Abstract
The NAC family is a multigene family that present uniquely in plants and whose members are involved in many important cellular processes including abiotic stress tolerance. In this study, sequences of two ATNAC3-related genes (SlNAC3) were identified in the tomato genome using different bioinformatics approaches. Phylogenetic analysis clustered 84 tomato identified NAC proteins into 19 different subfamilies that included 5 subfamilies for stress-related NAC genes with SlNAC3 members clustered with previously characterized ATNAC3 members from Arabidopsis. Gene expression analysis of SlNAC3 genes indicated that both of them are expressed in response to drought and salinity stress conditions. The over-expression of two stress-related SlNAC3 in tomato plants resulted in enhanced drought and salt tolerance when compared with wild type plants. The identified stress-related NAC genes could be a useful tool to improve tomato productivity under stress conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcription factors (TFs) are highly important cellular proteins that regulate gene expression processes in living organisms. These proteins play important regulatory roles in every aspect of cellular activity in prokaryotic and eukaryotic organisms (Charoensawan et al. 2010). Transcription factors show sequence-specific DNA binding activity to regulatory elements commonly found in promoter regions of targeted genes (Agarwal et al. 2011). Therefore, they are capable of activating or repressing the transcription process of targeted genes involved in a range of different cellular functions. Plant genomes include a few thousands genes that encode TFs, and these make up, on average, ~ 2 % of their total genome size (Riechmann and Ratcliffe 2000). For instance, Arabidopsis and poplar genomes encode ~ 1,550 and 2,900 TFs, which constitute 6.2 and 6.4 % of their total encoding genes, respectively. Furthermore, TFs can be divided into families on the basis of their structural features. These features mainly include conserved DNA-binding domains, activation or repression domains and oligomerization domains (Agarwal et al. 2011). In plants, several TF families were characterized and found to play a major role in different aspects of plant growth and development.
One of the major plant specific TF families is the NAC gene family, which plays a major role in different aspects of plant growth and development (Puranik et al. 2012). It is widely distributed in the plant kingdom where it is found in monocotyledons, dicotyledons and gymnosperms (Olsen et al. 2005). For instance, in the genomes of rice, Arabidopsis, soybean and poplar more than one hundred genes were identified to encode NAC TFs in each plant species (Le et al. 2011; Pinheiro et al. 2009; Hu et al. 2010; Nuruzzaman et al. 2010; Ooka et al. 2003). The NAC gene name abbreviates the initial letters of the petunia NAM gene (No Apical Meristem), the ATAF1/ATAF2 genes (Arabidopsis Thaliana Activation Factor1/2) and the CUC gene (CUp-shaped Cotyledon) from Arabidopsis (Ooka et al. 2003).
This family was originally characterized by the consensus sequence of the N-terminal DNA binding domain, commonly conserved in all family members (Aida et al. 1997). The C-terminal part of the NAC proteins however reveal diverse domain structure between the family members and are thought to be responsible for protein–protein interaction and transcription activation or repression (Grant et al. 2010; Guo and Gan 2006; Jensen et al. 2010; Kjaersgaard et al. 2011; Shan et al. 2012). In plants, NAC gene family members have divergent functions and they are involved in different developmental and physiological processes (Le et al. 2011; Pinheiro et al. 2009). The NAC family members are expressed in various tissues, as well as at different developmental stages and in response to different stimuli (Olsen et al. 2005; Hu et al. 2010; Grant et al. 2010). They are involved in shoot apical meristem development, lateral root development, xylogenesis, leaf senescence, embryo development, floral morphogenesis, grain nutrient remobilization, shoot branching determination and plant defense responses to both biotic and abiotic stresses (Hu et al. 2010; Grant et al. 2010; Mao et al. 2007; Hu et al. 2006; Hao et al. 2011; Ohnishi et al. 2005; Shen et al. 2009). Three Arabidopsis NAC genes (AtNAC019, AtNAC055 and AtNAC072), which are induced in response to drought, high salinity and abscisic acid, were shown to bind promoter regions of several stress-responsive genes (Tran et al. 2004). The over-expression of these genes in Arabidopsis plant resulted in improved tolerance against drought stress. The drought-inducible rice NAC gene (SNAC1) was found to play a major role in drought tolerance and its over-expression resulted in drought tolerance of field grown rice plants (Hu et al. 2006). Likewise, the over-expression of another stress-related NAC gene, OsNAC045, in rice showed enhanced drought and salt tolerance (Zheng et al. 2009). In another study, the overexpression of SbSNAC1 from sorghum conferred drought tolerance in transgenic Arabidopsis (Lu et al. 2013). In chickpea, Cicer arietinum, the CarNAC5 gene was induced by drought, heat, wounding, salicylic acid (SA) and indole-3-acetic acid (IAA) treatments (Peng et al. 2009). In soybean, Glycine max, GmNAC20 and GmNAC11 gene over-expression resulted in enhanced salt and freezing tolerance, and improved salt tolerance respectively (Hao et al. 2011). In barley, the overexpression of HvSNAC1 gene was found to improve drought tolerance of field grown plants (Al Abdallat et al. 2014) and to confer resistance to Ramularia leaf spot (McGrann et al. 2014). For these reasons, such genes are considered promising targets for use in plant genetic improvement for the development of plants tolerant to adverse environmental conditions.
