
-
Transformed roots
- UV:
-
Ultraviolet
- WS:
-
Withania somnifera
References
Alam N, Hossain M, Mottalib M, Sulaiman S, Gan S, Khalil M (2012) Methanolic extracts of Withania somnifera leaves, fruits and roots possess antioxidant properties and antibacterial activities. BMC Complement Altern Med 12:1–8. https://doi.org/10.1186/1472-6882-12-175
Bandyopadhyay M, Jha S, Tepfer D (2007) Changes in morphological phenotypes and withanolide composition of Ri-transformed roots of Withania somnifera. Plant Cell Rep 26:599–609. https://doi.org/10.1007/s00299-006-0260-0
Banerjee S, Naqvi AA, Mandal S, Ahuja PS (1994) Transformation of Withania somnifera (L.) Dunal by Agrobacterium rhizogenes: infectivity and phytochemical studies. Phytother Res 8:452–455. https://doi.org/10.1002/ptr.2650080803
Bansal M, Kumar A, Reddy MS (2014) Influence of Agrobacterium rhizogenes strains on hairy root induction and ‘bacoside A’ production from Bacopa monnieri (L.) Wettst. Acta Physiol Plant 36:2793–2801. https://doi.org/10.1007/s11738-014-1650-5
Bécard G, Fortin JA (1988) Early events of vesicular–arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol 108:211–218. https://doi.org/10.1111/j.1469-8137.1988.tb03698.x
Chatterjee S, Srivastava S, Khalid A, Singh N, Sangwan RS, Sidhu OP, Roy R, Khetrapal CL, Tuli R (2010) Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Phytochem 71:1085–1094. https://doi.org/10.1016/j.phytochem.2010.04.001
Chaurasia ND, Sangwan NS, Sabir F, Misra L, Sangwan RS (2012) Withanolide biosynthesis recruits both mevalonate and DOXP pathways of isoprenogenesis in Ashwagandha Withania somnifera L. (Dunal). Plant Cell Rep 31:1889–1897. https://doi.org/10.1007/s00299-012-1302-4
Conlan XA, Stupka N, McDermott GP, Barnett NW, Francis PS (2010) Correlation between acidic potassium permanganate chemiluminescence and in vitro cell culture assay: physiologically meaningful antioxidant activity. Anal Methods 2:171–173. https://doi.org/10.1039/B9AY00242A
Cvetanović A, Švarc-Gajić J, Zeković Z, Savić S, Vulić J, Mašković P et al (2015) Comparative analysis of antioxidant, antimicrobiological and cytotoxic activities of native and fermented chamomile ligulate flower extracts. Planta 242:721–732. https://doi.org/10.1007/s00425-015-2308-2
Danphitsanuparn P, Boonsnongcheep P, Boriboonkaset T, Chintapakorn Y, Prathanturarug S (2012) Effects of Agrobacterium rhizogenes strains and other parameters on production of isoflavonoids in HR of Pueraria candollei Grah. Ex Benth. var. candollei. Plant Cell Tissue Organ Cult 111:315–322. https://doi.org/10.1007/s11240-012-0196-8
Dehdashti SM, Acharjee S, Kianamiri S, Deka M (2017) An efficient Agrobacterium rhizogenes-mediated transformation protocol of Withania somnifera. Plant Cell Tiss Organ Cult 128:55–65. https://doi.org/10.1007/s11240-016-1081-7
Dewir YH, Chakrabarty D, Lee SH, Hahn EJ, Paek KY (2010) Indirect regeneration of Withania somnifera and comparative analysis of withanolides in in vitro and greenhouse grown plants. Biol Plant 54:357–360. https://doi.org/10.1007/s10535-010-0063-6
Doner LW, Becard G (1991) Solubilization of gellan gels by chelation of cations. Biotechnol Tech 5:25–28. https://doi.org/10.1007/BF00152749
Francis PS, Costin JW, Conlan XA, Bellomarino SA, Barnett JM, Barnett NW (2010) A rapid antioxidant assay based on acidic potassium permanganate chemiluminescence. Food Chem 122:926–929. https://doi.org/10.1016/j.foodchem.2010.02.050
Gai QY, Jiao J, Luo M, Wei ZF, Zu YG, Ma W, Fu YJ (2015) Establishment of hairy root cultures by Agrobacterium rhizogenes mediated transformation of Isatis tinctoria L. for the efficient production of flavonoids and evaluation of antioxidant activities. PLoS ONE 18:e0119022. https://doi.org/10.1371/journal.pone.0119022
Galano A, Mazzone G, Alvarez-Diduk R, Marino T, Alvarez-Idaboy RJ, Russo N (2016) Food antioxidants: chemical insights at the molecular level. Annu Rev Food Sci Technol 7:15.1–15.18. https://doi.org/10.1146/annurev-food-041715-033206
