Abstract
Passiflora pohlii Mast. is a wild species native to Brazil, with potential agronomic interest. Although there are few studies on this particular species, recent works described several biological activities in other species of the genus. The goal of this work was to establish adventitious roots cultures from stem and root segments excised from in vitro-grown plants of P. pohlii, as well as to perform phytochemical analyses and evaluate the antioxidant potential of extracts obtained from the in vitro materials, in comparison with in vivo-grown plants. The influence of different parameters (type of explant, culture systems, light, and type and concentrations of auxins) on the induction of adventitious roots was evaluated. Internodal segments showed the best rhizogenesis induction on solidified medium supplemented with 2.7 µM NAA, whereas root segments showed the highest proliferation rate on liquid medium supplemented with 2.85 µM IAA, both in the absence of light. TLC analysis indicated the presence of saponins in extracts from all in vivo and in vitro-derived materials. The antioxidant potential was determined by DPPH and TLC-DPPH assays. The highest antioxidant activities were observed in extracts from primary and secondary roots of in vivo-grown plants. The characterization of the phytochemical profile of the in vitro and in vivo materials, as well as their pharmacological potential are reported for the first time for this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Evaluation of different culture systems and types and concentrations of auxins for the establishment of adventitious root cultures, and analysis of phytochemical profile and antioxidant activity of Passiflora pohlii Mast.
Introduction
The genus Passiflora (Passifloraceae) comprises approximately 560 species, with tropical and subtropical distribution. Brazil is considered an important origin center of the genus, with 150 known species (Bernacci et al. 2013). In spite of the great diversity, few species are commercially cultivated, mainly Passiflora edulis, P. edulis f. flavicarpa and P. alata. In addition, several species are used in folk medicine and some of them are present as official drugs in the pharmacopeias of several countries (Dhawan et al. 2004).
Pharmacological properties of extracts from different plant materials of Passiflora species have been addressed to their anxiolytic, sedative, analgesics, anti-inflammatory, antihyperglycemic and antioxidant activities, which have been associated to the presence of a number of compounds such as flavonoids, alkaloids, polyphenols and saponins, revealed by phytochemical studies carried out during the last decades (Dhawan et al. 2004; Gosmann et al. 2011).
Passiflora pohlii Mast. is a wild species native to Brazil, mainly found in the Atlantic Forest, Cerrado and Pantanal (Bernacci et al. 2013), which are regions highly impacted by human actions. It has an important agronomic potential due to its tolerance to soil-borne pathogens from genus Phytophthora, which cause damages to the passion fruit culture (Junqueira et al. 2005). However, to our knowledge, there are no phytochemical or pharmacological studies for this species.
Biotechnological methods are considered important tools for large-scale production of uniform and healthy plants, under controlled conditions. These techniques also provide an alternative for the production of high-value phytochemicals, which may be limited by seasonal variations, environmental factors, and unsustainable natural harvest (Wilson and Roberts 2012). Adventitious root cultures are considered a promising approach for producing bioactive molecules, since plant roots are naturally capable to synthesize and storage a great diversity of secondary metabolites and proteins, and may display similar phytochemical patterns of the in vivo-derived roots (Bais et al. 2001; Flores et al. 1999). Moreover, when compared to unorganized cultures, like calluses and cell suspensions, adventitious roots are considered to be more stable, and to accumulate greater amount of secondary metabolites in the intercellular spaces, which facilitate their isolation (Sivakumar 2006). These cultures can be achieved either by genetic transformation with Agrobacterium rhizogenes or manipulating the in vitro culture conditions. In general, non-transformed roots are more suitable for secondary metabolites production, since they are free from opine like substrates, which may be lethal to mammalian cells (Choi et al. 2000).
Although many adventitious root cultures are used for several cultivated species, the mechanisms that control rhizogenesis are poorly understood (Verstraeten et al. 2013). In general, the development of these roots is a complex process, controlled by hormone signals, specially auxins, and environmental factors, involving cellular dedifferentiation, in which predetermined cells change the morphogenetic route to give rise to the root primordia. At natural conditions, adventitious root formation is generally preceded by a rapid increase of ethylene concentration, mainly due to the physical retention of this hormone in the submerse root, increasing tissue sensibility to endogenous auxins (George et al. 2008; Hasan and Hussein 2013). However, under in vitro conditions, ethylene release is related to the mechanical injuries caused by the excision of explants, thus providing the necessary signals to capture both endogenous and exogenous auxins for the formation of unorganized tissue or adventitious roots and shoots (George et al. 2008).
Several authors reported the influence of medium consistency (Blidar et al. 2011; De la Viña et al. 2001; Kadota et al. 2001; Cui et al. 2011; Scherwinski-Pereira et al. 2012), as well as the type and concentration of plant growth regulators in root induction and proliferation capacity of the cultures (Sudha and Seeni 2001; Nandagopal and Kumari 2007; Hasan and Hussein 2013). Successful adventitious root systems have been developed for secondary metabolite production of several medicinal plants, including Echinacea angustifolia (Wu et al. 2006), Panax ginseng C.A. Meyer (Paek et al. 2009) and Hypericum perforatum L. (Cui et al. 2011).
