Abstract
Withania somnifera is an important medicinal plant that contains withanolides as bioactive compounds. We have investigated the effects of macroelements and nitrogen source in hairy roots of W. somnifera with the aim of optimizing the production of biomass and withanolide A content. The effects of the macroelements NH4NO3, KNO3, CaCl2, MgSO4 and KH2PO4 at concentrations of 0, 0.5, 1.0, 1.5 and 2.0× strengths and of nitrogen source [NH4 +/NO3 − (0.00/18.80, 7.19/18.80, 14.38/18.80, 21.57/18.80, 28.75/18.80, 14.38/0.00, 14.38/9.40, 14.38/18.80, 14.38/28.20 and 14.38/37.60 mM)] in Murashige and Skoog medium were evaluated for biomass and withanolide A production. The highest accumulation of biomass (139.42 g l−1 FW and 13.11 g l−1 DW) was recorded in the medium with 2.0× concentration of KH2PO4, and the highest production of withanolide A was recorded with 2.0× KNO3 (15.27 mg g−1 DW). The NH4 +/NO3 − ratio also influenced root growth and withanolide A production, with both parameters being larger when the NO3 − concentration was higher than that of NH4 +. Maximum biomass growth (148.17 g l−1 FW and 14.79 g l−1 DW) was achieved at NH4 +/NO3 − ratio of 14.38/37.60 mM, while withanolide A production was greatest (14.68 mg g−1 DW) when the NH4 +/NO3 − ratio was 0.00/18.80 mM. The results are useful for the large scale cultivation of Withania hairy root culture for the production of withanolide A.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Withania somnifera, also known as ashwagandha, Indian ginseng and winter cherry is an important medicinal plant in ayurvedic medicine, the traditional medicinal system of India (Gupta and Rana 2007). It has been used as a tonic and antistress supplement. Pharmacological activities include physiologic and metabolic restoration, antiarthritic, antiaging, nerve tonic, cognitive function improvement in geriatric states, and recovery from neurodegenerative disorders (Bhattacharaya et al. 2002; Dhuley 2000). Various alkaloids, withanolides and sitoindosides have been isolated from this plant. Of the various withanolides reported, withaferin A and withanone are customary major withanolides of the plant whereas the amount of withanolide A is usually very low (Zhao et al. 2002). Recently, withanolide A has attracted interest due to its strong neuropharmacological properties of promoting outgrowth and synaptic reconstruction (Kuboyama et al. 2005; Tohda et al. 2005a, Tohda et al. 2005b). Withanolide A is therefore an important candidate for the therapeutic treatment of neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, convulsions, cognitive function impairment, as it is able to reconstruct neural networks (Tohda et al. 2005a).
Plant cell and organ culture systems are promising methodologies for obtaining valuable plant-specific metabolites (Verpoorte et al. 2002). Cell and organ cultures have a higher rate of metabolism than field-grown plants because the initiation of cell and organ growth in culture leads to the rapid proliferation of cells/organs and to a condensed biosynthetic cycle (Ramachandra Rao and Ravishankar 2002). Further, plant cell/organ cultures are not limited by environmental, ecological and climatic conditions, and the cells/organs can thus proliferate at higher growth rates than the cultivated whole plant. Several biotechnological advances have been made in tissue culture methodologies that have improved secondary metabolite production, such as the optimization of cultural conditions, the selection of high-producing strains of lines, precursor feeding, elicitation, metabolic engineering, transformed root cultures, micropropagation, and bioreactor cultures, among others (Sarin 2005). Production of withanolide A has been reported in hairy root cultures of W. somnifera (Murthy et al. 2008). In an earlier publication, we reported that withanolide A production depends on carbon source and medium pH in hairy root culture of W. somnifera (Praveen and Murthy 2012). Further enhancement of biomass and withanolide A accumulation can also be achieved by manipulation of the medium composition. To date, there have been no reports on the effect of macro elements and nitrogen source on the biomass accumulation and withanolide A production in hairy root cultures of W. somnifera. Therefore, we have investigated the effects of different macro elements and nitrogen source on hairy root growth of W. somnifera in terms of biomass accumulation and withanolide A production.
