Abstract
Stable lines of hairy roots were established from leaf explants of Bacopa monnieri using different strains (A4, R1000, SA79, MTCC 532 and MTCC 2364) of Agrobacterium rhizogenes. The efficiency of hairy roots induction of these strains varied significantly and the maximum transformation frequency (75 %) was observed in case of strain SA79 using leaf explants followed by internode (55 %) in the presence of acetosyringone. Different parameters such as cell density of Agrobacterium suspension, co-cultivation period and infection time influenced the root induction frequency. Maximum frequency of root induction was obtained with bacterial density of 0.6 OD600, 2 days of co-cultivation period and 10 min of infection time. Integration of T-DNA in the genome of hairy roots was confirmed by PCR amplification of rolB gene. Elimination of Agrobacterium from the established root cultures was ascertained by amplifying the DNA fragment specific to 16S rDNA and virD gene. All lines of hairy roots except strain A4 induced showed higher growth rate and accumulated higher levels of ‘bacoside A’ than the untransformed roots. Maximum biomass accumulation (6.8 g l−1) and ‘bacoside A’ content (10.02 mg g−1 DW) were recorded in case of the hairy root line induced by strain MTCC 2364.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacopa monnieri (L.) Wettst, family Scrophulariaceae (common name brahmi) is the only source of bacosides, which possesses legendary reputation as a memory vitalizer with a proven nootropic action (Anonymous 1988). The major chemical entity responsible for its memory-facilitating action is ‘bacoside A’, which is usually known to co-occur with ‘bacoside B’ (Chatterji et al. 1965), latter differing only in optical rotation (Rastogi 1990). Bacopa monnieri is also reported to be beneficial for treatment of cardiac, respiratory and neurological disorders (Russo and Borrelli 2005). Brahmi-based herbal drugs such as ‘Mentat’, ‘Memory Plus’ and ‘Memory Perfect’, rich in ‘bacoside A’ are gaining popularity in both developed and developing countries (Pase et al. 2012). Because of these reasons, B. monnieri has been prioritised as the second most important Indian medicinal plant with a potential of further research and development (Rajani 2008). Further, an increased demand of raw material is resulting in over-exploitation of the plant from the natural habitat (Tiwari et al. 1998), and now many workers consider this plant as an important candidate for conservation (Sharma et al. 2012; Tripathi et al. 2012).
The production of secondary metabolites using biotechnological tools has been explored for many medicinal plant species, and these are considered helpful in conservation of these plants (Tiwari et al. 2007; Thimmaraju et al. 2008). Some preliminary efforts were made for in vitro production of ‘bacoside A’ and ‘bacoside B’ using cell suspension cultures (Rahman et al. 2002). The major constraint with cell suspension culture is gradual loss of ability of these cultures to produce bioactive molecules (Srivastava and Srivastava 2007). The conventional roots were found to be slow growing and have a short life span (Giri and Narasu 2000). Agrobacterium rhizogenes-induced hairy roots offer a promising system due to their stable growth and ability to produce secondary metabolites in plant growth regulators free medium (Sevon and Oksman-Caldentey 2002). Further, faster growth, short doubling time, ease of maintenance and ability to synthesise a range of phytochemicals offer additional advantages (Flores et al. 1999).
Previous reports indicated that lines of hairy roots induced by different strains of A. rhizogenes tend to behave differently in terms of growth, morphology and production of secondary metabolites (Lemcke and Schmulling 1998). Therefore, screening of established lines of hairy roots would be important for the selection of a promising line (Sujatha et al. 2013; Giri et al. 2001). Not many reports are available on A. rhizogenes-mediated hairy root establishment of B. monnieri except one preliminary study (Majumdar et al. 2011), wherein ‘bacoside A’ production from shoot buds and callus derived from hairy roots was reported. A close survey of literature did not reveal any report on establishment of stable hairy root lines and production of ‘bacosides’ from B. monnieri. Therefore, the present study was aimed to induce several lines of stable hairy roots of B. monnieri using different strains of A. rhizogenes and subsequent selection of the hairy roots capable of faster growth, stable morphology and potential for production of higher levels of ‘bacoside A’.