Detection, quantification, isolation, and identification of such phytochemical compounds are commonly carried out by chromatography techniques, like thin layer chromatography (TLC) and high pressure liquid chromatography (HPLC). Although HPLC techniques, such as HPLC–UV or HPLC/ESI-MS, provide rich information on compounds structures, which can be obtained from the MS spectra (Ha et al. 2006; Sun et al. 2007), TLC technique allows the determination of large number of samples simultaneously, thus being considered an easy, fast and low cost approach for the identification, characterization and quality assurance of plant compounds (Wagner and Bladt 2001; Birk et al. 2005).
In a previous study with P. pohlii we established efficient protocols for shoot regeneration and callus induction from stem explants of in vitro plants (Merhy 2014; Merhy et al. 2014). Here, we describe the induction and proliferation of adventitious roots of P. pohlii from stem and root explants, and investigate the effects of different culture systems and type and concentration of auxins on these processes, also exploring their phytochemical composition and antioxidant potential.
Materials and methods
Plant material and culture conditions
In vitro-grown plants of P. pohlii cultured on solidified ½ MSM medium (Monteiro et al. 2000), supplemented with 3 % (w/v) sucrose, and solidified with 0.7 % (w/v) agar (Merck) for 3 years were used as sources of explants.
Basal media consisted of MSM salts, containing MS (Murashige and Skoog 1962), vitamins, 3 % (w/v) sucrose, and solidified with 0.7 % (w/v) agar. MSM medium formulation is a modification of MS medium based on the composition of leaves of field-grown plants of P. edulis f. flavicarpa. It presents reduced concentrations of nitrogen, potassium, zinc, boron and chloride, associated to increased concentrations of calcium, magnesium, sulfur, iron, manganese, copper, sodium and EDTA, and does not contain iodine. The reduction of chloride is provided by changing the calcium source from calcium chloride to calcium nitrate. The pH for all media was adjusted to 5.8 and growth regulators were added at different concentrations before autoclaving for 15 min at 121 °C. All inorganic chemicals used were P.A. grade, and biochemical reagents were of the best grade available from Sigma Chemical Co.
Cultures were maintained in a growth chamber at 25 ± 2 °C in the dark or under a 16 h photoperiod, using a total irradiance of 46 μmol m−2 s−1 provided by cool-white fluorescent lamps.
Induction of adventitious roots from stem segments
Nodal (0.5 cm) and internodal segments (1.5 cm) excised from in vitro-grown plants were inoculated on MSM solid medium supplemented with different concentrations of naphthaleneacetic acid (NAA) (0.54, 2.7, 5.4 µM), indole-3-acetic acid (IAA) (0.57, 2.85, 5.7 µM) or indole-3-butyric acid (IBA) (0.49, 2.45, 4.9 µM). Alternatively, explants were inoculated on liquid medium (static or under agitation, 100 rpm) or placed onto paper bridges (discs of Whatman® No. 1 filter paper supported by glass beads) using 15 mL of MSM medium with the same range of the above auxins concentrations. For control, stem explants were inoculated on medium without growth regulators (MSM0). Four flasks (11 × 5.5 cm) closed with polypropylene caps containing five explants placed horizontally were used per treatment and each experiment was repeated twice. Cultures were maintained for 60 days in the presence or absence of light. After this period, root fresh and dry weights were determined. For biomass determination, the harvested roots were washed with running water, and then rinsed with distilled water. Fresh weight (FW) was determined after blotting the washed roots on filter paper. Dry weight was obtained after drying roots at 60 °C to constant weight (approximately 24 h). Biomass accumulation was calculated as the ratio between final and initial values of fresh and dry weights.
For the evaluation of the proliferative capacity of the adventitious roots, 1 g of roots derived from the internodal segments cultured on solid MSM medium supplemented with 2.7 µM NAA, in the absence of light, were transferred to fresh medium of the same composition, using three different culture systems: liquid medium under agitation, paper bridges over liquid medium or double-phase medium (10 mL liquid medium over 20 mL solid medium). Four flasks (11 × 5.5 cm) were used per treatment and each experiment was repeated twice. Cultures were maintained for 60 days in the absence of light. After this period, root fresh and dry weights were determined and biomass accumulation was calculated as described above.
Root multiplication from root segments
Root segments (2 cm) without lateral roots were excised from in vitro-grown plants and cultured in 250 mL Erlenmeyer flasks containing 50 mL of liquid MSM0 or MSM supplemented with NAA (0.54, 2.7, 5.4 µM), IAA (0.57, 2.85, 5.7 µM) or IBA (0.49, 2.45, 4.9 µM). Five flasks closed with a double blade aluminum cap and containing three explants were used per treatment and the experiments were repeated twice. Cultures were maintained on a rotatory shaker (100 rpm), in the presence or absence of light for 60 days. After this period, root fresh and dry weights were evaluated as described above.
Extract preparation
Extracts from dry roots excised from in vivo and in vitro plants, as well as from adventitious roots derived from internodal and root segments were prepared separately, using 40 % ethanol under reflux (1:10, plant:solvent, w/v) for 1 h, as described by Birk et al. (2005). The extracts were then dried in hot water bath (90 °C), and sample solutions (10 mg/mL) were prepared ultrasonically using methanol as solvent.