Recently, the NAC gene family has been analyzed comprehensively in different plant species including Arabidopsis, rice, soybean, poplar, potato and tomato (Le et al. 2011; Hu et al. 2010; Nuruzzaman et al. 2010; Ooka et al. 2003; Singh et al. 2013; Kou et al. 2014; Su et al. 2014). In tomato, few NAC genes have been characterized to date and the functions of most of them remain to be elucidated (Yang et al. 2011; Kou et al. 2014; Su et al. 2014). Since the NAC gene family encompasses members involved in both biotic and abiotic stress tolerance and in growth and developmental events, it is necessary to isolate and characterize stress-related NAC gene from tomato in order to decipher the potential for using them in tomato improvement with particular emphasis on tolerance against abiotic stresses.
In this study, the characterization of the two members of ATNAC3-related members in tomato (SlNAC3) was performed using modern bioinformatics and molecular tools. Twelve identified stress-related NAC members genes were subjected to multiple sequence alignment (MSA) and phylogenetic analysis to determine their relatedness and functional grouping with previously characterized members in other plants. Promoter analysis of the identified stress-related NAC members in tomato was carried out in order to identify conserved cis-elements responsible for their expression under stress conditions. Finally, the over-expression of SlNAC3 genes in tomato plants resulted in enhanced tolerance against drought and high salt stresses.
Materials and methods
Plant material, growth conditions and stress treatments
Tomato cultivar “Moneymaker” was used for the drought and salinity gene expression analysis experiments. Transgenic tomato lines overexpressing SlNAC3 members were used for performance analysis in comparison with Moneymaker plants. For gene expression experiments, tomato seeds were soaked in water for 2 days at 25 °C and then washed with sterilized water before sowing into small pots (10 cm diameter × 10 cm depth) filled with acid washed sand. After germination, tomato seedlings were placed under controlled conditions (continuous 25 °C temperature, photoperiod of 16 h light/8 h dark with 80 µmol m−2 s−1 photon flux density) and irrigated daily with fixed volume of Hoagland solution. For drought treatment, 2-week-old tomato seedlings were subjected to water withholding for 3, 5 and 7 days. For salt treatment, tomato seedling roots were submerged in saline water (300 mM NaCl) for 1, 3, 5 and 10 h. Well-watered plants were included as a control for both stress conditions. For each treatment, three replicates were used and for each replicate young leaves were harvested from three plants. To evaluate transgenic lines performance under drought conditions, 2-week-old seedlings (20 plants) of wild type and transgenic lines grown under controlled conditions were subjected to water withholding conditions for 21 days and the wilting in the treated plants was monitored. For high salt stress tolerance test, the germination of tomato seeds in petri dishes containing 0.5 % NaCl was performed as described previously (Singh et al. 2012).
Identification of NAC genes in tomato
To identify NAC genes in the tomato genome, the SGN database (http://solgenomics.wur.nl) was the main source of retrieving the NAC genes as it is considered the main depository of tomato proteins and DNA sequences. Initially, the retrieval of tomato NAC genes was based on a similarity search using Basic Local Alignment Search Tool (BLAST) that relies on previously identified NAC proteins from model plants such as Arabidopsis, rice and poplar. For this purpose, the Arabidopsis and rice NAC protein sequences were downloaded from the Plant Transcription Factors Database (http://plntfdb.bio.uni-potsdam.de/v3.0/) while the poplar NAC proteins were obtained from Hu et al. (2010). The retrieved Arabidopsis, rice and poplar NAC proteins were blasted using different algorithms against different SGN databases: SGN tomato combined database, ITAG2.3 predicted cDNA and ITAG2.3 predicted protein sequences. The blast parameters were set with an e-value cut-off of 1e−10 and with 15 maximum hits to show. The retrieved tomato NAC DNA sequences were verified for the existence of a true NAC domain by performing a Blast search against the InterProScan database (Quevillon et al. 2005).
Phylogenetic analysis
Phylogenetic analysis of NAC proteins retrieved from tomato was performed to study the grouping and relatedness to other NAC proteins from different plant species. For this purpose, the retrieved NAC full length sequence or NAC domain amino acid sequences were subjected to multiple sequence alignment and phylogenetic analysis with previously characterized NAC family proteins from Arabidopsis, rice, poplar and soybean. Multiple sequence alignment (MSA) and phylogenetic analysis were carried out using the ClustalW algorithm (Thompson et al. 1997) embedded in the MEGA5 software (Tamura et al. 2011) by employing the neighbor-joining (NJ) method with a bootstrapping value of 10,000 as described previously (Pinheiro et al. 2009; Hu et al. 2010; Nuruzzaman et al. 2010). The tree branch repeatability in the phylogenetic tree is represented by the bootstrapping value that measures “confidence limits” on phylogenies.
Promoter analysis and identification of conserved motifs
For MSA analysis, the Clustal X program (version 2.0; Thompson et al. 1997) was used. The determination of five NAC sub-domains was based on Ooka et al. (2003) and the Conserved Domain Database (CDD) (http://www.ncbi.nlm.nih.gov/cdd). The complete downstream sequences from the NAC domain were investigated for potential putative motifs based on Ooka et al. (2003). The shading of consensus motif sequences was marked using the GeneDoc program (Version 2.7.000, NRBSC, USA).
For promoter analysis, the upstream sequences (~2,000 bp) of each identified tomato stress-related NAC gene were retrieved from the SGN web server. The upstream sequences were analyzed for the identification of regulatory cis-elements important for gene expression under stress conditions using the following databases: PLACE; Plant cis-acting regulatory elements (http://www.dna.affrc.go.jp/PLACE/; Higo et al. 1999), PLANTCARE; Plant cis-Acting regulatory elements (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/;(Lescot et al. 2002)).