Materials and methods
Plant material, chemicals, glassware
Based on in vitro morphogenetic response and ‘bacoside A’ content, Bacopa monnieri (L.) Wettst. accession BM6 was selected for the present study (Bansal et al. 2014). All routine chemicals were purchased from HiMedia Laboratories (Mumbai, India); growth regulators and antibiotics were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Unless otherwise mentioned, all experiments were conducted in 300 ml glass culture bottles (Kasablanka, Mumbai, India) containing 50 ml of Murashige and Skoog (MS) (Murashige and Skoog 1962) medium. The pH of medium was adjusted to 5.8 before autoclaving at 121 °C for 20 min.
Establishment of aseptic cultures
Cultures of B. monnieri were established using nodal explants according to the procedure described earlier (Aggarwal et al. 2013). Following surface disinfection, nodal segments were trimmed from the cut ends and cultured on MS medium containing 58 mM sucrose and gelled with 0.7 % (w/v) agar (basal MS medium) and supplemented with 2.5 µM benzyladenine (BA). Cultures were incubated at 25 ± 1 °C under 16-/8-h light/dark cycle with a light intensity of 42 µmol m−2 s−1 (inside the culture vessel) provided by cool white fluorescent lamps (Philips, India Ltd, Mumbai). The cultures were sub-cultured at 3-week interval on basal MS medium supplemented with 2.5 µM BA. Three-week-old expanded leaves from the elongated microshoots were used as a source of explants (leaf and internodal segments) for further experimentation.
Bacterial cultures
Five strains of A. rhizogenes, viz., R1000, SA79, A4 (obtained from Professor A. K. Srivastava, Indian Institute of Technology Delhi, India) MTCC 532, MTCC 2364 (procured from Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India) were used in the present study. The bacterial strains were grown on yeast mannitol agar (YMA) medium (10 g l−1 glucose, 10 g l−1 yeast extract, 1 g l−1 ammonium sulphate, 0.25 g l−1 di-potassium phosphate, 15 g l−1 agar and pH 6.8) at 28 °C overnight. Single colony from overnight-grown cultures was picked and inoculated in yeast mannitol broth (YMB) and incubated at 28 ± 2 °C on orbital shaker (220 rpm) for 24 h. Bacterial cells were pelleted by centrifugation (4,000×g, 2 min) and re-suspended in YMB liquid medium with or without 100 µM acetosyringone to attain the desired OD600.
Establishment of hairy root cultures
Leaf and internode explants obtained from the microshoots of B. monnieri were injured by gently pricking (about 100 pricks per leaf) with a sterile needle dipped in bacterial suspension before submerging these in a suspension of A. rhizogenes (OD600 of 0.6) for different time periods (0–20 min). The infected explants were blotted on sterile blotting paper to remove excess bacteria and were co-cultivated for 0–4 days on basal MS medium. After co-cultivation, the explants were washed with sterile distilled water containing 500 mg l−1 ampicillin to remove bacteria and subcultured on basal MS medium containing ampicillin (500 mg l−1). During subsequent subculture cycles, concentration of ampicillin was reduced stepwise to 250 mg l−1, 100 mg l−1 and then it was completely eliminated from the medium.
Molecular analysis
Integration of T-DNA in the nuclear genome of hairy roots was confirmed using polymerase chain reaction (PCR) by amplifying a DNA fragment specific to rolB gene (an important gene of T-DNA of Ri plasmid) from the genomic DNA of established root cultures. Genomic DNA from roots was isolated using CTAB method (Doyle and Doyle 1990), and plasmid DNA of A. rhizogenes was isolated using a rapid boiling method (Holmes and Quigley 1981). The PCR mixture consisted of 20.0 ng of DNA, 1.0 U of Taq DNA polymerase (Larova, Teltow, Germany), 100 µmol dNTP’s mixture, 2.0 µl reaction buffer (10×), 10 nmol each primer and Milli-Q water (Millipore India, Bangalore) was added to make a final volume of 20 µl. Amplification conditions were: an initial denaturation at 94 °C for 5 min followed by 31 cycles of 95 °C for 1 min, 56 °C for 1 min and 72 °C for 1 min with a final extension of 72 °C for 10 min. A fragment of 380 bp specific to rolB gene was amplified using specific primer pair (forward primer 5-GCTCTTGCAGTGCTAGATTT-3 and reverse primer 5-GAAGGTGCAAGCTACCTCTC-3).