Johny L, Cahill D, Conlan X, Adholeya A (2015) In vitro and in situ screening systems for morphological and phytochemical analysis of Withania somnifera germplasms. Plant Cell Tiss Organ Cult 120:1191–1202. https://doi.org/10.1007/s11240-014-0673-3
Kalra R, Kaushik N (2017) Withania somnifera (Linn.) Dunal: a review of chemical and pharmacological diversity. Phytochem Rev. https://doi.org/10.1007/s11101-017-9504-6
Karadag A, Ozcelik B, Saner S (2009) Review of methods to determine antioxidant capacities. Food Anal Methods 2:41–60. https://doi.org/10.1007/s11240-014-0673-3
Khedgikar V, Kushwaha P, Gautam J, Verma A, Changkija B, Kumar A et al (2013) Withaferin A: a proteasomal inhibitor promotes healing after injury and exerts anabolic effect on osteoporotic bone. Cell Death Dis 4:e778. https://doi.org/10.1038/cddis.2013.294
Kim YJ, Wyslouzil BE, Weathers PJ (2002) Secondary metabolism of hairy root cultures in bioreactors. In Vitro Cell Dev Biol Plant 38:1–10. https://doi.org/10.1079/IVP2001243
Kuboyama T, Tohda C, Komatsu K (2005) Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br J Pharmacol 144:961–971. https://doi.org/10.1038/sj.bjp.0706122
Kumar V, Desai D, Shriram V (2014) Hairy root induction in Helicteres isora L. and production of diosgenin in hairy roots. Nat Prod Bioprospect 4:107–112. https://doi.org/10.1007/s13659-014-0011-9
Madina BR, Sharma LK, Chaturvedi P, Sangwan RS, Tuli R (2007) Purification and characterization of a novel glucosyltransferase specific to 27b-hydroxy steroidal lactones from Withania somnifera and its role in stress responses. Biochem Biophys Acta 1774:1199–1207. https://doi.org/10.1016/j.bbapap.2007.06.015
Magalhaes LM, Segundo MA, Reis S, Lima JLFC. (2008) Methodological aspects about in vitro evaluation of antioxidant potential. Anal Chim Acta 613:1–19. https://doi.org/10.1016/j.aca.2008.02.047
McDermott GP, Conlan XA, Noonan LK, Costin JW, Mnatsakanyan M, Shalliker RA et al (2011a) Screening for antioxidants in complex matrices using high performance liquid chromatography with acidic potassium permanganate chemiluminescence detection. Anal Chim Acta 684:134–141. https://doi.org/10.1016/j.aca.2010.10.046
McDermott GP, Francis PS, Holt KJ, Scott KL, Martin SD, Stupka N et al (2011b) Determination of intracellular glutathione and glutathione disulfide using high performance liquid chromatography with acidic potassium permanganate chemiluminescence detection. Anal 136:2578–2585. https://doi.org/10.1039/c1an00004g
Moon JK, Shibamoto T (2009) Antioxidant assays for plant and food components. J Agric Food Chem 57:1655–1666. https://doi.org/10.1021/jf803537k
Mukundan U, Hjortso MA (1990) Thiophene content in normal and transformed root cultures of Tagetes erecta: a comparison with thiophene content in roots of intact plants. J Exp Bot 41:1497–1501. https://doi.org/10.1093/jxb/41.11.1497
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Murthy HN, Dijkstra C, Anthony P, White DA, Davey MR, Power JB, Hahn EJ, Paek KY (2008) Establishment of Withania somnifera hairy root cultures for the production of withanolide A. J Int Plant Biol 50:975–981. https://doi.org/10.1111/j.1744-7909.2008.00680.x
Nagella P, Thiruvengadam M, Jung SJ, Murthy HN, Chung IM (2013) Establishment of Gymnema sylvestre hairy root cultures for the production of gymnemic acid. Acta Physiol Plant 35:3067–3073. https://doi.org/10.1007/s11738-013-1327-5
Niederländer HAG, Van Beek TA, Bartasiute A, Koleva II (2008) Antioxidant activity assays on-line with liquid chromatography. J Chromatogr A 1210:121–134. https://doi.org/10.1016/j.chroma.2008.09.061
Nopo-Olazabal C, Hubstenberger J, Nopo-Olazabal L, Medina-Bolivar F (2013) Antioxidant activity of selected stilbenoids and their bioproduction in hairy root cultures of muscadine grape (Vitis rotundifolia Michx.). J Agric Food Chem 23:11744–11758. https://doi.org/10.1021/jf400760k