Quantitative real-time RT-PCR analysis
For RT-qPCR analysis, total RNA was isolated from leaf samples taken from treated plants as described above. Gene-specific primers pairs for three selected stress-related NAC genes and SlActin (used as the reference internal control for relative gene expression analysis) were designed using Primer 3 Software (Rozen and Skaletsky 2000; Table 1). The amplification of the target genes was carried out using the GoTaq® qPCR Master Mix Kit (Promega, Madison, USA), and real-time detection of products was performed using Mini-Opticon Real Time PCR System (BioRad, Hercules, CA). All cDNA samples were analyzed in triplicate, and the cDNA was derived from at least two biological replicates. Thermal cycling conditions consisted of 40 cycles of 94 °C for 30 s, 58 °C for 30 s and 72 °C for 45 s, plus a final extension at 72 °C for 5 min. The relative changes in gene expression were quantified as described in Vandesompele et al. (2002).
Isolation of SlNAC3 members and plant transformation
Based on the obtained DNA sequence of two SlNAC3 members, gene-specific primer pairs for Solyc07g063410 (410Fwd: 5′-ATGGGTGTTCAAGAAATGGATC-3 and 410Rev: 5′-TTACCCAGTAAAACCCATATTTAC-3′) and Solyc12g013620 (620Fwd: 5′-ATGGGTGTTCAAGAAAAAGATCC-3′ and 620Rev: 5′-CTACTGCTTGAACCCGAGATTTA-3′) were designed. To isolate the full length cDNA of the corresponding stress-related SlNAC3 genes, 2 week old tomato seedling cv. Moneymaker were subjected to drought stress by withholding water for 7 days. Total RNA was isolated from stressed leaf tissue using SV Total RNA Isolation System Kit (Promega, Madison, USA) following the manufacturer’s instructions. The isolated RNA was used to synthesize a first strand cDNA library using the SuperScript® First-Strand Synthesis System (Invitrogen, USA) and oligo T(18) primer following the manufacturer’s instructions. The full-length cDNAs of the two SlNAC3 members were amplified using PCR in a 25 µl reaction mixture containing 5 µl of cDNA as a template, 2.5 µl of dNTPs (100 µM), 5 µl of 5 × PCR buffer, 0.5 µM of each primer and 0.25 µl of 5 U/µl GoTaq DNA polymerase (Promega, Madison, Wisconsin). The PCR conditions were 94 °C for 5 min, followed by 40 cycles of 94 °C for 30 s, 55 °C for 1 min, and 72 °C for 1 min, and a final 10 min extension at 72 °C. The amplified PCR products were separated in a 1 % agarose gel and stained with ethidium bromide. Positive PCR products were extracted from agarose gels using Wizard® SV Gel and PCR Clean-Up System (Promega, Madison, Wisconsin) and cloned into pGEM®-T Easy Vector System (Promega, Madison, Wisconsin) following manufacturer’s instructions. Positive recombinant plasmids that contained the full-length cDNAs were fully sequenced using an ABI 3730XL machine by Macrogen (Seoul, Korea).
For transgenic plant generation, the full length cDNAs of SlNAC3 were introduced into the binary plasmid pCABIMA1302 by replacing the GFP gene with the crossponding SlNAC3 gene at the NcoI and BstEII sites. The introduced Solyc07g063410 and Solyc12g013620 cDNAs were under the control of a CaMV 35S promoter. The binary plasmids harboring Solyc07g063410 and Solyc12g013620 cDNAs were used for Agrobacterium tumefaciens-mediated transformation of the Moneymaker cultivar at the Ralph M. Parsons Foundation Plant Transformation Facility at UC Davis (http://ucdptf.ucdavis.edu/). Transgenic seeds from T1 plants were selected on MS medium containing 50 mg/l kanamycin and lines showing 3:1 segregation for the antibiotic resistance were selected to get the T2 progeny plants. T2 plants seeds were further analyzed for transgene existence using PCR, segregation for antibiotic resistance plants and gene expression levels using RT-qPCR analysis. In addition, transgene copy number was estimated using RT-qPCR using neomycin phosphoril-transferase II (nptII) gene specific primers and the SlActin (Solyc03g078400) gene as internal control as described previously (Shepherd et al. 2009). Two T2 homozygous lines having single insertion event (reflected in nptII to SlActin ratios close to 1 as calculated in (Shepherd et al. 2009)) and showing high levels of transgene expression were selected and used for the stress experiments described above.
Results and discussion
Identification of stress-related NAC genes in tomato
A homology search was performed by using all previously identified NAC-protein members from Arabidopsis (126 AtNACs), rice (145 OsNACs) and poplar (163 PtNACs). The search resulted in the identification of 84 NAC-domain proteins in the tomato genome. All retrieved sequences were checked by thorough filtering and removal of redundant sequences manually. In addition, all unique protein sequences showing high similarity to NAC family members were subjected to InterProScan search using a cut-off e-value of 1e−10 to verify the presence of the NAM domain and to validate their identity (Quevillon et al. 2005). This step validated the existence of the NAC domain in the 84 NAC genes identified in tomato using the described approach. Detailed information on NAC gene family members in tomato is listed in Supplementary Table 1.