Elimination of bacteria from the plant tissue was confirmed by the PCR amplification of a fragment of DNA of about 440 bp specific to virD1 gene using a primer pair (forward primer 5-TGTCGCAAGGCAGTAAG-3 and reverse primer 5-CAAGGAGTCTTTCAGCATG-3) specific to virD1 gene. Similarly, PCR amplification of 16S rDNA fragment of about 1,500 bp was also carried out using forward primer 5-AGAGTTTGATCCTGGCTCAG-3 and reverse primer 5-ACGGGCGGTGTGTTC-3 (Weisburg et al. 1991). Genomic DNA and/or plasmid from bacteria were used as positive control. Amplification conditions were same as mentioned above. The amplified products were separated on 1.0 % (w/v) agarose gel and viewed using UV transilluminator (BioRad, CA, USA) following ethidium bromide staining.
Growth and morphology of hairy root cultures
The growth characteristics of hairy roots generated using five different strains of A. rhizogenes were evaluated on basal MS medium devoid of ampicillin. Roots from untransformed microshoots were used as control. Four root tips (approx. 1 cm long) from roots induced by each bacterial strain, harvested from 3-week-old root cultures were transferred to the same medium (20 ml) in 9-cm petri-plates and cultured for 30 days. Root elongation and the number of roots per centimetre on the primary roots (lateral root density) of various root lines were recorded.
Growth performance (biomass accumulation) of various lines of hairy roots induced by each strain of A. rhizogenes along with control (untransformed) roots was also studied. Fifty mg of actively growing hairy roots from 30-day-old culture was transferred to 250 ml Erlenmeyer’s flask containing 30 ml of basal MS medium. All cultures were incubated in dark at 25 ± 2 °C on a gyratory shaker at 60 rpm. After 30 days of culture, the growth of hairy roots was assessed in terms of biomass and the ‘bacoside A’ content.
Determination of root biomass
The roots were separated from the medium. Their fresh weight (FW) was determined after these were washed with distilled water. Excess of water was removed by blotting before taking fresh weight. Dry weight (DW) was recorded after these roots were dried at 60 °C till constant weight was attained. The growth ratio (GR) was determined by dividing the dry weight of root biomass harvested by the dry weight of inoculum.
Extraction and quantification of ‘bacoside A’
Samples were extracted and purified as described earlier (Bansal et al. 2014). Briefly, samples (dried roots) were powdered using blender. Powdered samples (1.0 g each in triplicate) were soaked in 10.0 ml water for 24 h. These were filtered through glass wool and filtrates were discarded. The residues were extracted with 20.0 ml of aqueous ethanol (95 %, v/v) for 3 days. These were then filtered through glass wool. The extraction of residue was repeated three times (×20 ml) with (95 %, v/v) ethanol. Filtrates from three extractions were pooled and dried in vacuo. Residues were reconstituted in 1.0 ml methanol and filtered through 0.45-μm pore size filters (Millipore-Carrigtwohill, Ireland) prior to quantification using high-performance liquid chromatography (HPLC).
Quantification of ‘bacoside A’ content in purified extracts was carried out using reverse phase HPLC (Waters Corporation, USA) equipped with high-pressure binary pump system (515), diode array detector (2998) and Rheodyne injector with 20 μl sample loop. Samples (20 μl) were injected through injector into SunfireTM C18 column, 250 × 4.6 mm i.d. particle size 5.0 μm (Waters, Ireland) and elution was carried out in an isocratic mode with a mobile phase consisting of aqueous acetonitrile (65:35 v/v) containing phosphoric acid (0.2 %, v/v; pH 3.0) at a flow rate of 1.0 ml min−1. Column eluates were monitored with online PDA detector set at 205 nm. Quantifications were carried out using external standard curves plotted by taking known quantities of standard compounds (individually ‘bacoside A3’, ‘bacopaside II’ and ‘bacosaponin C’) (Sigma Chemical Co., St. Louis, MO, USA).
Statistical analysis
All experiments were performed in triplicate and repeated three times and values were expressed as mean ± standard error. Data were analysed using analysis of variance (ANOVA) and the means were compared using Duncan’s multiple range test (P ≤ 0.05) using CoStat 6.4 software (CoHort Software, Pacific Grove USA).