Nourozi E, Hosseini B, Hassani A (2014) A reliable and efficient protocol for induction of hairy roots in Agastache foeniculum. Biologia 69:870–879. https://doi.org/10.2478/s11756-014-0382-8
Ooi CT, Syahida A, Stanslas J, Maziah M (2013) Efficiency of different Agrobacterium rhizogenes strains on hairy roots induction in Solanum mammosum. World J Microbiol Biotechnol 29:421–430. https://doi.org/10.1007/s11274-012-1194-z
Pandey V, Niranjan A, Atri N, Chandrashekhar K, Mishra M, Trivedi P et al (2014) WsSGTL1 gene from Withania somnifera, modulates glycosylation profile, antioxidant system and confers biotic and salt stress tolerance in transgenic tobacco. Planta 239:1217–1231. https://doi.org/10.1007/s00425-014-2046-x
Patel N, Patel P, Kendurkar VS, Thulasiram HV, Khan MB (2015) Overexpression of squalene synthase in Withania somnifera leads to enhanced withanolide biosynthesis. Plant Cell Tissue Organ Cult 122:409–420. https://doi.org/10.1007/s11240-015-0778-3
Prajapati ND, Purohit SS, Sharma AK, Kumar T (2007) A handbook of medicinal plants. Agrobios, Jodhpur
Praveen N, Murthy HN (2012) Synthesis of withanolide A depends on carbon source and medium pH in hairy root cultures of Withania somnifera. Ind Crop Prod 35:241–243. https://doi.org/10.1016/j.indcrop.2011.07.009
Praveen N, Murthy HN (2013) Withanolide A production from Withania somnifera hairy root cultures with improved growth by altering the concentrations of macro elements and nitrogen source in the medium. Acta Physiol Plant 35:811–816. https://doi.org/10.1007/s11738-012-1125-5
Ramawat KG, Goyal S (2008) The Indian herbal drugs scenario in global perspectives. In: Merillon JM (ed) Bioactive molecules and medicinal plants. Springer, Berlin, pp 325–347
Ray S, Ghosh B, Sen S, Jha S (1996) Withanolide production by root cultures of Withania somnifera transformed with Agrobacterium rhizogens. Planta Med 62:571–573. https://doi.org/10.1055/s-2006-957977
Sabir F, Mishra S, Sangwan SR, Jadaun SJ, Sangwan SN (2013) Qualitative and quantitative variations in withanolides and expression of some pathway genes during different stages of morphogenesis in Withania somnifera Dunal. Protoplasma 250:539–549. https://doi.org/10.1007/s00709-012-0438-y
Sangwan RS, Chaurasia ND, Misra LN, Lal P, Uniyal GC, Sharma R, Sangwan NS, Suri KA, Qazi GN, Tuli R (2007) Phytochemical variability in commercial herbal products and preparations of Withania somnifera (Ashwagandha). Curr Sci 86:461e465
Sharada M, Ahuja A, Suri KA, Vij SP, Khajuria RK, Verma V et al (2007) Withanolide production by in vitro cultures of Withania somnifera (L.) and its association with differentiation. Biol Plant 51:161–164
Sharma P, Padh H, Shrivastava N (2013) Hairy root cultures: a suitable biological system for studying secondary metabolic pathways in plants. Eng Life Sci 13:62–75. https://doi.org/10.1002/elsc.201200030
Sheludko Y, Gerasymenko I (2013) Biosynthetic potential of hairy roots for production of new natural products. In: Biotechnology for Medicinal Plants. Springer, Berlin, pp 241–262
Shi SY, Zhanga YP, Jianga XY, Chena XQ, Huanga KL, Zhoub HH et al (2009) Coupling HPLC to on-line, post-column (bio) chemical assays for high-resolution screening of bioactive compounds from complex mixtures. Trends Anal Chem 28:865–877. https://doi.org/10.1016/j.trac.2009.03.009
Sil B, Mukherjee C, Jha S, Mitra A (2015) Metabolic shift from withasteroid formation to phenylpropanoid accumulation in cryptogein-cotransformed hairy roots of Withania somnifera (L.) Dunal. Protoplasma 252:1097–1110. https://doi.org/10.1007/s00709-014-0743-8
Singh P, Guleri R, Angurala A, Kaur K, Kaur K, Kaul SC, Wadhwa R, Pati PK (2017) Addressing challenges to enhance the bioactives of Withania somnifera through organ, tissue, and cell culture based approaches. Biomed Res Int. https://doi.org/10.1155/2017/3278494 2017.