In this study, the followed methodology resulted in the identification of 84NAC gene family members in the tomato genome. Recently, 74 putative NAC genes were identified in tomato genome using a different approach based on BLAST search using 10 previously reported NACs sequences (Kou et al. 2014). In another study, 104 NAC genes were identified in the tomato genome using a similar approach described in this study with some differences (Su et al. 2014). The discrepancy in numbers of tomato NAC proteins could be attributed to databases sources and the e-value cutoff where Su et al. (2014) used the NCBI tomato protein database with a BLAST cutoff value of 1e−03 while the BLAST search in this study was against the SGN protein and cDNA sequences database using an e-value cutoff of 1e−10. In addition, all protein sequences identified by BLAST were considered in their study while the best 15 hits were selected here. In potato plant, Singh et al. (2013) used a Hidden Markov Model (HMM) search with a cut-off e-value of 1.0 using the NAM domain (PF02365) to retrieve 110 potato NAC genes. However, 17 potato NAC proteins were found to lack conserved NAC sub-domain regions and were described as NAC-like proteins (Singh et al. 2013). In rice, Nuruzzaman et al. (2010) used the NAM/NAC keywords and the Pfam (PF02365) domain amino acid sequence blast search against the NCBI and different rice databases. In poplar, Hu et al. (2010) also used the keyword search of Pfam NAC domain (PF02365) at the Phytozome database and the NAM keyword search against the NCBI nucleotide database. In Arabidopsis, Ooka et al. (2003) performed a sequence retrieval approach using the NAC InterPro domain. Therefore, the adoption of different approaches will result in variable numbers of identified NAC genes in different organisms including tomato.
Phylogenetic analysis of NAC gene family members in tomato
The phylogenetic relationships and the functional relatedness of the identified tomato NAC proteins were analyzed using two different approaches. In the first approach, combined phylogenetic trees of tomato NAC proteins were aligned with reference homologous NACs with known functions from other plant species as described previously (Hu et al. 2010; Nuruzzaman et al. 2010; Ooka et al. 2003; Fang et al. 2008; Yang et al. 2011; Rushton et al. 2008). The phylogenetic analysis was based on either full-length protein sequence or the NAC domain amino acid sequence. Using this approach, the phylogenetic trees resulted in the distribution of 55 tomato NAC proteins into 12 phylogenetic subfamilies (data not shown). This result was in general agreement with Kou et al. (2014) and Su et al. (2014) where the identified tomato NAC proteins were divided into 12 subfamilies. In the second approach, combined phylogenetic trees were constructed based on either full-length protein sequence or the NAC domain amino acid sequence aligned separately with the entire NAC family members of four different model plant species (Arabidopsis, rice, and poplar). The results of this analysis matched very well with the previous phylogenetic trees obtained using homologous NACs with some minor exceptions (data not shown). Moreover, additional tomato NAC proteins that were not clustered in phylogenetic trees after the first approach became affiliated to defined groups.
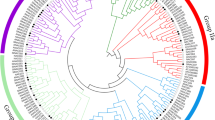
Based on the phylogenetic results produced using the two described approaches, a new phylogenetic tree was constructed using the full length amino acid sequences of tomato NAC proteins with newly selected reference homologous NACs from different plant species (Fig. 1). This approach resulted in the distribution of 83 tomato NAC proteins into 19 subfamilies with one tomato NAC (Solyc06g068580) protein remaining unlinked to any subfamily. The phylogenetic analysis of 136 potato NAC proteins with NAC proteins from Arabidopsis and rice identified 18 subfamilies (Singh et al. 2013), which is consistent with the results of the phylogenetic analysis in this study.
The distribution of the tomato NAC phylogenetic subfamilies was in general agreement with previous studies (Hu et al. 2010; Nuruzzaman et al. 2010; Ooka et al. 2003; Fang et al. 2008; Yang et al. 2011; Rushton et al. 2008). For instance, 11 subfamilies were related to Arabidopsis NAC phylogeny analysis that included the ANAC011, ATNAC3, NAC1, NAC2, NAP, ONAC022, OsNAC7, OsNAC8, SENU5, TERN and TIP (Ooka et al. 2003). Three subfamilies were named based on the rice NAC proteins phylogeny analysis that included CUC, ONAC4 and ONAC6 (Nuruzzaman et al. 2010; Fang et al. 2008). Two subfamilies were named based on soybean phylogeny that included ATAF1 and NAM-B1 (Nuruzzaman et al. 2010). One subfamily, NAC-K like, was named based on poplar NAC phylogeny analysis (Hu et al. 2010). Noticeably, a unique phylogenetic subfamily, called Solanaceae-specific, was identified that included 10 tomato NACs. The Solanaceae-specific NACs from tomato were clustered with the previously identified tobacco TNAC134 gene (Fig. 1), which is a member of the previously described Solanaceae-specific NAC genes (Rushton et al. 2008). The Solanaceae specific NAC subfamily appears to be restricted to the Solanaceae family as their members were absent in Arabidopsis, rice, soybean and poplar genomes but present in tomato, pepper, and potato genomes (Singh et al. 2013;, Rushton et al. 2008). Interestingly, the Solanaceae-specific NACs from potato included 36 members and 50 members in tobacco compared to 26 members in Su et al. (2014) tomato NAC study and 10 members in the current study. Therefore, the major difference in tomato NAC genes number between the Su et al. (2014) analysis and the current study could be attributed to the Solanaceae-specific subfamily members.