Results
Establishment of hairy root cultures
Hairy roots were induced from leaf and internode segments using five different strains of A. rhizogenes. Strain SA79 was found to be more effective for induction of hairy roots as compared to other strains from both leaf and intermodal segments. Maximum root induction was recorded from leaf explants (52 %) when infected with strain SA79, while root induction was minimum from internodal segments infected with strain MTCC 532 (Table 1). Among all the five strains used, transformation frequency from leaf explants was higher as compared to internodal segments. Since maximum root induction was observed from leaf explants infected with strain SA79, hence these were used to study the effect of various factors on root induction in subsequent experiments.
Effect of various factors on root induction
Various factors such as bacterial density, incorporation of acetosyringone in infection medium, and co-cultivation period were studied. Incorporation of 100 µM acetosyringone in the medium used for re-suspension of bacterial pellet increased frequency of root induction in both leaf and internodal explants (Table 1). The number of days required for induction of roots was also significantly reduced from 15–18 to 3–8 days (Table 1). The bacterial density of suspension used for infection of leaf explants also influenced root induction (Fig. 1a). Among five different densities of bacterial suspension (OD600 0.2, 0.4, 0.6, 0.8 and 1.0) tested for the infection, maximum frequency of hairy root induction (in 62 % leaf explants) was observed when a bacterial suspension of OD600 0.6 was used (Fig. 1a). The time period of infection with A. rhizogenes also influenced the frequency of root induction. Among the different infection time periods tested (0, 5, 10, 15 and 20 min), maximum frequency of leaf explants (78 %) was induced to root when infection time of 10 min was given (Fig. 1b). Similarly, co-cultivation period also influenced root induction from leaf explants (Fig. 1c). A co-cultivation period of 2 days was found to be suitable for root induction from 61.9 % of leaf explants.
Molecular analysis
The rolB gene is one of the major parts of T-DNA of A. rhizogenes and plays an important role in root induction. Integration of T-DNA in the genome of hairy roots of B. monnieri was confirmed by PCR amplification of DNA fragment of 380 bp specific to rolB gene (Fig. 2a). Genomic DNA from various lines of putative hairy roots showed amplification of this fragment, indicating the presence of rolB gene (Fig. 2a). Amplification was not observed from untransformed roots (negative control). Size of DNA fragment amplified from the Ri plasmid (positive control) was similar to the one amplified from hairy roots (Fig. 2a). Further, elimination of bacteria from the tissues was ascertained by testing the presence of DNA fragments specific to virD1 gene and 16S rDNA in genomic DNA from various lines of hairy roots by PCR amplification. Amplification of DNA fragments specific to virD1 gene and 16S rDNA was not observed from genomic DNA of various lines of hairy roots (Fig. 2b, c) indicating the absence of bacterial DNA contaminations. However, a fragment of about 440 bp specific to virD1 gene (Fig. 2b) and 1,500 bp specific to 16S rDNA (Fig. 2c)was amplified from bacterial plasmid DNA and chromosomal DNA, respectively. These results suggest the absence of Agrobacterium in the established root cultures.
Amplification of a rolB gene b virD1 gene c 16S rRNA in hairy root lines of B. monnieri. Lane 1 = 1,000 bp DNA ladder, lane 2 amplification from plasmid DNA (chromosomal DNA of A. rhizogenes in case of 16S rRNA), lane 3 = negative control (DNA from untransformed roots), lane 4–8 hairy roots induced by A. rhizogenes strains (A4, MTCC 532, R1000, SA 79, MTCC 2364, respectively)
Morphology and growth characteristics
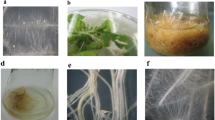
At the end of the culture period, hairy root lines established using different strains of A. rhizogenes varied in morphology and growth pattern based on root elongation, lateral root density and root thickness (Fig. 3). Roots induced by strains MTCC 2364 and MTCC 532 were thin (Fig. 3a, b), whereas hairy roots induced by strains R1000 and SA79 were thick (Fig. 3c, d). Strain A4-induced hairy roots were thin in morphology and also developed intervening callus masses (Fig. 3e). Lateral root density varied significantly among the lines of hairy root induced by various strains of A. rhizogenes. Maximum density of lateral roots was observed in roots induced by strain MTCC 2364 (3.6 roots per cm), while the minimum lateral root density was recoded with A4 (2.0 roots per cm). Root elongation per cycle also varied significantly amongst lines of roots induced by different strains of A. rhizogenes. Maximum root elongation (4.5 cm per subculture cycle of 30 days) was observed in hairy roots induced by strain MTCC 2364 (Fig. 4). The maximum root elongation and lateral root density observed in roots induced by strain MTCC 2364 were positively correlated with higher biomass accumulation by these roots.