Materials and methods
Hairy root culture
Withania somnifera (Indian ginseng) was transformed by Agrobacterium rhizogenes. Explants from seedling cotyledons and young leaves were inoculated with A. rhizogenes strain R1601, and hairy roots were induced from these explants. The transgenic status of hairy roots was confirmed by PCR using nptII and rolB specific primers and, subsequently, by Southern analysis for the presence of nptII and rolB genes in the genomes of transformed roots (Murthy et al. 2008). The hairy root cultures were initiated by culturing 500 mg W. somnifera hairy roots in 250 ml Erlenmeyer’s flasks each containing 50 ml of MS medium (Murashige and Skoog 1962) supplemented with 3 % sucrose (Murthy et al. 2008). The initial medium pH was adjusted to 5.8 ± 0.2 before autoclaving (at 121 °C and 1.2 kg cm2 pressure for 15 min), and the cultures were kept under continuous agitation at 110 rpm in an orbital shaker (Orbitek, Scigenics, Chennai, India) and incubated at 25 ± 2 °C with a 16 h photoperiod (40 μmol m−2 s−1) provided by 40 W white fluorescent lamps (Philips, Kolkata, India). The roots were subcultured every 15 days.
Effect of varying concentrations of macro elements in the culture medium
Five hundred milligram of hairy roots was cultured in 250 ml Erlenmeyer’s flasks containing 50 ml of MS medium supplemented with 3 % sucrose. The levels of NH4NO3, KNO3, CaCl2, MgSO4 and KH2PO4 in the MS medium were each varied at 0, 0.5, 1.0, 1.5 and 2.0× strengths, respectively, of the normal concentration.
Effects of nitrogen source on hairy root growth and withanolide A production
Hairy root cultures were established as described above, and the medium was altered with respect to nitrogen source; the following ratios of NH4 +/NO3 − (mM/mM) were used: 0.00/18.80, 7.19/18.80, 14.38/18.80, 21.57/18.80, 28.75/18.80, 14.38/0.00, 14.38/9.40, 14.38/18.80, 14.38/28.20 and 14.38/37.60. All the cultures were kept under continuous agitation at 110 rpm in an orbital shaker (Orbitek) and incubated as described for the initiation of hairy root cultures. Root biomass growth and withanolide A productivity were measured after 4 weeks of culture.
Determination of root biomass
The roots were separated from the media by passing them through a 0.45 μm stainless steel sieve (Sigma, USA). Their fresh weights (FW) were determined after they were washed with distilled water and the excess surface water blotted away. Dry weights (DW) were recorded after the roots were dried at 60 °C till constant weight is recorded. The growth rate was determined as GR = (harvested dry weight-inoculated dry weight)/inoculated dry weight.
Extraction and HPLC analysis
Extraction and HPLC analysis of withanolide A was carried out by following the method of Ganzera et al. (2003). Hundred milligram of powdered hairy root material was extracted with 2 ml of methanol by sonication for 20 min after centrifugation (5 min at 3,000 rpm), the extracts were combined and diluted with equal volume of methanol. The samples were filtered through a 0.45 μm nylon membrane filters and then subjected for the HPLC analysis. The withanolide fractions were analyzed using HPLC system (Waters 2487, Milford, CT, USA) equipped with Phenomenex C18, 5 μm (4.6 × 250 mm) column. The mobile phase was a mixture of reagent alcohol and water (80:20, v/v) at flow rate of 1 ml/min and the column temperature was maintained at 30 °C. The detection wave length was set at 230 nm. The injection volume was 20 μl. The chromatography system was equilibrated by the mobile phase. Withanolide A standard was obtained from Chromadex Inc. (Laguna Hills, CA, USA). All the experiments were set up in a completely randomized design, and the data were subjected to Duncan’s multiple range test using SPSS software version 9.0.