Based on the phylogenetic analysis, the functional relatedness of several tomato NACs with reference homologous NACs from other plant species was revealed allowing fifteen subfamilies to be functionally predicted (Fig. 1). Based on the predicted functions of the reference homologous NACs from other plant species, five out of the nineteen subfamilies were predicted to include 12 stress-related tomato NACs. The five stress-related subfamilies included four subfamilies with previously characterized members from other plant species (ATAF1, ATNAC3, NAMB1 and NAP) while the remaining subfamily contains three putatively stress-related NACs that were specific to tomato (Fig. 1).. Further analysis of the tomato stress NAC subfamilies revealed that three NACs were grouped with the ATAF1 gene, which is induced in Arabidopsis by drought, high-salinity, mechanical wounding and Botrytis cinerea infection (Lu et al. 2007; Wu et al. 2009). This subfamily contains two NAC genes (Solyc04g009440 (also known as SlNAC1) and Solyc06g060230 (also known as SlNAM1)) that were found to be induced by high salt stress (Yang et al. 2011). In another study, the Solyc04g009440 (SlNAC1) gene was found to be induced by the Tomato Leaf Curl Virus and it was implicated in viral DNA replication (Selth et al. 2005). Moreover, Solyc04g009440 (SlNAC1) was speculated to have important roles in mediating cross-talk between different stresses and hormonal signaling (Ma et al. 2013). The second stress NAC subfamily, ATNAC3, included two NACs that showed similarity to ANAC055 and ANAC072 (also known as RD26 for Responsive to Desiccation 26) genes, which are induced by salt and drought stresses and confer drought tolerance in Arabidopsis (Tran et al. 2004) and tomato (Su et al. 2014). The third subfamily, NAM-B1 contain two NACs that clustered with the NAM-B1 gene, which is known for its role in regulating grain protein content and in accelerating senescence in wheat (Uauy et al. 2006). The NAM-B1 subfamily contains a previously characterized NAC gene, Solyc07g063420 that was found to be repressed by salt, drought stress and ABA treatments (Han et al. 2012). The NAP subfamily included two NACs that showed similarity to the AtNAP gene, which is known to regulate cell division and cell expansion in flower organs (Sablowski and Meyerowitz 1998). Moreover, AtNAP gene expression was associated with leaf senescence and programed cell death responses (Guo and Gan 2006). This subfamily contains one NAC gene, Solyc05g007770 (also known as SlNAC2), that was previously studied and found to be induced by the virulence factor Conoratine produced by Pseudomonas syringae (Uppalapati et al. 2008). The 12 stress-related NAC genes identified in this study were selected and subjected to further analysis in order to clarify their roles in abiotic stress tolerance in tomato.
Promoter analysis and motifs identification
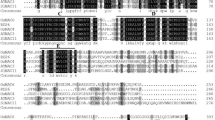
To support the phylogenetic findings and functional predictions of the stress-related NAC genes in tomato, a promoter and motif analysis were performed. For motif analysis, the 12 stress-related NAC genes were investigated for common motifs by performing MSA for each subfamily with their reference proteins from the phylogenetic analysis (Supplementary Figure 1). In all members, the NAC domain was located at the N-terminal region and was tightly conserved among all members with its defined sub-domains (A-E) structure, which is consistent with previous reports (Puranik et al. 2012; Ooka et al. 2003). The MSA results with reference NACs showed conserved motifs in C-terminal region, also known as the transcription activation region, which may indicate biological relevance (Supplementary Figure 1). The SlNAC3 proteins were the richest in number of identified motifs in C-terminal region that were previously characterized in ATNAC3 members of Arabidopsis and rice orthologous (Ooka et al. 2003).
For promoter analysis, cis-regulatory elements were identified in DNA sequences upstream of their putative start codons in the 12 stress-related NAC genes using different promoter analytical tools. Such analysis was carried out to identify cis-acting regulatory elements (CARE) in the upstream DNA sequences that are involved in regulation of gene expression under stress conditions. For this purpose, genomic DNA sequences, located 2 kb upstream of putative start codons were retrieved using the genome Browser tool at SOL Genomics Network (SGN; http://solgenomics.wur.nl/). Thereafter, transcription start sites (TSS) and key regulatory elements in the proximal or core promoter region including TATA box and CAAT box were identified (Supplementary Table 2) using the TSSP predication tool (www.Softberry.com, RegSite Plant DB, Softberry Inc. (Shahmuradov et al. 2003)).
To identify key regulatory elements or CARE involved in stress inducible expression, the distal promoter regions of the 12 stress-related NAC genes were subjected to a comprehensive promoter analysis using two CARE databases: Plant cis-acting regulatory elements [PLACE: http://www.dna.affrc.go.jp/PLACE/; (Higo et al. 1999)] and Plant cis-Acting regulatory elements [PLANTCARE: http://bioinformatics.psb.ugent.be/webtools/plantcare/html/; (Lescot et al. 2002)]. Using this approach, different abiotic stress-related CAREs like MYB, DRE/C-repeat, ABA-responsive and LTR (Low-Temperature Responsive element) were identified in several stress-related tomato NAC promoter regions (Table 2). The data might indicate a major role for the identified stress-related NAC genes in regulating their gene expression in response to different stresses in tomato.
Expression patterns of tomato stress-related NAC genes
The expression patterns of tomato stress-related NAC genes in response to drought and salinity treatments were analyzed using quantitative real-time PCR (RT-qPCR). In addition, the gene expression patterns of the stress-related NAC genes was compared with the patterns of the stress-inducible gene, Solyc10g075090, a phospholipid transfer protein from tomato also known as Le16 [Lycopersicon esculentum protein 16; (Plant et al. 1991)]. The Le16 gene is known to be highly induced by drought stress and to a lesser extent by high salt stress. The RT-qPCR was performed on all identified 12 stress-related NAC genes and Le16 in response to drought and salinity treatments. No induction in response to drought and salinity treatment was observed for the NAM-B1 subfamily members, Solyc07g063420 and Solyc10g006880, and for the three tomato NAC genes belonging to the stress-related putative subfamily (Fig. 2a, b). The observed pattern of Solyc07g063420 was inconsistence with previous results where gene expression repression was observed after salt, drought stress and ABA treatments (Han et al. 2012). On the contrary, the Solyc04g009440 (SlNAC1), Solyc06g060230 and Solyc11g017470, all belong to ATAF1 subfamily showed induction in response to salinity and drought treatments (Fig. 2a, b). As shown in Fig. 2a, b, the induction patterns of NAC genes belonging to the ATAF1 subfamily varied in response to stress treatments where Solyc11g017470 had the highest expression levels after drought and salinity stress followed by Solyc04g009440 (SlNAC1). This was in general agreement with previous results where Solyc04g009440 (SlNAC1) showed strong induction in response to different abiotic stresses that included drought and salinity (Yang et al. 2011; Ma et al. 2013; Su et al. 2014). Similarly, the NAP subfamily members, Solyc04g005610 and Solyc05g007770 (SlNAC2), and the ATNAC3 subfamily, Solyc07g063410 and Solyc12g013620, were found to be responsive to the drought and salinity treatments (Fig. 2a, b). This is in general agreement with the induction patterns of both SlNAC3 genes in response to PEG and NaCl treatments observed by Su et al. (2014).