The effect of strains of A. rhizogenes on the root elongation and lateral root density of established hairy roots grown on basal MS medium (without ampicillin). Values sharing same uppercase letters (root elongation) and lowercase letters (lateral root density) within the series are not significant at P ≤ 0.05
Growth analysis
All lines of hairy roots accumulated higher biomass (g l−1) over the untransformed roots except hairy root line established using strain A4 (Table 2). However, there were significant differences with respect to biomass accumulation by various lines of hairy roots. Maximum biomass accumulation (6.8 g l−1) was recorded in the cultures of hairy roots induced by strain MTCC 2364, which showed about tenfold increment in dry weight of roots (Table 2) and also showed about 5.5-fold more biomass accumulation than untransformed roots.
‘Bacoside A’ content
The ‘bacoside A’ content varied significantly amongst the various lines of hairy roots established using different strains of A. rhizogenes. An important observation of the present study is higher levels of ‘bacoside A’ content accumulated by most of the lines of hairy root as compared to untransformed roots (Table 3). Hairy root line induced by strain MTCC2364 accumulated about fivefold higher (10.02 mg g−1 DW) ‘bacoside A’ content than untransformed roots. The ‘bacoside A’ content in roots induced by strain A4 (1.25 mg g−1 DW) was also lower as compared to untransformed roots.
Discussion
The objectives of the present study were to establish lines of hairy roots of B. monnieri using various strains of A. rhizogenes and to study subsequently the potential of ‘bacoside A’ production of these lines of hairy roots. Further, various factors influencing the establishment of hairy roots were also investigated. Earlier, there was only one report on the induction of hairy roots of B. monnieri using A. rhizogenes. However, these roots developed callus and differentiated shoots (Majumdar et al. 2011). In the present study, five stable lines of hairy roots were established using different strains of A. rhizogenes. Till date, literature survey did not reveal any report on the establishment of stable hairy root lines of B. monnieri thus this seems to be the first report on the establishment of stable lines of hairy roots and subsequently assessment of ‘bacoside A’ production by established lines of these roots.
In the present study, hairy roots were established from B. monnieri, an important endangered medicinal plant of India with the aim to exploit these roots for the production of ‘bacoside A’. Such roots have been established from many important medicinal plants for the production of active principle (Sujatha et al. 2013; Tiwari et al. 2007; Giri et al. 2001) and these roots have also played important role in the conservation of these plant species (Tiwari et al. 2007; Thimmaraju et al. 2008). Root induction potential of the various strains of A. rhizogenes varied significantly (Table 1) and maximum root induction was observed in case of strain SA79 followed by strain R1000. This could be due to differential virulence of these strains and/or host specificity (Zehra et al. 1999; Porter 1991). Although, higher virulence of strain R1000 and strain SA79 for root induction have been reported earlier in many plants (Tiwari et al. 2007; Tao and Li 2006), yet some studies reported that strain A4 is more efficient in root induction (Ooi et al. 2013; Giri et al. 2001).
In the present study, higher percent of leaf explants were induced to root as compared to internodal segments. Explant-dependent response of root induction following A. rhizogenes has been reported earlier in some plants (Alpizar et al. 2006; Kang et al. 2006). In the present study, roots were induced at a high frequency from leaf explants (Table 1). This could be due to reported higher number of competent cells produced from these explants, which are ideal targets for hairy root induction (Potrykus 1990).
Various factors namely bacterial density, acetosyringone, infection time and co-cultivation period influenced hairy root induction. Earlier, these factors have been studied and found to influence the process of T-DNA delivery to the plant tissues (Aggarwal et al. 2013). Addition of acetosyringone in the bacterial cell suspension before infection increased the transformation frequency in the present study (Table 1). These results are in line with the earlier reports (Aggarwal et al. 2013; Barik et al. 2005). Amongst different bacterial cell suspension densities tested, maximum root induction was observed at cell density of 0.6 OD600 (Fig. 1a). These results are in line with the earlier reports (Jian et al. 2009; Barik et al. 2005). At higher bacterial densities, the explants were observed to turn necrotic which may be due to increased production of toxic compounds due to bacterial overgrowth (Sonia et al. 2007). Investigating on the effect of different infection times, it was found that an infection time of 10 min is optimum for the root induction from the leaf explant (Fig. 1b). The varying effect of infection time on root induction in different plants has been reported earlier (Aggarwal et al. 2013; Jun et al. 2007). Co-cultivation period also influenced root induction from leaf explant significantly (Fig. 1c). A co-cultivation period of 2 days was found to induce the roots in maximum percent of explants. Earlier, the effect of co-cultivation period on T-DNA delivery has been reported in various plants (Aggarwal et al. 2013; Karthikeyan et al. 1996).