TLC analysis
For TLC analysis, each sample was directly applied on silica gel-coated TLC aluminium plates (Si gel 60 UV254nm, Marcherey–Nagel, 20 × 20 cm plates). Flavonoid analysis was carried out using AcOEt:formic acid:AcOH:H2O (100:11:11:26, v/v) as the mobile phase (Wagner and Bladt 2001). Two identical TLC plates were prepared: one plate was sprayed using a vanillin-sulfuric acid solution (1 % ethanolic vanillin and 10 % sulfuric acid) before heating (100 °C) for 5–10 min, and the other one was sprayed using 1 % methanolic solution of diphenylboryloxyethylamine (Sigma Aldrich®), followed by spraying with 5 % (w/v) PEG 4000 (Natural Product Reagent—polyethyleneglycol) as colour reagents. Spots were observed under visible light and UV light (365 nm).
Saponins were analyzed using CHCl3:AcOH:MeOH:H2O (60:32:12:18, v/v) as the mobile phase (Wagner and Bladt 2001). TLC plates were then sprayed using anisaldehyde-H2SO4 before heating (100 °C) for 5–10 min, and visualized under visible light.
Determination of antioxidant potential
DPPH assay
Free radical scavenging capacity was measured using the DPPH assay, according to Brand-Williams et al. (1995). Briefly, 25 μL of extracts diluted in 100 % methanol (1, 5, 10, 20, 30, 40, 50 g L−1) were added to a 60 µM DPPH MeOH solution (975 μL). The DPPH solution in the absence of the extract was used as negative control and MeOH was used as blank. The samples were incubated for 2 h in the dark at room temperature, and the decrease in absorbance was spectrophotometrically quantified at 515 nm (Shimadzu UV-B382). Measurements were performed in triplicate. The percentage of DPPH reduction was calculated using the following equation:
where A0 is the absorbance of the control and A1 is the absorbance in the presence of the extract.
The EC50, which corresponds to the extract concentration (g L−1) required for quenching 50 % of the initial DPPH radicals under a given experimental condition, was determined graphically (Brand-Williams et al. 1995). The same procedure was carried out with methanol solution of quercetin (0.01–0.15 g L−1), which was used as standard.
Qualitative DPPH assay on TLC
TLC was used to separate constituents of extracts as described above. To detect antioxidant activity, plates were sprayed with 0.2 % DPPH solution in methanol, and maintained in the dark for 1 h. The presence of antioxidant compounds was detected by yellow spots against a purple background (Masoko and Eloff 2007).
Statistical analysis
Roots and callus formation rates were expressed as percentage of responsive explants. All experiments were repeated twice, using four replicates each containing five explants. Data were subjected to analysis of variance (ANOVA) and comparisons of means were carried out with Tukey–Kramer comparisons test at 0.05 % significance level using the software GraphPad Instat.
Results
Adventitious root formation from stem segments
Stem segments of P. pohlii cultured on MSM supplemented with different concentrations of NAA, IBA or IAA, using the four culture systems tested, showed the formation of adventitious roots, at distinct frequencies (Table 1). In the presence of IBA or IAA, explants gave rise to small and thin roots at low frequencies (data not shown), while calluses and adventitious roots were observed in response to NAA, irrespective of the culture system.
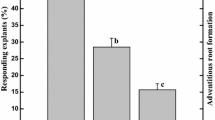
Internodal segments cultured on liquid MSM medium (static or under agitation) showed root and callus formation at low rates. However, when cultured on solid MSM medium or paper bridges over liquid medium supplemented with NAA 2.7 µM, in the dark, the same explants showed 100 % rhizogenesis, with low rates of callus formation (20 %; Table 1). Root formation occurred directly from the explants, after 12 days of culture. White and thin adventitious roots originated directly from the surface of the explants, turning into yellow and thick roots during the culture (Fig. 1a, b). Although the same regeneration pattern was observed in both culture systems, highest biomass accumulation was observed when explants were cultured on solid MSM medium (Table 2).
Adventitious root cultures of P. pohlii from stem and root segments excised from in vitro plants obtained in response to different NAA concentrations, light and distinct culture systems. a Adventitious roots from internodal segments cultured on MSM solid medium supplemented with 2.7 µM NAA for 60 days, in the dark; b Adventitious roots from internodal segments cultured on paper bridge over liquid MSM medium supplemented with 2.7 µM NAA for 60 days, in the dark; c Adventitious roots from nodal segments cultured on MSM solid medium supplemented with 0.54 µM NAA for 60 days, in the dark; d Root multiplication on double-phase MSM medium supplemented with 2.7 µM NAA, after 60 days in the dark; e Root multiplication on paper bridge over liquid MSM medium supplemented with 2.7 µM NAA, after 60 days in the dark; f–h Adventitious root proliferation from root segments cultured on liquid MSM medium supplemented with f 2.85 µM IAA, g 4.9 µM IBA and h 5.4 µM NAA, maintained under agitation for 60 days in the dark. Bar 1 cm
Nodal segments showed low rates of rhizogenesis, except when cultured on solid MSM medium supplemented with 0.54 µM NAA (Fig. 1c) or on paper bridges over liquid MSM medium supplemented with 2.7 µM NAA (90 and 80 %, respectively; Table 1). However, since these systems also resulted in high callus formation (100 %), they were not selected for further experiments. In addition, nodal explants cultured in the presence of different concentrations of IAA or IBA, showed only callogenesis in all culture systems (data not shown).