Gene expression analysis of tomato stress-related NAC genes in response to different stress conditions. a Expression levels of 12 stress-related NAC genes in leaves of tomato plants subjected to water withholding (WH) conditions for 3, 5 and 7 days compared to well-watered plants (control). b Expression levels of 12 stress-related NAC genes in leaves of tomato plants subjected to 300 mM NaCl for 1, 3, 5 and 10 h compared to non-stressed plants (control). The stress responsive Le16 (Solyc10g075090) gene was included as a control. The bars are standard deviations (SD) of three technical repeats
Recently, two ATNAC3-related genes in potato (PGSC0003DMG400019294 (StNAC072) and PGSC0003DMG400015342 (StNAC101)), was found to have high induction levels in response to different abiotic stresses including salt and mannitol treatments (Singh et al. 2013), which indicate a conserved role of the ATNAC3 subfamily in stress tolerance in Solanaceae plants.
Overexpression of ATNAC3 subfamily members improves drought and salt tolerance in tomato
To validate the bioinformatics and gene expression results in predicting the stress-related NAC genes in tomato, members of the uncharacterized SlNAC3 subfamily, Solyc07g063410 and Solyc12g013620, were selected to validate their role in mediating tolerance against drought stress. For this purpose, transgenic plants overexpressing Solyc07g063410 and Solyc12g013620 full length CDS were generated. Positive transgenic lines overexpressing the targeted genes were confirmed for the transgene copy number and gene expression levels by RT-qPCR analysis (Fig. 3). Positive transgenic lines with single transgene copy and overexpressing Solyc07g063410 and Solyc12g013620 showed growth retardation phenotypes where transgenic plants were shorter, with smaller leaf area and shorter internodes when compared with the wild type plants (Fig. 4a). These pleiotropic phenotypes were more pronounced in transgenic lines overexpressing Solyc12g013620 when compared with lines overexpressing Solyc07g063410. Such variation in growth retardation phenotypes was also observed in transgenic Arabidopsis plants overexpressing three ATNAC3-related genes (ANAC019, ANAC055 and ANAC072) (Tran et al. 2004).
Analysis of transgenic lines overexpressing tomato SlNAC3 subfamily members under drought conditions. a Performance of 2-week-old seedlings of transgenic lines overexpressing Solyc07g063410 (SlNAC056) and Solyc12g013620 (SlNAC081) and wild type under normal conditions. b Performance of transgenic lines overexpressing Solyc07g063410 (SlNAC056) and Solyc12g013620 (SlNAC081) and wild type after 21 days of water withholding. c Germination of transgenic lines overexpressing Solyc07g063410 (SlNAC056) and Solyc12g013620 (SlNAC081) and wild type after 14 days of sowing on 0.5 % NaCl
To analyze stress tolerance in the transgenic tomato lines, 2-week-old seedlings growing in sand culture were subjected to drought conditions by withholding water for 21 days and salt stress by germinating seeds directly on 0.5 % NaCl. Wild type plants were included as controls and their performance under the same conditions were compared with that of the transgenic lines. Under drought conditions, nearly all non-transgenic plants had a wilted appearance indicating that they were suffering from water deficit (Fig. 4b). However, transgenic tomato plants overexpressing Solyc07g063410 and Solyc12g013620 genes showed delayed wilting and enhanced tolerance to drought conditions when compared with non-transgenic plants (Fig. 4b). Furthermore, transgenic tomato plants overexpressing Solyc07g063410 and Solyc12g013620 genes had higher germination percentages (after 14 days of sowing) on 0.5 % NaCl solution when compared with wild type plants (Fig. 4c). However, under such conditions, the transgenic plants showed slower growth rates and yellow cotyledons appearance when compared with non-stressed plants. The drought tolerance behavior of SlNAC3 genes was similar to their counterparts in Arabidopsis where the overexpression of ANAC019, ANAC055 and ANAC072 conferred drought tolerance (Tran et al. 2004). Recently, the overexpression Solyc04g009440 (SlNAC1) in tomato plants enhanced chilling tolerance through the maintenance of higher oxygen-evolving enzymatic activities that lowered ion leakage and malondialdehyde content in stressed transgenic plants when compared with non-transgenic plants (Ma et al. 2013). These results confirm that the bioinformatics approach used in this study was successful in predicting the functions of stress-related NAC genes in tomato and uncovered their potential role in enhancing tomato growth under stress conditions.
In conclusion, 12 stress related NAC genes were identified in tomato using different bioinformatics and molecular approaches. The phylogenetic analysis clustered them into 5 different subfamilies with their functions predicated from their counterparts in other plants species. The phylogenetic analysis identified a new stress related subfamily specific to tomato with no clear role in stress tolerance till now. Promoter analysis of the stress-related NACs identified key regulatory elements involved in stress inducible expression. Furthermore gene expression analysis of stress-related NACs showed inducible expression patterns to different stresses including drought and salinity of different members. The overexpression of two selected stress-related SlNAC3 genes in tomato resulted in enhanced drought tolerance when compared with non-transgenic plants. Finally, the comprehensive analysis described in this study provides valuable information on the putative functions of the identified stress-related tomato NAC genes, which will help in the future improvement of the tomato crop as an important commodity in the horticultural industry.