Results and discussion
Effect of macro elements on hairy root growth and withanolide A production
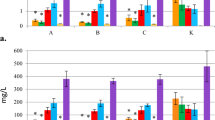
The effect of different macroelements and different concentrations of these macroelements play an important role in biomass accumulation, and secondary metabolite production in suspension culture systems (Wu and Zhong 1999). In our study, the highest accumulation of biomass (139.42 g l−1 FW and 13.11 g l−1 DW) was recorded in the medium containing 2.0× concentration of KH2PO4, followed by that containing 2.0× KNO3, (137.87 g l−1 FW and 13.69 g l−1 DW) (Table 1). The highest production of withanolide A content was recorded in the medium with 2.0× KNO3, (15.27 mg g−1 DW), followed by 2.0× KH2PO4 (14.68 mg g−1 DW) (Fig. 1).
Withanolide A content in Withania hairy root cultures as affected by different concentrations of macroelements. Hairy roots (500 mg) were cultured in 250 ml Erlenmeyer flasks containing 50 ml of MS medium supplemented with 3 % sucrose for 4 weeks. Data represents mean values ± SE of three replicates; each experiment was repeated twice. Means with common letters are not significantly different at P ≤ 0.05 according to Duncan’s multiple range test
Effect of NH4NO3 on hairy root growth and withanolide A production
Medium supplemented with 0.5× NH4NO3 was responsible for maximum hairy root biomass (127.65 g l−1 of FW and 12.74 g l−1 of DW) with a growth rate of 10.11 and resulted in the optimal production of withanolide A (14.37 mg g−1 DW). Yu et al. (2001) also reported that 0.5× concentration of NH4PO3 in SH medium favored the biomass accumulation and the NH4 + free medium resulted in the maximum production of ginsenoside from adventitious roots of ginseng. Murthy and Praveen (2012) also reported that 0.5× concentration of NH4NO3 in the MS medium favored the biomass accumulation and withanolide A production from adventitious roots of W. somnifera.
Effect of KNO3 on hairy root growth and withanolide A production
Higher strength of 2.0× KNO3 was responsible for optimal accumulation of biomass (137.87 g l−1 FW and 13.69 g l−1 DW) and withanolide-A content (15.27 mg g−1 DW). Similar results were reported with the adventitious root cultures of W. somnifera for the production of withanolide A (Murthy and Praveen 2012). Whereas, Yu et al. (2001) reported that 1.0× KNO3 resulted in the highest accumulation of biomass, and 2.0× strength medium favored ginsenoside production from adventitious root suspension cultures of Panax ginseng.
Effect of CaCl2 on hairy root growth and withanolide A production
Among different concentrations of CaCl2 tested, 2.0× strength resulted in the highest accumulation of biomass (133.18 g l−1 FW and 13.28 g l−1 DW) and withanolide A (12.62 mg g−1 DW). Similar to our results, 2.0× CaCl2 favored the highest biomass accumulation and ginsenoside production from ginseng adventitious root culture (Yu et al. 2001) and withanolide A production from W. somnifera adventitious root culture (Murthy and Praveen 2012).
Effect of MgSO4 on hairy root growth and withanolide A production
A higher strength of 1.5× MgSO4 favored the highest accumulation of biomass (134.85 g l−1 FW and 13.50 g l−1 DW), and 2.0× MgSO4 favored the highest production of withanolide A (14.17 mg g−1 DW). Similarly, 1.5× MgSO4 favored the highest accumulation of biomass, whereas 2.0× MgSO4 favored the maximum production of ginsenoside (Yu et al. 2001).
Effect of KH2PO4 on hairy root growth and withanolide A production
Among various concentrations of KH2PO4 tested, 2.0× favored the highest accumulation of biomass (139.42 g l−1 FW and 13.11 g l−1 DW) and withanolide A (14.68 mg g−1 DW; Table 1, Fig. 1). Similar to our results, higher concentrations of KH2PO4 favored the biomass accumulation and withanolide A production in adventitious root cultures of W. somnifera (Murthy and Praveen 2012). In cell suspension cultures of P. ginseng and P. quinquefolium, a low initial concentration of phosphate in the medium sufficiently promoted both cell growth and ginsenoside accumulation (Liu and Zhong 1998). Mantell and Smith (1983) reported that lack of phosphate stimulated secondary metabolite biosynthesis. Therefore, optimizing macro elements concentration, especially for nitrogen and phosphate in the culture media is a key step towards higher production of secondary metabolites in plant cell, tissue and organ cultures.