Morphology and growth pattern of roots established using different strains of A. rhizogenes were found to vary significantly (Fig. 3). Maximum lateral root density and root elongation were observed in hairy roots induced by strain MTCC 2364 (Fig. 4). It has been suggested that, different morphotypes obtained using different A. rhizogenes strains may be due to differential expression of T-DNA genes, variable copy numbers and positional integration of T-DNA in the host genome (Cho et al. 1998). Earlier, such variations in root morphology were reported in Gentiana macrophylla (Tiwari et al. 2007) and Beta vulgaris (Thimmaraju et al. 2008). The growth and biomass accumulation of different lines of hairy root induced by different strains of A. rhizogenes also varied significantly (Table 2). Hairy roots induced by strain MTCC 2364 showed higher growth and accumulated about fivefold more biomass as compared to untransformed roots. The maximum lateral root density and root elongation were also observed in roots induced by strain MTCC 2364. The lowest growth was recorded in hairy roots induced by strain A4 (Table 2). Difference in the growth of roots induced by different strains of A. rhizogenes has also been reported earlier (Ooi et al. 2013; Tiwari et al. 2007).
The content of ‘bacoside A’ also varied significantly amongst different lines of hairy roots and most of the lines of roots induced by various strains accumulated higher levels of ‘bacoside A’ (Table 3). The accumulations of higher levels of ‘bacoside A, in hairy roots could be due to the effect of T-DNA integration on the secondary metabolite production as reported in many other plant species (Bulgakov 2008; Shkryl et al. 2008). ‘Bacoside A’ content was highest in hairy roots induced from strain MTCC 2364, which accumulated about 4.5-fold more ‘bacoside A’ as compared to untransformed roots. Although, the variations in secondary metabolite content in hairy roots induced by different strains of A. rhizogenes have also been reported earlier (Giri et al. 2001), yet the higher levels observed in the present study is a significant observation. Such root lines can be utilised for the efficient production of active principle. A line of hairy roots induced by strain A4 was found to accumulate lowest levels of ‘bacoside A’ (lower than that of untransformed roots). Earlier, strain A4-induced roots in B. vulgaris also showed lower accumulation of secondary metabolite and biomass than the roots induced by the strain LMG-150 (Thimmaraju et al. 2008). Further, the production of ‘bacoside A’ in the cell suspension cultures of B. monnieri has also been studied by Rahman et al. (2002), who reported the accumulation of ‘bacoside A’ by cell suspension cultures comparable to hairy root cultures. However, the growth rate of hairy root cultures as compared to cell cultures was higher, thus providing higher yield of ‘bacoside A’ than cell suspension cultures on one hand and stability of hairy root cultures for the production of secondary metabolites over a period of time provides an additional advantage (Flores et al. 1999).
In the present study, for the first time, stable lines of hairy roots were established from B. monnieri using different strains of A. rhizogenes. Some of the lines of roots induced showed significantly higher growth rate (5.5-fold) and also accumulated higher ‘bacoside’ content (4.5-fold) over the untransformed root cultures. Such fast growing roots with higher levels of ‘bacoside A’ have the potential of up-scaling in the bioreactors for the production of ‘bacoside A’. This can be helpful to reduce the pressure on the wild populations.
Author contribution
Ms. Mahima Bansal (Project Fellow) has been involved in performing the various experiments, data analysis and writing the initial draft of manuscript. Dr. Anil Kumar has been involved in the designing and execution of various experiments and finalized the manuscript for submission to journal. Prof. M. Sudhakara Reddy has been involved in designing and data analysis of experiments related to the molecular markers. Corrected the final version of manuscript submitted to journal.