References
Agarwal P, Kapoor S, Tyagi AK (2011) Transcription factors regulating the progression of monocot and dicot seed development. BioEssays 33:189–202
Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M (1997) Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell 9:841–857
Al Abdallat AM, Ayad JY, Abu Elenein JM, Al Ajlouni Z, Harwood WA (2014) Overexpression of the transcription factor HvSNAC1 improves drought tolerance in barley (Hordeum vulgare L.). Mol Breed 33:401–414
Charoensawan V, Wilson D, Teichmann SA (2010) Genomic repertoires of DNA-binding transcription factors across the tree of life. Nucleic Acids Res 38:7364–7377
Fang Y, You J, Xie K, Xie W, Xiong L (2008) Systematic sequence analysis and identification of tissue specific or stress-responsive genes of NAC transcription factor family in rice. Mol Genet Geno 280:547–563
Grant EH, Fujino T, Beers EP, Brunner AM (2010) Characterization of NAC domain transcription factors implicated in control of vascular cell differentiation in Arabidopsis and populus. Planta 232:337–352
Guo Y, Gan S (2006) AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J 46:601–6012
Han Q, Zhang J, Li H, Luo Z, Ziaf K, Ouyang B, Wang T, Zhibiao Y (2012) Identification and expression pattern of one stress-responsive NAC gene from Solanum lycopersicum. Mol Biol Rep 39:1713–1720
Hao YJ, Wei W, Song QX, Chen HW, Zhang YQ, Wang F, Zou HF, Lei G, Tian AG, Zhang WK, Ma B, Zhang JS, Chen SY (2011) Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. Plant J 68:303–313
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27:297–300
Hu H, Dai M, Jialing Y, Xiao B, Li X, Zhang Q, Xiong L (2006) Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci 103:12987–12992
Hu R, Guang Q, Kong Y, Kong D, Gao Q, Zhou G (2010) Comprehensive analysis of NAC domain transcription factor gene family in Populus trichocarpa. BMC Plant Biol 10:1–23
Jensen MK, Kjaersgaard T, Petersen K, Skriver K (2010) NAC genes: time-specific regulators of hormonal signalling in Arabidopsis. Plant Signal Behav 5:907–910
Kjaersgaard T, Jensen MK, Christiansen MW, Gregersen P, Kragelund BB, Skriver K (2011) Senescence associated barley NAC (NAM, ATAF1,2, CUC) transcription factor interacts with radical-induced cell death 1 through a disordered regulatory domain. J Biol Chem 286:35418–35429
Kou X, Wang S, Wu M, Guo R, Xue Z, Meng N, Tao X, Chen M, Zhang Y (2014) Molecular characterization and expression analysis of NAC family transcription factors in tomato. Plant Mol Biol Rep 32:501–516
Le DT, Nishiyama R, Watanabe Y, Mochida K, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP (2011) Genome-wide survey and expression analysis of the plant-specific NAC transcription factor family in soybean during development and dehydration Stress. DNA Res 18:263–276
Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327
Lu PL, Chen NZ, An R, Su Z, Qi BS, Ren F, Chen J, Wang XC (2007) A novel drought-inducible gene, ATAF1, encodes a NAC family protein that negatively regulates the expression of stress responsive genes in Arabidopsis. Plant Mol Biol 63:289–305
Lu M, Zhang D, Shi Y, Song Y, Wang T, Li Y (2013) Expression of SbSNAC1, a NAC transcription factor from sorghum, confers drought tolerance to transgenic Arabidopsis. Plant Cell Tiss Organ Cult 115:443–455
Ma NN, Zuo YQ, Liang XQ, Yin B, Wang GD, Meng QW (2013) The multiple stress-responsive transcription factor SlNAC1 improves the chilling tolerance of tomato. Physiol Plant 149:474–486
Mao C, Ding W, Wu Y, Yu J, He X, Shou H, Wu P (2007) Overexpression of a NAC-domain protein promotes shoot branching in rice. New Phytol 176:288–298
McGrann GR, Steed A, Burt C, Goddard R, Lachaux C, Bansal A, Corbitt M, Gorniak K, Nicholson P, Brown JK (2014) Contribution of the drought tolerance-related Stress-responsive NAC1 transcription factor to resistance of barley to Ramularia leaf spot. Mol Plant Pathol. doi:10.1111/mpp.12173
Nuruzzaman M, Manimekalai R, Sharoni AM, Satoh K, Kondoh H, Ooka H, Kikuchi S (2010) Genome wide analysis of NAC transcription factor family in rice. Gene 465:30–44
Ohnishi T, Sugahara S, Yamada T, Kikuchi K, Yoshiba Y, Hirano HY, Tsutsumi N (2005) OsNAC6, a member of the NAC gene family, is induced by various stresses in rice. Genes Genet Syst 80:135–139
Olsen AN, Ernst HA, Leggio LL, Skriver K (2005) NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci 10:79–87
Ooka H, Satoh K, Doi K, Nagata T, Otomo Y, Murakami K, Matsubara K, Osato N, Kawai J, Carninci P, Hayashizaki Y, Suzuki K, Kojima K, Takahara Y, Yamamoto K, Kikuchi S (2003) Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res 10:239–247
Peng H, Cheng HY, Yu XW, Shi QH, Zhang H, Li JG, Ma H (2009) Characterization of a chickpea (Cicer arietinum L.) NAC family gene, CarNAC5, which is both developmentally- and stress regulated. Plant Physiol Biochem 47:1037–1045