Effects of nitrogen source on hairy root growth and withanolide A production
Nitrogen concentration affects the level of proteinaceous or amino acid products in cell suspension cultures. The ratio of the ammonia/nitrate-nitrogen and overall levels of total nitrogen markedly affect the production of secondary plant products. In the present study, maximum root biomass (148.17 g l−1 FW and 14.79 g l−1 DW) was achieved at a NH4 +/NO3 − ratios of 14.38/37.60 mM, followed by 7.19/18.80 mM of NH4 +/NO3 − ratios which produced the biomass of 140.45 g l−1 FW and 13.29 g l−1 DW (Table 2). The maximum production of withanolide A (14.68 mg g−1 DW) was found with an NH4 +/NO3 − ratios of 0.00/18.80 mM, followed by 7.19/18.80 mM of NH4 +/NO3 − ratios which produced the withanolide A content of 12.56 mg g−1 DW (Fig. 2). In contrast, root growth and the accumulation of withanolide A were severely inhibited when the NH4 + concentration was increased. Liu and Zhong (1997) also reported increased root growth of P. ginseng at low NH4 +/NO3 − ratios, as well as the greatest ginsenoside productivity in the absence of NH4 +. Maximum levels of saponin and polysaccharide were also obtained when NO3 − was the sole nitrogen source (Zhong and Wang 1998). These results suggest that the nitrate and ammonium ions have differential effects on secondary metabolism in plant cell and tissue cultures. Many reports have confirmed that root growth as well as secondary metabolite accumulation is promoted under the influence of NO3 − in hairy root cultures of Artemisia annua (Wang and Tan 2002) and in cell suspension cultures of W. somnifera, where the biomass accumulation was maximum at lower concentration of NH4 + and the withanolide A production was increased under the influence of NO3 − (Nagella and Murthy 2011). Whereas in Camptotheca acuminate cell suspension cultures, the cell dry weight was improved in the medium with the higher NO3 −/NH4 + ratio (60:0 mM), and for secondary metabolism, a high ratio of NH4 +/NO3 − (5:1) was favourable for camptothecin production (Pan et al. 2004).
Withanolide A content in hairy root cultures as affected by different ratio of NH4 +/NO3 − in the MS medium. Hairy roots (500 mg) were cultured in 250 ml Erlenmeyer flasks containing 50 ml of MS medium supplemented with 3 % sucrose for 4 weeks. Data represents mean values ± SE of three replicates; each experiment was repeated twice. Means with common letters are not significantly different at P ≤ 0.05 according to Duncan’s multiple range test
Conclusion
It is evident from our present study on flask scale hairy root cultures from W. somnifera that both the biomass and secondary metabolite accumulation were influenced by macro elements and ammonia-nitrate ratio. The highest accumulation of biomass was recorded in the medium with 2.0× concentration of KH2PO4 and withanolide A was recorded in the medium with 2.0× concentration of KNO3. The moderate concentration of 14.38 mM of NH4 + with higher concentration of 37.60 mM of NO3 − favoured the highest accumulation of biomass and maximum production of withanolide A was recorded in the absence of NH4 + with moderate concentration of NO3 − (18.80 mM). The above results are useful for the large scale cultivation of Withania hairy root culture for the production of withanolide A.
Author contributions
H. N. Murthy provided the idea and supervised the research work, N. Praveen designed and performed the experiments. H. N. Murthy and N. Praveen wrote the manuscript and approved it.