References
Aggarwal D, Jaiswal N, Kumar A, Reddy MS (2013) Factors affecting genetic transformation and shoot organogenesis of Bacopa monnieri (L.) Wettst. J Plant Biochem Biotechnol 22:382–391
Alpizar E, Dechamp E, Espeout S, Royer M, Lecouls AC, Nicole M, Bertrand B, Lashermes P, Etienne H (2006) Efficient production of Agrobacterium rhizogenes-transformed roots and composite plants for studying gene expression in coffee roots. Plant Cell Rep 25:959–967
Anonymous (1988) Wealth of India, raw materials. Council of Scientific and Industrial Research (CSIR), New Delhi
Bansal M, Kumar A, Reddy MS (2014) Diversity among wild accessions of Bacopa monnieri (L.) Wettst. and their morphogenetic potential. Acta Physiol. doi:10.1007/s11738-014-1493-0
Barik DP, Mohapatra U, Chand PK (2005) Transgenic grasspea (Lathyrus sativus L.): factors influencing Agrobacterium-mediated transformation and regeneration. Plant Cell Rep 24:523–531
Bulgakov VP (2008) Functions of rol genes in plant secondary metabolism. Biotechnol Adv 26:318–324
Chatterji N, Rastogi RP, Dhar ML (1965) Chemical examination of Bacopa monnieri Wettst.: parti-isolation of chemical constituents. Indian J Chem 3:24–29
Cho HJ, Widholm JM, Tanaka N, Nakanishi Y, Murooka Y (1998) Agrobacterium rhizogenes-mediated transformation and regeneration of the legume Astragalus sinicus (Chinese milk vetch). Plant Sci 138:53–65
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissues. Focus 12:13–15
Flores HE, Vivanco JM, Loyola-Vargas VM (1999) Radicle biochemistry: the biology of root-specific metabolism. Trends Plant Sci 4:220–226
Giri A, Narasu ML (2000) Transgenic hairy roots: recent trends and applications. Biotechnol Adv 18:1–22
Giri A, Ravindra ST, Dhingra V, Narasu ML (2001) Influence of different strains of Agrobacterium rhizogenes on induction of hairy roots and artemisinin production in Artemisia annua. Curr Sci 81:378–382
Holmes DS, Quigley M (1981) A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem 114:193–197
Jian B, Hou W, Wu C, Liu B, Liu W, Song S, Bi Y, Han T (2009) Agrobacterium rhizogenes-mediated transformation of Superroot-derived Lotus corniculatus plants: a valuable tool for functional genomics. BMC Plant Biol 9:78
Jun YX, Qing Y, Qin J, Ming LY, Feng ZY, Ke G, Zhan WD (2007) Optimization of Agrobacterium-mediated transformation parameters for sweet potato embryogenic callus using beta -glucuronidase (GUS) as a reporter. Afr J Biotechnol 6:2578–2584
Kang HJ, Anbazhagan VR, You XL, Moon HK, Yi JS, Choi YE (2006) Production of transgenic Aralia elata regenerated from Agrobacterium rhizogenes-mediated transformed roots. Plant Cell Tissue Org Cult 85:187–196
Karthikeyan AS, Sarma KS, Veluthambi K (1996) Agrobacterium tumefaciens-mediated transformation of Vigna mungo (L.) Hepper. Plant Cell Rep 15:328–331
Lemcke K, Schmulling T (1998) A putative rol B gene homologue of Agrobacterium rhizogenes TL-DNA has different morphogenetic activity in tobacco than rolB. Plant Mol Biol 15:423–434
Majumdar S, Garai S, Jha S (2011) Genetic transformation of Bacopa monnieri by wild type strains of Agrobacterium rhizogenes stimulates production of bacopa saponins in transformed calli and plants. Plant Cell Rep 30:941–954
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Ooi CT, Syahida A, Stanslas J, Maziah M (2013) Efficiency of different Agrobacterium rhizogenes strains on hairy roots induction in Solanum mammosum. World J Microbiol Biotechnol 29:421–430
Pase MP, Kean J, Sarris J, Neale C, Scholey AB, Stough C (2012) The cognitive-enhancing effects of Bacopa monnieri: a systematic review of randomized, controlled human clinical trials. J Altern Complem Med 18:647–652
Porter RR (1991) Host range and implication of plant infection by Agrobacterium rhizogenes. Crit Rev Plant Sci 10:387–421
Potrykus I (1990) Gene transfer to plants: assessment and perspectives. Physiol Plant 79:125–134