The proliferative capacity of the adventitious roots induced from internodal segments cultured on solid MSM medium supplemented with 2.7 µM NAA was evaluated on liquid medium under agitation, paper bridges over liquid medium or double-phase medium, with the same conditions of the induction phase. Roots maintained on liquid medium under agitation did not show increase of the biomass. On the other hand, root multiplication was observed on paper bridges, and in the double-phase system (Fig. 1d, e). The highest dry matter accumulation (0.334 g) was achieved from adventitious roots maintained on double-phase medium when compared to those maintained onto paper bridges (0.112 g) (Fig. 2). In addition, the material maintained in the double-phase system was morphologically similar to the roots induced from the stem segments. Newly formed roots were thin and white, turning into thick and yellowish during the culture. Root proliferation started after 7 days of culture, in darkness, and this multiplication capacity continued up to 60 days of culture, covering all medium surface. High proliferative capacity was sustained over repeated subcultures.
Root multiplication from root segments
Root segments excised from in vitro-grown plants of P. pohlii and cultured in the different culture systems tested (solid medium, paper bridges over liquid medium or double-phase medium) showed low proliferative capacity, independent of the type and concentration of the growth regulator used. On the contrary, a significant biomass increase was observed when root explants were cultured for 60 days on MSM liquid medium supplemented with IAA, IAB or NAA, and maintained under agitation (100 rpm) for 60 days, in the darkness (Fig. 1f–h).
The highest multiplication rate was achieved from explants cultured in the presence of 2.85 µM IAA (0.203 g dry weight), although no statistical differences were observed when compared to the other treatments (Fig. 3). Yellowish and thin roots were observed from these explants after 7 days of culture, with subsequent elongation after 12 days. On the other hand, roots obtained in response to NAA were smaller and thicker, similar to those obtained from the stem segments in response to the same growth regulator (Fig. 1h).
TLC analysis
TLC analysis of extracts from the different materials of P. pohlii using both vanillin-sulfuric acid solution and NP/PEG as color reagents did not reveal flavonoid-related spots (data not shown). On the other hand, saponin analysis of TLC plates showed that all extracts presented similar chromatographic profiles, with orange (RF = 0.58) and dark-blue spots (RF = 0.27–0.36), except for the extract from adventitious roots derived from internodal segments cultured on MSM medium supplemented with 2.70 µM NAA (Fig. 4). It was also possible to verify two other dark-brown and red spots (RF = 0.44 and 0.48, respectively) in extracts from primary and secondary roots from in vivo-grown plants.
Chromatograms of extracts of different plant materials of P. pohlii for saponins identification. 1-secondary roots of in vivo plants, 2-primary roots of in vivo plants, 3-roots of in vitro-grown plants, 4-leaves of in vivo plants, 5-leaves of in vitro-grown plants, 6-adventitious roots obtained from internodal segments, 7-adventitious roots obtained from roots segments. Mobile phase: chloroform:AcOH:MeOH:H2O (60:32:12:8 v/v), sprayed with anisaldehyde-H2SO4 before heating (100° C)
Determination of antioxidant potential
DPPH assay
The evaluation of the antioxidant potential of the extracts from different in vivo and in vitro materials of P. pohlli revealed the presence of substances with antioxidant activity, since all samples showed radical scavenging capacity (Table 3).
The EC50 values ranged from 446.70 to 0.18 g L−1. The highest antioxidant potentials were observed in extracts from primary and secondary roots excised from in vivo plants, which showed the lowest EC50 values (0.18 and 3.05 g L−1, respectively). On the other hand, extracts from adventitious roots derived from internodal segments displayed the lowest radical scavenging capacity (EC50 = 446.70 g L−1; Table 3).
Qualitative DPPH assay on TLC
The TLC-DPPH screening method indicated the presence of antioxidant compounds only in extracts from roots excised from in vivo-grown plants, after spraying the TLC plates with DPPH. The most prominent compounds were at the bottom of the TLC plate, and at RF = 0.2, indicating very polar substances. The degree of activity of all the samples tested was determined qualitatively from observation of the yellow color intensity (Fig. 5).
Chromatograms of extracts of different plant materials of P. pohlii for qualitative antioxidant evaluation. 1-secondary roots of in vivo plants, 2-primary roots of in vivo plants, 3-roots of in vitro-grown plants, 4-leaves of in vivo plants, 5-leaves of in vitro-grown plants. Mobile phase: chloroform:AcOH:MeOH:H2O (60:32:12:8 v/v), sprayed with 0.2 % DPPH solution
Discussion
Adventitious root cultures are often employed for the production of secondary metabolites, since these organs are potential sources of a great diversity of bioactive molecules (Bais et al. 2001; Flores et al. 1999). In this work, we established efficient protocols for the induction of adventitious roots from internodal and root segments of P. pohlii, in response to NAA and IAA, respectively, using different culture systems. Although most studies reports the auxin IBA as the most efficient for rhizogenesis, regardless the type of explants (Ludwig-Müller et al. 2005; Ling et al. 2009; Hasan and Hussein 2013), the highest rates of induction of adventitious roots from stem and root explants of P. pohlii were observed in response to NAA and IAA, respectively, at different concentrations and culture systems. However, roots obtained from both explants on media with NAA were morphologically different from those obtained in response to IAA or IBA, and displayed occasional callus formation. These aspects were also observed by Sudha and Seeni (2001) and Nandagopal and Kumari (2007), in adventitious roots cultures of Decalepsis arayalpathra and Cichorium intybus L., respectively.