References
Bhattacharaya SK, Bhattacharya D, Sairam K, Ghosal S (2002) Effect of Withania somnifera glycowithanolides on rat model of tardie dyskinesia. Pytomedicine 9:167–170
Dhuley JN (2000) Adaptogenic and cardioprotective action of aswagandha in rats and frogs. J Ethanopharmacol 70:57–63
Ganzera M, Choudhary MI, Khan IA (2003) Quantitative HPLC analysis of withanolides in Withania somnifera. Fitoterapia 74:68–76
Gupta GL, Rana AC (2007) Withania somnifera (Ashwagandha): a review. Pharmacog Rev 1:129–136
Kuboyama T, Tohda C, Komatsu K (2005) Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br J Pharmacol 144:961–971
Liu S, Zhong JJ (1997) Simultaneous production of ginseng saponin and polysaccharide by suspension cultures of Panax ginseng: nitrogen effects. Enz Microb Technol 21:518–524
Liu S, Zhong JJ (1998) Phosphate effect of production of ginseng saponin and polysaccharide by cell suspension cultures of Panax ginseng and Panax quinquefolium. Process Biochem 33:69–74
Mantell SH, Smith H (1983) Cultural factors that influence secondary metabolite accumulation in plant cell and tissue cultures. In: Mantell SH, Smith H (eds) Plant Biotechnology. Society for Experimental Biology Seminar Series 18. Cambridge University Press, Cambridge, 75–108
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy HN, Praveen N (2012) Influence of macro elements and nitrogen source on adventitious root growth and withanolide A production in Withania somnifera (L.) Dunal. Nat Prod Res 26:466–473
Murthy HN, Dijkstra C, Anthony P, White DA, Davey MR, Power JB, Hahn EJ, Paek KY (2008) Establishment of Withania somnifera hairy root cultures for the production of withanolide A. J Int Plant Biol 50:975–981
Nagella P, Murthy HN (2011) Effects of macroelements and nitrogen source on biomass accumulation and withanolide A production from cell suspension cultures of Withania somnifera (L.) Dunal. Plant Cell Tiss Organ Cult 104:119–124
Pan XW, Xu HH, Liu X, Gao X, Lu YT (2004) Improvement of growth and camptothecin yield by altering nitrogen source supply in cell suspension cultures of Camptotheca acuminate. Biotechnol Lett 26:1745–1748
Praveen N, Murthy HN (2012) Synthesis of withanolide A depends on carbon source and medium pH in hairy root cultures of Withania somnifera. Ind Crops Prod 35:241–243
Ramachandra Rao S, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotech Adv 20:10–153
Sarin R (2005) Useful metabolites from plant tissue cultures. Biotechnology 4:79–93
Tohda C, Kuboyama T, Komatsu K (2005a) Search for natural products related to regeneration the neuronal network. Neurosignals 14:34–45
Tohda C, Komatsu K, Kuboyama T (2005b) Scientific basis of anti-dementia drugs of constituents from ashwagandha (Withania somnifera). J Tad Med 22:176–182
Verpoorte R, Contin A, Memelink J (2002) Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1:13–25
Wang JW, Tan RX (2002) Artemisinin production in Artemisia annua hairy root cultures with improved growth by altering the nitrogen source in the medium. Biotechnol Lett 24:1153–1156
Wu Y, Zhong JJ (1999) Production of ginseng and its bioactive components in plant cell culture: current technological and applied aspects. J Biotechnol 68:89–99
Yu KW, Gao WY, Hahn EJ, Paek KY (2001) Effects of macro elements and nitrogen source on adventitious root growth and ginsenoside production in ginseng (Panax ginseng C. A. Meyer). J Plant Biol 44:179–184
Zhao J, Nakamura N, Hattori M, Kuboyama T, Tohda C, Komatsu K (2002) Withanolide derivatives from roots of Withania somnifera and their neurite outgrowth activities. Chem Pharm Bull 50:760–765
Zhong JJ, Wang SJ (1998) Effects of nitrogen source on the production of ginseng saponins and polysaccharide by cell cultures of Panax quinquefolium. Proc Biochem 33:671–675
Acknowledgments
Authors are thankful to Department of Biotechnology (DBT-KUD-IPLS program BT/PR14555/INF/22/126/2010) and University Grants Commission [Project No. F. No. 41-423/2012 (SR)], New Delhi for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K.-Y. Paek.
Rights and permissions
About this article
Cite this article
Praveen, N., Murthy, H.N. Withanolide A production from Withania somnifera hairy root cultures with improved growth by altering the concentrations of macro elements and nitrogen source in the medium. Acta Physiol Plant 35, 811–816 (2013). https://doi.org/10.1007/s11738-012-1125-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1125-5