Rahman LU, Verma PC, Singh D, Gupta MM, Banerjee S (2002) Bacoside production by suspension cultures of Bacopa monnieri (L.) Pennell. Biotechnol Lett 24:1427–1429
Rajani M (2008) Bacopa monnieri, a nootropic drug. In: Ramawat KG, Merillon JM (eds) Bioactive molecules and medicinal plants. pp 175–195
Rastogi RP (1990) Compendium of Indian medicinal plants, vol 1. CSIR, New Delhi, pp 118–122
Russo A, Borrelli F (2005) Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine 12:305–317
Sevon N, Oksman-Caldentey KM (2002) Agrobacterium rhizogenes mediated transformation: root cultures as a source of alkaloids. Planta Med 68:859–868
Sharma N, Satsangi R, Pandey R, Singh R, Kaushik N, Tyagi RK (2012) In vitro conservation of Bacopa monnieri (L.) using mineral oil. Plant Cell Tiss Org Cult 111:291–301
Shkryl YN, Veremeichik GN, Bulgakov VP, Tchernoded GK, Mischenko NP, Fedoreyev SA, Zhuravlev YN (2008) Individual and combined effect of the rol A, B and C genes on anthraquinone production in Rubia cordifolia transformed calli. Biotechnol Bioeng 100:118–125
Sonia Saini R, Singh RP, Jaiswal PK (2007) Agrobacterium tumefaciens-mediated transfer of Phaseolus vulgaris a-amylase inhibitor-1 gene into mungbean: Vigna radiata (L.) Wilczek using bar as selectable marker. Plant Cell Rep 26:187–198
Srivastava S, Srivastava AK (2007) Hairy root culture for mass-production of high-value secondary metabolites. Crit Rev Biotechnol 27:29–43
Sujatha G, Zdravkovic-Korac S, alic DC, Flaminic G, Ranjitha Kumaria BD (2013) High-efficiency Agrobacterium rhizogenes-mediated genetic transformation in Artemisia vulgaris: Hairy root production and essential oil analysis. Ind Crop Prod 44:643–652
Tao J, Li L (2006) Genetic transformation of Torenia fournieri L. mediated by Agrobacterium rhizogenes. South Afr J Bot 72:211–216
Thimmaraju R, Venkatachalam L, Bhagyalakshmi N (2008) Morphometric and biochemical characterization of red beet (Beta vulgaris L.) hairy roots obtained after single and double transformations. Plant Cell Rep 27:1039–1052
Tiwari V, Singh BD, Tiwari NK (1998) Shoot regeneration and somatic embryogenesis from different explants of Brahmi Bacopa monniera (L.) Wettst. Plant Cell Rep 17:538–543
Tiwari RK, Trivedi M, Guang ZC, Guo GQ, Zheng GC (2007) Genetic transformation of Gentiana macrophylla with Agrobacterium rhizogenes: growth and production of secoiridoid glucoside gentiopicroside in transformed hairy root cultures. Plant Cell Rep 26:199–210
Tripathi N, Chouhan DS, Saini N, Tiwari S (2012) Assessment of genetic variations among highly endangered medicinal plant Bacopa monnieri (L.) from Central India using RAPD and ISSR analysis. 3. Biotech 2:327–336
Weisburg WA, Barns SM, Pelletier DA, Lane DJ (1991) 16S rDNA amplification for phylogenetic study. J Bacteriol 173:697–703
Zehra M, Banerjee S, Sharma S, Kumar S (1999) Influence of Agrobacterium rhizogenes strains on biomass and alkaloid productivity in hairy root lines of Hyoscyamus muticus and H. albus. Planta Med 65:60–63
Acknowledgments
Authors are thankful to University Grant Commission (UGC), Govt. of India, New Delhi for the financial assistance. Professor A. K. Srivastava, Indian Institute of Technology, New Delhi is thanked for providing some of the strains of Agrobacterium rhizogenes and Professor Desh Bir Sharma is thanked for editing the English of the ms. Thanks are also due to TIFAC-CORE, Thapar University Patiala for the facilities to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K.-Y. Paek.
Rights and permissions
About this article
Cite this article
Bansal, M., Kumar, A. & Sudhakara Reddy, M. Influence of Agrobacterium rhizogenes strains on hairy root induction and ‘bacoside A’ production from Bacopa monnieri (L.) Wettst.. Acta Physiol Plant 36, 2793–2801 (2014). https://doi.org/10.1007/s11738-014-1650-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1650-5