The better efficiency for root induction in the absence of light was also observed in this work, except for the internodal segments cultured on liquid medium supplemented with 2.70 µM NAA, under agitation. This result is consistent with those reported for D. arayalpathra (Sudha and Seeni 2001), Eleutherococcus sessiliflorus (Jin-Wook et al. 2003) and C. intybus L. (Nandagopal and Kumari 2007), and can be related to the inhibitory effect of light on the activity of peroxidase and endogenous phenolic compounds, necessary to the metabolism of auxins and rhizogenesis induction (Druart et al. 1982).
Another important parameter evaluated in this work was the effect of different culture systems on the establishment of in vitro root cultures, which was specific for each explant type. Internodal segments showed the best root induction rates on medium solidified with agar, while root segments had higher proliferation rate in liquid medium under agitation. The success of liquid medium systems for adventitious root multiplication has already been reported by several authors (Sudha and Seeni 2001; Nandagopal and Kumari 2007; Cui et al. 2011) and can be assigned to the increased aeration, mitigation of the effects of the polarity and greater availability of water and nutrients for the whole explant surface (Adelberg and Toler 2004). However, the high root induction rates observed from stem explants cultured on solid medium may be attributed, in addition to the particular endogenous composition of each segment, to the presence of the gelling agent in the culture media, which may form a mesh that limits the access to nutrients and growth regulators, thus stimulating root development in order to overcome their unavailability in areas of the medium near the explants (Blidar et al. 2011; George et al. 2008).
Even though the induction of adventitious roots from internodal segments was successfully achieved in solid medium, the use of double-phase medium seemed to be more efficient for their multiplication. This behavior might be associated with the need for a physical support offered by the basal layer of solid medium, which allows the explants to be partially submerged into the medium, facilitating gas exchange between the tissue and the culture environment (George et al. 2008). Another advantage of the double-phase medium is the reduction of the manipulation during in vitro culture, since it is possible to add only fresh liquid medium, eliminating the need of periodic subcultures (Scherwinski-Pereira et al. 2012). Double-phase systems have been successfully reported for the micropropagation of japanese pear (Kadota et al. 2001) and pineapple (Scherwinski-Pereira et al. 2012). On the other hand, although filter paper bridges over liquid medium also act as a physical support, allowing the explants to be partially in contact with the liquid medium, with suitable gas exchange rates, this system was not as effective when compared to the use of the double-phase medium, probably due to the filter paper acting as a physical barrier, hindering the uptake of nutrients and growth regulators (De la Viña et al. 2001). Similar results were described by Savio et al. (2012) for the micropropagation of H. perforatum L., where the explants cultivated in liquid media under paper bridges showed low rates of development and multiplication, with tissue necrosis.
The phytochemical profile and antioxidant potential of the adventitious root cultures of P. pohlii, as well as the roots excised from in vivo and in vitro-grown plants, were also evaluated in this work. Different bioactive substances have already been described in Passiflora, including alkaloids, saponins and polyphenols, predominantly C-glycoside flavonoids (Dhawan et al. 2004). Flavonoids and saponins are considered suitable substances to be used as chemical markers in the genus due to their great structural diversity and chemical stability, allowing the differentiation of similar species, and contributing to the standardization of crude drugs (Gosmann et al. 2011).
TLC analysis was chosen as a preliminary technique in order to detect flavonoids and saponins in extracts of different plant materials of P. pohlii, since it is a valuable fast, low cost method for the identification of phytochemicals (Birk et al. 2005). Although no flavonoid-related spots could be detected among the tested materials, all extracts of in vivo and in vitro-derived materials of P. pohlii showed saponin regions indicated by the formation of red-violet, blue and dark-brown spots after revealing with anisaldehyde-sulphuric acid reagent, except for the extract from adventitious roots derived from internodal segments cultured on MSM medium supplemented with 2.70 µM NAA. The anisaldehyde reacts with the sulphuric acid giving colored products in the presence of aglycones (Wagner and Bladt 2001; Oleszek et al. 2008).
The evaluation of the antioxidant potential of the different plant materials of P. pohlii was another aspect of this work reported for the first time. The antioxidant activity of extracts of other Passiflora species has already been confirmed by in vivo (Rudnicki et al. 2007a; Rasool et al. 2011; Silva et al. 2013) and in vitro assays (Ali et al. 2010; Rudnicki et al. 2007b), and has been associated with the presence of C-glycoside flavonoids. These natural antioxidants, which may display great structural diversity, have been widely used in the last years due to their beneficial biological effects, especially the prevention of cardiovascular and neurodegenerative diseases (Kris-Etherton et al. 2002; Nilsson et al. 2005). In addition, they have also been exploited by the cosmetic industry (Gesztesi and Da Luz 2007).