Pinheiro GL, Marques CS, Costa MDBL, Reis PAB, Alves MS, Carvalho CM, Fietto LG, Fontes EPB (2009) Complete inventory of soybean NAC transcription factors: sequence conservation and expression analysis uncover their distinct roles in stress response. Gene 444:10–23
Plant AL, Cohen A, Moses MS, Bray EA (1991) Nucleotide sequence and spatial expression pattern of a drought- and abscisic acid-induced gene of tomato. Plant Physiol 3:900–906
Puranik S, Sahu PP, Srivastava PS, Prasad M (2012) NAC proteins: regulation and role in stress tolerance. Trends Plant Sci 17:369–381
Quevillon E, Silventoinen V, Pillai S, Harte N, Mulder N, Apweiler R, Lopez R (2005) InterProScan: protein domains identifier. Nucleic Acids Res 33:W116–W120
Riechmann JL, Ratcliffe OJ (2000) A genomic perspective on plant transcription factors. Curr Opin Plant Biol 3:423–434
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Rushton PJ, Bokowiec MT, Han S, Zhang H, Brannock JF, Chen X, Laudeman TW, Timko MP (2008) Tobacco transcription factors: novel insights into transcriptional regulation in the Solanaceae. Plant Physiol 147:280–295
Sablowski RW, Meyerowitz EM (1998) A homolog of NO APICAL MERISTEM is an immediate target of the floral homeotic genes APETALA3/PISTILLATA. Cell 92:93–103
Selth LA, Dogra SC, Rasheed MS, Healy H, Randles JW, Rezaian MA (2005) A NAC domain protein interacts with tomato leaf curl virus replication accessory protein and enhances viral replication. Plant Cell 17:311–325
Shahmuradov IA, Gammerman AJ, Hancock JM, Bramley PM, Solovyev VV (2003) PlantProm: a database of plant promoter sequences. Nucleic Acids Res 31:114–117
Shan W, Kuang JF, Chen L, Xie H, Peng HH, Xiao YY, Li XP, Chen WX, He QG, Chen JY, Lu WJ (2012) Molecular characterization of banana NAC transcription factors and their interactions with ethylene signalling component EIL during fruit ripening. J Exp Bot 63:5171–5187
Shen H, Yin Y, Chen F, Xu Y, Dixon RA (2009) A Bioinformatic analysis of NAC genes for plant cell wall development in relation to lignocellulosic bioenergy production. Bioenerg Res 2:217–232
Shepherd CT, Moran Lauter AN, Scott MP (2009) Determination of transgene copy number by real-time quantitative PCR. Methods Mol Biol 526:129–134
Singh J, Sastry EV, Singh V (2012) Effect of salinity on tomato (Lycopersicon esculentum Mill.) during seed germination stage. Physiol Mol Biol Plants 18(1):45–50
Singh AK, Sharma V, Pal AK, Acharya V, Ahuja PS (2013) Genome-wide organization and expression profiling of the NAC transcription factor family in potato (Solanum tuberosum L.). DNA Res 20:1–21
Su H, Zhang S, Yin Y, Zhu D, Han L (2014) Genome-wide analysis of NAM-ATAF1,2-CUC2 transcription factor family in Solanum lycopersicum. J Plant Biochem Biotechnol. doi:10.1007/s13562-014-0255-9
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thompson JD, Higgins DG, Gibson TJ (1997) ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tran LSP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2004) Isolation and functional analysis of Arabidopsis stress inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 16:2481–2498
Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J (2006) A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314:1298–1301
Uppalapati SR, Ishiga Y, Wangdi T, Urbanczyk-Wochniak E, Ishiga T, Mysore KS, Bender CL (2008) Pathogenicity of Pseudomonas syringae pv. Tomato on tomato seedlings: phenotypic and gene expression analyses of the virulence function of Coronatine. Mol Plant Microbe Interact 21:383–395
Vandesompele J, Katleen DP, Pattyn F, Bruce P, Van Roy N, Paepe AD, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–12
Wu Y, Deng Z, Lai J, Zhang Y, Yang C, Yin B, Zhao Q, Zhang L, Li Y, Yang C, Xie Q (2009) Dual function of Arabidopsis ATAF1 in abiotic and biotic stress responses. Cell Res 19(11):1279–1290
Yang R, Deng C, Ouyang B, Ye Z (2011) Molecular analysis of two salt-responsive NAC family genes and their expression analysis in tomato. Mol Biol Rep 38:857–863
Zheng X, Chen B, Lu G, Han B (2009) Overexpression of a NAC transcription factor enhances rice drought and salt tolerance, Biochemical and Biophysical. Biochem Biophys Res Commun 379:985–989
Acknowledgments
We gratefully acknowledge Miss Shireen Qasrawi, Mrs. Samar Misbeh and Mrs. Tamara Qudah for their technical assistance. We thank Dr. Wendy Harwood for critically reading the manuscript, her helpful comments and encouraging remarks. This work was supported in part by a grant from the Deanship of Scientific Research, University of Jordan and in part by a grant from the Ford Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. M. Al-Abdallat and M. A. Ali-Sheikh-Omar contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Al-Abdallat, A.M., Ali-Sheikh-Omar, M.A. & Alnemer, L.M. Overexpression of two ATNAC3-related genes improves drought and salt tolerance in tomato (Solanum lycopersicum L.). Plant Cell Tiss Organ Cult 120, 989–1001 (2015). https://doi.org/10.1007/s11240-014-0652-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0652-8