Sivanandhan G, Arun M, Mayavan S, Rajesh M, Jeyaraj M, Dev GK et al (2012a) Optimization of elicitation conditions with methyl jasmonate and salicylic acid to improve the productivity of withanolides in the adventitious root culture of Withania somnifera (L.) Dunal. Appl Biochem Biotech 168:681–696. https://doi.org/10.1007/s12010-012-9809-2
Sivanandhan G, Rajesh M, Arun M, Jeyaraj M, Dev GK, Manickavasagam M, Selvaraj N, Ganapathi A (2012b) Optimization of carbon source for hairy root growth and withaferin A and withanone production in Withania somnifera. Nat Prod Commun 7:1271–1272
Sivanandhan G, Dev GK, Jeyaraj M, Rajesh M, Arjunan A, Muthuselvam M, Manickavasagam M, Selvaraj N, Ganapathi A (2013) Increased production of withanolide A, withanone, and withaferin A in hairy root cultures of Withania somnifera (L.) Dunal elicited with methyl jasmonate and salicylic acid. Plant Cell Tissue Organ Cult 114:121–129. https://doi.org/10.1007/s11240-013-0297-z
Srivastava S, Conlan XA, Adholeya A, Cahill DM (2016) Elite hairy roots of Ocimum basilicum as a new source of rosmarinic acid and antioxidants. Plant Cell Tissue Organ Cult 126:19–32. https://doi.org/10.1007/s11240-016-0973-x
Sujatha G, Zdravković-Korać S, Ćalić D, Flamini G, Kumari BR (2013) High-efficiency Agrobacterium rhizogenes mediated genetic transformation in Artemisia vulgaris: hairy root production and essential oil analysis. Ind Crop Prod 44:643–652. https://doi.org/10.1016/j.indcrop.2012.09.007
Thimmaraju R, Venkatachalam L, Bhagyalakshmi N (2008) Morphometric and biochemical characterization of red beet (Beta vulgaris L.) hairy roots obtained after single and double transformations. Plant Cell Rep 27:1039–1052. https://doi.org/10.1007/s00299-008-0527-8
Thiruvengadam M, Praveen N, John KMM, Kim SH, Chung I (2014) Establishment of Momordica charantia hairy root cultures for the production of phenolic compounds and determination of their biological activities. Plant Cell Tissue Organ Cult 118:545–557. https://doi.org/10.1007/s11240-014-0506-4
Triplett BA, Moss SC, Bland JM, Dowd MK (2008) Induction of hairy root cultures from Gossypium hirsutum and Gossypium barbadense to produce gossypol and related compounds. In Vitro Cell Dev Biol Plant. 44:508–517. https://doi.org/10.1007/s11627-008-9141-2
Vaishnavi K, Saxena N, Shah N, Singh R, Manjunath K, Uthayakumar M et al (2012) Differential activities of the two closely related withanolides, withaferin A and withanone: bioinformatics and experimental evidences. PLoS ONE 7:e44419. https://doi.org/10.1371/journal.pone.0044419
Verma PC, Singh D, Lu R, Gupta MM, Banerjee S (2002) In vitro studies in Plumbago zeylanica: rapid micropropagation and establishment of higher plumbagin yielding hairy root cultures. J Plant Physiol 159:547–552. https://doi.org/10.1078/0176-1617-00518
Vyas P, Mukhopadhyay K (2014) Development of a rapid and high frequency Agrobacterium rhizogenes mediated transformation protocol for Ocimum tenuiflorum. Biol 69:765–770. https://doi.org/10.2478/s11756-014-0375-7
Wang Y, Weathers PJ (2007) Sugars proportionately affect artemisinin production. Plant Cell Rep 26:1073–1081. https://doi.org/10.1007/s00299-006-0295-2
Weremczuk-Jeżyna I, Grzegorczyk-Karolak I, Frydrych B, Królicka A, Wysokińska H (2013) Hairy root of Dracocephalum moldavica: rosmarinic acid content and antioxidant potential. Acta Physiol Plant 35:2095–2103. https://doi.org/10.1007/s11738-013-1244-7
Acknowledgements
The authors acknowledge Deakin University, Australia, and The Energy and Resources Institute for financial assistance and infrastructure support. Leena Johny was the recipient of a Deakin University postgraduate scholarship.
Author information
Authors and Affiliations
Contributions
LJ conducted all experiments and wrote the first draft of the manuscript. XAC supervised the experiments, contributed to understanding analytical tools, to data interpretation and paper drafting. AA and DMC developed the scientific question, supervised the experiments and contributed to drafting the manuscript. All authors read, reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Our work complies with the ethical rules applicable for this journal.
Additional information
Communicated by Ali R. Alan.
Rights and permissions
About this article
Cite this article
Johny, L., Conlan, X.A., Adholeya, A. et al. Growth kinetics and withanolide production in novel transformed roots of Withania somnifera and measurement of their antioxidant potential using chemiluminescence. Plant Cell Tiss Organ Cult 132, 479–495 (2018). https://doi.org/10.1007/s11240-017-1344-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1344-y