Several techniques have been used for the determination of the antioxidant potential of plant extracts, and the most common is the DPPH assay. In Passiflora, this assay has already been used for the evaluation of the antioxidant capacity of extracts from leaves (Ferreres et al. 2007; Sunitha and Devaki 2009; Sasikala et al. 2011; Saptarini et al. 2013; Lugato et al. 2014; Ramaiya et al. 2014), fruits (Ali et al. 2010; Sasikala et al. 2011; Zeraik and Yariwake 2012; Simirgiotis et al. 2013; Gil et al. 2014) and seeds (Malacrida and Jorge 2012), in addition to in vitro-derived materials (Antognoni et al. 2007; Lugato et al. 2014). On the other hand, there are only a few reports on the determination of antioxidant potential of the roots of the Passiflora species. Sasikala et al. (2011) used the DPPH assay for the evaluation of the antioxidant potential of root extracts of P. foetida obtained with different solvents, and the highest rate of radical inhibition was observed with the extract in petroleum ether (13.97 %), followed by the extracts prepared with ethanol (11.52 %) and hot water (11.2 %). In this work, the highest antioxidant activity was observed in the ethanolic extracts of primary and secondary roots of in vivo-grown plants, which showed the highest values of DPPH quenching (91 % in both extracts) and, thus, the lowest values of EC50 (0.18 and 3.07 g L−1 respectively).
One of the main disadvantages of spectrophotometric methods to evaluate the quenching ability of free radicals is associated with the impossibility to determine which substances in the extract are responsible for the antioxidant activity (Ciesla et al. 2012). Therefore, the DPPH assay using TLC plates may be employed as an alternative for a qualitative analysis, since it allows the localization and identification of substances with antioxidant potential (Masoko and Eloff 2007). Most of the TLC-DPPH assays reported antioxidant activities associated with the presence of polyphenols, especially flavonoids and phenolic acids, since these substances act as free radical scavengers, due to their ability to donate hydrogen or electrons (Ciesla et al. 2012). However, the efficiency of phenolic compounds as antiradicals or antioxidants is diverse and may depend on several factors, including the number of hydroxyls, the ligation site and the position of these on the aromatic ring (Sroka and Cisowski 2003). In this work, the lowest EC50 value and, thus, the highest antioxidant activity was observed in extracts of primary and secondary roots of in vivo-grown plants. These results were corroborated by the high intensity of the spots detected in the same samples by the TLC-DPPH assay, using saponin-specific conditions. In view of these results, we suggest that the capacity of quenching free radicals observed in the extracts of roots of in vivo-grown plants of P. pohlii is probably related to the presence of saponins, which antioxidant activity has already been demonstrated by several authors (Sur et al. 2001; Francis et al. 2002; Chen et al. 2014).
The in vitro root culture strategies described in this work were successfully applied for P. pohlii. Saponins that are present in the roots showed antioxidant activity when analysed by DPPH and TLC-DPPH assays. These results demonstrate, for the first time, the biotechnological and pharmacological potential of this species.
References
Adelberg J, Toler J (2004) Comparison of agar and thin-film liquid system for micropropagation of ornamental Alocasia and Colocasia. HortSci 39(5):1088–1109
Ali MA, Devi LI, Nayan V, Chanu KV, Ralte L (2010) Antioxidant activity of fruits available in Aizawl market of Mizoram, India. Int J Biol Pharm Res 1(2):76–81
Antognoni F, Zheng S, Pagnucco C, Baraldi R, Poli F, Biondi S (2007) Induction of flavonoid production by UV-B radiation in Passiflora quadrangularis callus cultures. Fitoter 78:345–352
Bais HP, Loyola-Vargas VM, Flores HE, Vivanco JM (2001) Root specific metabolism: the biology and biochemistry of underground organs. In Vitro Cell Dev Biol-Plant 37(6):730–741
Bernacci LC, Cervi AC, Milward-De-Azevedo MA, Nunes TS, Imig DC, Mezzonato AC (2013) Passifloraceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB12506. Accessed 10 Nov 2013
Birk CD, Provensi G, Gosmann G (2005) TLC fingerprint of flavonoids and saponins from Passiflora species. J Liq Chromatogr Relat Technol 28:2285–2291
Blidar CF, Ardelean A, Turcus V (2011) Efficient initiation of in vitro culture at wheat. Fasc Biol 18(2):176–181
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Food Sci Technol 28:25–30
Chen Y, Miao Y, Huang L, Li J, Sun H, Zhao Y, Yang J, Zhou W (2014) Antioxidant activities of saponins extracted from Radix Trichosanthis: an in vivo and in vitro evaluation. BMC Complement Altern Med 14:1–8
Choi SM, Son SH, Yun SR, Kwon OW, Seon JH, Paek KY (2000) Pilot-scale culture of adventitious roots of ginseng in a bioreactor system. Plant Cell, Tissue Organ Cult 62(3):187–193
Ciesla L, Kryszen J, Stochmal A, Oleszek W, Waksmundzka-Hajnos M (2012) Approach to develop a standardized TLC-DPPH test for assessing free radical scavenging properties of selected phenolic compounds. J Pharm Biomed Anal 70:126–135
Cui X, Murthy HN, Jin Y, Yim Y, Kim J, Paek K (2011) Production of adventitious root biomass and secondary metabolites of Hypericum perforatum L. in a balloon type airlift reactor. Bioresour Technol 102:10072–10079
De La Viña G, Barceló-Muñoz A, Pliego-Alfaro F (2001) Effect of culture media and irradiance level on growth and morphology of Persea americana Mill microcuttings. Plant Cell, Tissue and Organ Cult 65:229–237
Dhawan K, Dhawan S, Sharma A (2004) Passiflora: a review update. J Ethnopharmacol 94:1–23
Druart P, Kevers C, Boxus P, Gaspar T (1982) In vitro promotion of root formation by apple shoots through darkness effect on endogenous phenols and peroxidases. Zeitschrift für Pflanzenphysiologie 108(5):429–436
Ferreres F, Sousa C, Valentão P, Andrade PB, Seabra RM, Gil-Izquierdo A (2007) New C-deoxyhexosyl flavones and antioxidant properties of Passiflora edulis leaf extract. J Agric Food Chem 55(25):10187–10193
Flores HE, Vivanco JM, Loyola-Vargas VM (1999) “Radicle” biochemistry: the biology of root-specific metabolism. Trends Plant Sci 4(6):220–226
Francis G, Kerem Z, Makkar HPS, Becker K (2002) The biological action of saponins in animal systems: a review. Br J Nutr 88:587–605
George EF, Hall MA, De Klerk G (2008) Plant propagation by tissue culture. Springer, Dordrecht
Gesztesi LJ, Da Luz PM (2007) Procede pour la preparation d’um extrait vegetal de Passiflora alata et utilization dudit extrait dans dês compositions cosmetiques et pharmaceutiques. Patent FR2920310. August 3
Gil M, Restrepo A, Millán L, Alzate L, Rojano B (2014) Microencapsulation of banana passion fruit (Passiflora tripartita var. mollissima): a new alternative as a natural additive as antioxidant. Food Nutr Sci 5:671–682
Gosmann G, Provensi G, Comunello LN, Rates SMK (2011) Composição química e aspectos farmacológicos de espécies de Passiflora L. (Passifloraceae). Rev Bras Bioc 9(1):88–99
Ha YW, Na YC, Seo JJ, Kima SN, Linhardt RJ, Kim YS (2006) Qualitative and quantitative determination of ten major saponins in Platycodi Radix by high performance liquid chromatography with evaporative light scattering detection and mass spectrometry. J Chromatogr A 1135:27–35
Hasan N, Hussein S (2013) Adventitious root induction of Labisia pumila in respond to plant growth regulators and different type of explant. Sci News-Lett 7(1):9–18
Jin-Wook S, Cha-Gyun S, Yong-Eui C (2003) Mass production of adventitious roots of Eleutherococcus sessiliflorus through the bioreactor culture. J Plant Biotechnol 5(3):187–191
Junqueira NTV, Braga MF, Faleiro FG, Peixoto JR, Bernacci LC (2005) Potencial de espécies silvestres de maracujazeiro como fonte de resistência a doenças. In: Faleiro FG, Junqueira NTV, Braga MF (eds) Passionfruit: germplasm and breeding, 1st edn. Embrapa Cerrados, Planaltina, pp 81–108
Kadota M, Imizu K, Hirano T (2001) Double-phase in vitro culture using sorbitol increases shoot proliferation and reduces hyperhydricty in Japanese pear. Sci Hortic 89:207–215
Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Kang SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD (2002) Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med 113:71–88
Ling APK, Kok KM, Hussein S, Ong SL (2009) Effects of plant growth regulators on adventitious roots induction from different explants of Orthosiphon stamineus. Am-Eurasian J Sustain Agric 3(3):493–501
Ludwig-Müller J, Vertocnik A, Town CD (2005) Analysis of indole-3-butyric acid-induced adventitious root formation on Arabidopsis stem segments. J Exp Bot 56(418):2095–2105
Lugato D, Simão MJ, Garcia R, Mansur E, Pacheco G (2014) Determination of antioxidant activity and phenolic content of extracts from in vivo plants and in vitro materials of Passiflora alata Curtis. Plant Cell, Tissue Organ Cult 118:339–346
Malacrida CR, Jorge N (2012) Yellow passion fruit seed oil (Passiflora edulis f. flavicarpa): physical and chemical characteristics. Braz Arch Biol Technol 55(1):127–134
Masoko P, Eloff JN (2007) Screening of twenty-four south african combretum and six Terminalia species (Combretaceae) for antioxidant activities. Afr J Tradit Complement Altern Med 4(2):231–239
Merhy TSM (2014) Tissue culture, in vitro conservation and assessment of genetic stability of Passiflora pohlii Mast. Thesis, Federal University of Rio de Janeiro
Merhy TSM, Vianna MG, Garcia RO, Pacheco G, Mansur E (2014) Cryopreservation and assessment of genetic stability by RAPD and ISSR of Passiflora pohlii. Cryo Lett 35(3):204–215
Monteiro ACBA, Higashi EN, Gonçalves AN, Rodriguez APM (2000) A novel approach for the definition of the inorganic medium components for micropropagation of yellow passion fruit (Passiflora edulis Sims. f. flavicarpa Deg.). In Vitro Cell Dev Biol-Plant 36(6):527–531
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Nandagopal S, Kumari BDR (2007) Effectiveness of auxin induced in vitro root culture in chicory. J Cent Eur Agric 8(1):73–80
Nilsson J, Pillai D, Önning G, Persson C, Nilsson A, Akesson B (2005) Comparison of the 2,2′-azinobis-3-ethylbenzotiazo-line-6-sulfonic acid (ABTS) and ferric reducing anti-oxidant power (FRAP) methods to assess the total antioxidant capacity in extracts of fruits and vegetables. Mol Nutr Food Res 49:239–246
Oleszek W, Kapusta I, Stochmal A (2008) TLC of triterpenes (including saponins). In: Hajnos MW, Sherma J, Kowalska T (eds) Thin layer chromatography in phytochemistry, 1st edn. Taylor & Francis Group, Boca Raton, pp 519–541
Paek KY, Murthy HN, Hahn EJ (2009) Establishment of adventitious root cultures of Echinacea purpurea for the production of caffeic acid derivatives. Methods Mol Biol 547:3–16
Ramaiya SD, Bujang JS, Zakaria MH (2014) Assessment of total phenolic, antioxidant, and antibacterial activities of Passiflora species. Sci World J 2014:1–10
Rasool SN, Jaheerunnisa S, Jayaveera KN, Suresh Kumar C (2011) In vitro callus induction and in vivo antioxidant activity of Passiflora foetida L. leaves. Int J App Res Nat Prod 4(1):1–10
Rudnicki M, Silveira MM, Pereira TV, Oliveira MR, Reginatto FH, Dal-Pizzol F, Moreira JCF (2007a) Protective effects of Passiflora alata extract pretreatment on carbon tetrachloride induced oxidative damage in rats. Food Chem Toxicol 45:656–661
Rudnicki M, Oliveira MR, Pereira TV, Reginatto FH, Dal-Pizzol F, Moreira JCF (2007b) Antioxidant and antiglycation properties of Passiflora alata and Passiflora edulis extracts. Food Chem 100:719–724
Saptarini NM, Wardati Y, Juliawati R (2013) Antioxidant activity of extract and fraction of yellow passion fruit (Passiflora flavicarpa) leaves. Int J Pharm Pharm Sci 5(2):194–196
Sasikala V, Saravanan S, Parimelazhagan T (2011) Analgesic and anti-inflammatory activities of Passiflora foetida L. Asian Pac J Trop Med 4(8):600–603
Savio LEB, Astarita LV, Santarém ER (2012) Secondary metabolism in micropropagated Hypericum perforatum L. grown in non-aerated liquid medium. Plant Cell, Tissue Organ Cult 108(3):465–472
Scherwinski-Pereira JE, Lima ECA, Silva TL, Mesquita AGG, Maciel SA, Costa FHS (2012) Double-phase culture system for large scale production of pineapple. Plant Cell, Tissue Organ Cult 109:263–269
Silva JK, Cazarin CBB, Colomeu TC, Batista AG, Meletti LMM, Paschoal JAR, Bogusz Júnior S, Furlan MF, Reyes FGR, Augusto F, Maróstica Júnior MR, Zollner RL (2013) Antioxidant activity of aqueous extract of passion fruit (Passiflora edulis) leaves: in vitro and in vivo study. Food Res Int 53:882–890
Simirgiotis MJ, Schmeda-Hirschmann G, Bórquez J, Kennelly EJ (2013) The Passiflora tripartita (Banana Passion) fruit: a source of bioactive flavonoid C-glycosides isolated by HSCCC and characterized by HPLC-DAD-ESI/MS/MS. Mol 18:1672–1692
Sivakumar G (2006) Bioreactor technology: a novel industrial tool for high-tech production of bioactive molecules and biopharmaceuticals from plant roots. Biotechnol J 1:1419–1427
Sroka Z, Cisowski W (2003) Hydrogen peroxide scavenging antioxidant and anti-radical activity of some phenolic acids. Food Chem Toxicol 41:753–758
Sudha CG, Seeni S (2001) Establishment and analysis of fast-growing normal root culture of Decalepis arayalpathra, a rare endemic medicinal plant. Curr Sci 81(4):371–374
Sun F, He Q, Shi P, Xiao P, Cheng Y (2007) Characterization and identification of tripernoid saponins in crude extracts from Clematis spp. by high performance liquid chromatography/eletrospray ionization with multi-stage tandem mass spectrometry. Rapid Commun Mass Spectrom 21:3743–3750
Sunitha M, Devaki K (2009) Antioxidant activity of Passiflora edulis Sims leaves. Indian J Pharm Sci 71(3):310–311
Sur P, Chaudhuri T, Vedasiromoni JR, Gomes A, Ganguly DK (2001) Antiinflamatory and antioxidant property of saponins of tea [Camellia sinensis (L) O. Kuntze] root extract. Phytother Res 15:174–176
Verstraeten I, Beeckman T, Geelen D (2013) Adventitious root induction in Arabidopsis thaliana as a model for in vitro root organogenesis. Methods Mol Biol 959:159–175
Wagner H, Bladt S (2001) Plant drug analysis: a thin layer chromatography atlas. Springer, Berlin
Wilson SA, Roberts SC (2012) Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol J 10(3):249–268
Wu C, Dewir YH, Hahn E, Paek K (2006) Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. J Plant Biol 49(3):193–199
Zeraik ML, Yariwake JH (2012) Analysis of passion fruits rinds (Passiflora edulis): isoorientin quantification by HPTLC and evaluation of antioxidante (radical scavenging) capacity. Quim Nova 35(3):541–545
Acknowledgments
The authors acknowledge the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) for financial support. Mariela Simão is a recipient of a scholarship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). E. Mansur is a recipient of a research fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simão, M.J., Fonseca, E., Garcia, R. et al. Effects of auxins and different culture systems on the adventitious root development of Passiflora pohlii Mast. and their ability to produce antioxidant compounds. Plant Cell Tiss Organ Cult 124, 419–430 (2016). https://doi.org/10.1007/s11240-015-0904-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0904-2